HTLV-1 Tax Stimulates Molecular Events in Various Cancers
Article Information
Wanyi Zhu1, Igor F Tsigelny2, 3, Valentina L Kouznetsova2*
1MAP Program, San Diego Supercomputer Center, UC San Diego, San Diego, CA, USA
2San Diego Supercomputer Center, UC San Diego, La Jolla, CA, 92093, USA
3Department of Neurosciences, UC San Diego, San Diego, CA, USA
*Corresponding Author: Valentina L Kouznetsova, San Diego Supercomputer Center, UC San Diego, La Jolla, CA, 92093, USA
Received: 16 December 2020; Accepted: 26 December 2020; Published: 04 January 2021
Citation: Wanyi Zhu, Igor F Tsigelny, Valentina L Kouznetsova. HTLV-1 Tax Stimulates Molecular Events in Various Cancers. Fortune Journal of Health Sciences 4 (2021): 160-190.
View / Download Pdf Share at FacebookAbstract
The human T-lymphotropic viruses (HTLV) are a family of retroviruses that causes adult T-cell leukemia/lymphoma (ATL). The objective of this study is to elucidate the host genes affected by the HTLV-1 Tax protein, find how host genes are affected, and how this influence is related to several cancer pathways. The DAVID program was used to examine genes affected by Tax, and the gene list was significantly enriched: pancreatic cancer, chronic and acute myeloid leukemia, small cell lung cancer, prostate cancer, non-small cell lung cancer, colorectal cancer, glioma, melanoma, and bladder cancer. The influence of Tax on genes involved in these pathways was studied and the effects on the progression of the pathway were deduced. HTLV-1’s Tax protein contributes epigenetically to the development of different cancers by altering normal transcription and translation, cell cycle signaling systems, and tumor suppressing mechanisms. The discovered effects of Tax on the NF-κB, MAPK, Cyclin-CDK, ErbB, Jak-STAT, VEGF, TGF-β, PI3K-Akt, and β-catenin pathways active during the progression of pancreatic cancer, chronic myeloid leukemia, small cell lung cancer, and colorectal cancer indicates that HTLV-1’s effects are not limited to ATL, can activate cancer-specific biomarkers including ZEB-1, ZEB-2, EZH2, E2F, TRIM33, GLUT1, HK2, PKM2, and LDHA, and can mimic the cancer-specific oncogenes BCR-ABL, K-Ras, and APC. Four signaling networks were elucidated to represent these signaling events, creating a useful representation of an HTLV-1 infection’s role in different cancers, which can aid in identifying and characterizing HTLV-1-associated cancers, determining prognosis, and selecting effective treatments for patients.
Keywords
Human T-lymphotropic virus type 1 (HTLV-1); Molecular mechanisms; Signaling network; Pancreatic cancer; Chronic myeloid leukemia (CML); Small cell lung cancer (SCLC); Colorectal cancer (CRC); Tax; Carcinogenesis
Human T-lymphotropic virus type 1 (HTLV-1) articles; Molecular mechanisms articles; Signaling network articles; Pancreatic cancer articles; Chronic myeloid leukemia (CML) articles; Small cell lung cancer (SCLC) articles; Colorectal cancer (CRC) articles; Tax articles; Carcinogenesis articles, Human T-lymphotropic virus type 1 (HTLV-1) articles Human T-lymphotropic virus type 1 (HTLV-1) Research articles Human T-lymphotropic virus type 1 (HTLV-1) review articles Human T-lymphotropic virus type 1 (HTLV-1) PubMed articles Human T-lymphotropic virus type 1 (HTLV-1) PubMed Central articles Human T-lymphotropic virus type 1 (HTLV-1) 2023 articles Human T-lymphotropic virus type 1 (HTLV-1) 2024 articles Human T-lymphotropic virus type 1 (HTLV-1) Scopus articles Human T-lymphotropic virus type 1 (HTLV-1) impact factor journals Human T-lymphotropic virus type 1 (HTLV-1) Scopus journals Human T-lymphotropic virus type 1 (HTLV-1) PubMed journals Human T-lymphotropic virus type 1 (HTLV-1) medical journals Human T-lymphotropic virus type 1 (HTLV-1) free journals Human T-lymphotropic virus type 1 (HTLV-1) best journals Human T-lymphotropic virus type 1 (HTLV-1) top journals Human T-lymphotropic virus type 1 (HTLV-1) free medical journals Human T-lymphotropic virus type 1 (HTLV-1) famous journals Human T-lymphotropic virus type 1 (HTLV-1) Google Scholar indexed journals Molecular mechanisms articles Molecular mechanisms Research articles Molecular mechanisms review articles Molecular mechanisms PubMed articles Molecular mechanisms PubMed Central articles Molecular mechanisms 2023 articles Molecular mechanisms 2024 articles Molecular mechanisms Scopus articles Molecular mechanisms impact factor journals Molecular mechanisms Scopus journals Molecular mechanisms PubMed journals Molecular mechanisms medical journals Molecular mechanisms free journals Molecular mechanisms best journals Molecular mechanisms top journals Molecular mechanisms free medical journals Molecular mechanisms famous journals Molecular mechanisms Google Scholar indexed journals Signaling network articles Signaling network Research articles Signaling network review articles Signaling network PubMed articles Signaling network PubMed Central articles Signaling network 2023 articles Signaling network 2024 articles Signaling network Scopus articles Signaling network impact factor journals Signaling network Scopus journals Signaling network PubMed journals Signaling network medical journals Signaling network free journals Signaling network best journals Signaling network top journals Signaling network free medical journals Signaling network famous journals Signaling network Google Scholar indexed journals Pancreatic cancer articles Pancreatic cancer Research articles Pancreatic cancer review articles Pancreatic cancer PubMed articles Pancreatic cancer PubMed Central articles Pancreatic cancer 2023 articles Pancreatic cancer 2024 articles Pancreatic cancer Scopus articles Pancreatic cancer impact factor journals Pancreatic cancer Scopus journals Pancreatic cancer PubMed journals Pancreatic cancer medical journals Pancreatic cancer free journals Pancreatic cancer best journals Pancreatic cancer top journals Pancreatic cancer free medical journals Pancreatic cancer famous journals Pancreatic cancer Google Scholar indexed journals Chronic myeloid leukemia (CML) articles Chronic myeloid leukemia (CML) Research articles Chronic myeloid leukemia (CML) review articles Chronic myeloid leukemia (CML) PubMed articles Chronic myeloid leukemia (CML) PubMed Central articles Chronic myeloid leukemia (CML) 2023 articles Chronic myeloid leukemia (CML) 2024 articles Chronic myeloid leukemia (CML) Scopus articles Chronic myeloid leukemia (CML) impact factor journals Chronic myeloid leukemia (CML) Scopus journals Chronic myeloid leukemia (CML) PubMed journals Chronic myeloid leukemia (CML) medical journals Chronic myeloid leukemia (CML) free journals Chronic myeloid leukemia (CML) best journals Chronic myeloid leukemia (CML) top journals Chronic myeloid leukemia (CML) free medical journals Chronic myeloid leukemia (CML) famous journals Chronic myeloid leukemia (CML) Google Scholar indexed journals Small cell lung cancer (SCLC) articles Small cell lung cancer (SCLC) Research articles Small cell lung cancer (SCLC) review articles Small cell lung cancer (SCLC) PubMed articles Small cell lung cancer (SCLC) PubMed Central articles Small cell lung cancer (SCLC) 2023 articles Small cell lung cancer (SCLC) 2024 articles Small cell lung cancer (SCLC) Scopus articles Small cell lung cancer (SCLC) impact factor journals Small cell lung cancer (SCLC) Scopus journals Small cell lung cancer (SCLC) PubMed journals Small cell lung cancer (SCLC) medical journals Small cell lung cancer (SCLC) free journals Small cell lung cancer (SCLC) best journals Small cell lung cancer (SCLC) top journals Small cell lung cancer (SCLC) free medical journals Small cell lung cancer (SCLC) famous journals Small cell lung cancer (SCLC) Google Scholar indexed journals Colorectal cancer (CRC) articles Colorectal cancer (CRC) Research articles Colorectal cancer (CRC) review articles Colorectal cancer (CRC) PubMed articles Colorectal cancer (CRC) PubMed Central articles Colorectal cancer (CRC) 2023 articles Colorectal cancer (CRC) 2024 articles Colorectal cancer (CRC) Scopus articles Colorectal cancer (CRC) impact factor journals Colorectal cancer (CRC) Scopus journals Colorectal cancer (CRC) PubMed journals Colorectal cancer (CRC) medical journals Colorectal cancer (CRC) free journals Colorectal cancer (CRC) best journals Colorectal cancer (CRC) top journals Colorectal cancer (CRC) free medical journals Colorectal cancer (CRC) famous journals Colorectal cancer (CRC) Google Scholar indexed journals Tax articles Tax Research articles Tax review articles Tax PubMed articles Tax PubMed Central articles Tax 2023 articles Tax 2024 articles Tax Scopus articles Tax impact factor journals Tax Scopus journals Tax PubMed journals Tax medical journals Tax free journals Tax best journals Tax top journals Tax free medical journals Tax famous journals Tax Google Scholar indexed journals Carcinogenesis articles Carcinogenesis Research articles Carcinogenesis review articles Carcinogenesis PubMed articles Carcinogenesis PubMed Central articles Carcinogenesis 2023 articles Carcinogenesis 2024 articles Carcinogenesis Scopus articles Carcinogenesis impact factor journals Carcinogenesis Scopus journals Carcinogenesis PubMed journals Carcinogenesis medical journals Carcinogenesis free journals Carcinogenesis best journals Carcinogenesis top journals Carcinogenesis free medical journals Carcinogenesis famous journals Carcinogenesis Google Scholar indexed journals
Article Details
Introduction
The involvement of HTLV-1 in different types of cancer has been implicated, with the main protein involved in cancerogenesis of host cells being Tax. However, more information is needed on the specific genes affected by Tax in cancer and their subsequent interactions with each other to promote cancer progression. Thus, this study aims to elucidate interactions between viral and host proteins during an HTLV-1 infection and determine both specific properties and the comprehensive effect of HTLV-1 in different cancers.
Human T-lymphotropic Virus
The HTLVs (human T-lymphotropic viruses) are retroviruses that cause adult T-cell leukemia/lymphoma (ATL) [1]. There are four types of HTLV that have been identified (HTLV-1, HTLV-2, HTLV-3, HTLV-4) [1]. Viral proteins encoded in the HTLV-1 genome play a role in the proliferation and survival of the infected cells [2]. Its genome includes the Gag, Pol, and Env genes, which encode structural proteins that aid in replication. Rex is the regulator of viral mRNA splicing. Tax controls the expression of viral and cellular genes through several pathways: CREB/ATF, NF-κB, AP-1 and SRF [3]. It is considered to play a central role in the process leading to ATL, and modulates cellular processes by binding with proteins, transcriptional activation and repression, and dysregulating cellular processes, including the cell cycle and the maintenance of genomic integrity, which promotes cell proliferation and resistance to apoptosis [4].
