Phytochemical Screening and in vitro Antioxidant Evaluation of Ajuga iva
Article Information
Mohamed H Ladjimi, Karima Lahbib, Zaineb Ben Barka*, Hanène Ben Miled, Khémais Ben Rhouma, Mohsen Sakly, Olfa Tebourbi
Laboratory of Integrated Physiology LR17ES02, Faculty of Science of Bizerte, University of Carthage, 7021, Jarzouna, Tunisia
*Corresponding Author: Zaineb Ben Barka, Laboratory of Integrated Physiology LR17ES02, Faculty of Science of Bizerte, University of Carthage, 7021, Jarzouna, Tunisia
Received: 16 December 2020; Accepted: 28 December 2020; Published: 30 December 2020
Citation: Mohamed H Ladjimi, Karima Lahbib, Zaineb Ben Barka, Hanène Ben Miled, Khémais Ben Rhouma, Mohsen Sakly, Olfa Tebourbi. Phytochemical Screening and in vitro Antioxidant Evaluation of Ajuga iva. Journal of Pharmacy and Pharmacology Research 4 (2020): 164-175.
View / Download Pdf Share at FacebookAbstract
Oxidative stress was associated with several diseases. Many epidemiologic studies showed that antioxidant supplementation decreases the state of oxidative stress. The present work aims to study the in vitro antioxidant activity of different parts of Ajuga iva aqueous extract (AIE) simultaneously with a phytochemically analyze. The antioxidant activities of AIE were evaluated in terms of total antioxidant capacity evaluated by the phosphomolybdenum essay and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical method, as well as the ferric ion reducing power (FRP) and ferrous ion chelating (FIC) assays. All extracts showed a potent antioxidant activity and a powerful scavenging capacity in comparison with the ascorbic acid (AA) used as reference molecule. Importantly, Ajuga iva leaves exhibited the highest activity in comparison with stems and roots.
Keywords
Ajuga iva; Antioxidant; Phytochemical; Leaves; Stems; Roots
Ajuga iva articles; Antioxidant articles; Phytochemical articles; Leaves articles; Stems articles; Roots articles
Article Details
1. Introduction
Antioxidant therapy has gained immense importance. Antioxidants are considered as a first line therapy that protect organisms from highly reactive free radicals, especially oxygen derived ones called ROS (Reactive Oxygen Species), which are capable of oxidizing biomolecules causing human diseases [1]. Electron acceptors, such as molecular oxygen, react rapidly with free radicals to become radicals themselves. A series of free radical-mediated chain reaction processes are also associated with lipid peroxidation, which leads to several types of biological damage [2]. Therefore, many investigations directed toward finding naturally occurring antioxidants of plant origin. The selection of natural products, such as plants used in ethno medicine, and the screening of their pharmacological activity may provide identification of newer drugs for the treatment of various diseases.
Ajuga iva (Ai) is one of the largest genera of the Lamiaceae family with 301 species all over the world [3]. Ajuga species are used in traditional medicine all over the world against some illness such as antitumor [4-6], neuroprotective effects [7], anti-inflammatory [8], antioxidant [9,10], psychotropic [11] , antidiabetic [12] , antibacterial [13] analgesic [14], antioxidant [15,16], hypoglycemic [17] and vasorelaxant [18] activities among others biological effects. Ajuga genus contains many important bioactive compounds like anthocyanins, diterpenoids, sterols, ionones, iridoids, phenylethanol and flavonoid glycosides [19-21]. Even though, several bioactive compounds have been well studied on some Ajuga species, there is not enough data on their phytochemical and biological activities. To the best of our knowledge, although Ajuga species are well mentioned in the literature for their wide range of pharmacologic activities, Ajuga iva has been less evaluated. Therefore, we report in this study, the phytochemical analyze (phenolic contents, flavonoids and proanthocyanidins) and evaluate the in vitro antioxidant activity of the different AIE.
2. Materials and methods
2.1. Chemicals
All reagents were purchased from Sigma Aldrich, (St Louis, US-MI). Solvents were from Panreac (Barcelona, ES), Carlo Erba, Strada Rivoltana (Rodano, IT) and were of the highest analytical grade. The Folin-Ciocalteu reagent was from Merck (Darmstadt, DE).
