Efficacy of Anti-VEGF Therapy in Diabetic Macular Edema-Bashundhara Eye Hospital & Research Institute Experience
Article Information
Saleh Ahmed1, Moutushi Islam2, Tasruba Shahnaz3, RubinaAkther4, Bilkis Akhter5, S M Monowarul Islam6
1Professor Saleh Ahmed, Department of Vitreo- Retina, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh
2Associate Professor Dr.Moutushi Islam, Department of Cornea &Vitreo-Retina, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh
3Dr.Tasruba Shahnaz, Consultant, Department of Vitreo-Retina, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh
4Dr.Rubina Akther, Senior Consultant, Department of Vitreo-Retina, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh
5Dr. Bilkis Akhter, Medical Officer, Department of Vitreo-Retina, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh
6Professor Dr. S.M. Monowarul Islam, Senior Consultant, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh
*Corresponding author: Professor Saleh Ahmed, Department of Vitreo- Retina, Bashundhara Eye Hospital & Research Institute, Dhaka, Bangladesh.
Received: 12 December 2023; Accepted: 29 December 2023; Published: 10 January 2024
Citation: Saleh Ahmed, Moutushi Islam, Tasruba Shahnaz, RubinaAkther, Bilkis Akhter, SM Monowarul Islam. Efficacy of Anti-VEGF Therapy in Diabetic Macular Edema-Bashundhara Eye Hospital & Research Institute Experience. Journal of Ophthalmology and Research. 7 (2024): 05-12.
View / Download Pdf Share at FacebookAbstract
Introduction: Diabetic macular edema (DME) is a common eye complication of diabetes mellitus (DM) that may cause severe visual impairment if left unattended. Vascular endothelial growth factor (VEGF) is an important factor in ocular homeostasis. Anti-VEGF therapy is an effective tool in the management of DME. Aim: The study aimed to evaluate the efficacy of different types of anti- VEGF therapy in DME regarding the improvement of best corrected visual acuity (BCVA) and the reduction of central macular thickness (CMT).
Methods: A retrospective study was conducted in Bashundhara Eye Hospital & Research Institute, from January, 2018 to March, 2022. A total of 416 patients having DME who received ≥3 anti-VEGF therapy (monthly interval) for reduced vision BCVA <20/40 & CMT >275 μm were enrolled in the study.
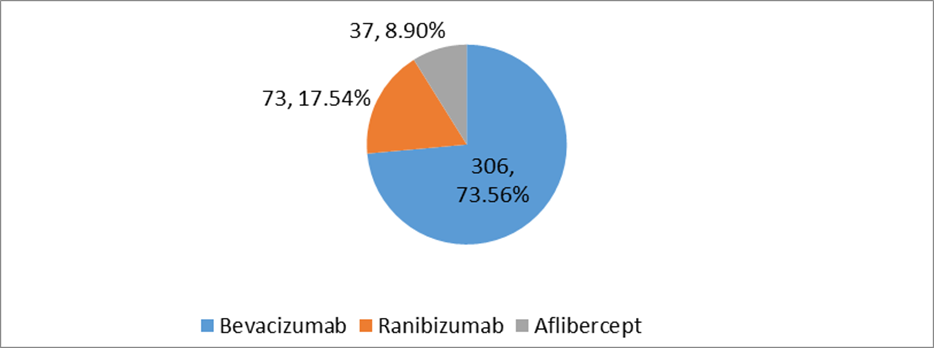
Result: More than half of the study population were female 61.77%. The single eye was affected by 80% of patients and the both eye was affected by 20% of patients. The majority of the patients 73.56% received Bevacizumab, 17.54% of patients were administered Ranibizumab, and one-tenth 8.90% of the patients received Aflibercept. The mean number of anti-VEGF injections applied to the patients was 6±SD. Around half of the study population 52.3 % was injected more than thrice, and the rest of the patients were injected with anti-VEGF three times during the study period. Only three 0.72% patients received Anti-VEGF injections up to 11 doses. After the application of injection, the visual acuity (VA) was improved in 93.26% patients. After the application of three consecutive Anti-VEGF injections, the visual acuity (VA) was improved in one-eighty patients 46.40% and after the application of more than three anti-VEGF therapy, the vision was improved in two hundred eight patients 53.60%. The mean CMT level of the study population after three consecutive anti-VEGF therapy (n=180) was 280±17.02μm. Mean CMT level of study population after more than three anti-VEGF therapy (n=208) was 250±7.42 μm.
