Indications, Visual Outcomes and Complications of Boston Keratoprosthesis; a Systematic Review
Article Information
Mohammed Al Fayyadh1, Majed Al Subaie2, Abdullah Al Rajhi3, Khalid Al Arfaj4
1Departement of Ophthalmology, Prince Mutaib bin Abdulaziz Hospital, Aljouf, Saudi Arabia
2Department of Ophthalmology, Dhahran Eye Specialist Hospital, Dhahran, Saudi Arabia
3Departement of Ophthalmology, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
4Departement of Ophthalmology, College of Medicine, King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
*Corresponding author: Mohammed Al Fayyadh, Departement of Ophthalmology, Prince Mutaib bin Abdulaziz Hospital, AL jouf, Saudi Arabia
Received: 29 December 2021; Accepted: 07 January 2022; Published: 12 January 2022
Citation: Mohammed Al Fayyadh, Majed Al Subaie, Abdullah Al Rajhi, Khalid Al Arfaj. Indications, Visual Outcomes and Complications of Boston, Keratoprosthesis; a Systematic Review. Journal of Ophthalmology and Research 5 (2022): 1-19
View / Download Pdf Share at FacebookAbstract
Over many years the development in Boston keratoprosthesis (B-KPro) and its design and post-operative management has much improvement. Recently, the use of Boston has increased signify-cantly. It became a reasonable option for patients with corneal disease and poor prognosis for a traditional penetrating keratoplasty. Immune systems diseases, deep corneal vascularization, compound injuries or limbal stem-cell deficiencies (LSCD), the grafts are more likely to be rejected are the most indications of B-KPro. In this review, systematic literature search about indications, outcomes, complications and the retention rates over a period of 6 years from 2015 to 2021. The studies published in English .The outcomes are good along Intermediate and long-term but the risk of serious complications after B-KPro making frequent lifelong follow up, monitoring and treatment a must.
Keywords
Boston keratoprosthesis, KPro, B-KPro, corneal transplantation, limbal stem cell deficiency
Boston keratoprosthesis articles, KPro articles, B-KPro articles, corneal transplantation articles, limbal stem cell deficiency articles
Article Details
1. Intruduction
Corneal illnesses are the second main source of visual deficiency around the world, cataract being the most common [1-3]. Corneal transplantation is extremely effective at re-establishing vision, giving positive results in many complicated or severe conditions. However, in certain condition, such as immune system diseases, deep corneal vascularization, compound injuries or limbal stem-cell deficiencies (LSCD), the grafts are more likely to be rejected [1,4]. Keratoprosthesis (KPro) often allows for visual restoration in conditions where transplantation of the cornea fails to offer a good prognosis [5]. The Boston keratoprosthesis or B-KPro graft, in the current ophthalmic trend, has shown extensive utility and is globally acclaimed to be effective. Globally, some 36 million people have been established to be blind. Corneal illness is amongst the leading five causes of blindness, the top four being listed as cataract, refractive errors (uncorrected), glaucoma and AMD (age-related macular degeneration). Also, worldwide, bilateral blindness or visual impairment has been estimated at 4.9 million people and unilaterally, 23 million. The concept of a prosthetic or artificial cornea has gained traction with the populations, bringing in hope of normal or semi-normal vision [6].
In 1974, after examining the first case series of patients who had the type 1 B-KPro implant placed, the FDA approved the design and utilization in further similar circumstances. B-KPro has undergone various changes and improvements since its inception in order to improve the surgeon's confidence as well as the postoperative outcomes. Corneal transplants, i.e. keratoplasty (penetrating, anterior lamellar, or endothelial), is effective in re-establishing visual acuity. Keratoprosthesis implantation entails removing the cornea to its full thickness and replacing it with an artificial cornea. An artificial cornea is a notion that has been around for over 200 years [7]. Many of these impaired patients are found in developing countries where resources are always scarce, leading to a necessity of affordable quality of treatment by keratoprosthesis. Although Nussbaum is proffered to have conducted the first human transplant of KPro in 1855, many contradict this by putting forth that an ophthalmologist, Guillaume Pellier de Quengsy's brother, may have been the first to perform such a procedure in 1789 during the French Revolution.