HTLV’s Presence in Different Cancers
HTLV-1 has been found to be involved in different types of cancer. Some of the cancers are: pancreatic cancer, chronic myeloid leukemia, bladder cancer, biliary tract cancer, esophageal cancer, gastric cancer, colorectal cancer, liver cancer, lung cancers, and glioblastoma [5-7,9]. HTLV-1 proviral DNA has been associated with inflammation of these organs and has been found in their cancer cells, but the mechanisms of inflammation and carcinogenesis as well as interactions among viral and cellular proteins in the pathways leading to different cancers are largely unknown.
HTLV-1 and Pancreatic Cancer
The majority of pancreatic cancer cases are exocrine, occurring in cells lining the pancreatic duct that either form glands or surround an empty space, and a small percentage of cases are endocrine, which occur in the hormone-producing islet of Langerhans cells [8]. HTLV-1’s presence in pancreatic cancer has been found before [9], and cases have been reported of ATL patients experiencing acute pancreatitis, but the reason is not certain [10]. Patients with acute pancreatitis are twice as likely to develop pancreatic cancer [11]. BRG1 is an essential oncoprotein in both the acute myeloid leukemia cell cycle and pancreatic ductal adenocarcinoma tumor initiation [12].
HTLV-1 and Chronic Myeloid Leukemia
Chronic myeloid leukemia (CML) is a cancer of myeloid cells, which produce red blood cells, platelets, and most types of white blood cells [13]. It originates in hematopoietic stem cells (HSCs) and is characterized by the accumulation of apparently normal myeloid cells [14]. HSCs give rise to two cell lines: myeloid cell lines, which include monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes, and platelets; and lymphoid cell lines [15], which include T cells, the cells involved in ATL. Cancer in myeloid cells progresses from the chronic phase to blast crisis, which has a poor prognosis [16]. Blast crisis occurs when blast cells exceed 20% or 30% (based on different definitions) of all the cells in the blood or bone marrow [17], which results in hyperviscous blood and a relative reduction of other cell types [18]. Myeloid cells have been shown to be targets of HTLV-1 and may exhibit impaired function, with HTLV-1 DNA having been detected in hematopoietic stem cells [19], but there is no information on the mechanisms of HTLV-1-induced CML.
HTLV-1 and Small Cell Lung Cancer
A small percentage of lung cancers (10-15%) are small cell lung cancers (SCLC), which grow and spread more quickly than non-small cell lung cancers and are more prone to relapse [20]. A SCLC patient’s cells were found to have serum with high levels of soluble interleukin-2 receptors (IL2-R) [21]. IL2-R is found on activated T-cells' that are stimulated to proliferate by IL2, and even three IL2-R-positive myeloid cell lines have been identified [22]. It is possible that HTLV-1 is the cause of IL2-R’s presence on SCLC cell surfaces [22], and Tax has been found to induce the expression of IL2-R on T-cells [23]. Interleukin-2 is an immune cytokine signaling molecule, and IL2-R is a biomarker for inflammatory diseases, including pathogen-caused inflammatory liver diseases [24], one of which is hepatitis B, listed in Table 2. HTLV-I is involved in HTLV-I associated T-cell bronchioloalveolitis [25], and pulmonary lymphocytosis has been found to occur in HTLV-I carriers [26], and no explanation exists to explain why HTLV-1 is associated with pulmonary inflammation.
HTLV-1 and Colorectal Cancer
Cancers of the colon and rectum are the fourth leading cause of cancer-related deaths worldwide [27], with about 65% of colorectal cancer (CLC) cases being unrelated to family history or genetic predisposition [28]. Mutation of adenomatous polyposis coli (APC), a tumor suppressor mutated in 80% of CLC cases [29], is the first step in transforming normal colorectal epithelium to adenoma [30], which is a benign epithelial glandular tumor. Three major conditions can develop during the transition between adenoma and carcinoma: chromosomal instability (CIN), where several structural or numerical chromosomal changes happen; microsatellite instability (MSI), where DNA mismatch repair functions incorrectly and results in many point mutations, small deletions, and insertions near nucleotide repeat tracts; and CpG island methylator phenotype (CIMP), which is associated with epigenetic instability involving DNA hypomethylation or hypermethylation [31]. These conditions alter gene expression in tumor suppressing pathways, including KRAS, BRAF, TP53, MLH1, MSH2, BAX, and TGFBR [32]. CRC is the result of cumulative genetic and epigenetic changes that transform normal cells into tumors, where certain mutations are highly prevalent in certain stages [31]. For example, Wnt/β-catenin signaling is active in adenomas, and K-Ras activity and the loss of p53 are involved in the transition to invasive and malignant behaviors [31]. HTLV-1-infected lymphocytes can be transferred when infants ingest maternal milk, implying a transmission route through the digestive tract, and a study found that infectious HTLV-1 virions are able to cross the intestinal epithelium through transcytosis and infect underlying dendritic cells [33]. Infection with HTLV-1 and CRC development have been compared before [9], but combination of specific effects of HTLV-1 on the CRC pathway is unknown. Genes affected by Tax protein [34] are presented in Table 1.
Table 1: Genes Affected By Tax Protein
Methods
The methods used are illustrated in Figure 1 diagram. DAVID (Database for Annotation, Visualization and Integrated Discovery) [35] combines functional genomic annotations and allows for the visualization of gene and protein pathways and interactions [36]. Genes from Table 1 were submitted to DAVID with functional annotation to search for KEGG pathways in which the gene list in Table 1 is enriched.
The EASE Score, a modified Fisher Exact test, is calculated by DAVID to determine the degree to which the submitted genes are expressed in the resulting pathways [37].

Table for Fisher’s Exact test (left) [37], where 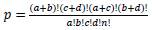 based on values in a 2×2 contingency table, or
based on values in a 2×2 contingency table, or 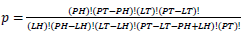 in terms of gene-enrichment. Modified table for EASE Score (right) [37], where the resulting p-value is larger and more conservative, and
in terms of gene-enrichment. Modified table for EASE Score (right) [37], where the resulting p-value is larger and more conservative, and 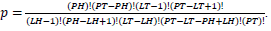 .
.
Population Total (PT) is the total number of genes in a genome, Population Hits (PH) is the number of genes in a selected pathway of the genome, List Total (LT) is the number of genes in a submitted list, and List Hits (LH) is the number of genes from the submitted list that belong in the selected pathway [37].
To be more certain that the results were not due to random error, the Benjamini-Hochberg Procedure was used by DAVID to calculate adjusted p-values based on the EASE Score p-value. The adjusted p-values from the Benjamini–Hochberg Procedure reduce the false discovery rate [38]. To calculate adjusted p-values, the EASE Score p-values are ordered from smallest to largest, given ranks of increasing consecutive natural numbers starting from 1, and EASE Score p-values are compared to 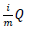 , where i is the rank, m is the number of tests, and Q is the false discovery rate [39]. All p-values lower than the largest p-value that satisfies
, where i is the rank, m is the number of tests, and Q is the false discovery rate [39]. All p-values lower than the largest p-value that satisfies 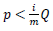 are considered significant.
are considered significant.
Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database that maps the functions of biological systems [40]. DAVID indicated several KEGG pathways, which are depicted in the results along with their EASE Score p-values and Benjamini-Hochberg p-values. Initially submitted genes from Table 1 and their surrounding genes in the pathway were studied to investigate Tax’s effects on different pathway genes, their connections to each other, their effects on the progression of the pathway, and their relationship with specific cancers. Based on the KEGG pathways, information from existing literature was used to deduce properties of HTLV-1-associated cancers by comparing the molecular mechanisms of HTLV-1’s Tax protein in ATL with the carcinogenesis pathways containing genes that respond to Tax in pancreatic cancer, CML, SCLC, and CRC and finding connections between the two. To depict the analysis of KEGG pathways, four novel signaling networks were elucidated, consisting of cancer-specific proteins and their interactions with Tax and each other.
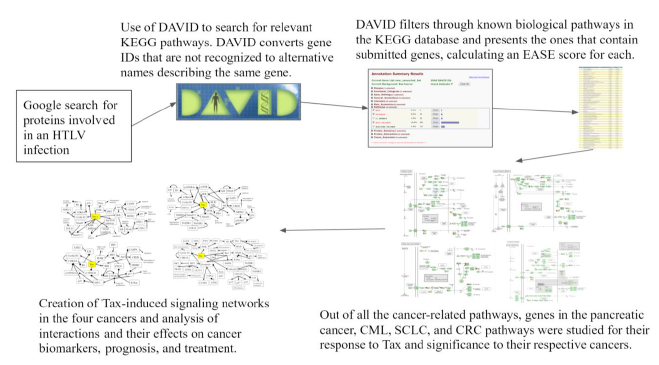
Figure 1: Flowchart of Methods. A flowchart of the tools and methods used in this study
Results and Discussion
Table 2: Signaling Pathways Affected by Tax. Based on the list of genes and proteins from Table 1 entered into DAVID, this table lists several pathways that contain genes and proteins affected by Tax in order of smaller p-values to larger p-values from the EASE Score.
|
Pathway |
P-Value |
Benjamini |
|
4.30E-27 |
3.20E-25 |
|
|
6.20E-18 |
3.10E-16 |
|
|
2.10E-11 |
6.20E-10 |
|
|
2.90E-08 |
6.20E-07 |
|
|
5.50E-08 |
1.00E-06 |
|
|
1.90E-07 |
3.20E-06 |
|
|
2.30E-07 |
3.50E-06 |
|
|
5.70E-07 |
7.70E-06 |
|
|
3.10E-06 |
3.30E-05 |
|
|
4.20E-06 |
4.10E-05 |
|
|
8.50E-06 |
7.90E-05 |
|
|
2.30E-05 |
1.80E-04 |
|
|
3.60E-05 |
2.70E-04 |
|
|
5.40E-05 |
3.80E-04 |
|
|
5.70E-05 |
3.90E-04 |
|
|
4.70E-04 |
2.40E-03 |
|
|
5.10E-04 |
2.60E-03 |
|
|
6.50E-04 |
3.00E-03 |
|
|
8.70E-04 |
3.60E-03 |
|
|
1.30E-03 |
5.20E-03 |
|
|
2.30E-03 |
8.70E-03 |
|
|
3.00E-03 |
1.00E-02 |
|
|
1.20E-02 |
3.30E-02 |
|
|
1.30E-02 |
3.60E-02 |
|
|
1.70E-02 |
4.70E-02 |
|
|
2.20E-02 |
5.80E-02 |
Pathways have EASE Score p-values that are lower than 0.05 and lower than their Benjamini-Hochberg adjusted p-values. The cancer-related pathways are emphasized and significantly associated with the genes that Tax influences, including pancreatic cancer, chronic and acute myeloid leukemia, small cell lung cancer, prostate cancer, non-small cell lung cancer, colorectal cancer, glioma, melanoma, and bladder cancer (Table 2).
