Juvenile Trabecular Ossifying Fibroma (TrJOF) Orginating from Lateral Fronto-Orbital Bone with Secondary Aneurysmal Bone Cyst (ABC) Formation: Report of an Unusual Case and Literature Review
Article Information
Isra Abdulla1, Ali Basalamah1, Omar alwhaibi2, Ammar Alrikabi3, Ahmad Al Boukai4, Sherif Elwatidy1
1Department of Surgery, Division of Neurosurgery, King Saud University, Medical City
2 Department of Surgery, Division of Neurosurgery, King Saud University
3Department of pathology, King Saud University
4Department of Radiology, King Saud University
*Corresponding Author: Isra Abdulla, Neurosurgery Intern, Department of Surgery, Division of Neurosurgery, King Saud University, King Saud University Medical City, Riyadh, Saudi Arabia
Received: 22 June 2021; Accepted: 1 July 2021; Published: 13 July 2021
Citation: Isra Abdalla, Ali Basalamah, Omar alwhaibi, Ammar Alrikabi, Ahmad Al Boukai, Sherif Elwatidy. Juvenile Trabecular Ossifying Fibroma (Jtrof) Orginating from Lateral Fronto-Orbital Bone with Secondary Aneurysmal Bone Cyst (ABC) Formation: Report of an Unusual Case and Literature Review. Journal of Ophthalmology and Research 4 (2021): 205-220.
View / Download Pdf Share at FacebookAbstract
Juvenile trabeculated Ossifying Fibroma (JTrOF) is commonly arising from the maxilla and the mandibular bones, while Psammomatoid Juvenile Ossifying Fibroma (PsJOF) commonly arises in the sinonasal and calvarial bones. In our paper, we described a 12-year-old pediatric female who presented with a right fronto-orbital disfiguring swelling. She underwent total resection, peripheral osteotomy, and reconstruction. Pathology reported a TrJOF associated with Aneurysmal Bone Cyst (ABC) formation. TrJOF rarely originates from superolateral wall orbital bone, and it is exceedingly rare when it is associated with ABC formation. Pathologically, it is difficult to distinguish TrJOF from other FOLs due to similarities and overlap. The ideal treatment is total surgical excision and reconstruction. The Surgical resection is challenging as regards functional and cosmetic outcomes.
Keywords
Juvenile ossifying fibroma; Aneurysmal bone cyst; Trabecular ossifying fibroma; Orbital tumors; Craniofacial surgery
Juvenile ossifying fibroma articles; Aneurysmal bone cyst articles; Trabecular ossifying fibroma articles; Orbital tumors articles; Craniofacial surgery articles
Article Details
Abbreviations:
FOLs- Fibro-osseous lesions; TrJOF: Trabecular juvenile ossifying fibroma; PsJOF-Psammomatoid juvenile ossifying fibroma; JOF-Juvenile ossifying fibroma; OF- Ossifying fibroma; COF- cemento-ossifying fibroma; FD- Fibrous dysplasia; OFD- Osteofibrous dysplasia; ABC-aneurysmal bone cyst.
1. Introduction
Juvenile trabeculated Ossifying Fibroma (TrJOF) is one of the benign fibro-osseous lesions (FOLs) that may occur anywhere in the skeleton predominately affecting jaw bones (the maxilla and the mandible “gnathic”). It is extraordinarily rare that (TrJOF) involves the paranasal sinuses, orbital bones, and other extra-gnathic bones [1-3]. An aneurysmal bone cyst (ABC) is an expansile blood-filled multi-cystic lesion that commonly affects children. It's arising de novo in most cases or secondary to another osseous lesion [4, 5] Herein, we report an exceedingly rare case of TrJOF originating from the fronto-orbital bone (calvarial) with a secondary ABC formation.