Experts believe that Nussbaum’s surgery using a quartz crystal implant may have been the first reported human KPro surgery. However, history shows that Keratoprosthesis is a two-century-old notion that was originally fully detailed during the French Revolution, but attention decreased after Eduard Zirm, in 1905, conducted the first full-thickness penetrating keratoplasty in human, successfully [8-10]. For many, corneal transplantation is a blessing that could allow an opportunity for sight. For some, however, this may not be possible owing to the hostile ocular environment, which makes it difficult for the ocular graft to survive. Graft rejection is common in several eye conditions, such as aniridia, severe chemical burns, or even autoimmune illnesses. Keratoprosthesis can provide hope and the prospect of eyesight restoration in such instances [4]. Recent decades have seen the development of several KPros, of which only three are currently in utilization for clinical practice. These are the Osteo Odonto KPro (OOKP), the Boston type 1 KPro, and the Boston type 2 KPro. With Claes Dohlman showering his lifetime supervision on the Boston KPro, this prosthetic has progressed from a simple innovative concept reaching the status of a well-established technology in the last 50 years. The "Nut-and-Bolt" design, also known as the screw design, has become redundant and no longer used. In the current transplant, a collar button design has been implemented with a snap-on, 2-piece architecture containing the donor corneal transplant sandwiched between two plates. A central opening in the donor cornea is passed by an optic stem on the front plate made of poly-methyl-methacrylate. The porous titanium back plate allows aqueous humor to supply the donor cornea with nutrients and hydration [11]. In the older versions, a second titanium ring was employed to lock the back plate, which is again not needed in the latest design. The device is sutured firmly into eye of the recipient during the corneal trephination, just as a penetrating corneal graft. Short duration topical steroids in addition to lifelong prophylactic antibiotic eye drops are typically given. It is necessary to indefinitely wear a soft contact lens as part of the postoperative treatment. The Boston KPro can be used mainly in eyes with appropriate blinking function (wet eyes) while in dry, non-blinking eyes, the modified OOKP is the alternative [12].
This device was given commercial FDA approval in 1992. From then on, its utility has steadily expanded over the past 20 years, not only in the United States but also worldwide. In Boston, MA, and other locations throughout the world, an active keratoprosthesis research program continues to foster device innovation [13-15]. The most often employed artificial cornea i.e. keratoprosthesis remains at present, the Boston Keratoprosthesis (KPro). It's a procedure for treating corneal disease that doesn't respond to regular penetrating keratoplasty (PKP) or corneal transplantation. The continuous research and subsequent advancements in design along with the insisted upon enhanced postoperative care have delivered improved outcomes. This has catalyzed an exponential increase in the usage of the device in recent years [16-19].
2. Development and Advancement of the Boston KPro Type 1
First introduced in the 1970s by Dr. Dohlman, the Boston Type I Keratoprosthesis is at present, the most commonly employed keratoprosthesis device, both in the US and globally. B-KPro boasts a collar button design, consisting of three parts: a front plate with an optical stem, a corneal allograft button and a back plate. Typically, the front and back plates are shaped using medical-grade PMMA (poly-methyl-methacrylate). These sandwich a corneal graft and are secured with a titanium locking ring. Once the device assembly is complete, a partial-thickness trephination is done on the host cornea and full-thickness resection completed using curved corneal scissors. The keratoprosthesis is then secured to host tissue using interrupted or running sutures [1, 2, 13]. The power of the B-KPro is decided by two elements, namely, the radius of curvature of the optical surface (set at 3.5–3.7 mm central diameter) and the front plate (5mm central diameter).
