Safety of Chloroquine Associated with Azithromycin in Patients with Coronavirus Disease 2019
Article Information
Houda Nassih1*, Karima El Fakiri2, Rabiy El Qadiry1, Aicha Bourrahouat1, Imane Ait Sab1, Noureddine Rada2, Ghizlane Draiss2, Mohammed Bouskraoui2
1Pediatric Unit “B”, Department of Pediatrics, Child and Mother Hospital, Mohammed VI University Hospital Center, Marrakesh Medical and Pharmacy Faculty, Caddy Ayad University, City of Marrakesh, Morocco
2Pediatric Unit “A”, Department of Pediatrics, Child and Mother Hospital, Mohammed VI University Hospital Center, Marrakesh Medical and Pharmacy Faculty, Caddy Ayad University, City of Marrakesh, Morocco
*Corresponding Author: Houda Nassih, Pediatric Unit “B”, Department of Pediatrics, Child and Mother Hospital, Mohammed VI University Hospital Center, Marrakesh Medical and Pharmacy Faculty, Caddy Ayad University, City of Marrakesh, Morocco, PB: 40150
Received: 20 June 2020; Accepted: 01 July 2020; Published: 19 August 2020
Citation: Houda Nassih, Karima El Fakiri, Rabiy El Qadiry, Aicha Bourrahouat, Imane Ait Sab, Noureddine Rada, Ghizlane Draiss, Mohammed Bouskraoui. Safety of Chloroquine Associated with Azithromycin in Patients with Coronavirus Disease 2019. Journal of Pharmacy and Pharmacology Research 4 (2020): 58-66.
View / Download Pdf Share at FacebookAbstract
Objectives: To assess the safety of chloroquine and azithromycin in patients with coronavirus disease 2019.
Methods: We conducted a prospective study, including 19 adults infected with SARS-CoV-2 virus.
Results: The mean age was 33,6 years, and the sex ratio was 2:17. There were no severe toxicity cases. QTc prolongation was seen in two cases, and hypokalemia in one case. Treatment withdrawal was efficient in these three cases. Meanwhile, the majority of side effects were milder ones, mainly gastrointestinal and neuropsychiatric signs, responding well to symptomatic measures.
Conclusions: The association of chloroquine and azithromycin seems to be safe in patients with coronavirus disease 2019. However, caution and appropriate monitoring are necessary.
Keywords
COVID-19; Chloroquine; Azithromycin; QTc prolongation
COVID-19 articles, Chloroquine articles, Azithromycin articles, QTc prolongation articles
Article Details
Introduction
Chloroquine, which belongs to the 4-aminoquinoline group of compounds, has been one of the most widely used antimalarials for some 60 years. It is a generic antiviral agent that has shown effectiveness against the SARS-CoV-2 virus, and in this time of pandemic, physicians are trying any plausible approach to therapy. In Morocco, the Ministry of health applied in early march 2020 a nationwide protocol of treatment of all confirmed adult cases of coronavirus disease 2019 (COVID-19). The protocol consisted of an association of chloroquine or hydroxychloroquine with azithromycin as a first-line treatment. With this heightened use, it may be prudent to reflect on the risks of therapy.