2.2. Plant material and preparation of the extract
Ajuga iva was collected during March 2015 from Ain Draham (TN). Organs (roots, stems and leaves) were oven-dried at 37°C and grounded with electric blinder. Powder (50 g) were homogenized in 300 ml of methanol (50%, v/w) and maintained on magnetic stirring (24 h, 25°C). The mixture was centrifuged (4500 rpm/10 min) and the upper layer was lyophilized. The extraction yields were 8%, 4%, 2.12 % for roots, stems and leaves respectively.
2.3. Phytochemical study
2.3.1. Determination of total phenolics content
75 µl of AIE was added to 125 µl of Folin-Ciocalteu reagent and, then, to 500 µl of ddH2O water. The mixture was shaken and stabilized for 3 min before adding 1250 µl of Na2CO3 (7%) and adjusting the final volume with ddH2O to 3 ml. After incubation for 90 min in dark, the absorbance was measured at 760 nm [22]. Results were expressed as mg of Gallic Acid Equivalent /g Dry Weight of the AIE (mgGAE/gDW AIE).
2.3.2. Determination of total flavonoids content
Flavonoids were estimated according to the method described by Falleh [22]. 250 µl of AIE were mixed with 75 µl of NaNO2 (5%, w/v) for 6 min, before adding 150 µl of AlCl3, 6H2O (10%, w/v). After incubation for 5 min at 25°C, 500 µl of NaOH (1 M) were added and the mixture was adjusted to 2.5 ml with ddH2O. Absorbance was measured at 510 nm. Results were expressed as mg Catechin Equivalent/g of Dry Weight of the AIE (mgCE/gDW AIE).
2.3.3. Determination of proanthocyanidins content
The proanthocyanidins content was determined according to the method of Falleh [22]. 50 µl of AIE were mixed with 3 ml of vanillin (4%, w/v). Then, 1.5 ml of hydrochloric acid (12 M) was added to the mixture. After incubation for 15 min at 25°C, absorbance was measured at 500 nm. Results were expressed as mg Catechin Equivalent/g of Dry Weight of the AIE (mgCE/gDW AIE).
2.4. Antioxidant study
2.4.1. Total antioxidant activity (TAC)
Total antioxidant capacity can be calculated by the method described by Prieto [23] 0.1 ml of sample solution at different concentrations (0.01-1 mg/ml) was combined with 1 ml of reagent (0.6 M of sulfuric acid, 28 mM of sodium phosphate and 4 mM of ammonium molybdate). The tube is capped and incubated in a boiling water bath at 95°C for 90 min. After cooling the sample to room temperature, the absorbance of the aqueous solution is measured at 695 nm against blank in UV spectrophotometer. A typical blank solution contained 1 ml of reagent solution and the appropriate volume of the same solvent used for the sample and it is incubated under same conditions as rest of the sample. For samples of unknown composition, antioxidant capacity can be expressed as:
(%) = [(Atest - Acont)/Acont] x 100
Where Atest is the absorbance of the sample in the presence of the extract and Acont is the absorbance of the control. The result was expressed as IC50.
2.4.2. DPPH radical scavenging assay
The DPPH radical scavenging assay employed here is as described by Braca [24]. The reduction capability of DPPH radical is determined by the decrease in absorbance at 517 nm induced by antioxidants. Ascorbic acid (AA) is the reagent used as standard. The sample is able to reduce the stable radical DPPH to the yellow-colored diphenylpicrylhydrazine [25]. Experimentally, various dilutions of the methanolic solution extractor standard (0.01-1 mg/ml, in triplicate) were added to DPPH solution (0.035 mg/ml). The absorbance of the mixture was taken at 517 nm with methanol as blank. A control sample with no added test compounds was also analyzed. Radical scavenging activity was expressed as a percentage and calculated using the formula:
% Scavenging = [(Acont-Atest)/Acont] x 100
Where Acont is the absorbance of the control, and Atest is the absorbance of the sample in the presence of the extract. The result was presented as IC50.