The B-KPro can be availed in either a single standard pseudo-phakic power or an aphakic power at customized axial lengths (range 16–31 mm in increments of 1 mm). The front edge is refined during the manufacturing process carefully, in order to avoid the sensation of a foreign body ensuring a smooth blend between the poly-methyl-methacrylate and donor cornea. The central stem comprises an intraocular segment and a locking interface. The intraocular segment has a flat interface allowing the passage of light rays without bending. The locking interface secures the back plate. In the original design, two and a half turn threads were present for screwing the back plate in place. 2003 marked a turning point in the screws when a titanium locking ring was added to secure the back plate in position, thereby preventing later intraocular unscrewing of the plate [3, 4, 9, 12]. In 2007, another revolutionized newer stem having no threads was introduced. This thread less design was anticipated to eliminate corneal graft damage in the process of screwing, show easier application by the ophthalmic surgeons and bring about inexpensive manufacture of the device possible owing to the process of moulding rather than machining. The back plate has shown evolution and progress over the past two decades. Early cycles of the back plate comprised of a strong 8 mm PMMA. In any case, the tall frequency (over 50%) of sterile keratolysis watched with this show was thought to be auxiliary to diminished wholesome bolster from the fluid humor to the giver cornea. This driven perception brought about the improvement of a fenestrated back plate. Sixteen circular gaps (1.17 mm distance across each) in an 8.5 mm measured back plate and eight circular gaps (1.3 mm breadth each) in a 7.0 mm measured back plate were included to the plan permitting fluid to reach the join.
This alteration brought about in a diminish in keratolysis to roughly 10% of cases [20]. Right now, the back plate is accessible in two materials, the initial PMMA and more current titanium demonstrates. PMMA is a dormant and well-tolerated fabric with long-term secure intraocular utilize. Titanium too gives amazing tissue resistance in organic inserts and has numerous extraordinary properties, counting tall resistance to erosion, softness and quality. These characteristics permit the titanium back plate to be more slender (titanium back plate has an edge thickness of 0.25 mm compared to 0.8 mm central and 0.6 mm fringe thickness within the PMMA back plate).
Titanium is non-magnetic; hence patients can be subjected to attractive reverberation imaging. Besides, the titanium back plate can be colored through electrochemical anodization to progress cosmesis. In 2014, the click-on adaptation was presented counting a titanium backplate that clicks onto the stem without the required for a locking ring. At first, the most advantage of the titanium back plate was thought to be a decrease in retroprosthetic film (RPM) arrangement. Todani and colleagues detailed a diminished recurrence in RPM arrangement from 31.2% with the PMMA back plate to 13% with titanium at 6-month follow-up. Be that as it may, a case-matched control think about by Talati and colleagues detailed no statistically noteworthy contrast within the recurrence of outwardly critical RPM in titanium and PMMA back plate bunches at 12 months (35% and 30%, separately). Furthermore, Taniguchi and colleagues as of late detailed that not one or the other the fabric or the measure of the B-KPro back plate had a noteworthy effect on point life structures [21].
A minimal expense simulation of artificial cornea, named the Auro KPro, based on the same design is currently manufactured by Auro-lab in India [22, 23]. Although proof is still generally restricted, results seem similar to those of the B-KPro. The FDA propagated another B-KPro model in 2019: The Lucia keratoprosthesis. This plan decreased assembling expenses and allowed for a solitary titanium back plate 7.75 mm in width. Furthermore, the spiral petaloid shape of the back plate and anodization to earthy coloured shading assist with working on superficial appearance [22, 24]. Generally, patients who have a past filled with numerous bombed PKs are possibility for a keratoprosthesis relocate. Different signs incorporate serious keratitis or visual surface illness coming about because of limbal undifferentiated cell disapp-ointment, for example, aniridia, Stevens-Johnson condition, visual cicatricial pemphigoid and subst-ance injury [7, 20, 25]. The Boston Sort II Kerato-prosthesis may be a comparable contraption with a more drawn out optic planning to reach out through an opening made within the upper eyelid. It is appeared for the foremost genuine cicatrizing visual surface malady [18, 26]. Current literature boasts plentiful retrospective studies exploring at the short-term outcomes of Boston KPro surgeries. The general consensus is that the Boston KPro shows good visual outcomes, retention rates, and fewer complications. However, there is less evidence for medium-term (2–5 years) and long-term (>5 years) effects as follow-up rarely extends up to or beyond 5 years. Accordingly, the goal of this study was to comprehensively investigate the Boston type 1 KPro's effectiveness by reporting on visual and retention findings over the years, from short term to long term, as well as to review its postoperative sequelae.