Patients and Methods
We conducted a prospective study in one unit of Mohammad VI university hospital of Marrakesh from march 02, 2020, to April 01, 2020. Patients were included in our study if their age was more than 15-year-old, if they were infected with SARS-COV-2 virus, and if they had acute side effects to chloroquine (CHQ) and azithromycin (AZT) association. We aimed to assess the severity of CHQ+AZT toxicity in these patients. COVID-19 cases were defined by exposure history, rarely by clinical manifestations as fever, respiratory symptoms (cough, flu syndrome, sore throat, runny nose, and sneezing) or digestive symptoms (vomiting, nausea, and diarrhea). The severity of COVID-19 was defined based on clinical features into five categories:
- Asymptomatic infection
- Mild form with symptoms of acute upper respiratory tract infection
- Moderate form with pneumonia
- Severe form with acute respiratory distress syndrome or respiratory failure
- Critical form with myocardial injury, acute kidney injury, coagulation dysfunction and shock
RT-PCR obtained confirmation of SARS-CoV-2 infection on nasopharyngeal swab in all cases. We collected patients’ data regarding age, gender, past medical history, number of confirmed contacts, symptoms of COVID-19, and their duration. CT scan was indicated in moderate, severe, and critical COVID-19 cases. Laboratory workup (including CBC, CRP, procalcitonin, urea, creatinine, ALT, AST, PTT, aPTT, D-Dimer, ferritin, LDH, CPK, fibrinogen, and serum electrolytes) were conducted in all patients before the beginning and at the sixth day of treatment. An electrocardiogram (ECG) was performed in all patients before treatment. Treatment of COVID-19 consisted in CHQ (500 mg twice a day for ten days), associated to AZT (500mg the first day, then 250 mg daily for six days), vitamin C (1 g twice a day for ten days) and zinc (90 mg twice a day for ten days) supplementations, and low molecular weight heparin (3500 UI daily until recovery). Blood tests (serum electrolytes) and ECG controls were carried out daily when treatment toxicity was suspected. CHQ+AZT side effects (SE) were classified into three-wide groups:
- Mild side effects: acute onset of clinical signs of toxicity (such as nausea, vomiting, diarrhea, gastralgia, cutaneous rash, erythema multiforme, vertigo, headache, diplopia), with normal ECG and normokalemia (3,5-4,0 mmol/L);
- Moderate side effects: acute onset of clinical signs of toxicity, with prolonged corrected QT interval (QTc) or inverted T wave on ECG, or mild hypokalemia (2,8-3,5 mmol/L);
- Severe side effects: arrhythmia, prolonged QRS, cardiovascular failure with shock and cardiac arrest, respiratory distress, convulsions, coma, or severe hypokalemia (<2,8 mmol/L).
Management of side effects consisted of symptomatic measures (antiemetics, potassium supplementation, probiotics, anxiolytics, proton pump inhibitors). A switch from CHQ to hydroxychloroquine (HCHQ) (200 mg three times a day to complete ten days of treatment) was indicated when patients had mild SE not responding well to symptomatic measures. A withdrawal of all treatments (CHQ, HCHQ, and AZT) was indicated in cases with moderate and severe SE. RT-PCR controls were done according to a preset schedule (Table 1). Two consecutive negative RT-PCR results were necessary to declare COVID-19 recovery.