2.4.3. Hydrogen peroxide (H2O2) scavenging capacity
The ability of the extract to scavenge H2O2 was determined according to the method of Dingeon [26]. A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (pH 7.4), then a methanolic solution of the extract at various concentrations (0.01-1 mg/ml) was added. Absorbance of hydrogen peroxide at 230 nm was determined 19 min later against a blank solution containing the phosphate buffer without hydrogen peroxide. The percentage of hydrogen peroxide scavenging was calculated from the equation:
% Scavenging = [(Acont-Atest)/Acont] x 100
Where Acont is the absorbance of the control, and Atest is the absorbance of the sample in the presence of the extract. The result was expressed as IC50.
2.4.4. Hydroxyl radical (OH°) scavenging activity
The hydroxyl radical scavenging activity of extract was measured by the method described by Halliwell et al. [27] and compared with that of AA. It was determined by measuring the competition between deoxyribose and the test compounds in scavenging hydroxyl radicals generated from the Fe3+/ascorbate/EDTA/H2O2 system. Attack of the hydroxyl radicals on deoxyribose led to the formation of thiobarbituric acid-reactive substances (TBARS) which were measured by the method of Ohkawa et al. [28]. The percentage of hydroxyl radical scavenging was calculated from the equation:
% Scavenging = [(Acont - Atest)/Acont] x 100
Where Acont is the absorbance of the control, and Atest is the absorbance of the sample in the presence of the extract. The result was expressed as IC50.
2.4.5. Superoxide anion (O2°-) scavenging activity
The superoxide anion scavenging activity of the plant extracts can be measured as described by Robak and Gryglewski [29]. The superoxide anion is generated in 3.0 ml of Tris-HCl buffer (16 mM, pH 8.0), containing 0.5 ml of nitro blue tetrazolium (NBT) (0.3 mM), 0.5 ml of NADH (0.936 mM) solution, 1.0 ml of extract and 0.5 ml of Tris-HCl buffer (16 mM, pH 8.0). The reaction is initiated by adding 0.5 ml of phenazine methosulfate (PMS) solution (0.12 mM) to the mixture, incubated at 25°C for 5 min and then the absorbance is measured at 560 nm against a blank sample. The percentage of O2°- scavenging was calculated from the equation:
% Scavenging = [(Acont-Atest)/Acont] x 100
Where Acont is the absorbance of the control, and Atest is the absorbance of the sample in the presence of the extract. The result was expressed as IC50.
2.4.6. Ferric reducing power (FRP)
The reducing power of the extract was determined according to the method of Oyaizu [30] and compared with AA. Experimentally, a methanolic solution of the extract (1 ml) at various concentrations (0.01-1 mg/ml) was mixed with phosphate buffer (0.2 M) and potassium ferricyanide (1%). The mixture was incubated at 50°C for 20 min. Aliquots of trichloroacetic acid (10%) were added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer (2.5 ml) was mixed with distilled water and a freshly prepared ferric chloride solution (0.1%). The absorbance was measured at 700 nm. Ascorbic acid was used as standard. A control sample was prepared without adding standard or extract.
Increased absorbance of the reaction mixture indicates increase in reducing power. The percent increase in reducing power was calculated using the following formula:
Increase inreducing power (%) = [(Atest-Acont)/Acont] x 100
Where Atest is the absorbance of the sample in the presence of the extract and Acont is the absorbance of the control. The result was expressed as IC50.
2.4.7. Ferrous ion chelating (FIC) activity
The FIC ability of the extract was determined according to the method of Singh and Rajini [31]. A methanolic solution of the extract (1.0 ml) at various concentrations (0.01-1 mg/ml) was added to 1.0 ml of FeSO4 (0.1 mM) and 1.0 ml of ferrozine (0.25 mM). The tubes were shaken well and left to stand for 10 min. The absorbance was measured at 562 nm. The ability of each sample to chelate ferrous ions was calculated relative to the control consisting of only iron ferrozine, using the following formula:
% FIC = [(Acont-Atest)/Acont] x 100
Where Acont is the absorbance of the control, and Atest is the absorbance of the sample in the presence of the extract. The result was expressed as IC50.