3. Methodology
3.1 Search Strategy
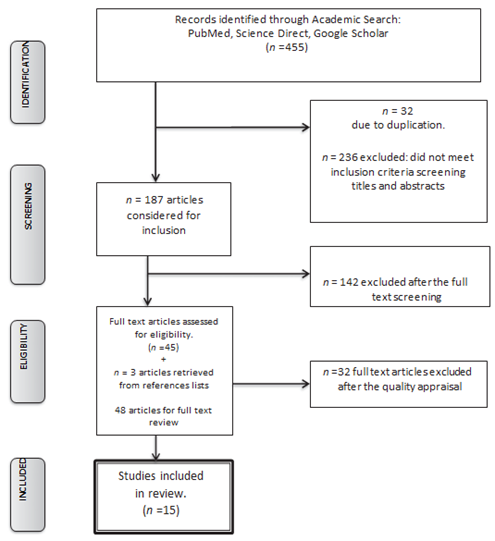
A systematic search strategy to identify and retrieve the relevant literature was developed, the components of the review specifically established as Boston keratoprosthesis, its indications, complications, visual outcomes and retention rate. A review of literature through PubMed, Science direct, and Google scholar databases using relevant medical subject heading terms (MeSH) such as “Boston Keratoprosthesis”, “KPro” and “B-KPro” yielded around 455 results of which only 187 were relevant from 2015 to 2021. (Table 1, Fig 1). The searches were limited to studies published in English.
Of the 455 citations; the creators surveyed the abstracts of these articles and chosen 187 that tended to the B-KPro. Letters, publications, case reports, surveys, histopathology reports, and research facility considers were avoided from these abstracts, and 48 full-text articles were looked into for pertinence. Of these 48 articles, 15 met the consideration criteria based on think about plan and the number of eyes detailed within the think about. Review surveys were constrained to thinks about that included 10 eyes or more. The commentators were not veiled to the names of the distributions or their reviewers.
3.2 Data Analysis and Findings
Data was extracted using a specifically designed data extraction table (Table 1). The data groups analyzed included the objective, settings, and sample, strategy and key findings.
TABLE 1: Summary of Studies Regarding Boston Keratoprosthesis
BCVA = best-corrected visual acuity, RCs = retinal complications, LSCD = limbal stem cell deficiency
These studies were further analyzed in depth in terms of the indications for the keratoprosthesis, the visual outcomes both long term and short term, the safety of the implant, its complications (if any) and also the satisfaction of the patients receiving the treatment.
4. Results
The Boston keratoprosthesis was once thought of as a last resort surgery. Currently, commonly used, with its modified enhancements in design, selection of patients and quality of care postoperatively. The use of B-KPro has been well accepted in the cases of failed PKP; aniridia; as well as ocular trauma including chemical burns, herpes keratitis, corneal dystrophies and so on [32, 42]. Table 2 show the various indications, outcomes and the retention rates of the Keratoprosthesis over a period of 6 years from 2015, to 2021. With more than 1000 eyes analysed over 15 studies, the mean retention rate was found at around 78%. Most studies indicated that the visual acuity improves to 20/200 or better. The single study with pediatric population showed that the outcomes in children were not as in adults. Children have low outcome with keratoprosthesis and they showed more complications.
TABLE 2: Studies Discussing the Indications, Visual Outcomes and Retention Rates of Keratoprosthesis
BCVA = best-corrected visual acuity, RC = Retinal complications, NRC = No retinal complications, LP = Light perception, LSCD = limbal stem cell deficiency, AV = Average, NA = Not Available.
Whereas most cases showed a positive long-term outcome with the Boston keratoprosthesis, many cases showed a side effect, with several complications in the long run. As Table 3 show, the most commonly seen anterior segment complications were Retro prosthetic membrane (45.93%), Glaucoma (21.16%), Infectious keratitis (17.31%) and corneal melt/necrosis (20.33%).
TABLE 3: Studies Discussing the Anterior Segment Complications of Boston Keratoprosthesis
The commonly seen posterior segment complications with the Boston keratoprosthesis across the fifteen studies were Retinal detachment (15.23%), Endophthalmitis (11.85%), Epiretinal membrane (10%) Cystoid Macula Edema (10%) Sterile vitritis(6.26%) Choroidal detachment (5.43%) Hypotony (22%) and others such as posterior capsule opacification and extrusion of device. See Table 4.