Conclusion: The visual outcomes in this retrospective analysis appear to be comparable to previously reported outcomes in routine clinical practice. Our analysis provides information about the effectiveness of anti- VEGF treatment in routine clinical practice in Bashundhara Eye Hospital & Research Institute. Consecutive ≥5 anti-VEGF therapy for DME with reduced vision may be implemented in the management of patients in order to achieve better vision.
Keywords
Diabetic Macular Oedema (DME), Vascular Endothelial Growth Factor (VEGF), Diabetic Retinopathy (DR), Central Macular Thickness (CMT), Optical Coherence Tomography (OCT).
Diabetic Macular Oedema (DME) articles; Vascular Endothelial Growth Factor (VEGF) articles; Diabetic Retinopathy (DR) articles; Central Macular Thickness (CMT) articles; Optical Coherence Tomography (OCT) articles.
Article Details
1. Introuduction
Diabetic macular oedema (DME) is termed as the accumulation of excess fluid in the extracellular space within the retina, sp; in the macular area.It can be characterized by thickening of the oedema of the macula that can evolve at any stage of diabetic retinopathy.It can cause severe blindness if left untreated and undiagnosed. However, it usually takes several years for DME to reach a stage where it could harm eyesight [1]. The initial stage of DME is called background retinopathy which means there are several microscopic bulges also called microaneurysms that may cause the vessels to leak small amounts of blood into the retinas [2]. Many scientists have suggested, the treatment of DME which may include focal/grid laser photocoagulation, ocular steroids, intravitreal anti-vascular endothelial growth factor (VEGF) drugs, and vitreoretinal surgery. The alteration of the blood-retinal barrier and increased permeability of retinal vessels are the main cause of DME [3]. VEGF causes the extracellular accumulation of fluid from the intravascular compartment by disturbing the intercellular tight junctions normally present in retinal endothelial cells. The avenues confined between activation of the VEGF receptor and VEGF gene transcription are the main focus of the new therapeutic tactics based on the use of VEGF antagonists. VEGF Trap, bevacizumab, ranibizumab, and pegaptanib are molecules that directly constrain the VEGF protein [4]. There are some signs and symptoms of diabetic macular oedema, such as; blurred vision, double vision, a sudden increase in eye floaters, seeing colours that look washed out or faded, and vision loss [5]. Some studies revealed that people who have type-1 diabetes are substantially affected by DME compared to others who may have type-2 diabetes. More than 21 million people are universally affected by DME and approximately one in every 14 people with diabetes has some extent of DME [6]. In Bangladesh, more than 6.9 million people are suffering from DME and the number is continuously increasing each year [7]. The prevalence of DME is frequently seen in middle-aged (40) to old people (>40) in contrast with the young generation [8]. The disclosure of vascular endothelial growth factor (VEGF) and the subsequent progress of new indicators targeting the VEGF pathway have made a pathogenesis-oriented approach possible for DME therapy. These signalling particles modulate vasculogenesis during developmental embryogenesis and angiogenesis in later stages such as in ocular diseases[9]. In the eye, VEGF is an essential factor in ocular homeostasis. It may be produced by both vascular endothelial cells or pericytes, Müller cells, retinal pigment epithelium, retinal neurons, and astrocytes, and even by non-pigmented ciliary epithelium [10]. Increased expression of VEGF was found to be an important factor in the pathogenesis of many ocular diseases. Nowadays anti-VEGF therapy is an effective and powerful implement in the complications of uncontrolled diabetes mellitus which results in DME [11]. Perhaps, anti-VEGF therapy is not only enough in regulating macular oedema in all cases. Distinct consequences among the DME patients treated with anti-VEGF reflect the multifactorial pathogenic disease, where not only VEGF but many other factors also play roles.The study aimed to evaluate the efficacy of different types of anti-VEGF therapy in the improvement of Visual Acquity (VA) and the reduction of CMT.