2.5. Statistical analysis
Statistical analyses were performed using one-way ANOVA followed by a Tukey post-hoc test (GraphPad Prism 5, GraphPad soft-ware, Inc, San Diego, US-CA). Statistical significance of the differences between organs was accepted at a p < 0.05. Data are expressed as mean ± standard error of the mean (SEM).
3. Results
3.1. Phytochemical study
We report in the present investigation on the efficiency of an extract of different organs (leaves, stems, roots) of Ajuga iva as antioxidant agents. First, we outlined the composition of such Lamiaceae and as expected, leaves extract showed the highest total phenolics compounds at a value 340.12 ± 39.70 mgGAE/gDW in comparison with other organs (roots 235.95 ± 13.31 mgGAE/gDW and stems 202.67 ± 22.94 mgGAE/gDW AIE, p < 0.05). In addition, data showed that leaves are more rich than roots and stems on flavonoids (leaves 41.01 ± 0.39 mgCE/gDW vs roots 37.39 ± 0.59 mgCE/gDW, p < 0.001 vs stems 29.97 ± 0.13 mgCE/gDW, p < 0.001) and proanthocyanidins (leaves 307.54 ± 3.50 mgCE/gDW vs roots 209.21 ± 5.98 mgCE/gDW, p < 0.001 vs stems 183.76 ± 11.16 mgCE/gDW, p < 0.001) (Table 1). Results were in accordance with those obtained with Göger et al. [32].
Table 1: Content of total phenolics, flavonoids and proanthocyanidins
|
Polyphenols content (mgGAE/gDW) |
Flavonoids content (mgCE/gDW) |
Proanthocyanidins content (mgCE/gDW) |
|
|
AIE leaves |
340.12 ± 39.70 |
41.01 ± 0.39 |
307.54 ± 3.50 |
|
AIE stems |
202.67 ± 22.94* |
29.97 ± 0.13*** |
183.76 ± 11.16*** |
|
AIE roots |
235.95 ± 13.31 |
37.39 ± 0.59*** |
209.21 ± 5.98*** |
Values are expressed as mean ± SEM of a triplicate measurement. DW: dry weight, GAE: gallic acid equivalent, CE: catechin equivalent, AIE: 50% methanolic (leaves, stems or roots) extract of Ajuga iva. *P<0.05, **P<0.01, ***P<0.001 vs AIE leaves (ANOVA, Tukey post hoc test).
3.2. Antioxidant study
In the second part of this work, we focused our efforts to study the effect of Ajuga iva aqueous extract as an antioxidant agent by measuring the IC50 in mg/ml. It is important to mention that all Ajuga iva organ antioxidant activities are significant lower than that of AA considers as reference.
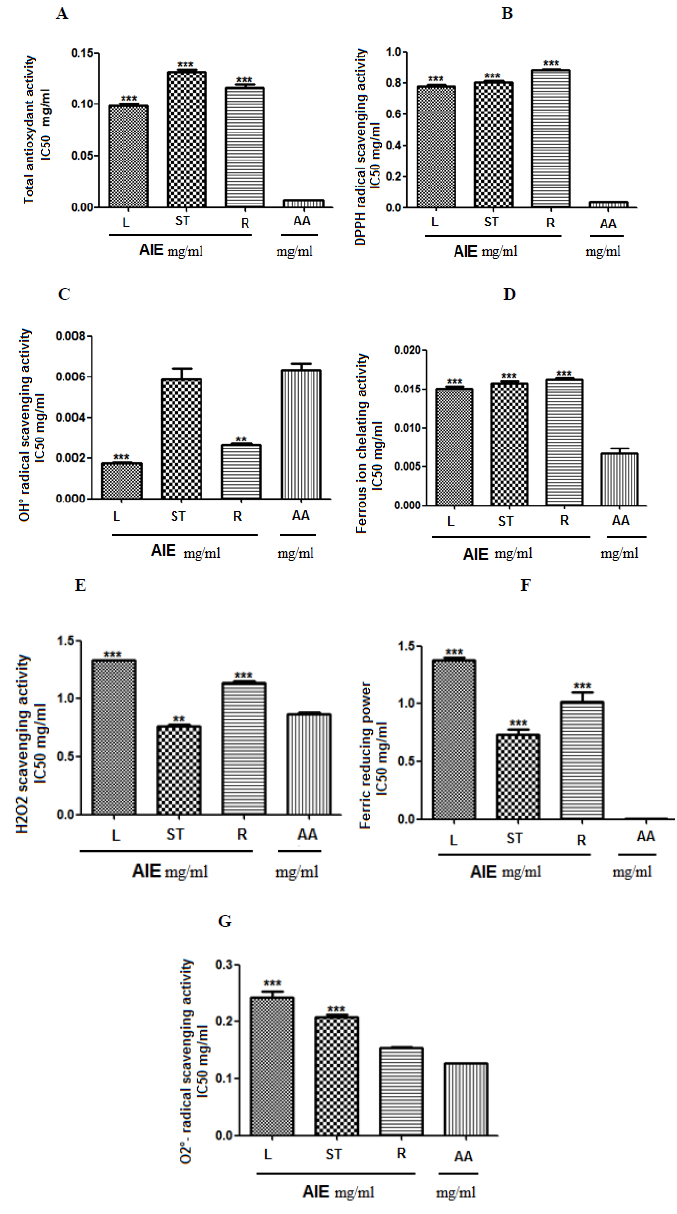