2. Case Report
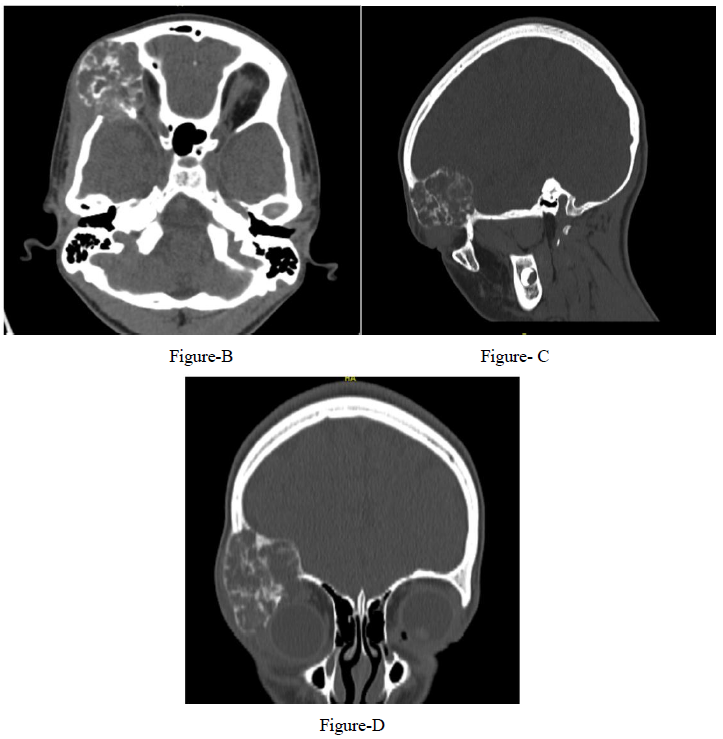
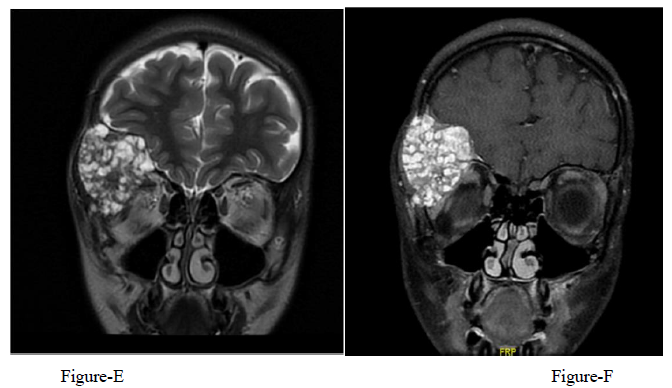
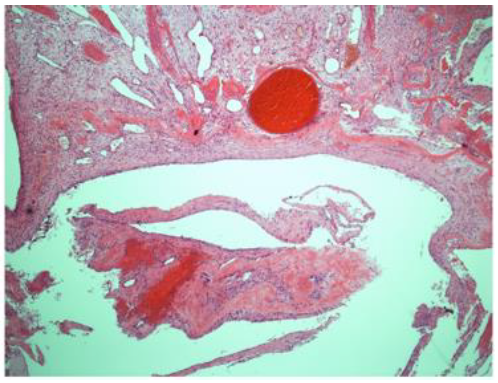
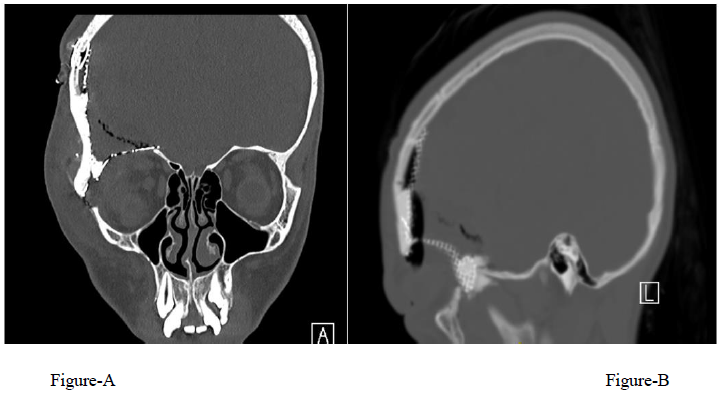
A 12-year-old girl presented with a 1-year history of right fronto-orbital swelling involving the superior and lateral borders of the orbit. The lesion was slowly increasing in size over 2 years then stabilized. The patient did not have any previous history of trauma and did not report any visual problems. Physical examination revealed a right orbital disfiguring swelling (Figure 1-A), hard in consistency and tender when exerting pressure over it, otherwise, the examination was unremarkable with no neurological deficits. Computed tomography (CT) scan of the Brain and Orbit showed an expansile multiseptated bony lesion in the right frontal bone extending into the right orbital superolateral wall. The lesion measures approximately 4 x 3.6 x 4.2 cm (AP, transverse and craniocaudal dimensions). It exerts a mass effect on the right orbital structures with subsequent proptosis. Moreover, a marked thinning of the cortex of the involved bone with areas of cortical breach intracranially and intraorbitally (Figure B, C, D) Magnetic resonance imaging (MRI), the tumor appeared multicystic, well-circumscribed with small areas of fluid-fluid levels (Figure 1-E, F). focal osteoblastic and osteoclastic activity with osteoid formation seen at the periphery as well. Postcontrast images showed diffuse intense heterogeneous enhancement with areas of high perfusion on rCBV and few small foci of diffusion restriction. The differential diagnosis based on radiological findings were fibro-osseous tumors including (osteo-fibrous dysplasia, fibrous dysplasia, ossifying fibromas, and osteoblastoma), intraosseous hemangioma, and aneurysmal bone cyst.