Table 1: Schedule of RT-PCR controls in patients with COVID-19
|
Days from diagnosis |
Tests |
Indication |
|
1st |
RT-PCR for SARS-CoV2 on nasopharyngeal swab |
To confirm diagnosis |
|
9th |
To assess recovery |
|
|
10th |
To confirm recovery if PCR9 is negative |
|
|
14th |
To assess recovery if PCR9 is positive |
|
|
15th |
To confirm recovery if PCR14 is negative |
|
|
24th |
To assess recovery if PCR14 is positive |
|
|
29th |
To confirm recovery if PCR24 is negative |
Results
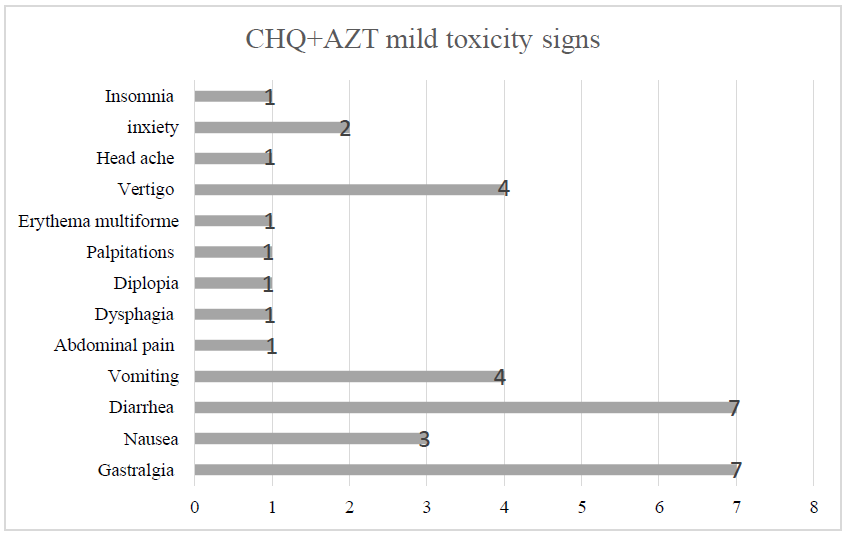
Out of 19 patients enrolled in our study, we had seventeen females (89,5%) and two males (10,5%). Sex ratio was 0,12. The mean age was 33,6 years, with interquartile range from 17 to 77 years. Distribution according to age groups was as follows: 11 cases (57,9%) under 35-year-old, 7 cases (36,8%) between 35 and 55-year-old, and one case (5,3%) over the age of 55-year-old. All patients had a history of close contact with COVID-19 confirmed family members. Seven patients (36,8%) were asymptomatic at diagnosis. Meanwhile, the remaining 12 symptomatic cases (63,2%) were all classified as being mild COVID-19 forms. The median time from illness onset to diagnosis was two days. Most frequent signs were dry cough and anosmia (36,8%), followed by myalgia and sore throat (15,8%); then arthralgia, headache, and rhinorrhea (10,5%) (Table 2). Physical examination was unremarkable in all cases, and no lower respiratory involvement or severe and critical forms were noted; that is why no radiological exploration was necessary. All ECGs before treatment were normal, except for one case of sensual bradycardia in a 46-year-old woman who was euthyroid and had normal serum electrolytes levels. Blood tests on the first day of treatment noted mostly high levels of LDH (47,4%), ALT (26,3%), ferritin (21%), and D-Dimers (15,8%), while five patients (26,3%) had normal tests. Meanwhile, laboratory workup on the sixth day was normal in the majority (68,4%) of cases, while four cases (21%) had high levels of LDH (Table 2). No case of severe SE of CHQ+AZT was noted. Meanwhile, three cases (15,8%) had moderate toxicity indicating treatment withdrawal (Table 2). The first case (patient 12) was a 37-year-old woman, obese with a BMI=34, who developed by the fourth day of treatment incoercible vomiting with hypokalemia leading to QTc prolongation. The second case (patient 18) was a 16-year-old boy who developed isolated QTc prolongation also by the fourth day of treatment. The last case (patient 19) was a 32-year-old woman who developed vomiting leading to hypokalemia also by the fourth day of treatment. Clinical, laboratory, and electric abnormalities improved one day after treatment withdrawal in the three cases. The 16 remaining cases (84,2%) had mild toxicity, mainly gastrointestinal symptoms, followed by neuropsychiatric ones (Figure 1). Twelve of those patients responded well to symptomatic measures. Therefor treatment withdrawal was not necessary. However, a switch to HCHQ was necessary in four of them. After what, tolerance of HCHQ was excellent, and no more side effects were noted. RT-PCR became negative after 11,5 days on average. Surprisingly, we noticed it was slightly shorter in patients with moderate toxicity compared to those with the mild one (respectively 10 and 13 days) (Table 2).