Pancreatic Cancer
The PI3K-Akt signaling pathway is one of the most commonly activated signaling pathways in pancreatic cancer [41]. Interference with the PI3K-Akt signaling pathway marks the transition of a normal duct to Pancreatic Intraepithelial Neoplasia 1-A and 1-B (PanIN-1A and PanIN-1B). This is when epithelial lesions, or an abnormal growth of squamous cells, appear on ductal epithelium [42]. In the PI3K-Akt signaling pathway, the PI3K complex is responsible for the activation of Akt through the activation of PIP3 [43]. By activating PI3K, Tax causes Akt to increase the activity of two cell-survival-related proteins: CREB, a transcription factor that causes the transcription of survival genes [43]; and MDM2, which inhibits p53 [43]. Akt phosphorylates CASP9 [44], which is a caspase essential in apoptosis [45], and this phosphorylation downregulates the apoptotic activity of the enzyme [46]. The effect of Akt on these genes induces cell proliferation. Akt also stimulates the NF-κB pathway by phosphorylating IKK α and β, which induces the degradation of IκB, phosphorylating the p65 subunit of NF-κB (p65/RelA-p50) so that the p65/RelA-p50 dimer translocates into the nucleus and regulates transcriptional activity [47]. This activated transcriptional activity results in products that inhibit components of the apoptotic machinery in normal and cancerous cells [48]. Additionally, cells expressing an overactive p65 subunit of NF-κB showed an elevated expression of ZEB-1 and ZEB-2 and increased ZEB-1 promoter activity, which are linked with cell cycle progression or survival and a epithelial to mesenchymal transition (EMT) phenotype [49]. EMT is when epithelial cells lose their cell polarity and cell-cell adhesion [50], and it is shown to be widely associated with the invasiveness of pancreatic cancer [51]. Thus, the activation of NF-κB, ZEB-1, and ZEB-2 by Tax supports pancreatic cancer progression. In addition to Akt activation, Tax’s direct binding to IKK amplifies the same effects. Meanwhile, excessive input from PI3K will result in the hyperactivation of Rac, which is implicated in tumorigenesis and metastasis [52], so Rac activation by Tax is another association with pancreatic cancer (Figure 2A).
As the cells transition into PanIN-2 and PanIN-3, changes happen in the pathways that regulate the cell cycle, leading to uncontrolled proliferation, increased survival, and genomic instability, which can also be induced by Tax. This is when dysplasia and cribriforming can be seen in epithelial cells [42]. In the p16-cyclin D1-CDK4/6-Rb pathway, cyclin D1 activates CDK4, which then phosphorylates (and inhibits) the Rb protein, leading to cell cycle progression, and p16 inhibits CDK4, keeping Rb hypophosphorylated (active) and preventing cell cycle progression [53]. Cyclin D1 promotes the cell cycle’s progression through the G1 phase by enhancing NDR1/2 kinase activity [54], and the overexpression of cyclin D1 is commonly seen in cancer [55]. The activation of cyclin D1 by Tax contributes to cancer, and the inhibition of p16 by Tax prevents the inhibition of CDK4, reinforcing the cell cycle’s progression through the G1 phase. The loss of the CDK4/6 inhibitor CDKN2A, which encodes p16, is a signature genetic event in pancreatic ductal adenocarcinoma [56], the most common type of exocrine pancreatic cancer that constitutes 95% of cases [57]. Although CDKN2A is not “lost” in an HTLV-1 infection, its gene product is suppressed by Tax and a similar effect is achieved.
Tax binds to and inhibits Smad2/3 and Smad4 in the TGF-β signaling pathway [58]. Smads are receptors and signal transducers of the transforming growth factor beta (TGF-β) superfamily, which play a role in cell development and growth [59]. The pathway induces cell cycle arrest and apoptosis, a tumor-suppressing function [60]. The result of Tax inhibition on the TGF-β pathway is an increased likelihood of developing pancreatic cancer, as abnormal TGF-β signaling is considered a primary EMT inducer, and the abrogation of TGF-β signalling switches cells towards a cohesive migratory phenotype [61]. Inhibition of p16 is usually an early effect of pancreatic cancer while p53 inhibition is a late stage effect [41]. Figure 2A shows the activation of the ErbB pathway, Jak-STAT pathway, and VEGF pathway in the intermediate stages, and these pathways promote resistance to apoptosis, with VEGF promoting angiogenesis, the growth of new blood vessels. The inhibition of the TGF-β pathway is shown near the end of non-viral pancreatic cancer development. Tax is able to bind to and interact with proteins in these molecular pathways and induce the changes simultaneously. A block-diagram of Tax interactions affecting pancreatic cancer are presented on Figure 2B.
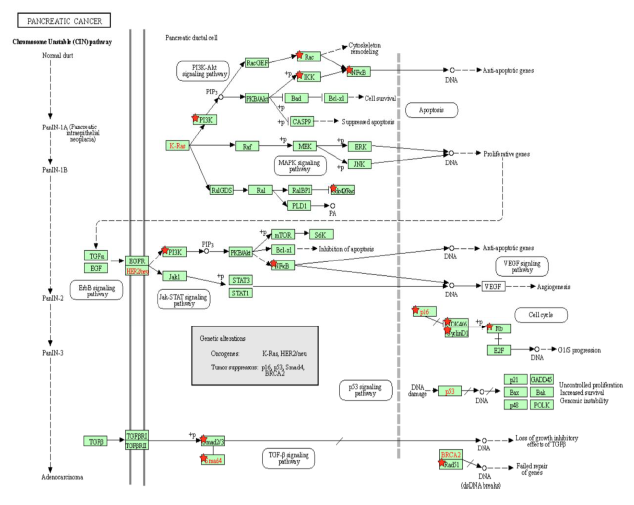
Figure 2A: Pancreatic Cancer Pathway. A KEGG pathway depicting the cellular events that take place during pancreatic cancer. Genes from Table 1 are marked with red stars.
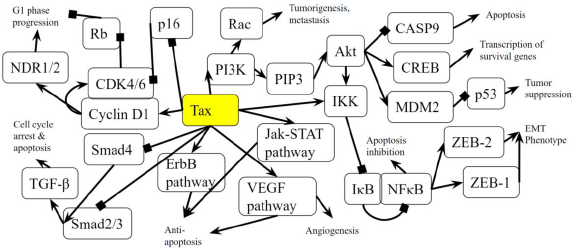
Figure 2B: Role of Tax in Pancreatic Cancer Pathway. A diagram that represents the analysis of the individual protein-protein interactions in the pathway (Figure 2A) as well as existing literature, and the combined picture of their connections to each other and to pancreatic cancer is novel. Arrows indicate the activation of a protein by its preceding protein, and lines ending with squares indicate the inhibition of a protein by its preceding protein.
Chronic Myeloid Leukemia
Activation of the BCR-ABL tyrosine kinase is a crucial causing factor of CML [16], and the BCR-ABL oncogene is the primary target that must be blocked to prevent CML [62]. The activation of PI3K is an essential signaling mechanism in ABL leukemogenesis [63], where BCR-ABL activates the PI3K/mTOR pathway, increasing the production of reactive oxygen species [64], which damages DNA and the cell division process. In this case, Tax is able to replace the effects of the BCR-ABL oncogene by activating the PI3K/mTOR pathway. As said before, this PI3K activation increases the activity of proliferative proteins CREB and MDM2, and downregulates the activity of apoptotic protein CASP9. AKT phosphorylates and activates mTOR, which causes cyclinD1 and CDK to bind together and promote cell division [65]. IKK activates NF-κB through Akt activation by PI3K, as well as through the direct binding of Tax with IKK. BCR-ABL1 activates lymphoid and myeloid leukemogenesis through the expression of IKK [66], supporting the idea that Tax’s activating effect on IKK contributes to chronic myeloid leukemia in a manner similar to BCR-ABL1. KEGG diagram of this pathway is presented on Figure 3A and on Figure 3B is elucidated a block diagram of Tax interactions affecting CML.
The activity of BCR/ABL is necessary but not enough to induce CML though [67], and a combination of many gene mutations is needed for CML to develop. However, Tax’s effects are not limited to those of BCR/ABL either. p14ARF is a tumor suppressing protein that is encoded by the same locus (CDKN2A) as p16, which also known as p16INK4a, but p14ARF uses a different reading frame [68]. p14ARF is epigenetically silenced in ATL [68], and both p14ARF and p16INK4a promoter methylation occur often in leukemias and lymphomas [69], and p14ARF and p16INK4a mRNA levels are significantly low in CML patients [70]. Overexpression of MDM2 causes the degradation of p14ARF [71], and since Tax increases MDM2 activity through the PI3K-Akt pathway, HTLV-1 is involved in CML through the indirect inhibition of p14ARF. p14ARF is negatively regulated by p53 [71], and Tax inhibits the activity of p53 through PI3K as well. It is unknown whether Tax can directly bind to p14ARF because p14ARF shares no structural homology with p16INK4a [72]. However, Tax binds directly to p16INK4a and inhibits it, preventing the inhibition of CDK4/6 by p16INK4a and allowing CDK4/6 to inhibit the tumor suppressor Rb [72].
The activation of mTOR, the inhibition of p16INK4a, and Tax’s direct binding to the cyclin D/CDK complex all stimulate cyclin/CDK activity. Increased cyclin/CDK activity hyperphosphorylates Rb, which binds to and suppresses E2F in the hypophosphorylated state [73]. The resulting separation of Rb and E2F increases E2F activity and allows it to transcribe more genes necessary in the S phase, leading to cell cycle progression [73]. A study examined Rb expression in CML cells undergoing blast crisis and found that all the megakaryoblastic crisis cases lacked the expression of the Rb encoded protein, which suggests that CML transformation megakaryoblastic is associated with the loss of Rb [74]. Megakaryoblastic crisis is a rare subtype of blast crisis, and its cause is unknown [75]. Tax’s inhibitory effect on Rb activity is a propelling factor of CML entering the megakaryoblastic crisis.