2.1 Surgical Technique
After taking consent from the family, the patient was taken for total resection and reconstruction. Steps of surgery are described below.
- General anesthesia, supine position, head fixed in a 3- point fixator and rotated to the left about 40 degrees with slight extension.
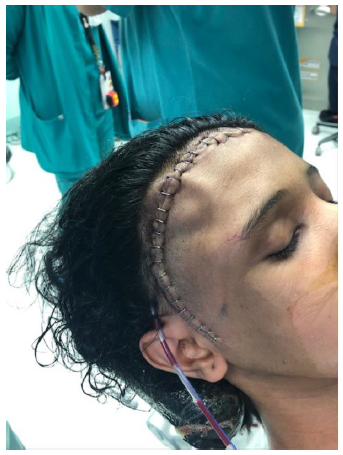
- Curvilinear skin incision extending from the just above the zygomatic arch half a cm anterior to the tragus, extending upward behind the hair line to cross the midline, Figure 2-A.
- Skin, temporalis fascia and pericranium were elevated as one flap, temporalis muscle detached subperiosteally. We exposed the frontal, temporal bone, and orbit, we dissected the periorbital fascia completely from the orbital bones.
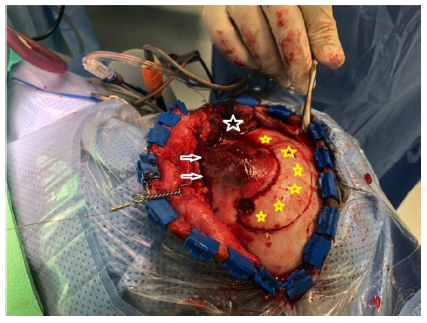
- We did a strip craniotomy around the tumor (leaving a thin rim of healthy bone around the tumor), Figure 2 B.
- The dura was carefully and completely dissected from the involved bone.
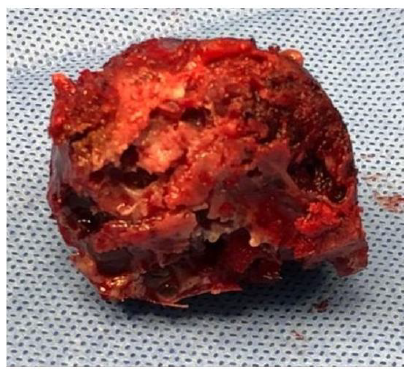
- With the oscillating saw and craniotome we removed the whole tumor en-bloc Figure 2-C, the resected part included the superior and lateral orbital walls with the superior and lateral orbital rim
- We used split calvarial bone grafts fixed together with long titanium microplate and screws to reconstruct the superior and lateral rim of the orbit, it took a lot of efforts and trials to achieve symmetry of both orbits.
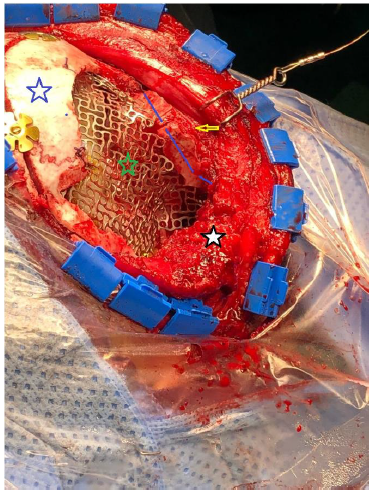
- We used titanium mesh to cover defects in the roof and lateral wall of the orbit as well as the skull defect, Figure 2-D. The Titanium mesh was finally covered with bone cement (Hydroxy Apatite) and contoured to merge with the normal bone, Figure 2-E.
- The wound was closed in layers as routine with suction drain, Figure 2-F.
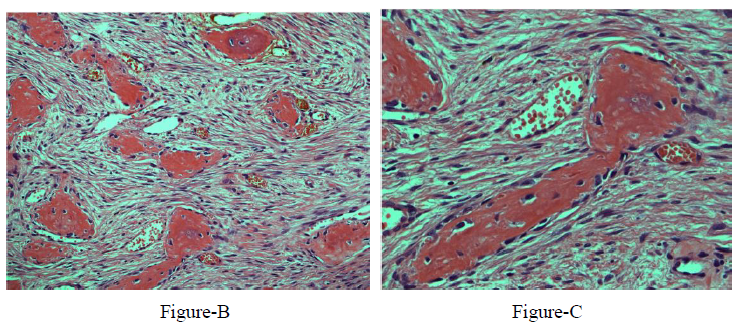
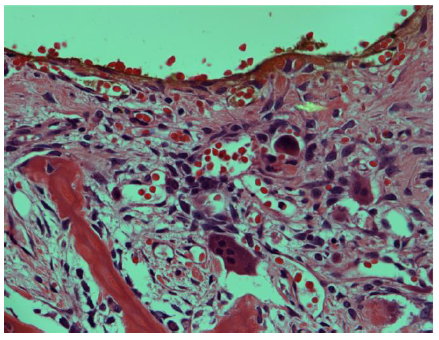
The final histopathology report was consistent with trabeculated ossifying fibroma with a secondary multiloculated aneurysmal bone cyst. In addition, there was a focal osteoblastic and osteoclastic activity with osteoid formation at the periphery of the lesion Figure-3.