2. Methods:
A retrospective study was conducted in the Bashundhara Eye Hospital & Research Institute,a tertiary eye hospital, Dhaka,Bangladesh. Between January 2018 to March 2022, a total of 416patients (atotalof444 eyes)havingDME who received ≥3 anti-VEGF therapy (monthly interval) due to reduced vision BCVA <20/40 & CMT >270 µm were enrolled in this study . History and clinical examination findings were noted in a case record form. Data were collected from medical records at the time of the first to eleventh (as given monthly for follow up) anti-VEGF injection including demographics, medical and ophthalmic history, RBS, HbA1C, visual acuity (VA) bySnellenchart,Centralmacular thickness (CMT)by3D optical coherence tomography (3D-OCT) and Intraocular pressure (IOP) by Goldmann Applanation Tonometer & Air Puff Tonometer. During the study period, patients were administered three types of anti-VEGF (Bevacizumab, Ranibizumab, Aflibercept) with the dose of 1.25mg/0.05ml, 0.3mg/0.05ml & 2mg/0.05mlrespectively (at one month interval).The information was kept confidential only to be used for the study purpose.
Inclusion criteria:
- Patients with a variable duration of DM
- Patients with the central macular thickness (CMT) >275 um
- Patients with reduced vision BCVA <20/40
- Patients having DME who received ≥3 anti-VEGF therapy
- DME with Previous or No H/O of administration of anti-VEGF therapy
Exclusion criteria:
- Patients with reduced vision due to HTN, macular scar, macular ischemia, ocular inflammation, CKD or another organ failure or severe anaemia
- Patients with DME received photocoagulation
Data Analysis:
The study coordinators performed random checks to verify data collection processes. Completed data forms were reviewed, edited, and processed for computer data entry. Statistical analyses were carried out by using the Statistical Package for Social Sciences version 26.0 for Windows (SPSS Inc., Chicago, Illinois, USA). The quantitative observations will be indicated by frequencies and percentages.
3. Results:
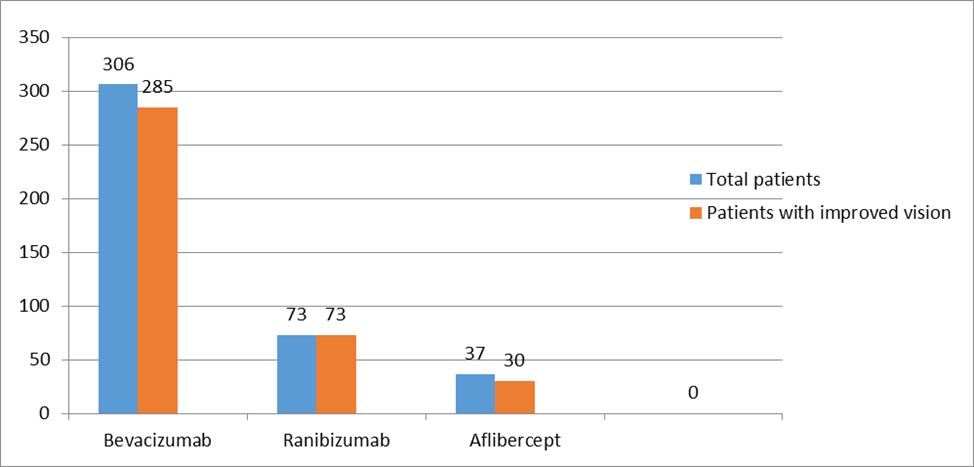
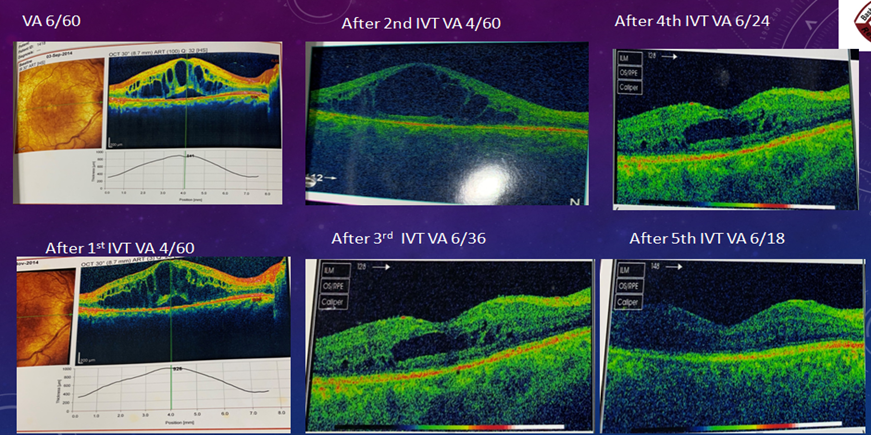
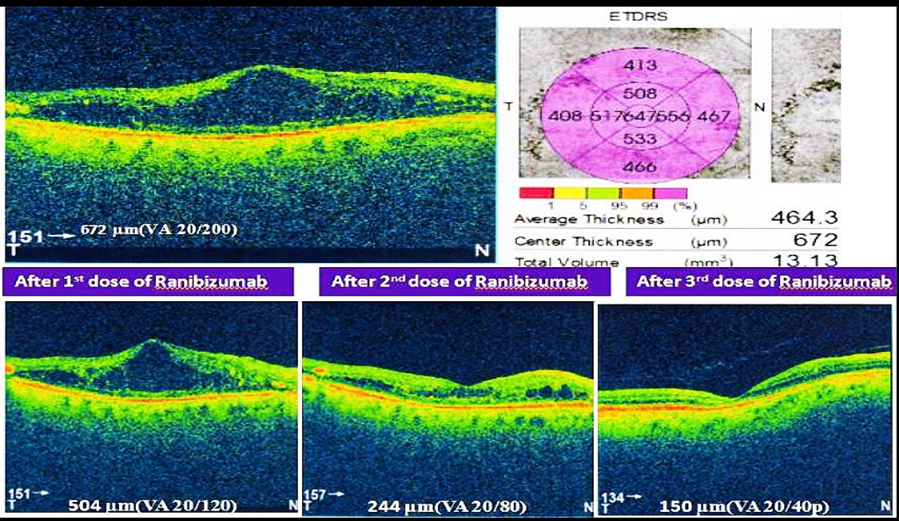
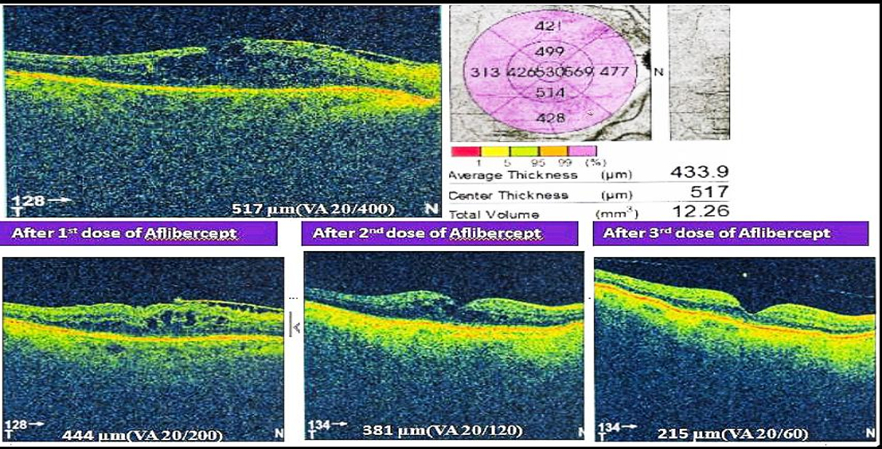
Total number of 416 patients (N=416) were included in this study. Their age range was 35-90 (mean age 58.83).Among them,159(38.22%) were males and 257(61.77%) were females.Two-fifth of the patients (170,40.86%) had glycosylated hemoglobin levels of 9 to 12%, and one-fourth of patients (106,25.48%) had glycosylated hemoglobin of more than 12%. Single eye was affected in 80% (333) of patients and only 20% (83) patients were affected by both eyes. 67.3% of patients had RBS levelswereless than 11.1% and 32.7% of patients had RBS were more than 11.1 mmol/L[Table-I].Half of the study population (228,54.8%) were diagnosed with NPDR. 37.9% of patients were diagnosed