TABLE 4: Studies Discussing the Posterior Segment Complications of Boston Keratoprosthesis
4. Discussion
For several many years, prosthetic keratoplasty become reserved for treatment of eyes that had corneal blindness due to severe corneal opacification, especially case in which patients had poor visual diagnosis. Results of B-KPro surgical treatments have been often seemed with combined perspectives as to protection and viable visual effects. Many researches evaluate brief-term efficacy and safety results but did not look for long term effects. Keeping this in mind, the objective of this assessment was to evaluate the outcomes and complications of the B-KPro for treatment of corneal diseases not suitable for corneal keratoplasty.
Snellen chart measured visual acuity was the mainstay investigation the papers involved and ranged from 20/100 to light perception before surgery. Visions post-operative ranged from 20/20 to no light perception. Five articles found a range of 45- 90% of eyes seeing 20/200 or better [33, 35 37 39, 43]. Seven reported best-corrected visual acuity by Snellen chart of 20/40 or better in 10-40% eyes [28, 31, 33, 35, 37, 39, 43], and four articles [27, 28, 30, 39] reported 20/50 or better vision in 40-70% eyes.
These articles elucidate that the B-KPro device can lead to acceptable restoration of vision in conditions of pre-op corneal opacification. Another finding was that consistency standards with Boston KPro went from 65-100% in thirteen works [18, 23, 41, 43, 25, 27-30, 37-39]. From these a general result proportion of normal maintenance of 88% can be refreshed. Three articles [28, 35, 38] announced degrees of consistency of 90% or better, while just one article [29] revealed a definite maintenance of 100%. Although these numbers seem great, a considerable most of these examinations were found to have generally short subsequent occasions, going from 8.5 to 21 months.
The review with the longest development of 60 months observed a degree of consistency of 100% [29]. Each of the articles evaluated for complications, of which Retroprosthetic membrane was the most well-known. The incidence of Retroprosthetic membrane range from 1% to 65% (mean SD, 30.019.0%) in 16 articles [13, 18, 30-32, 37, 41, 43, 19-21, 23, 25-28]. Glaucoma was the second complication, with a range from 2.4% to 64.0% (mean SD, 27.518.1%), followed by corneal melts and keratitis.
The posterior segment complication was less common than anterior segment. The rate of endophthalmitis range from 0% to 12.5% (mean SD, 4.64.6%) in the 15 articles that detailed its event. Although good visual recovery and low retention rates have been reported for the B-KPro, caution should still be exercised when implanting the device in patients with certain conditions that are prone to keratolysis, because these can lead to an increased risk. In particular, patients with mucous membrane pemphigoid, toxic epidermal necrolysis, Ecto-dermal dysplasia, and Stevens-Johnson syndrome should be monitored very closely after implantation of the B-KPro device [10, 13, 34].
Neurotrophic keratitis, regular epithelial imperfections, herpetic eye infections can be regarded as relative contraindications to the B-KPro. The Type II KPro is probably considered as a superior treatment desire for these conditions. Glaucoma one of the important complications of B-KPro, which is the second most common complications. A number of authors performed B-KPro surgery in conjunction with glaucoma drainage device implantation, either simultaneously or sequentially [15, 22, 24, 28, 29]. As Measuring the IOP in patients who had KPro difficult, Tonometry measurements are challenging.
Digital palpation of the globe still the common method used for measuring IOP. So, all patients with the B-KPro should be followed up for glaucoma, with frequent visual field and optic nerve assessment [15]. Conclusions that B-KPro is an alternative option for patients who corneal disease with poor prognosis to corneal keratoplasty. 89% of patients had a visual acuity of 20/200 or better after B-KPro. Although this visual restoration after B-KPro, it has a side effects like Retroprosthetic membrane which maximum happen in the first 2 years. There are no enough researches about the use and safety of B-KPro in children. Patients with autoimmune conditions such as mucous membrane pemphigoid and Stevens-Johnson syndrome, as well as conditions associated with neurotrophic corneas and chronic epithelial defects, had higher rates of severe complications resulting from the resulting from the BI-KPro device.