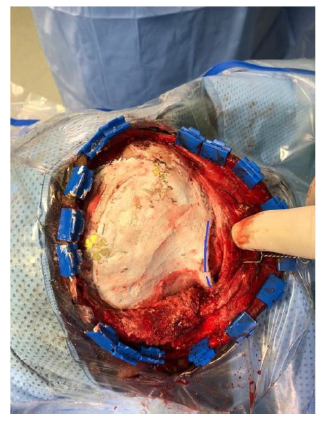
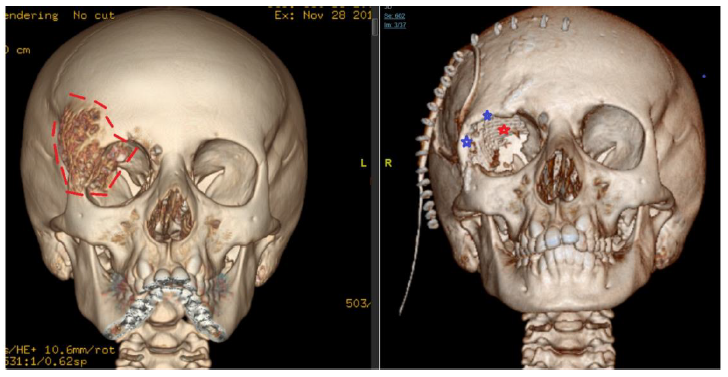
Postoperative course was uneventful with no postoperative complications. Postyoperative CT scan showed no residual tumor and satisfactory reconstruction, Figure-4. One year after surgery, the patient was doing well, there was no delyed complications and she was happy with the cosmetic outcome, Figure 5.
-Reconstruction of superior and lateral orbital rim using split calvarial graph (blue line).
-Reconstruction of skull defect using titanium mesh (green star).
-Strip craniotomy (white star).
-Globe (yellow arrow).
- Area resected (outline in red dashed line)
- Reconstruction of superior and lateral orbital rim (blue stars)
- Reconstruction of the orbital roof with titanium mesh (red star)
3. Discussion
Fibro-osseous lesions (FOLs) are a group of benign conditions arising when the bone is replaced by connective tissue. In 2008, Eversole et al classified FOLs of the craniofacial complex into five categories comprising; bone dysplasias, cemento-osseous dysplasias, inflammatory/reactive processes, metabolic disease, and neoplastic lesions. The juvenile ossifying fibroma (OF) was considered a subtype of neoplastic lesions [6]. According to the 4th edition of WHO classification of the head and neck tumors which was released in 2017, OF was classified under the fibro-osseous and osteochondromatous lesions subcategory of the Odontogenic and maxillofacial bone tumors, furthermore, OF can be subclassified into Juvenile Ossifying Fibroma and cemento-ossifying fibroma [7].
OF is thought to arise from mesenchymal blast cells. It is seen in pediatrics and adults with a female predilection [8]. Juvenile Ossifying Fibroma (JOF) is a term used to describe the OF variant occurring in young patients with a mean age of 11 years old. It typically involves craniofacial bones, and it comprises two distinct histopathological types including psammomatoid juvenile ossifying fibroma (PsJOF) and TrJOF [1, 9-11]. In literature, TrJOF was first described in 1965 by Reed et al under the general name of JOF while PsJOF was 1st described by Benjamin in 1938 [9]. It is usually presenting at an earlier age on average being 8-12 years while PsJOF presenting on average between 16-33 years[1, 2, 9]. The cemento-ossifying fibroma (COF) was 1st described by Menzel in 1872. COF is peaking at the 3rd to 4th decades of life with female predominance [10].
In the craniofacial bones, TrJOF predominately arises from the maxilla and mandibular areas (gnathic), and it's extremely rare to arise from any other extraganthic areas [9, 12]. Table 1 summarizes all reported extragnathic cases of Tr JOF.
In the presented case the TrJOF originated from the lateral fronto-orbital bone wall.