A study found that levels of TGF-β type II receptor (TGFβR2) (p = 0.012) and Smad4 (p = 0.043) were significantly low in patients with CML, and that caused reduced tumor suppressive effects in CML [76]. In the TGF-β signaling pathway, TGF-β binds to a TGFβR2, which forms a complex with TGFβR1, and the resulting complex phosphorylates a receptor-regulated Smad (R-Smad) so that it forms an active Smad signaling complexes that regulate the transcription of genes related to apoptosis and immune function [77]. One such complex is between Smad2/3 and TRIM33, which is a protein that controls gene expression, and the Smad2/3-TRIM33 complex stimulates the differentiation of hematopoietic progenitors [78]. Tax’s inhibition of Smad4 and Smad2/3 prevents Smad2/3-TRIM33 complex activity. Since CML involves blast phase cells failing to differentiate and causing a rapid accumulation of non-functional cells in the bone marrow and blood [79], Smad inhibition by Tax is a causing factor that contributes to differentiation failure through TRIM33. Interestingly, a knockout of the TRIM33 gene in premalignant pancreatic progenitors produces the same effects as a SMAD4 knockout [78,80], indicating their similar role in pancreatic tumor suppression as well as in CML.
EVI1 gene overexpression affects BCR–ABL to induce CML, and one of the transcription factors that EVI1 recruits is HDAC, a transcriptional repressor [81]. Inhibiting HDAC alleviates EVI1-mediated repression of TGF-β signaling [81]. HDAC inhibition is a proposed treatment for hematological cancers, especially CML, and the method is undergoing trial studies [82], so HDAC plays a role in EVI1 tumorigenesis. HDAC has an opposing role though: it has been shown to negatively regulate Tax activity by competing with CBP to bind with Tax [83]. CBP controls cell growth, cell division, and differentiation [84], so HDAC reduces these functions. Thus, in hematological cancers that do not involve HTLV-1, HDAC inhibitors may be an effective treatment, but since HDAC downregulates the oncogenic viral protein Tax, inhibiting HDAC may, to some extent, work against treatment of CML cases involving HTLV-1.
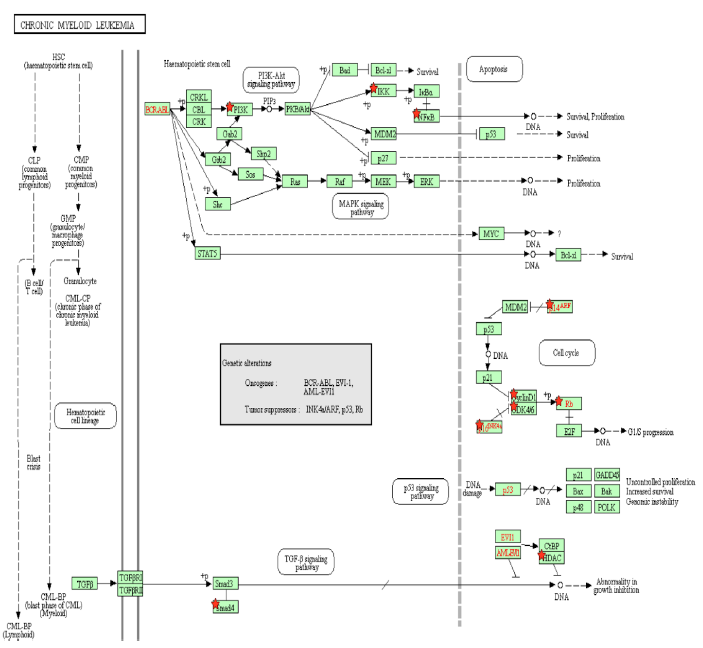
Figure 3A: Chronic Myeloid Leukemia Pathway. A pathway depicting the cellular events that take place during chronic myeloid leukemia. Genes from Table 1 are marked with red stars.
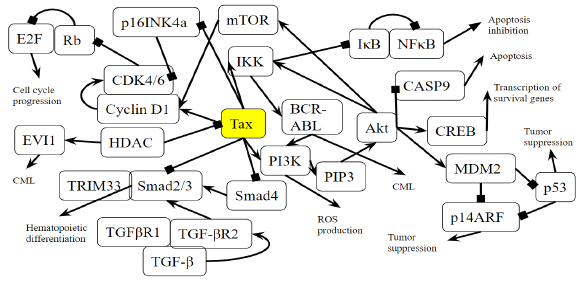
Figure 3B: Role of Tax in Chronic Myeloid Leukemia Pathway. A novel diagram that represents the analysis of the individual protein-protein interactions in the pathway (Figure 3A) as well as existing literature, and the combined diagram of their connections to each other and to chronic myeloid leukemia is novel.
Small Cell Lung Cancer
p15INK4b is a CDK inhibitor, and when Tax binds to p15INK4b, it inactivates p15INK4b and restores CDK4 kinase activity [85]. The lack of p15Ink4b (CDKN2B) expression is frequently seen in blood disorders like acute myeloid leukemia and myelodysplastic syndromes [86], so it is a point of commonality between SCLC and CML. The dysregulation of p15INK4b in neuroendocrine lung tumors was found to happen regardless of p16INK4a and p14ARF status [87]. Neuroendocrine cells are spread throughout the human body, but are mainly found in the small intestine, pancreas, and lung bronchioles, and they help heal the epithelium after injury from infection [88]. Neuroendocrine cells may become overactive turn into cancerous cells, and a small portion of lung cancers are neuroendocrine [89]. Neuroendocrine tumors can form in different organs and the most common ones are gastrointestinal tumors [89], which relates to the colorectal cancer pathway that will be addressed later below. p15INK4b dysregulation by Tax is therefore a factor that can be common to pancreatic cancer, CML, SCLC, and gastrointestinal tumors. KEGG diagram of this pathway is presented on Figure 4A and on Figure 4B is elucidated a block diagram of Tax interactions affecting small-cell lung cancer.
SCLC cells have high levels of NF-κB activation, which is significantly associated with cancer advancement and poor prognosis, and inhibiting NF-κB prevents lung cancer cell survival and proliferation [90]. KRAS is an oncogene that accounts for 90% of Ras mutations in lung cancers, and KRAS activity, combined with p53 loss, collaboratively activate NF-κB in SCLC cells [90,91]. As Tax activates IKK, it prevents IκB from keeping NF-κB bound and inactive, which indicates that Tax’s role is similar to that of KRAS in that it activates NF-κB to induce SCLC cell proliferation. The functions of NF-κB and p53 are in contrast with each other: p53 induces apoptosis, and the stimulation of NF-κB promotes resistance to programmed cell death. [92]. A regulatory mechanism coordinates these opposing outcomes in a cellular decision-making event, where both p53 and NF-κB inhibit each other’s ability to stimulate gene expression through changing the relative levels of their transcription factors [92]. Thus, the activation of NF-κB inhibits p53 transactivation, so while p53 loss contributes to the activation of NF-κB in SCLC, Tax’s activation of NF-κB also inhibits p53, creating a positive feedback cycle that, again, supports SCLC cell proliferation.
Genomic alterations of the PI3K/AKT/mTOR pathway are distinguishable in SCLC, as well as a high prevalence of inactivating mutations in TP53 (the gene that codes for p53) and RB1 (the gene that codes for Rb), and an increase in MYC [93]. Specifically, in SCLC cells with a PI3K mutation, the inhibition of PI3K signaling led to apoptosis, and the inhibition of cell viability, transformation, and tumor growth, although in the same cells with PTEN loss, such an inhibition had no effect [94]. PI3K catalyzes PIP2 to form PIP3, and the lipid phosphatase PTEN induces the opposite effect by dephosphorylating PIP3 into PIP2 [95]. With PTEN opposing the proliferative functions of PI3K in SCLC, when the activation of PI3K is combined with the inhibition of PTEN, this removes the possibility of treatment through PI3K inhibition. This is exactly what Tax does: Table 2 indicates that Tax activates PI3K and its downstream signaling, and a study showed that Tax downregulates PTEN through the NF-κB pathway [96].
The mutation of RB1 is highly prevalent in SCLC. A study of SCLC cases identified such a mutation in 75% of patients [97]. While most SCLC TP53 mutations are caused by carcinogens from smoking, no evidence shows the same cause for RB mutations [98]. This is unusual, because SCLC is primarily caused by carcinogens from tobacco smoke, although the exact reason normal cells become cancerous cells is unknown [99]. It is unknown how often HTLV-1 Tax is associated with SCLC, but when it happens, the increased cyclin/CDK activity caused by Tax accounts for the lack of Rb activity in SCLC. The combination of TP53 and RB1 mutations accounts for over 90% of SCLC cases [100], and even though Tax does not mutate these genes, it interferes with the signaling of their gene products. A study found that EZH2 is upregulated in SCLC and is a promising therapeutic target [101]. It promotes SCLC by activating E2F [101], which Rb normally inhibits. Tax can support or even replace the tumorigenesis effects of EZH2 by inhibiting Rb and increasing the activity of E2F.
According to Figure 4A, MYC inhibits p15INK4b, activates CDK4/6-CyclinD1, and thus represses Rb. Specifically, MYC suppresses p16INK4a and p21 [102], both of which inhibit CDK4/6 [103]. The result is an active CDK4/6-CyclinD1 complex, and a downregulated Rb. MYC deregulation is frequently found in neuroblastoma, retinoblastoma, medulloblastoma, Wilm's tumors, prostate cancer with neuroendocrine differentiation, SCLC, Merkel cell carcinoma, and ovarian carcinoma [104]. Unlike other oncogenes such as RAS or EGFR, the MYC gene is usually not mutated in cancer, and its increased expression is either a result of amplification or deregulation [104]. Tax transactivates the MYC gene by altering the transcriptional activity of transcription factor NFkB [105]. This points towards epigenetic MYC activation as a commonality with an HTLV-1-based cause in many different cancers. In SCLC, MYC withdrawal in mice leads to tumor regression [104], highlighting the notable impact that Tax mediated dysregulation has in SCLC.
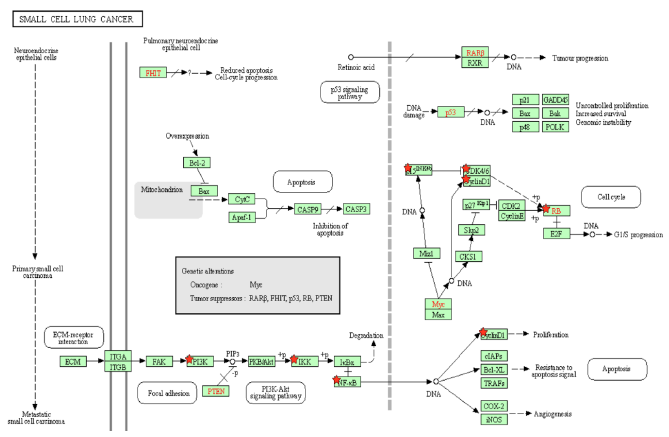
Figure 4A: Small Cell Lung Cancer Pathway. A pathway depicting the cellular events that take place during small cell lung cancer. Genes from Figure C are marked with red stars.