The common anterior segment complications after B-KPro implantation are Retro-prosthetic membrane and glaucoma, and for posteri-or segment are endophthalmitis and vitritis. The potential for complications increases with time after B-KPro surgery and with eyes that are predisposed to inflammatory effects around the backplate of the B-KPro device. This assessment does not take into account either the differences in B-KPro designs or newer modifications in care after surgery, such as the use of therapeutic contact lens and long-term fortified antibiotics.
5. Conclusion
The B-KPro often remains a patient’s only chance of visual recovery for cases with severe corneal disease and not suitable for corneal keratoplasty. The advances in its design and techniques have led to continuously improving results. However Complications occur. A high level of suspicion, frequent follow up, aggressive management can help decrease the occurrence of these complications. The ongoing research in B-KPro continues and has the potential to further improve outcomes.
Declaration of conflicting Interest
The authors declare that there is no conflict of interest.
References
- Kim MJ, Bakhtiari P, Aldave AJ. The international use of the boston type i keratoprosthesis. Int Ophthalmol Clin (2013).
- Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol (2012).
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ (2001).
- Lam FC, Liu C. The future of keratoprostheses (artificial corneae). Br. J. Ophthalmol (2011).
- Cortina MS, de la Cruz J. Keratoprostheses and artificial corneas: Fundamentals and surgical applications (2015).
- Avadhanam VS, Liu CSC. A brief review of Boston type-1 and osteo-odonto keratoprostheses. Br. J. Opht-halmol (2015).
- Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston Type I Keratoprosthesis. Improving Outcomes and Expanding Indications. Ophthalmology (2009).
- Armitage WJ, Tullo AB, Larkin DFP. The first successful full-thickness corneal transplant: A commentary on Eduard Zirm’s landmark paper of 1906. Br. J. Ophthalmol (2006).
- Priddy J, Bardan AS, Tawfik HS, Liu C. Systematic Review and Meta-Analysis of the Medium-and Long-Term Outcomes of the Boston Type 1 Keratoprosthesis. Cornea (2019).
- Chirila T V., Hicks CR. The origins of the artificial cornea: Pellier de Quengsy and his contribution to the modern concept of keratoprosthesis. Gesnerus (1999).
- Traish AS, Chodosh J. Expanding application of the boston type i keratoprosthesis due to advances in design and improved post-operative therapeutic strategies. Semin. Ophth-almol (2010).
- Avadhanam VS, Smith HE, Liu C. Keratoprostheses for corneal blindness: A review of contemporary devices. Clin. Ophthalmol (2015).
- Zarei-Ghanavati M, Liu C. Keratoprosthesis: Current choices and future development. Asia-Pacific J. Ophthalmol (2019).
- Zarei-Ghanavati M, Avadhanam V, Vasquez Perez A, Liu C. The osteo-odonto-keratoprosthesis. Curr. Opin. Ophthalmol (2017).
- Saeed HN, Shanbhag S, Chodosh J. The Boston keratoprosthesis. Curr. Opin. Ophthalmol (2017).
- Salvador-Culla B, Jeong KJ, Kolovou PE, Chiang HH, Chodosh J, Dohlman CH, et al. Titanium coating of the Boston keratoprosthesis. Transl Vis Sci Technol (2016).
- Bakshi SK, Graney J, Paschalis EI, Agarwal S, Basu S, Iyer G, et al. Design and Outcomes of a Novel Keratoprosthesis: Addressing Unmet Needs in End-Stage Cicatricial Corneal Blindness. Cornea (2020).
- Iyer G, Srinivasan B, Agarwal S, Ravindran R, Rishi E, Rishi P, et al. Boston Type 2 keratoprosthesis- mid term outcomes from a tertiary eye care centre in India. Ocul Surf (2019).
- Chhadva P, Cortina MS. Long-term outcomes of permanent keratoprosthesis. Curr. Opin. Ophthalmol (2019).
- Nonpassopon M, Niparugs M, Cortina MS. Boston type 1 keratoprosthesis: Updated perspectives. Clin Ophthalmol (2020).
- Taniguchi E V., Paschalis EI, Crnej A, Ren A, Colby KA, Chodosh J, et al. The Role of the Back Plate in Angle Anatomy with the Boston Type i Keratoprosthesis Cornea (2017).
- Basu S, Sureka S, Shukla R, Sangwan V. Boston type 1 based keratoprosthesis (Auro Kpro) and its modification (LVP Kpro) in chronic Stevens Johnson syndrome. BMJ Case Rep (2014).