A wide range of differential diagnoses was proposed based on the patent’s initial clinical and radiological presentation including OF, fibrous dysplasia (FD), ABC, osteoid osteoma, osteoblastoma, and osteosarcoma. The initial frozen section that was sent intraoperatively reported as FD. The final histopathological report was JOF of the trabecular subtype (TrJOF) with an associated ABC. Ossifying fibroma, FD, and osteofibrous dysplasia (OFD) are closely related, all are benign proliferative FOLs in which there is a replacement of normal bone with fibrous tissue [13]. They overlap clinically, histologically, and radiographically, with some key differentiating features.
Radiologically, FD typically exhibits a characteristically diffused ground-glass appearance that merges with the normal surrounding bone. It is polyostotic and usually has an irregular border and it may present as completely radiolucent or homogenously sclerotic lesions. On contrary to the FD, TrJOF, PsJOF and COF are monostotic and are usually associated with bony expansion, well-defined borders with central mixed lytic and osteoblastic areas, and spherical architecture, while FD maintains the same architecture of the involved bone. The ground-glass appearance is also seen occasionally with PsJOF, while TrJOF lacks this feature and manifests as scattered trabeculated calcifications within a primarily radiolucent structure [9]. Cemento-ossifying fibroma is usually not destructive and has an intact cortex on imaging while JOF typically demonstrates cortical thinning, disruption, and/or infiltration of surrounding tissue due to its more locally aggressive nature [7]. Furthermore, a hyperdense center surrounded by hypodensity (target-like appearance) may be seen in OF especially PsJOF and COF [14]. On MRI, OF is usually iso to hypo intense on T1 and has variable intensity on T2. Cysts, non-ossified regions, and mucocele are associated usually with a high T2 signal. Post-contrast administration, an enhancement is expected especially for the outer lesional layer and the septa, and due to the high osteoblastic activity, and the lesions demonstrate high Technetium-99 radioisotope uptake [7, 15]. On the other hand, FD usually has a low signal intensity on T2 weighted MRI and enhancement is seen on the expanded diploe only [10, 15].
Histopathologically, the FOLs may have similar microscopic features including the increase of fibroblastic-stroma, variable degrees of osteoid or cementum-like tissue, and variable degrees of calcified structures [10]. On the othedrhand, FD is commonly described with the appearance of chinse letters due to the thin curved bone trabeculae interspersed with fibroblast-like spindle cells, but it characteristically lacks the osteoclastic multinucleated giant cells seen in TrJOF along with anastomosing trabeculae of woven mature lamellar bone that is lined with osteoclasts and osteoblasts. Furthermore, FD has no osteoblasts rimming, less stromal cellularity, collagen, less vascularity, and maintains a similar pattern throughout the lesion. Both subtypes of JOF are unencapsulated infiltrating normal surrounding bone, with PsJOF having a typical curved or spherical ossicle (psammomatoid calcifications), while COFs are encapsulated, rimmed with osteoblasts, and have a variable ratio of bone/cementum-like tissue [7-9, 16]. Presence of areas of ABC in association with these FOLs may indicate rapid growth [17].
From a genetic point of view, FD has been proven to have a characteristic α subunit mutation of single-transducing G protein, which is lacking in OFs. PsJOF may have chromosomal breakpoints at Xq26 and 2q33 leading to (X;2) translocation. Few studies reported HRPT2 gene and germ-line mutation in COF. Although genetic studies of TrJOF are generally not well understood, MDM2 gene amplification was found in both TrJOF and PsJOF [16, 18].
The clinical course is another key aspect to differentiate JOFs. The majority of benign FOLs demonstrate slow progressive non-infiltrative mass effect over years. JOFs have an aggressive nature causing facial deformity occasionally. The initial asymptomatic growth may rarely exhibit symptoms. Other than local pain, paresthesia and deformity, symptoms are primarily related to the primary site of origin and invaded structures including but not limited to visual loss, proptosis, nasal blockage, anosmia, and hearing disturbance [1, 3, 19] The aggressive nature may be attributed to the young age of onset and the association of ABC. Continuous growth is expected if JOF is not treated [17]. Contrary to JOF, COF demonstrates a slow rate of growth and no reports of recurrence of malignant transformation [7, 10]. Several Investigators reported that OF originating from paranasal sinuses areas tend to be more aggressive when they are compared to those originating from the mandibular area while others have concluded a lack of relationship between location and aggressiveness [20].
FD, on the other hand, usually spontaneouslyregressesat puberty and can be observed if no local complications are associated. Table 3, summarizes radiological, histopathological, and genetic differences between fibrous FD and subtypes of OF.