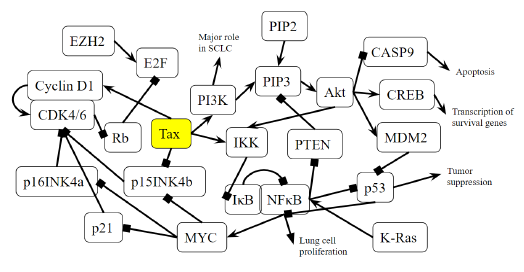
Figure 4B: Role of Tax in Small Cell Lung Cancer Pathway. A novel diagram that represents the analysis of the individual protein-protein interactions of the pathway (Figure 4A) as well as existing literature, and the combined diagram of their connections to each other and to SCLC is novel.
Colorectal Cancer
Tax activates the GTPase Rac1 and initiates the activation of many downstream signaling events that are related to cell proliferation, transcription, and an EMT phenotype in CRC. Rac1 activates PAK [106], a protein that is hyperstimulated in cancer. Rac1 also activates NFkB and members of the MAPK family [106], which are responsible for cell proliferation, differentiation, development, and inflammatory responses [107]. Rac1 also activates NOX1 and leads to the production of reactive oxygen species [106], which causes oxidative damage in DNA, causing mutations that lead to cancer [108]. Excessive ROS production causes the release of pro-inflammatory cytokines, and alters the microbiota, which are associated with chronic intestinal inflammation and an increased risk of colorectal cancer [109]. In mammalian and drosophila cells, Rac1 is necessary and sufficient to cause ROS-dependent intestinal stem cells proliferation and regeneration [110,111]. Because Tax activates Rac1, it causes the same downstream effects of NFkB, MAPK, and NOX1 activation, allowing an HTLV-1 infection to cause ROS production, gut microbiota alteration, and intestinal inflammation. KEGG diagram of this pathway is presented on Figure 5A and on Figure 5B is elucidated a block diagram of Tax interactions affecting colorectal cancer.
In the Wnt/β-catenin/TCF pathway, the Axin complex is composed of the scaffolding protein Axin, the tumor suppressor APC, and several others, and it degrades cytoplasmic β-catenin in the absence of Wnt [112]. Rac1 forms a complex with JNK2 and β-catenin to promote the nuclear translocation of β-catenin [112], so Rac1 opposes the effects of the Axin complex and activates β-catenin, which then activates the transcription of the proto-oncogenes MYC and CCND1 [113]. In cancer, deregulated β-catenin signaling in the intestinal epithelium’s tight junctions and adherens junctions occurs, which mediates uncontrolled proliferation [114]. An accumulation of β-catenin is usually the result of APC and CTNNB1 mutations that help β-catenin bypass degradation in the absence of Wnt [114], so Tax’s activation of Rac1 mimics APC mutations by preventing β-catenin degradation. As expected, Rac1 is overactive in CRC and associated with tumorigenesis, and CRC patients with an overactive Rac1 had shorter survival rates [115]. Rac1 is involved at multiple different steps in the progression of CRC, so the same can be said for Tax. The activation of Wnt/β-catenin signaling stimulates benign adenoma growth along with KRAS (gene that codes for K-RAS) mutations, and in cooperation with p53 inactivation, contribute to invasive and malignant behaviors in CRC [31]. β-catenin activation, KRAS mutation, and p53 inactivation through Rac1 and Wnt/β-catenin signaling combine to support the significant role of Tax in CRC.
PI3K signaling deregulation is a common feature in CRC [31] and its main contribution to tumorigenesis is made through growth factor signaling [31], regulating the proliferation of both normal and cancerous colon cells [116,117]. PIK3CA mutations arise late in the adenoma-carcinoma sequence, and are thought to be involved in invasion [118]. EGFR inhibition and MEK inhibition are therapies for reducing the oncogenic potential of tumors [119,120], but in many cases, especially KRAS-mutant CRC cells, resistance appears against these therapies [121,122]. Blocking the PI3K/AKT pathway is able to revert CRC resistance to EGFR inhibition and MEK inhibition [123]. Thus, Tax’s activation of PI3K may promote proliferation and invasion in late stages of CRC and contributes to CRC resistance against EGFR inhibition and MEK inhibition during treatment. Additionally, PI3K activates Akt, which then converts Bax from a pro-apoptotic protein to anti-apoptotic protein [124]. Bax protein plays a significant role in the cancerogenesis mechanism of CRC [125]. Similarly, Akt activation phosphorylates BAD and promotes cell survival [126], and an increased expression of phosphorylated BAD occurs in CRC cells, playing a role in tumorigenesis [127]. Because Tax activates PI3K, Tax can also initiate these downstream effects of Bax conversion and BAD phosphorylation in CRC.
Tax activates β-catenin through Rac1. MYC and CCND1 are transcriptional targets of β-catenin [128], both of which are oncogenic. The overexpression of Cyclin D1 increases during the adenoma to carcinoma sequence and is associated with the advanced tumor stage and a short survival period in CRC [129]. Even before tumor progression, Cyclin D1, CDKs and CDK inhibitors are significantly associated with the cell cycle transition into CRC [130]. Tax causes an increased transcription of Cyclin D1, so it plays a role in the adenoma to carcinoma sequence of CRC. MYC is overexpressed in up to 70–80% of CRC cases [130], making the eventual activation of MYC by Tax a significant contribution to CRC. CD36 is a tumor suppressor whose expression progressively decreases from adenomas to carcinomas and is associated with a poor CRC survival rate, and it normally causes the ubiquitination of GPC4, inhibiting β-catenin/c-Myc signaling and suppresses the expression of GLUT1, HK2, PKM2 and LDHA [128], genes involved in glucose transport and glycolysis, whose inhibition is an anticancer treatment [131]. Excessive glycolysis usage paired with lactic acid fermentation instead of oxidative phosphorylation is bioenergetically less efficient and requires increased glucose import, which are hallmarks of cancer known as the Warburg effect [132]. By activating β-catenin, Tax stimulates the expression of these downstream glycolytic genes and promotes an increase in glycolysis as the main source of energy, producing an effect similar to that of dysregulated CD36 and exhibiting the Warburg effect. Specifically in CRC, disrupting CD36 expression in both inflammation-induced CRC models and ApcMin/+ mice models increases CRC tumorigenesis [132].
TGF-β receptor type 2 (TGFBR2) mutations are found in CRCs with microsatellite instability (MSI), which are repeating stretches of DNA distributed throughout the genome that are prone to high mutation rate, accounting for 15% of CRC cases [133]. TGFBR2 mutations are also found in CRCs with loss of heterozygosity and chromosomal instability, in which unstable chromosomes or parts of chromosomes are duplicated or deleted, accounting for 85% of invasive CRC cases [133]. Most CRC cells with high levels of MSI have TGFBR2 mutations, because TGFBR2 carries microsatellite sequences [134]. As a result, a disruption of TGF-β signaling is a pivotal cause of many molecular subtypes of CRC pathogenesis [134]. TGFBR2 mutations occur in around 30% of CRCs and is the most common mechanism that results in altered TGF-β signaling [135]. In MSI CRCs, TGFBR2 frameshift mutations have been found in over 80% of cases [136], supporting its important role as a tumor suppressor especially in MSI CRC. TGFBR2 normally activates TGFBR1, which then phosphorylates Smad2 and Smad3, transcription factors that bind to Smad4 in response, and together, they regulate the transcription of TGF-β-responsive genes that are associated with malignancies [137]. Several TGF-β-responsive genes code for CDK inhibitors p15, p21, and p57 [138], So when Tax inhibits Smad2/3 and Smad4 and abrogates TGFBR2 mediated gene transcription, it leaves CDKs highly active. Since the reconstitution of TGFBR2 in MSI CRC cell lines decreases CDK4 activity, leading to a decrease in cellular proliferation [139], the lack of CDK inhibitors and resulting proliferation caused by Tax is a factor contributing to MSI in CRC. Besides that, TGFBR1*6A, a common hypomorphic variant of TGFBR1, has been found to account for 3% of all CRCs, a percentage that is higher than that of mismatch repair genes MLH1, MSH2, MSH6 and PMS2 [139], which already cause distinguishable cancer risk profiles [140], so the effect of Tax’s disruption in TGF-β signaling on CRC is comparable to that of established CRC biomarkers.
The genes SMAD2 and SMAD4 belong on chromosome 18q21, which is frequently affected by loss of heterozygosity (LOH) in MSI CRC [133]. This can cause SMAD4-deficient CRC, which has a low survival rate [133]. Additionally, the Cancer Genome Atlas database indicates SMAD4 as one of the most frequently mutated genes in CRC [141]. RNA sequencing comparing SMAD4-proficient and SMAD4-deficient mice epithelial colon cells showed an upregulation in many inflammation genes [142]. By inhibiting SMAD4, Tax produces an effect similar to that of LOH in MSI CRC and predicts a low survival rate. Ccl9 is an upregulated gene in a SMAD4-deficient intestinal tumor mouse model, and it cooperates with Ccr1 to recruit myeloid cells to promote invasion and metastases [143]. A similar situation of increased levels of myeloid-derived suppressor cells was found to promote human CRC progression [144]. An increased presence of myeloid-derived suppressor cells correlates with poor prognosis and reduced survival in cancer patients [145]. Upregulated Ccl9 and Ccr1 along with increased myeloid cell levels can be caused by Tax, leading to poor prognosis and reduced survival. Lastly, the loss of SMAD4 is a predictive biomarker for chemoresistance in 5-FU-based chemotherapy [133], indicating that Tax can contribute to chemoresistance in 5-FU-based chemotherapy.