We found that leaves exhibited a higher antioxidant power in total antioxidant capacity in comparison with stems and roots: the IC50 are 0.09; 0.13; 0.11 for leaves, stems and roots respectively; all vs 0.006 for AA, p < 0.001 (Figure A).
Similar results are found for: the DPPH scavenging activity (leaves 0.77; stems 0.80; roots 0.88; all vs AA 0.039, p < 0.001) (Figure B.), the OH° scavenging activity (leaves 0.001 vs AA 0.006, p < 0.001; stems 0.005 vs AA 0.006, no-significant (ns); roots 0.002 vs AA 0.006, p < 0.01) (Figure C) the FIC ability of plant extracts (leaves 0.015; stems 0.015; roots 0.016; all vs AA 0.006, p < 0.001) (Figure D).
However, stems are more effective on H2O2 radical scavenging (stems 0.76 vs AA 0.86, p < 0.01; both leaves 1.32 and roots vs AA 0.086, p < 0.001) (Figure E) and on FRP ability (stems 0.72; leaves 1.37; roots 1.02; all vs AA 0.00, p < 0.001) (Figure F).
Finally roots exhibited the lower antioxidant action in all the activities mentioned above, but were more active on O2°- scavenging in comparison with the other organs (roots 0.15 vs AA 0.12, ns.; both leaves 0.24 and stems 0.20 vs AA 0.12, p < 0.001) (Figure G).
Figures 1: Total antioxydant activity (A), DPPH radical scavenging activity (B), OH° radical scavenging activity (C), Ferrous ion chelating activity (D), H2O2 scavenging activity (E), Ferric reducing power (F), O2°- radical scavenging activity (F). Values are expressed as mean (IC50 mg/ml) ± SEM of a triplicate measurement. L: leaves, ST: stems, R: roots, AA: ascorbic acid. *P<0.05, **P<0.01, ***P<0.001 vs AA (ANOVA, Tukey post hoc test).
4. Discussion
Ajuga iva L. presented a high level of antioxidant activity in comparison with other species of medicinal plants as Thymelaea microphylla, Atriplex halimus or Artemisia herba-alba [13, 33-36]. According to the DPPH assay, our results confirmed previous studies showing an important total antioxidant activity of AIE and high reduction Ferric power [37]. The antioxidant activity of AIE could be attributed to their chemical composition [38]. The relationship between the antioxidant activity and their chemical profiles was previously reported [39]. Chemical studies on Ajuga iva aqueous extract have revealed the presence of several flavonoids and tannins, which corroborate with previous phytochemical studies on various Ajuga species [40-44].