with PDR. The majority of the patients (306,73.56%) were treated with Bevacizumab. Around one-fourth (73,17.54%) of patients were administered Ranibizumab, and one-tenth (37,8.90%) of the patient were given Aflibercept. [Figure 1]. Table III & IV shows improvement of BCVA & CMT level of all groups of patients after treatment which was significant (p <0.01). After application of different types of anti-VEGF, we observed the differences in outcome by Ranibizumab (73,100%), then Bevacizumab (285,93%) and Aflibercept (30,81%). Based on this observation,we may conclude that there is no statistically significant difference (p>0.05) among the agents we used (Table II & III).The mean number of anti-VEGF injections administered in patients was 6±SD with a minimum of 3 anti-VEGF shots and a highest of 11 anti-VEGF shots. Around half of the study population (220,52.88%) were injected more than three injections and rest were injected thrice during the study period. Moreover,three (0.72%) patients were injected with 11 doses of anti-VEGF injection. After application of Anti-VEGF injections, visual acuity (BCVA) was improved in three eighty-eight patients (93.26%). Remaining 28 (6.73%) patients were non-responsive to anti-VEGF therapy [Table VI]. Before application of injections to both eyes, among 83 study populations, mean best corrected visual acuity (BCVA) were 20/200, 20/120, 20/80and after application of injection mean BCVA was improved to 20/120, 20/80, 20/60, respectively. Before the application of injection in single eye,among 386 patients, BCVA was 20/200,20/120, 20/60, 20/40, CF. After the application of injection, BCVA was improved and it was 20/120, 20/60, 20/40,20/30,20/200, respectively. During the study period, 28(6.73%) of patients were not responsive to anti-VEGF therapy. Among them, 18 patients (64.3%) had BCVA was counting figure (CF) and 10(35.71%) patients had hand movement (HM). The visionwas improved in 180 patients (46.39%) after the application of 3consecutive injections. In addition, 208 patients’ vision was improved after administration of more than 3 anti-VEGF injection. The mean central macular thickness (CMT) level of the study population was 450 ± 28.39µm before the application of anti-VEGF therapy. The mean CMT level after 3 anti-VEGF therapy was 280± 17.02µm, the mean CMT level after more than 3 anti-VEGF therapy was 250 ± 7.42µm and the mean CMT level after anti-VEGF therapy for those whose vision was not improved was 350± 6.82µm.
Table I: Characteristics of the study population (N=416)
|
Age |
Mean Age 58.83±SD |
|||
|
Maximum=90 |
||||
|
Minimum=35 |
||||
|
Number(N=416) |
Percentage(%) |
|||
|
Gender |
Female |
257 |
61.77% |
|
|
Male |
159 |
38.22% |
||
|
Hypertension |
224 |
53.84% |
||
|
≤8% |
140 |
33.65% |
||
|
HbA1C |
9-12% |
170 |
40.86% |
|
|
>12% |
106 |
25.48% |
||
|
Affected eye |