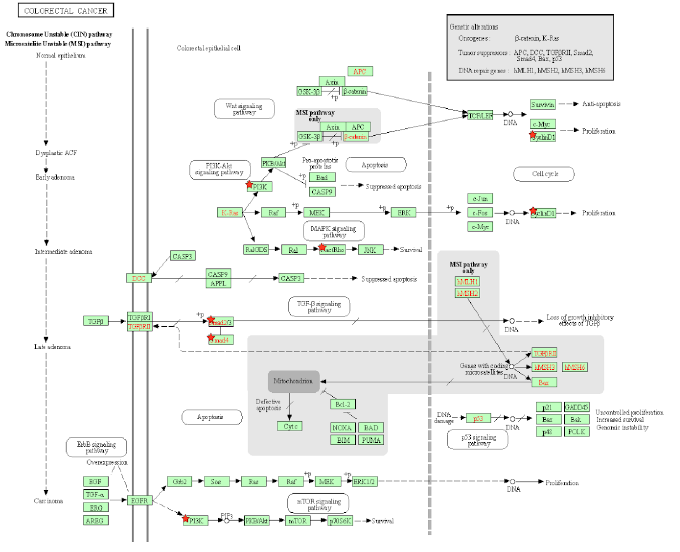
Figure 5A: Colorectal Cancer Pathway. A pathway depicting the cellular events that take place during colorectal cancer. Genes from Table 1 are marked with red stars.
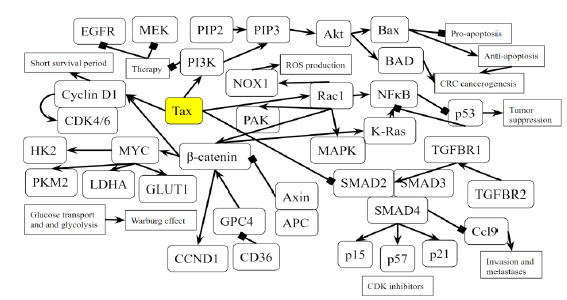
Figure 5B: Role of Tax in Colorectal Cancer Pathway. A novel diagram that represents the analysis of the individual protein-protein interactions of the pathway (Figure 5A) as well as existing literature, and the combined picture of their connections to each other and to SCLC is novel.
Discussion
In the PI3K pathway, Tax increases the activity of CREB1, MDM2, and RAC1, tumorigenesis genes, while downregulating CASP9 during the transition of a normal duct to PanIN-1A and PanIN-1B. Tax stimulates NF-κB transcriptional activity by phosphorylating IKK α and β, degrading IκB, and phosphorylating the p65 subunit of NF-κB. An overactive p65 subunit of NF-κB increases ZEB-1 and ZEB-2 expression, a characteristic of the EMT phenotype in pancreatic cancer. In the p16-cyclin D1-CDK4/6-Rb pathway, Tax activates CyclinD1, CDK4, and NDR1/2 kinase, leading to cell cycle progression, and Tax inhibits p16, whose gene is dysfunctional pancreatic ductal adenocarcinomas. The inhibition of Smad2/3 and Smad4 in the TGF-β pathway by Tax induces EMT and a may bring change towards a cohesive migratory phenotype in pancreatic cancer cells. Inhibition of p16 is usually an early effect of pancreatic cancer, while p53 inhibition is a late-stage effect [146]. Tax simultaneously affects proteins in pathways containing p16, p53, ErbB, Jak-STAT, VEGF, and TGF-β, which are normally mutated in different stages in the progression of pancreatic cancers not involving HLTV.
Tax is able to replace the effects of the BCR-ABL oncogene in CML by activating PI3K and its downstream targets, CREB1 and MDM2, and inhibiting CASP9. Through PI3K, Tax activates AKT and subsequently mTOR, which activates cyclinD1 and CDK in the cell division process. IKK is activated by Akt in BCR-ABL1-mediated leukemogenesis in CML, and Tax induces the same effects. Tax’s activation of the PI3K pathway activates MDM2, which inhibits p14ARF, a tumor suppressor, but Tax cannot initiate CML leukemogenesis through both p14ARF and p53 simultaneously. Tax inhibits p16INK4a and prevents the inhibition of CDK4/6, allowing CDK4/6 to inhibit the tumor suppressor Rb, which is associated with the megakaryoblastic crisis in CML. Rb also increases E2F activity to promote the progression of the S phase in the cell cycle. Tax prevents Smad2/3-TRIM33 complex activity, which prevents hematopoietic progenitors from differentiating in CML. EVI1 recruits HDAC, which represses TGF-β signaling, but it also negatively regulates Tax, so inhibiting HDAC may work against HLTV-positive cases of CML.
Tax suppresses p15INK4b, which allows for CDK4 activity, an event common to multiple cancers, including SCLC. Tax mimics the activity of K-Ras by activating IKK, leading to NF-κB activation, which along with p53 suppression, creates a cycle of increased NF-κB and inhibited p53 that furthers SCLC. Tax activates PI3K, which catalyzes PIP2 to form PIP3, and with Tax downregulating PTEN, PI3K inhibition is not a viable treatment in HLTV-involved SCLC. The increased cyclin and CDK activity caused by Tax inhibits Rb activity in SCLC, and Rb inactivity along with TP53 inactivity are exhibited in over 90% of SCLC cases. EZH2 is an upregulated gene in SCLC as it activates E2F, a gene that Rb normally inhibits. With Rb inhibited, Tax produces effects similar to EZH2 by increasing E2F activity. Tax activates MYC through NF-κB, and MYC suppresses p16INK4a and p21 and thus activates CDK4/6-CyclinD1 and represses Rb, playing a pivotal role in SCLC.
Tax activates Rac1 and downstream genes NFkB, KRAS, MAPK, NOX1, PAK, which are involved in the oncogenic process of CRC, and β-catenin, which activates GLUT1, HK2, PKM2, and LDHA, promoting the Warburg effect in CRC. Cyclin D1 is activated through both β-catenin and the TGFβ/SMAD pathway. These β-catenin related effects mimic the mutation of both APC, a tumor suppressor mutated in 80% of CLC cases, and CD36, a tumor suppressor that is highly associated with a poor survival rate in CRC. Tax opposes TGFβ signaling by inhibiting the activity of a complex between Smad2/3 and Smad4, resulting in excessive Ccl9 activity and reduced production of CDK inhibitors p15, p21, and p57. Tax activates PI3K, which combined with KRAS inactivity, renders EGFR inhibition and MEK inhibition therapies ineffective against CRC. PI3K activation also promotes apoptosis-resistance through Bax and BAD.
Tax influences many genes involved in pathways other than that of adult T-cell leukemia, contributing to the development of pancreatic cancer, CML, SCLC, and CRC, statistically significant pathways related to the HLTV Tax interactome by the EASE Score calculation and Benjamini–Hochberg Procedure. The specific gene interactions allow for greater clarity for researchers in developing drugs, for HTLV-1-infected cancer patients in selecting treatment options, and for potentially HLTV-infected individuals in identifying their cancers. As HTLV-1’s effects are not limited to ATL, cancer patients with pancreatic cancer, chronic and acute myeloid leukemia, small cell lung cancer, prostate cancer, non-small cell lung cancer, colorectal cancer, glioma, and melanoma should also get tested for HTLV-1.
HTLV-1 is a neglected public health problem: it is estimated to infect 10 to 20 million people worldwide, but is only associated with disease in around 5% of infected individuals [147]. This leaves 9.5 to 19 million people unaware of the existence or the significance of their infection. The discovery of associations between HTLV-1 and ten different cancers sheds light on the significance of being infected with HTLV-1, and this study created a large-scale explanation of why being infected with HTLV-1 increases the risk of four cancers that are remarkably different from ATL. Although evidence of HTLV-1’s presence in cancers other than ATL has been found before, the molecular events behind the conditions were not well-characterized until now. The elucidation of the molecular mechanisms in HTLV-1-associated cancers of the pancreas, myeloid tissue, lungs, and intestines can assist researchers and physicians in diagnosis, evaluating prognosis, and treatment.
Conflicts of interest:
The authors have declared that they have no conflicts of interest.
References
- Verdonck K, González E, Van Dooren S, et al. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. The Lancet Infectious Diseases 7 (2007): 266-281.
- Miyazato P, Matsuo M, Katsuya H, et al. Transcriptional and epigenetic regulatory mechanisms affecting HTLV-1 provirus. Viruses 8 (2016): 171.
- Azran I, Schavinsky-Khrapunsky Y, Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 1 (2004): 20.
- Yamagishi M, Fujikawa D, Watanabe T, et al. HTLV-1-mediated epigenetic pathway to adult T-cell leukemia–lymphoma. Frontiers in Microbiology 9 (2018): 1686.
- Dias A, Falcão LF, Falcão AS, et al. Human T lymphotropic virus and pulmonary diseases. Frontiers in Microbiology 9 (2018): 1879.
- Du G, Zhang W, Zhang Z, et al. HTLV-1-associated genes as potential biomarkers for endometrial cancer. Oncology Letters 18 (2019): 699-705.
- Hirata T, Nakamoto M, Nakamura M, et al. Low prevalence of human T cell lymphotropic virus type 1 infection in patients with gastric cancer. Journal of Gastroenterology and Hepatology 22 (2007): 2238-2241.
- Pancreatic Cancer [Internet]. Pancreatic Cancer: What is Pancreatic Cancer? Pancreatic Cancer Symptoms, Treatment, Diagnosis | Columbia University Department of Surgery. [cited 2020Dec4]. Available from: https://columbiasurgery.org/conditions-and-treatments/pancreatic-cancer
- Kouznetsova V, Chen S, Tsigelny I. HTLV-1 can be involved in acceleration of different nonhematological cancers. Journal of Cancer Research and Therapeutics 7 (2019): 1-8.
- Popescu M, Popov V, Popescu G, et al. Acute pancreatitis: the onset digestive manifestation, in a patient with adult T-cell leukemia/lymphoma. Rom J Morphol Embryol 53 (2012): 847-850.
- Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, et al. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology 154 (2018): 1729-1736.
- Alasiri A, Guerr JA, Hall WW, et al. Novel interactions between the human T-cell leukemia virus type 1 antisense protein HBZ and the SWI/SNF chromatin remodeling family: implications for viral life cycle. Journal of Virology 93 (2019): e00412-e00419.
- What Is Chronic Myeloid Leukemia?: Leukemia Types [Internet]. American Cancer Society. [cited 2020Dec5]. Available from: http://www.cancer.org/cancer/chronic-myeloid-leukemia/about/what-is-cml.html
- Perrotti D, Silvestri G, Stramucci L, et al. Cellular and molecular networks in chronic myeloid leukemia: the leukemic stem, progenitor and stromal cell interplay. Current Drug Targets 18 (2017): 377-388.
- Hematopoietic stem cell [Internet]. Wikipedia. Wikimedia Foundation; 2020 [cited 2020Dec5]. Available from: https://en.wikipedia.org/wiki/Hematopoietic_stem_cell.