Table 2: CHQ+AZT side effects in 19 patients with COVID-19
Discussion
As of Mai 24, 2020, more than 7433 cases and 199 deaths of COVID-19 have been reported in Morocco [1]. The Moroccan Ministry of health approved in early April 2020 a nationwide protocol using the association of CHQ or HCHQ with AZT in all adults testing positive for SARS-COV-2 [2]. CHQ is an old drug used historically for malaria, rheumatoid arthritis, and lupus erythematosus, among other less common disorders. It was synthesized in 1934 at Bayer, initially discarded but later clinically used in the 1940s [3]. CHQ’s most frequent toxicities are gastrointestinal, but their most serious is a retinopathy that is both dose and duration dependent [4]. CHQ is ion active, blocking IKr potassium channels. This pharmacologic action gives this drug the potential of causing life-threatening arrhythmias [5]. Electrocardiographic documented torsade de pointe with QTc interval prolongation was reported [6]. Their occurrence is more than likely dose-related, when the supposed ingested dose (SID) of CHQ is 2-3,5 g [7]. However, the risk could increase in case of association with factors such as hypokalemia, bradycardia, baseline long QTc, alterations in disposition which could increase systemic exposure (blood level of CHQ=2,5-5,0 mg/L), and other drugs like AZT (which is also QTc prolonging) [8,9]. The terminal half-life of CHQ is one to two months. Therefore a patient could remain at risk for recurrence of QTc interval prolongation and torsade de pointe for extended times after onset [10]. When the SID of CHQ exceeds 3,5 g, and its blood level is beyond 5 mg/L, multi-organ failure leading to death is very likely to occur. CHQ is 30-50% excreted renally as unchanged drug with the remaining fraction being metabolized through the liver. This profile sets up many anticipated drug interactions but also may increase the risk of toxicity in patients with renal dysfunction or advanced hepatic disease [5,6]. Preliminary evidence from studies suggests that the combination of CHQ and AZT may be more effective than CHQ alone for reducing viral burden in patients with COVID-19 [11]. This association might increase the risk of cardiovascular toxicity, and special attention with systematic screening in these patients is mandatory. In our series, the use of CHQ+AZT was complicated by QTc prolongation in two cases (10,5%), one of whom had associated hypokalemia. Fortunately, both patients recovered quickly (one day) after treatment withdrawal, and follow-up (one month) was unremarkable. Meanwhile, patients with mild toxicity took three more days to recover from COVID-19, despite receiving a more extended treatment duration.
Conclusion
Life-threatening arrhythmias associated with chloroquine appear to be rare. However, caution and appropriate monitoring are necessary. Baseline QTc interval should be measured, particularly in COVID-19 patients with additional risk factors, those with renal or hepatic disease, or those with concomitant use of other QTc interval-prolonging drugs such as azithromycin. Hypokalemia, hypomagnesemia, or hypocalcemia should be corrected, and QTc intervals should be monitored closely during therapy.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of Interests
The authors declare that there is no conflict of interest.
References
- The Official Coronavirus Portal in Morocco [Internet]. [cited 2020 May 12]. Available from: http://www.covidmaroc.ma/pages/Accueil.aspx (2020).
- Moroccan Ministry of health recommendations for the therapeutic management of confirmed COVID-19 cases [Internet]. [cited 2020 May 23]. Available from: http://www.covidmaroc.ma/Documents/2020/coronavirus/PS/Covid-19Prise%20en%20charge%20th%C3%A9rapeutique%20des%20cas%20confirm%C3%A9s%20(23mars2020).pdf (2020).
- Ben-Zvi I, Kivity S, Langevitz P, et al. Hydroxychloroquine: from malaria to autoimmunity. Clinical Reviews in Allergy & Immunology 42 (2012): 145-153.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmology 132 (2014): 1453-1460.
- Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clinical Toxicology 44 (2006): 173-175.
- O'Laughlin JP, Mehta PH, Wong BC. Life Threatening Severe QTc Prolongation in Patient with Systemic Lupus Erythematosus due to Hydroxychloroquine. Case Reports in Cardiology 2016 (2016): 4626279.
- Bauman JL, Bauernfeind RA, Hoff JV, et al. Torsade de pointes due to quinidine: observations in 31 patients. American Heart Journal 107 (1984): 425-430.
- Radke JB, Kingery JM, Maakestad J, et al. Diagnostic pitfalls and laboratory test interference after hydroxychloroquine intoxication: A case report. Toxicology Reports 6 (2019): 1040-1046.
- de Olano J, Howland MA, Su MK, et al. Toxicokinetics of hydroxychloroquine following a massive overdose. The American Journal of Emergency Medicine 37 (2019): 2264-2265.
- Bauman JL, Tisdale JE. Chloroquine and Hydroxychloroquine in the Era of SARS–CoV2: Caution on Their Cardiac Toxicity. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 40 (2020): 387-388.
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents (2020): 105949.


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 