Number(N=416) |
Percentage(%) |
||
|
Single Eye |
333 |
80% |
||
|
BE |
83 |
20% |
||
|
RBS |
<11.1mmol/L |
280 |
67.30% |
|
|
>11.1mmol/L |
136 |
32.70% |
||
Table II: Comparison of OCT level in µm across the 3 Types of Anti-VEGFs Used
|
Before treatment CMT(µm) |
After treatment CMT(µm) |
||||
|
3 types of Anti-VEGF |
Range |
Mean,SD |
Range |
Mean, SD |
p-value |
|
Ranibizumab |
301- 662 |
481,(5.24) |
146-415 |
280.5, (3.09) |
<0.001 |
|
Bevaciizumab |
295-761 |
675,(7.38) |
161-223 |
192, (6.19) |
<0.001 |
|
Aflibercept |
324-482 |
403, (2.19) |
170-266 |
218, (4.72) |
<0.001 |
Table III: Comparison of BCVA Before & After treatment by 3 Types of Anti-VEGFs Used
|
Before treatment BCVA in Decimal |
After treatment BCVA in Decimal |
||||
|
3 types of Anti-VEGF |
Range |
Mean, SD |
Range |
Mean, SD |
p-value |
|
Ranibizumab |
20/300- 20/80 (0.065-0.25) |
0.157, (0.23) |
20/40- 20/30 (0.50-0.65) |
0.575, (0.52) |
<0.001 |
|
Bevaciizumab |
20/300-20/60 (0.065-0.35) |
0.2075, (0.05) |
20/40-20/30 (0.50-0.65) |
0.575, (0.09) |
<0.001 |
|
Aflibercept |
20/300-20/60 (0.065-0.35) |
0.2075,(0.19) |
20/60-20/30 (0.35-0.65) |
0.50 (0.18) |
<0.001 |
*20/300 means Counting finger (CF
Table IV: Distribution of study population based on Improvement of vision (N=416)
|
Vision Improvement |
Number(N=416) |
Percentage(%) |
|
Vision improved |
388 |
93.26% |
|
Vision Not Improved |
28 |
6.73% |
4. Discussion
Ocular complications are consideredasmost debilitating health consequences for adults with DM. DME has historically been treated with laser photocoagulation and corticosteroid. In recent years, anti-VEGF therapy has turned up as a new standard of treatment for patients with DME. In current study, we tried to compare the outcome of different anti-VEGF agents in DME patients. The DRCR.net Protocol T compared the three medications for DME [32], on the possibility that one medication might be more effective in eyes with worse vision& higher macular thickness, possibly associated with higher VEGF levels and more active retinopathy. A pre-specified analysis was planned to compare results from two major subgroups: those with vision of 20/50 or worse and those with vision better than 20/50.The data from Protocol T showed that when the vision was better than 20/50, the efficacy of all three anti-VEGF medications for DME was similar.For severe DME with poor vision, the efficacy of Aflibercept is superior[33]. In this study, target population included according to inclusion criteria.So target BCVA of better than 20/40 and CMT level of<275µm.were considered as satisfactory level of improvement. Among thepatients,,aroundthree-fourths of the study population (306, 73.56%), were treated withBevacizumab injection. About one-fifth ofthe patients (73, 17.54%) and one-tenth of patients (37, 8.90%)were administered RanibizumabandAflibercept,respectively. Recent