- Ohmine K, Ota J, Ueda M, et al. Characterization of stage progression in chronic myeloid leukemia by DNA microarray with purified hematopoietic stem cells. Oncogene 20 (2001): 8249-8257.
- Blast crisis [Internet]. Blast crisis - Symptoms, diagnosis and treatment | BMJ Best Practice US. [cited 2020Dec5]. Available from: https://bestpractice.bmj.com/topics/en-us/1026
- Emergency Management of Blast Crisis [Internet]. Core EM. [cited 2020Dec5]. Available from: https://coreem.net/core/blast-crisis/
- Rocamonde B, Carcone A, Mahieux R, et al. HTLV-1 infection of myeloid cells: from transmission to immune alterations. Retrovirology 16 (2019): 45.
- What Is Lung Cancer?: Types of Lung Cancer [Internet]. American Cancer Society. [cited 2020Dec5]. Available from: http://www.cancer.org/cancer/lung-cancer/about/what-is.html
- Tisi E, Lissoni P, Angeli M, et al. Postoperative increase in soluble interleukin-2 receptor serum levels as predictor for early recurrence in non-small cell lung carcinoma. Cancer 69 (1992): 2458-2462.
- Matsuzaki H, Asou N, Kawaguchi Y, et al. Human T-cell leukemia virus type 1 associated with small cell lung cancer. Cancer 66 (1990): 1763-1768.
- Inoue J, Seiki M, Taniguchi T, et al. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 5 (1986): 2883-2888.
- Seidler S, Zimmermann HW, Weiskirchen R, et al. Elevated circulating soluble interleukin-2 receptor in patients with chronic liver diseases is associated with non-classical monocytes. BMC Gastroenterol 12 (2012): 38.
- Nomori H, Mori T, Iyama K, et al. Risk of Bronchioloalveolar Carcinoma in Patients with Human T-cell Lymphotropic Virus Type 1 (HTLV-I): Case-control Study Results. Annals of Thoracic and Cardiovascular Surgery 17 (2011): 19–23.
- Mori S, Mizoguchi A, Kawabata M, et al. Bronchoalveolar lymphocytosis correlates with human T lymphotropic virus type I (HTLV-I) proviral DNA load in HTLV-I carriers. Thorax 60 (2005): 138-143.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136 (2015): E359-E386.
- Burt R. Inheritance of Colorectal Cancer. Drug Discov Today Dis Mech 4 (2007): 293-300.
- Kwong LN, Dove WF. APC and its modifiers in colon cancer. Adv Exp Med Biol 656 (2009): 85-106.
- Causes of Colorectal Cancer: Is Colon Cancer Hereditary? [Internet]. American Cancer Society. [cited 2020Dec5]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/causes-risks-prevention/what-causes.html
- Zhang J, Roberts TM, Shivdasani RA. Targeting PI3K Signaling as a Therapeutic Approach for Colorectal Cancer. Gastroenterology 141 (2011): 50–61.
- Nguyen H, Duong HQ. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy (Review). Oncology Letters (2018).
- Martin-Latil S, Gnädig NF, Mallet A, et al. Transcytosis of HTLV-1 across a tight human epithelial barrier and infection of subepithelial dendritic cells. Blood 120 (2012): 572-580.
- Boxus M, Twizere JC, Legros S, et al. The HTLV-1 Tax interactome. Retrovirology 5 (2008): 76.
- DAVID Functional Annotation Bioinformatics Microarray Analysis. [cited 2020Dec5]. Available from: https://david.ncifcrf.gov/
- Dennis G, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4 (2003): R60.
- Functional Annotation Tool [Internet]. Help. [cited 2020Dec5]. Available from: https://david.ncifcrf.gov/helps/functional_annotation.html.
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 57 (1995): 289-300.
- Handbook of Biological Statistics [Internet]. Multiple comparisons - Handbook of Biological Statistics. [cited 2020Dec5]. Available from: http://www.biostathandbook.com/multiplecomparisons.html
- KEGG: Kyoto Encyclopedia of Genes and Genomes. [cited 2020Dec5]. Available from: http://www.genome.jp/kegg
- Polireddy K, Chen Q. Cancer of the Pancreas: Molecular Pathways and Current Advancement in Treatment. J Cancer 7 (2016): 1497-1514.
- Classification of Duct Lesions in the Pancreas [Internet]. Classification Of Duct Lesions In The Pancreas. 1999 [cited 2020Dec5]. Available from: http://www.path.jhu.edu/pancreas/professionals/DuctLesions.php
- PI3K-AKT Signaling Pathway [Internet]. PI3K-AKT Signaling Pathway - Creative Diagnostics. [cited 2020Dec5]. Available from: http://www.creative-diagnostics.com/PI3K-AKT-Signaling-Pathway.htm
- Cardone MH. Regulation of Cell Death Protease Caspase-9 by Phosphorylation. Science 282 (1998): 1318-1321.
- Database GCHG. CASP9 Gene (Protein Coding) [Internet]. GeneCards is a searchable, integrative database that provides comprehensive, user-friendly information on all annotated and predicted human genes. [cited 2020Dec5]. Available from: http://www.genecards.org/cgi-bin/carddisp.pl?gene=CASP9
- Sangawa A, Shintani M, Yamao N, et al. Phosphorylation status of Akt and caspase-9 in gastric and colorectal carcinomas. Int J Clin Exp Pathol 7 (2014): 3312-3317.
- Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer 125 (2009): 2863-2870.
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest 115 (2005): 2625-2632.
- Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26 (2007): 711-724.
- Epithelial–mesenchymal transition [Internet]. Wikipedia. Wikimedia Foundation; 2020 [cited 2020Dec5]. Available from: https://en.wikipedia.org/wiki/Epithelial%E2%80%93mesenchymal_transition
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell 148 (2012): 349-361.
- Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, et al. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal 24 (2012): 353-362.
- Peurala E, Koivunen P, Haapasaari KM, et al. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res 15 (2013): R5.
- Du Z, Tong X, Ye X. Cyclin D1 promotes cell cycle progression through enhancing NDR1/2 kinase activity independent of cyclin-dependent kinase 4. J Biol Chem 288 (2013): 26678-26687.
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 6 (2007): 24.
- Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 5 (2014): 6512-6525.
- Types of pancreatic cancer [Internet]. Pancreatic Cancer UK. 2020 [cited 2020Dec5]. Available from: http://www.pancreaticcancer.org.uk/information-and-support/facts-about-pancreatic-cancer/types-of-pancreatic-cancer/
- Lee DK, Kim BC, Brady JN, et al. Human T-cell lymphotropic virus type 1 tax inhibits transforming growth factor-beta signaling by blocking the association of Smad proteins with Smad-binding element. J Biol Chem 277 (2002): 33766-33775.
- SMAD (protein) [Internet]. Wikipedia. Wikimedia Foundation; 2020 [cited 2020Dec5]. Available from: https://en.wikipedia.org/wiki/SMAD_(protein).
- Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci 14 (2018): 111-123.
- Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 13 (2013): 788-799.
- Shah NP. Overriding Imatinib Resistance with a Novel ABL Kinase Inhibitor. Science 305 (2004): 399-401.
- Kharas MG, Fruman DA. ABL Oncogenes and Phosphoinositide 3-Kinase: Mechanism of Activation and Downstream Effectors. Cancer Research 65 (2005): 2047–2053.
- Kim JH, Chu SC, Gramlich JL, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood 105 (2005): 1717-1723.
- Xu F, Na L, Li Y, et al. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10 (2020): 54.
- Hsieh MY, Van Etten RA. IKK-dependent activation of NF-κB contributes to myeloid and lymphoid leukemogenesis by BCR-ABL1. Blood 123 (2014): 2401-2411.
- Ross TS, Mgbemena VE. Re-evaluating the role of BCR/ABL in chronic myelogenous leukemia. Mol Cell Oncol 1 (2014): e963450.
- Uenogawa K, Hatta Y, Arima N, et al. Azacitidine induces demethylation of p16INK4a and inhibits growth in adult T-cell leukemia/lymphoma. Int J Mol Med 28 (2011): 835-839.
- Kusy S, Larsen CJ, Roche J. p14ARF, p15INK4b and p16INK4a methylation status in chronic myelogenous leukemia. Leuk Lymphoma 45 (2004): 1989-1994.
- Cividin M, Ayrault O, Sorel N, et al. Expression of the cell cycle regulators p14(ARF) and p16(INK4a) in chronic myeloid leukemia. Leuk Res 30 (2006): 1273-1278.
- Vivo M, Matarese M, Sepe M, et al. MDM2-mediated degradation of p14ARF: a novel mechanism to control ARF levels in cancer cells. PLoS One 10 (2015): e0117252.
- Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry 50 (2011): 5566-5582.
- Gatza M, Watt J, Marriott S. Cellular Transformation by the HTLV-I Tax Protein, a Jack-of-All-Trades. Oncogene 22 (2003): 5141-5149.
- Towatari M, Adachi K, Kato H, et al. Absence of the human retinoblastoma gene product in the megakaryoblastic crisis of chronic myelogenous leukemia. Blood 78 (1991): 2178–2181.
- Bhattacharya JB, Gupta R, Samadhiya A. Acute megakaryoblastic blast crisis as a presentation manifestation of chronic myelogenous leukemia. Blood Res 52 (2017): 137-139.
- Shokeen Y, Sharma NR, Vats A, et al. Association between Altered Expression and Genetic Variations of Transforming Growth Factor β-Smad Pathway with Chronic Myeloid Leukemia. Int J Hematol Oncol Stem Cell Res 12 (2018): 14-22.
- Zi Z, Chapnick DA, Liu X. Dynamics of TGF-β/Smad signaling. FEBS Lett 586 (2012): 1921-1928.
- Massagué J, Xi Q. TGF-β control of stem cell differentiation genes. FEBS Lett 586 (2012): 1953-1958.
- Sloma I, Jiang X, Eaves A, et al. Insights into the stem cells of chronic myeloid leukemia. Leukemia 24 (2010): 1823-1833.
- Xi Q, Wang Z, Zaromytidou AI, et al. A poised chromatin platform for TGF-β access to master regulators. Cell 147 (2011): 1511-1524.
- Paquette RL, Nicoll J, Chalukya M, et al. Frequent EVI1 translocations in myeloid blast crisis CML that evolves through tyrosine kinase inhibitors. Cancer Genetics 204 (2011): 392–397.
- Fiskus W, Bhalla KN. Histone Deacetylase Inhibitors and Chronic Myeloid Leukemia. Journal-Histone Deacetylase Inhibitors and Chronic Myeloid Leukemia.
- Ego T, Ariumi Y, Shimotohno K. The interaction of HTLV-1 Tax with HDAC1 negatively regulates the viral gene expression. Oncogene 21 (2002): 7241-7246.