A proper initial diagnosis is suggested to guide the treatment plan7. Complete surgical excision of OF is recommended by the majority of authors [21]. According to a recent systematic review reported by Chrcanovic et al in 2019, enucleation and curettage alone are associated with a high recurrence rate, and this risk is significantly reduced when adjunctive management is added such as ostectomy. This study found that the treatment of choice for both JOF subtypes should be enucleation with peripheral osteotomy as it is associated with complete resection that renders recurrence [17]. Conservative approaches for lesions behaving benignly and not producing deformities such as curettage and osteoctomy may be attempted as 1st line management for these lesions [7, 22] The risk of recurrence for OF is considerably high ranging between 30-56% and this risk is significantly present in cases of incomplete resection [3, 20]. JOF may require removal multiple times to reach a cure. No malignant progression was reported to date [10, 23] On the other hand, COF tends not to recur after removal [7]. Interferon-alpha systemic therapy use was reported to decrease the rate of recurrence while radiotherapy is contraindicated due to the risk of malignant transformation [9]. In our case, upfront total resection, peripheral osteotomy, and reconstruction were attempted due to a relatively fast-growing rate (less than a year), painful locally deforming nature, and controversial radiological diagnosis.
ABC is another benign FOLs that is osteolytic, expansile in nature with connective tissue septations in between blood-filled spaces [4]. ABC may arise primarily or secondary to another bone lesion and it has been reported secondary to FD and JOF. The pathogenesis is not well understood. it was postulated that the formation of an ABC starts as myxoid change (myxofibrous cellular stroma overproduction) within a hemorrhagic stroma with osteoclastic giant cells and reactive bone that is gradually expanding and forming multiple cysts with thin fibrous septae. Moreover, due to local pressure, intracellular edema, weak supporting tissue, and maldeveloped vascularity, cysts may form, and blood may be pooled into these cystic cavities following small vessels rupture [12, 24-26]. when OF is associated with a fluid-fluid level, ABC is strongly suggested. However, this sign may be associated with other lesions such as simple bone cyst, cavernous haemangioma, osteosarcomas, and others [7].
ABC associated with JOF was reported predominantly with psammomatoid variant of JOF, while the association with TrJOF were reported in a total of 8 cases so far that are summarized in Table 2 [10, 12, 25-29].
|
No. of cases reported |
Reference / date |
Trabecular Juvenile Ossifying Fibroma (TrJOF) |
Location of origin |
|
1 |
Thomas et al. [14] |
TrJOF |
Orbital roof |
|
1 |
Nakagawa et al. [23] |
TrJOF |
Ethmoid sinus, orbit, and anterior cranial fossa |
|
1 |
Fakadej et al [21] |
TrJOF |
Ethmoid sinus |
|
1 |
Marvel JB et al. [30] |
TrJOF |
Ethmoid and sphenoid sinuses |
|
1 |
Tunc M et al. [20] |
TrJOF |
Lateral orbital wall. |
|
1 |
Caylakli F et al. [31] |
TrJOF |
Middle turbinate |
|
2 |
Antonio et al. [11] |
TrJOF |
Orbital roof. |
|
1 |
Yazici B et al. [10] |
TrJOF |
Lateral Orbit. |
|
1 |
Bohn OL et al. [32] |
TrJOF |
Nasal cavity and anterior cranial fossa |
|
1 |
B T Yang et al. [12] |
TrJOF |
Paranasal sinuses |
|
1 |
Marian Christoph Neidert et al. [33] |
TrJOF |
Sella |
|
1 |
Marglani OA et al. [34] |
TrJOF |
Nasal cavity and Paranasal sinuses |
|
1 |
Owosho AA et al. [24] |
TrJOF |
Ethmoid sinus |
|
1 |
Ciniglio Appiani M et al. [35] |
TrJOF |
Nasal cavity and Paranasal sinuses |
|
1 |
Bipasha Mukherjee et al. [9] |
TrJOF |
Superolateral orbital wall. |
|
1 |
Ta NH et al. [19] |
TrJOF |
Ethmoid sinus |
|
Total number: 17 |
Table 1: Confirmed Cases of Trabecular Juvenile Ossifying Fibroma (TrJOF) reported in the literature arising from the Skull excluding maxilla and mandible bones.
|
No |
Reference / date |
Diagnosis |
Location of origin |
|
1 |
Noffke CE et al. [27] |
TrJOF and ABC |
Mandible |
|
1 |
Yazici B et al. [10] |
TrJOF and ABC |
Lateral Orbit wall. |
|
1 |
Sankaranarayanan S et al. [28] |
TrJOF and ABC |
Maxilla. |
|
1 |
Silva CA et al. [25] |
TrJOF and ABC |
Maxilla. |
|
1 |
B T Yang et al. [12] |
TrJOF and ABC |
Paranasal sinuses |
|
2 |
Urs AB et al. [29] |
TrJOF and ABC |
Maxillae and Mandible |
|
1 |
Reddy AVS et al. [26] |
TrJOF and ABC |
Mandible. |
|
Total number: 8 |
Table 2:Confirmed Cases of Trabecular Juvenile Ossifying Fibroma (TrJOF) reported in the literature associated with aneurysmal bone cyst (ABC).