studies denote that there are three anti-VEGF agents that could be useful for DME :bevacizumab, ranibizumab and aflibercept. [12] Bevacizumab is a full-length murine monoclonal anti-VEGF antibody that has been humanized, which has the distinct advantage of the lowest cost of the available therapies. Ranibizumab is an affinity-enhanced antibody fragment developed from bevacizumab. The Fab fragment lacks the Fc portion and is similar with monovalent binding to VEGF, as opposed to the bivalent binding of the bevacizumab antibody but with higher affinity. [13] In addition, the VEGF –trap, aflibercept was created as an in-line fusion protein of VEGFR-1 and VEGFR-2 extracellular ligand-binding domains linked to the FC portion of human IgG1. [14]
In this study, 83 patients had DME in both eyes. Among them, the Visual Acuity (VA) of half of the patients’ right eye was 20/120 and the left eye was 20/200before administration of anti-VEGF therapy. They were improved to 20/60 and 20/120 respectively after injection. The recent clinical trials with anti-VEGF therapy have established similar improvement of vision for patients suffering from DME. [15]
In the present study, around half of the patients were applied anti-VEGF thrice in the study period and a significant improvement of vision was observed. A recent study reveals serum VEGF concentration after bevacizumab reduces plasma VEGF for up to 1 month after injection. [16] However, among the half-lives of three anti-VEGF, Bevacizumab has longer half life in vitreous . [17]
In the present study, twenty-eight patients (28, 6.73%) were unresponsive to anti-VEGF therapy. It could be due to poor control of DM with high HbA1C>11% during the treatment period. Occasionally, patients are poorly responsive to anti-VEGF therapy. Pathophysiology of macular edema that is independent of VEGF should be investigated. As there is a significant inflammatory component in the etiology of macular edema, steroids have been extensively evaluated as a treatment option. Many inflammatory cytokines have been found to be elevated in patients with DR. [18] Moreover, corticosteroids inhibit these inflammatory meditators while having some anti-VEGF activity considering anti-VEGF therapy impedes only VEGF itself. [19-27]
The mean CMT level of the study population was 450±28.39 µm before the application of anti-VEGF therapy. The mean CMT level after 3 anti-VEGF therapy was 280±17.02µm and the mean CMT level after more than 3 anti-VEGF therapy was 250.7±7.42µm. There was a study conducted among patients with DME at the Tokyo Medical University where the mean CMT of the study population was 514±SD µm and after one month of anti-VEGF therapy CMT reduced substantially to 299±SD µm. Although, the study found no significant correlation between visual acuity and reduction of CMT after one month of anti-VEGF therapy [28].