- Deshmukh S, Medhi J, Agarwal B, Sarma P, Gupta K. Experience with keratoprosthesis at a tertiary eye care center: A report from North-East India. TNOA J Ophthalmic Sci Res (2018).
- Malhotra C, Dhingra D, Jain A. Keratoprosthesis optic and carrier corneal graft “noncontact” as a cause of sterile stromal necrosis in a case of Auro KPro implantation. Indian J. Ophthalmol (2019).
- Raina K, Gupta A. Boston keratoprosthesis type 1 - indication, complication and visual outcomes. Int J Res Med Sci (2017).
- Basak S, Basak S. Recurrent cystoid macular edema following Boston keratoprosthesis type-II implantation: A treatment option. Indian J Ophthalmol (2020).
- Touma S, Harissi-Dagher M. Outcomes and complications of Boston keratoprosthesis type I implantation in unilateral versus bilateral corneal blindness. Can J Ophthalmol (2021).
- Nayman T, Bostan C, Szigiato AA, Harissi-Dagher M. Long-Term outcomes following primary versus secondary Boston keratoprosthesis type 1 implantation. Br J Ophthalmol (2021).
- Ono T, Mori Y, Nejima R, Iwasaki T, Fukuda M, Minami K, et al. Short-term Interim Results of Clinical Outcomes and Complications After Implantation of Boston Keratoprosthesis in Japanese Patients. Cornea (2020).
- Moshiri A, Safi M, Morse LS, Tang VD, Yiu G, Park SS, et al. Posterior Segment Complications and Impact on Long-Term Visual Outcomes in Eyes With a Type 1 Boston Keratoprosthesis. Cornea (2019).
- Fung SSM, Jabbour S, Harissi-Dagher M, Tan RRG, Hamel P, Baig K, et al. Visual Outcomes and Complications of Type I Boston Keratoprosthesis in Children: A Retrospective Multicenter Study and Literature Review. In: Ophthalmology (2018).
- Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC. Long-term Visual Outcomes and Complications of Boston Keratoprosthesis Type II Implantation. In: Ophthalmology (2017).
- Homayounfar G, Grassi CM, Al-Moujahed A, Colby KA, Dohlman CH, Chodosh J. Boston keratoprosthesis type i in the elderly. Br J Ophthalmol (2017).
- Aravena C, Bozkurt TK, Yu F, Aldave AJ. Long-term outcomes of the Boston Type I keratoprosthesis in the mana-gement of corneal limbal stem cell deficiency. Cornea (2016).
- Salvador-Culla B, Kolovou PE, Arzeno L, Martínez S, López MA. Boston keratoprosthesis type 1 in chemical burns. Cornea (2016).
- Gu J, Zhai J, Zhou S, Chen J. Boston Keratoprosthesis Outcomes in Severe Ocular Chemical Burns in Southern China: A Retrospective Study. Adv Ther (2016).
- Goins KM, Kitzmann AS, Greiner MA, Kwon YH, Alward WLM, Ledolter J, et al. Boston Type 1 keratoprosthesis: Visual outcomes, device retention, and complications. Cornea (2016).
- Noel CW, Isenberg J, Goldich Y, Conlon R, Teichman J, Rubinger DA, et al. Type 1 Boston keratoprosthesis: Outcomes at two Canadian centres. Can J Ophthalmol (2016).
- Rishi P, Rishi E, Koundanya V V., Mathur G, Iyer G, Srinivasan B. Vitreoretinal complications in eyes with Boston keratoprosthesis type I. Retina (2016).
- Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI, Lum F. Boston Keratoprosthesis: Outcomes and Complications: A Report by the American Academy of Ophthalmology. Ophthalmology (2015).
- Duignan ES, Nídhubhghaill S, Malone C, Power W. Long-term visual acuity, retention and complications observed with the type-I and type-II Boston keratoprostheses in an Irish population. Br J Ophthalmol (2016).
- Colby KA, Koo EB. Expanding indications for the Boston kerato-prosthesis. Curr Opin Ophthalmol (2011).


 Impact Factor: * 1.2
Impact Factor: * 1.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