|
FD |
TrJOF |
PsJOF |
COF |
|
|
“polyostotic” |
“monosotic” |
“monosotic” |
“monosotic” |
|
|
Histopathology |
Chinese letters appearance. Lack of osteoblastic rimming.Similar architecture to involved bone. |
Un-encapsulated infiltrating surrounding bone. Osteoclastic multinucleated giant cells, anastomosed with trabeculated lamellar bone. Osteoblastic rimming. |
Un-encapsulated infiltrating surrounding bone. Psammomatoid calcifications.Osteoblastic rimming. |
Encapsulated. Variable ratio of bone/cementum tissue.Osteoblastic rimming. |
|
Radiology |
Diffused ground-glass appearance. Radiolucent or homogeneously sclerotic. |
Scattered trabeculated calcifications within radiolucent structure. Locally aggressive with well-defined borders. |
Target-like appearance, occasionally ground-glass. Locally aggressive with well-defined borders. |
Target-like appearance. Non-destructive with intact cortex. |
|
(MRI) |
Low intensity (T1). Enhancement on expanding dipole (post-contrast) |
Iso to hypo intense (T1). Variable intensity (T2). Enhancement of outer layers and septae (post-contrast) |
Similar to TrJOF. High technetium-99 uptake. MDM2 gene amplification. |
|
|
Genetics |
α Submit Mutation of single-transducing G protein |
MDM2 gene amplification |
Chromosomal breakpoints at Xq26 and 2q33 leading to (X;2) translocation. |
HRPT2 gene and germ line mutations |
|
Course |
Spontaneous regression at puberty |
Locally aggressive. No spontaneous regression. May recur if incompletely resected. |
Similar to TrJOF. |
Slow growth rate. No recurrence or malignant transformation reported. |
Table 3: Comparison between subtypes of ossifying fibroma (OF) and fibrous dysplasia (FD).
4. Conclusion
TrJOF commonly arises from maxilla and mandibule, while PsJOF commonly arises in the sinonasal and calvarial bones. Pathologically, it is difficult to distinguish TrJOF from other FOLs due to similarities and overlap. The ideal treatment is total surgical excision and reconstruction. The Surgical resection is challenging as regards functional and cosemetic outcome.
References:
- El-Mofty S. Psammomatoid and trabecular juvenile ossifying fibroma of the craniofacial skeleton: two distinct clinicopathologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93 (2002): 296-304.
- Reed Rj, Hagy Dm. Benign Nonodontogenic Fibro-Osseous Lesions of The Skull; Report of Two Cases. Oral Surg Oral Med Oral Pathol 19 (1965): 214-227.
- Johnson LC, Yousefi M, Vinh TN, et al. Juvenile active ossifying fibroma. Its nature, dynamics, and origin. Acta Otolaryngol Suppl 488 (1991): 1-40.
- Wilson M, Snyderman C. Fibro-Osseous Lesions of the Skull Base in the Pediatric Population. J Neurol Surg B Skull Base 79 (2018): 31-36.
- Putnam A, Yandow S, Coffin CM. Classic adamantinoma with osteofibrous dysplasia-like foci and secondary aneurysmal bone cyst. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc 6 (2003): 173-178.
- Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol 2 (2008): 177-202.
- Kawaguchi M, Kato H, Miyazaki T, et al. CT and MR imaging characteristics of histological subtypes of head and neck ossifying fibroma. Dentomaxillofac Radiol 47 (2018): 20180085.
- Kendi AT, Kara S, Altinok D, Keskil S. Sinonasal ossifying fibroma with fluid-fluid levels on MR images. AJNR Am J Neuroradiol 24 (2003):1639-1641.
- Mukherjee B, Devi U, Agarkar S. Recurrent benign ossifying fibroma of the orbit - clinical, radiological profile and management options in a child [published correction appears in Orbit 38 (2019): 252-255.
- Yazici B, Yazici Z, Yalçinkaya U. Aneurysmal bone cyst secondary to ossifying fibroma in the orbit. Ophthalmic Plast Reconstr Surg 27(2011): e84-e85.
- Cruz AA, Alencar VM, Figueiredo AR, et al. Ossifying fibroma: a rare cause of orbital inflammation. Ophthalmic Plast Reconstr Surg 24 (2008): 107-112.
- Yang BT, Wang YZ, Wang XY, et al. Imaging study of ossifying fibroma with associated aneurysmal bone cyst in the paranasal sinus. Eur J Radiol 81 (2012): 3450-3455.