Diabetic retinopathy is the leading cause of visual impairment and preventable blindness and represents a significant socioeconomic cost for health care systems worldwide. Hence, new approaches beyond current standards of diabetes care are needed. Based on the crucial pathogenic role of vascular endothelial growth factor (VEGF) in the development of DME, intravitreal anti-VEGF agents have emerged as new treatments. Intravitreal administration of anti-VEGF agents is considered to defeat of anti-DME therapy. Anti-VEGF substance obtains very good results in many well-conducted randomized clinical trials. Regarding bevacizumab, ranibizumab, pegaptanib and aflibercept, short-term efficacy has been manifested by evidence that came from many randomized clinical trials. Perhaps, only ranibizumab has compatible long-term data. The role of laser therapy along with anti-VEGF therapy is still ambiguous. [29]The anti-VEGF therapy is an important invention in the treatment of DME. More studies should be carried out to elucidate the long-term results and the safety profile. [30]
Treatment of DME with VEGF inhibitors is well tolerated in the clinical practice setting. In this study,we included three agents (Bevacizumab, Ranibizumab & Aflibercept) for intra-vitreal injections and compared the results. We observed the differences in outcome but these differences were not statistically significant(Table-V).Though it is claimed that Ranibizumab& Aflibercept are superior to Bevacizumab.[31] Bevacizumab is more chosen by the patient due to it’s cost effectiveness. In this study, the study population was small. If similar observation is experienced in multiple studies with larger number of patients, a concrete idea will develop on which drug is better. Furthermore, there were few reports of adverse effects related to anti-VEGF therapy, although no reports of thromboembolic events related to anti-VEGF treatment.The lack of complications of anti-VEGF therapy also proved its favorable safety profiles in patients with DME. [30]Thus VEGF inhibitors have become the treatment of choice in DME because of their sanctuary as well as their effectiveness in reducing macular edema and improving visual acuity in patients with DME. Whilst the understanding of the pathogenesis and biomolecular pathways involved in DME continues to increase, it remains a major public health concern with a substantial disease burden. Furthermore, anti-VEGF therapy has revolutionized the management of DME patients and provided a new standard of care, current study shows that there remains a significant number of patients unresponsive to anti-VEGF medications. The underlying reason for not responding to anti-VEGF agents because of the presence of a distinct number of mediators and signaling pathways in the retina. Even if, at the present time, commercially available corticosteroids mystified the treatment of DME as it treats many inflammatory mediators. The promise of new, novel agents targeting other components of DME’s pathogenesis would provide persistently improvements in patients’ vision and quality of life. The expanding pharmacologic footsteps available in the retina subspecialty allow new prospects in DME patients.
5. Conclusion
The visual outcomes in this retrospective analysis appear to be comparable to previously reported outcomes in routine clinical practice. Our analysis provides some information about the effectiveness of anti-VEGF treatment in routine clinical practice atBashundhara Eye Hospital &institute.Our study conclude Bevacizumab is not inferior to other practiced Anti-VEGF agents in the treatment of DME. Consecutive ≥5 anti-VEGF therapy for DME with reduced vision may be implemented in the management of patients in order to achieve better visual outcomes
Recommendation:
Diabetic Retinopathy is a chronic condition; long-term data is needed to evaluate the efficacy of anti-VEGF therapy in DME. To get robust data, the multicenter study can be carried out. Consecutive ≥5 anti-VEGF therapy for DME with reduced vision may be implemented in the management of patients in order to achieve better visual outcomes.
Funding: No funding sources
Conflict of interest: None declared
Ethical approval: The study was approved by the Institutional Ethics Committee
References
- Bandello F, Parodi MB, Lanzetta P, et al. Diabetic macular edema. Macular Edema 47 (2010): 73-110.
- Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes 4 (2013): 290.
- Cunha-Vaz J. Mechanisms of retinal fluid accumulation and blood-retinal barrier breakdown. Macular edema 58 (2017): 11-20
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Progress in Retinal and Eye Res 34 (2013): 19-48.
- Shah SP, Patel M, Thomas D, et al. Factors predicting outcome of vitrectomy for diabetic macular oedema: results of a prospective study. British J Ophthalmol 90 (2006): 33-6.
- Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye & Vision 2 (2015): 1-25.
- Muqit MM, Kourgialis N, Jackson-deGraffenried M, et al. Trends in diabetic retinopathy, visual acuity, and treatment outcomes for patients living with diabetes in a fundus photograph–based diabetic retinopathy screening program in Bangladesh. JAMA 2 (2019): e1916285-e1916285.
- Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 132 (2014): 1334-40.
- Pozarowska D, Pozarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Central-European J Immunol 41 (2016): 311.
- Agrahari V, Agrahari V, Mandal A, et al. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert opinion on drug delivery 14 (2017): 1145-62.
- Azad T, Janse van Rensburg HJ, Lightbody ED, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Comm 9 (2018): 1-5.
- Titchenell PM, Antonetti DA. Using the past to inform the future: anti-VEGF therapy as a road map to develop novel therapies for diabetic retinopathy. Diabetes 62 (2013): 1808– 1815.
- Simó R, Sundstrom JM, Antonetti DA. Ocular anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 37 (2014): 893-9.
- Babiuch AS, Conti TF, Conti FF, et al. Diabetic macular edema treated with intravitreal aflibercept injection after treatment with other anti-VEGF agents (SWAP-TWO study): 6-month interim analysis. Int J Retina Vitreous 5 (2019): 1-8.
- Cheung N, Wong IY, Wong TY. Ocular antiVEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care 37 (2017): 900–905
- Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol 97 (2013): 454–459
- Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina 32 (2012): 434–457.
- Dong N, Xu B, Wang B, et al. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis 4 (2013): 1734-1746.
- Ung C, Borkar DS, Young LH. Current and emerging treatment for diabetic macular edema. Int Ophthalmol Clin 57 (2017): 165-177.
- Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diabetes Res. 2015;2015:794036. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmol 120 (2013): 2013-2022.
- Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmol 122 (2015): 2504-2513.
- Muether PS, Droege KM, Fauser S. Vascular endothelial growth factor suppression times in patients with diabetic macular edema treated with ranibizumab. Br J Ophthalmol 98 (2014): 179-181.
- Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 130 (2012): 972-979.
- Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmol 123 (2016): 2376-2385.
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmol 123 (2016): 1351-9.
- Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol 172 (2016): 72-79.
- Altinel MG, Acikalin B, Alis MG, et al. Comparison of the efficacy and safety of anti-VEGF monotherapy versus anti-VEGF therapy combined with subthreshold micropulse laser therapy for diabetic macular edema. Lasers in Med Sci 36 (2021): 1545-53.
- Imazeki M, Noma H, Yasuda K, et al. Anti-VEGF therapy reduces inflammation in diabetic macular edema. Ophthalmic Res 64 (2021): 43-9.
- Pacella F, Romano MR, Turchetti P, et al. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol 9 (2016): 1427.
- Blinder KJ, Dugel PU, Chen S, et al. Anti-VEGF treatment of diabetic macular edema in clinical practice: effectiveness and patterns of use (ECHO Study Report 1). Clin ophthalmol (Auckland, NZ) 11 (2017): 393.
- Hays D, Balinfy J. Eylea outperforms Avastine for diabetic macular edema with moderate or worse vision loss. Ophthalmic Res 301 (2016): 496-5248.
- Wells JA, Glassman AR, Ayala AR, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmol 123 (2018): 1351–9.
- Sun JK, Jampol LM. The Diabetic Retinopathy Clinical Research Network (DRCR.net) and ItsContributions to the Treatment of Diabetic Retinopathy. Ophthalmic Res 62 (2019).


 Impact Factor: * 1.2
Impact Factor: * 1.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