- CREBBP gene: MedlinePlus Genetics [Internet]. MedlinePlus. U.S. National Library of Medicine; 2020 [cited 2020Dec5]. Available from: https://medlineplus.gov/genetics/gene/crebbp/
- Suzuki T, Narita T, Uchida-Toita M, et al. Down-regulation of the INK4 Family of Cyclin-Dependent Kinase Inhibitors by Tax Protein of HTLV-1 through Two Distinct Mechanisms. Virology 259 (1999): 384-391.
- Humeniuk R, Rosu-Myles M, Fares J, et al. The role of tumor suppressor p15Ink4b in the regulation of hematopoietic progenitor cell fate. Blood Cancer J 3 (2013): e99.
- Chaussade L, Eymin B, Brambilla E, et al. Expression of p15 and p15.5 products in neuroendocrine lung tumours: relationship with p15INK4b methylation status. Oncogene 20 (2001): 6587-6596.
- What is neuroendocrine cancer? [Internet]. University of Iowa Hospitals & Clinics. 2019 [cited 2020Dec5]. Available from: https://uihc.org/health-topics/what-neuroendocrine-cancer
- Pulmonary Neuroendocrine Tumors [Internet]. Memorial Sloan Kettering Cancer Center. [cited 2020Dec5]. Available from: http://www.mskcc.org/cancer-care/types/lung/types/pulmonary-neuroendocrine-tumors
- Chen W, Li Z, Bai L, et al. NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci (Landmark Ed) 16 (2011): 1172-1185.
- Meylan E, Dooley AL, Feldser DM, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462 (2009): 104-107.
- Webster GA, Perkins ND. Transcriptional cross talk between NF-kappaB and p53. Mol Cell Biol 19 (1999): 3485-3495.
- Umemura S, Mimaki S, Makinoshima H, et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol 9 (2014): 1324-1331.
- Walls M, Baxi SM, Mehta PP, et al. Targeting Small Cell Lung Cancer Harboring PIK3CA Mutation with a Selective Oral PI3K Inhibitor PF-4989216. Clinical Cancer Research 20 (2013): 631-643.
- Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 4 (2009): 127-150.
- Fukuda RI, Tsuchiya K, Suzuki K, et al. HTLV-I Tax regulates the cellular proliferation through the down-regulation of PIP3-phosphatase expressions via the NF-κB pathway. Int J Biochem Mol Biol 3 (2012): 95-104.
- Bhateja P, Chiu M, Wildey G, et al. Retinoblastoma mutation predicts poor outcomes in advanced non small cell lung cancer. Cancer Med 8 (2019): 1459-1466.
- Kitamura H, Yazawa T, Sato H, et al. Small cell lung cancer: significance of RB alterations and TTF-1 expression in its carcinogenesis, phenotype, and biology. Endocr Pathol 20 (2009): 101-107.
- Small Cell Lung Cancer [Internet]. NORD (National Organization for Rare Disorders). 2019 [cited 2020Dec5]. Available from: https://rarediseases.org/rare-diseases/small-cell-lung-cancer/
- Meuwissen R, Linn SC, Linnoila R, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4 (2003): 181-189.
- Hubaux R, Thu KL, Coe BP, et al. EZH2 promotes E2F-driven SCLC tumorigenesis through modulation of apoptosis and cell-cycle regulation. J Thorac Oncol 8 (2013): 1102-1106.
- Rand TA, Sutou K, Tanabe K, et al. MYC Releases Early Reprogrammed Human Cells from Proliferation Pause via Retinoblastoma Protein Inhibition. Cell Reports 23 (2018): 361-375.
- Broude EV, Swift ME, Vivo C, et al. p21(Waf1/Cip1/Sdi1) mediates retinoblastoma protein degradation. Oncogene 26 (2007): 6954-6958.
- Brägelmann J, Böhm S, Guthrie MR, et al. Family matters: How MYC family oncogenes impact small cell lung cancer. Cell Cycle 16 (2017): 1489-1498.
- Duyao MP, Kessler DJ, Spicer DB, et al. Transactivation of the c-myc gene by HTLV-1 tax is mediated by NFkB. InMechanisms in B-Cell Neoplasia 1992 (1992): 421-424.
- Kotelevets L, Chastre E. Rac1 Signaling: From Intestinal Homeostasis to Colorectal Cancer Metastasis. Cancers (Basel) 12 (2020): 665.
- Zhang W, Liu H. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12 (2002): 9-18.
- Kumari S, Badana AK, G MM, et al. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark Insights 13 (2018): 1177271918755391.
- Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 138 (2010): 2101-2114.
- Myant KB, Cammareri P, McGhee EJ, et al. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell 12 (2013): 761-773.
- Myant KB, Scopelliti A, Haque S, et al. Rac1 drives intestinal stem cell proliferation and regeneration. Cell Cycle 12 (2013): 2973-2977.
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17 (2009): 9-26.
- Herbst A, Jurinovic V, Krebs S, et al. Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling. BMC Genomics 15 (2014): 74.
- Garcia MA, Nelson WJ, Chavez N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect Biol 10 (2018): a029181.
- Gao X, Xu W, Lu T, et al. MicroRNA-142-3p Promotes Cellular Invasion of Colorectal Cancer Cells by Activation of RAC1. Technol Cancer Res Treat 17 (2018): 1533033818790508.
- Sheng H, Shao J, Townsend CM, et al. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut 52 (2003): 1472-1478.
- Philp AJ, Campbell IG, Leet C, et al. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res 61 (2001): 7426-7429.
- Samuels Y. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 304 (2004): 554.
- Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 16 (2012): 15-31.
- Jokinen E, Koivunen JP. MEK and PI3K inhibition in solid tumors: rationale and evidence to date. Ther Adv Med Oncol 7 (2015): 170-180.
- Tsubaki M, Takeda T, Noguchi M, et al. Overactivation of Akt Contributes to MEK Inhibitor Primary and Acquired Resistance in Colorectal Cancer Cells. Cancers (Basel) 11 (2019): 1866.
- Bray S, Lee J, Kim ST, et al. Genomic characterization of intrinsic and acquired resistance to cetuximab in colorectal cancer patients. Sci Rep 9 (2019): 15365.
- Vitiello PP, Cardone C, Martini G, et al. Receptor tyrosine kinase-dependent PI3K activation is an escape mechanism to vertical suppression of the EGFR/RAS/MAPK pathway in KRAS-mutated human colorectal cancer cell lines. J Exp Clin Cancer Res 38 (2019): 41.
- Kale J, Kutuk O, Brito GC, et al. Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep 19 (2018): e45235.
- Pryczynicz A, Gryko M, Niewiarowska K, et al. Bax protein may influence the invasion of colorectal cancer. World J Gastroenterol 20 (2014): 1305-1310.
- Rokudai S, Fujita N, Hashimoto Y, et al. Cleavage and inactivation of antiapoptotic Akt/PKB by caspases during apoptosis. Journal of Cellular Physiology 182 (2000): 290-296.
- Kim MR, Jeong EG, Chae B, et al. Pro-Apoptotic PUMA and Anti-Apoptotic Phospho-BAD Are Highly Expressed in Colorectal Carcinomas. Dig Dis Sci 52 (2007): 2751-2756.
- Fang Y, Shen ZY, Zhan YZ, et al. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun 10 (2019): 3981.
- Albasri AM, Elkablawy MA, Ansari IA, et al. Prognostic Significance of Cyclin D1 Over-expression in Colorectal Cancer: An Experience from Madinah, Saudi Arabia. Asian Pac J Cancer Prev 20 (2019): 2471-2476.
- Huang CY, Tsai CW, Hsu CM, et al. The significant association of CCND1 genotypes with colorectal cancer in Taiwan. Tumor Biol 36 (2015): 6533-6540.
- Pelicano H, Martin D, Xu RH, et al. Glycolysis inhibition for anticancer treatment. Oncogene 25 (2006): 4633-4646.
- Fadaka A, Ajiboye B, Ojo O, et al. Biology of glucose metabolization in cancer cells. Journal of Oncological Sciences 3 (2017): 45-51.
- Itatani Y, Kawada K, Sakai Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int J Mol Sci 20 (2019): 5822.
- de Miranda NF, van Dinther M, van den Akker BE, et al. Transforming Growth Factor β Signaling in Colorectal Cancer Cells With Microsatellite Instability Despite Biallelic Mutations in TGFBR2. Gastroenterology 148 (2015): 1427-1437.
- Grady WM, Myeroff LL, Swinler SE, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res 59 (1999): 320-324.
- Takayama T, Miyanishi K, Hayashi T, et al. Colorectal cancer: genetics of development and metastasis. J Gastroenterol 41 (2006): 185-192.
- Xu Y, Pasche B. TGF-beta signaling alterations and susceptibility to colorectal cancer. Hum Mol Genet 16 (2007): R14-R20.
- Yan X, Xiong X, Chen Y-G. Feedback regulation of TGF-β signaling. Acta Biochimica et Biophysica Sinica 50 (2017): 37-50.
- Grady WM, Willis JE, Trobridge P, et al. Proliferation and Cdk4 expression in microsatellite unstable colon cancers with TGFBR2 mutations. Int J Cancer 118 (2006): 600-608.
- Ramsoekh D, Wagner A, van Leerdam ME, et al. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hered Cancer Clin Pract 7 (2009): 1-7.
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 (2012): 330-337.
- Means AL, Freeman TJ, Zhu J, et al. Epithelial Smad4 Deletion Up-Regulates Inflammation and Promotes Inflammation-Associated Cancer. Cell Mol Gastroenterol Hepatol 6 (2018): 257-276.
- Kitamura T, Kometani K, Hashida H, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet 39 (2007): 467-475.
- Inamoto S, Itatani Y, Yamamoto T, et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15-CCR1 Chemokine Axis. Clin Cancer Res 22 (2016): 492-501.
- Toor SM, Syed Khaja AS, El Salhat H, et al. Increased Levels of Circulating and Tumor-Infiltrating Granulocytic Myeloid Cells in Colorectal Cancer Patients. Front Immunol 7 (2016): 560.
- Klimovich B, Mutlu S, Schneikert J, et al. Loss of p53 function at late stages of tumorigenesis confers ARF-dependent vulnerability to p53 reactivation therapy. Proceedings of the National Academy of Sciences 116 (2019): 22288–22293.
- UpToDate. [cited 2020Dec5]. Available from: http://www.uptodate.com/contents/human-t-lymphotropic-virus-type-i-disease-associations-diagnosis-and-treatment.


 Impact Factor: * 5.814
Impact Factor: * 5.814 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 