- McCarthy EF. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol 7 (2013): 5-10.
- Thomas G, Kasper K. Ossifying fibroma of the frontal bone. Arch Otlaryngol 83 (1966): 69–72.
- Wakefield MJ, Ross AH, Damato EM, et al. Review of lateral orbital wall ossifying fibroma. Orbit 29 (2010): 317-320.
- Sakamoto A, Oda Y, Iwamoto Y, Tsuneyoshi M. A comparative study of fibrous dysplasia and osteofibrous dysplasia with regard to Gsalpha mutation at the Arg201 codon: polymerase chain reaction-restriction fragment length polymorphism analysis of paraffin-embedded tissues. J Mol Diagn 2 (2000): 67-72.
- Chrcanovic BR, Gomez RS. Juvenile ossifying fibroma of the jaws and paranasal sinuses: a systematic review of the cases reported in the literature. Int J Oral Maxillofac Surg 49 (2020): 28-37.
- Sawyer JR, Tryka AF, Bell JM, Boop FA. Nonrandom chromosome breakpoints at Xq26 and 2q33 characterize cemento-ossifying fibromas of the orbit. Cancer 76 (1995): 1853-1859.
- Ta NH, Addison A, Beigi B, Philpott C. Unilateral visual loss resulting from orbital encroachment of an ethmoidal juvenile trabecular ossifying fibroma. Ann R Coll Surg Engl 101 (2019): e111-e114.
- Tunc M, Char DH. Ossifying fibroma of the lateral orbital wall in an adult. Orbit 18 (1999): 291-293.
- Fakadej A, Boynton JR. Juvenile ossifying fibroma of the orbit. Ophthal Plast Reconstr Surg 12 (1996): 174–177.
- Abuzinada S, Alyamani A. Management of juvenile ossifying fibroma in the maxilla and mandible. J Maxillofac Oral Surg 9 (2010): 91-95.
- Nakagawa K, Takasato Y, Ito Y, et al. Ossifying fibroma involving the paranasal sinuses, orbit, and anterior cranial fossa: case report. Neurosurgery 36 (1995):1192–1195.
- Owosho AA, Hughes MA, Prasad JL, et al. Psammomatoid and trabecular juvenile ossifying fibroma: two distinct radiologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol 118 (2014): 732-738.
- Silva CAB, Silva AD, Soares JA de C, et al. Trabecular juvenile ossifying fibroma with aneurysmal bone cyst: a rare presentation. Pediatr Dent 33 (2011): 388-391.
- Reddy AVS, Reddy KRK, Prakash AR, et al. Juvenile ossifying fibroma with aneurysmal bone cyst: a case report. J Clin Diagn Res 8 (2014): ZD01-ZD02.
- Noffke CE. Juvenile ossifying fibroma of the mandible. An 8-year radiological follow-up. Dentomaxillofac Radiol 27 (1998): 363-366.
- Sankaranarayanan S, Srinivas S, Sivakumar P, et al. “Hybrid” lesion of the maxilla. J Oral Maxillofac Pathol 15 (2011): 299-302.
- Urs AB, Augustine J, Arora S, et al. Rare pediatric presentation of an aneurysmal bone cyst with trabecular juvenile ossifying fibroma and ossifying fibroma. Int J Pediatr Otorhinolaryngol 77 (2013): 576-580.
- Marvel JB, Marsh MA, Catlin FI. Ossifying fibroma of the mid-face and paranasal sinuses: diagnostic and therapeutic considerations. Otolaryngol Head Neck Surg 104 (1991): 803-808.
- Caylakli F, Buyuklu F, Cakmak O, et al. Ossifying fibroma of the middle turbinate: a case report. Am J Otolaryngol 25 (2004): 377-378.
- Bohn OL, Kalmar JR, Allen CM, et al. Trabecular and psammomatoid juvenile ossifying fibroma of the skull base mimicking psammomatoid meningioma. Head Neck Pathol 5 (2011): 71-75.
- Neidert MC, Woernle CM, Burkhardt JK, et al. Case report: Trabecular juvenile ossifying fibroma presenting as a sellar mass. J Neurol Surg A Cent Eur Neurosurg 74 (2013): 405-409.
- Marglani OA, Kassab MY, Raza SA. Minimally invasive endoscopic removal of a massive trabecular juvenile ossifying fibroma of the paranasal sinuses. Saudi Med J 35 (2014): 872-875.
- Ciniglio Appiani M, Verillaud B, Bresson D, et al. Ossifying fibromas of the paranasal sinuses: diagnosis and management. Acta Otorhinolaryngol Ital 35 (2015): 355-361.


 Impact Factor: * 1.2
Impact Factor: * 1.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