In our study, the phytochemical analysis of AIE showed the presence of several flavonoids. Since flavonoid have been reported to present antioxidant activity [45,46] it may be suggested that the antioxidant activity of Ai might be related in part to these compounds. Indeed, it is well established that flavonoids act as free radical scavenger [47] and that tannins have also antioxidant effects [48]. Moreover, the superoxide anion and hydroxyl radical could induce deleterious effects [49,50]. The present study showed the efficacy of the AIE to reduce oxidative stress parameters in vitro, due essentially to their richness of flavonoids and tannins, since it is well known that polyphenol compounds are potent natural antioxidants [51-53]. Our findings are confirmed by other investigations. Thus, Taleb-Senouci et al. [34] reported a high antioxidant effect of lyophilized AIE which is able to reduce the oxidative stress and prevent lipid peroxidation in vivo [15,35].
With regard to the mechanism of antioxidant activity, one can speculate that Ajuga iva extracts act as direct antioxidant agent by the scavenging ROS which may be attributable to their hydrogen-donating ability [54,55]. Moreover, AIE as iron chelators, exert a secondary antioxidant effect by chelating the bivalent iron necessary for the formation of hydroxyl radicals in the Fenton reaction [56].
These results suggested that AIE exhibit antioxidant activity may be exerted by both chelating metal transition ions as well as by scavenging free radicals. Observed biological activities could be also attributed not solely to the studied phenolic contents, but might be attributable to other unidentified substances or to synergistic interactions between identified ones.
5. Conclusion
The current study is the first to investigate various AIE for their phenolics composition and antioxidant activities in vitro. It gives scientific bases for use of this species as a potent source of natural antioxidant agents. Our results further made the link between phytochemically composition of plant organ extracts and their antioxidant capacity. Our results also emphasize the ability of AIE leaves, as scavenger and iron chelators, to exert a direct and secondary antioxidant effect by preventing the Fenton reaction.
References
- Folts JD. Potential health benefits from the flavonoids in grape products on vascular disease. Adv. Exp. Med. Biol. 505 (2002): 95-111.
- Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food and Chemical Toxicology 44 (2006): 198-206.
- Schreber JCD. Ajuga iva (L.) Plantarum Verticillatarum Unilabiatarum Genera et Species 15, (1774).
- Ben Jannet H, Chaari A, Bakhrouf A, et al. Structure antibacterial activity relationship of secondary metabolites from Ajuga pseudo iva Nat. Prod. Res. 203 (2006): 299-304.
- Israili ZH, Lyoussi B. Ethnopharmacology of the plants of genus Ajuga. Pakistan J. Pharm. 224 (2009): 425-462.
- Setif A. Antibacterial activity of extract of Ajuga iva and Teucriumpolium. Adv. Environ Bio. 52 (2011): 491-495.
- Guo P, Li Y, Xu J, et al. Bioactive neoclerodane diterpenoids from the whole plants of Ajuga ciliata J. Nat. Prod. 747 (2011): 1575-1583.
- Singh R, Patil SM, Pal G, et al. Evaluation of in vivo and in vitro anti-inflammatory activity of Ajuga bracteosa Wall ex Benth. Asian Pacific J. Trop Dis. 2 (2012): 404-407.
- Makni M, Haddar A, Kriaa W, et al. Antioxidant, free radical scavenging, and antimicrobial activities of Ajuga iva leaf extract. Int. J. Food Prop. 164 (2013): 756-765.
- Turkoglu S, Turkoglu I, Kahyaoglu M, et al. Determination of antimicrobial and antioxidant activities of Turkish endemic Ajuga chamaepitys Schreber subsp. euphratica PH Davis Lamiaceae. J. Med. Plant Res. 413 (2010): 1260-1268.
- Bennaghmouch L, Hajjaji N, Zellou A, et al. Etude pharmacologique d’Ajuga iva. Pharm. Fr. 59 (2001): 284.
- Wang JJ, Jin H, Zheng SL, et al. Phytoecdysteroids from Ajuga iva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Pharmacother. 96 (2017): 480-488.
- Medjeldi S, Bouslama L, Benabdallah A, et al. Biological activities, and phytocompounds of northwest Algeria Ajuga iva (L) extracts : Partial identification of the antibacterial fraction. Pathog. 121 (2018): 173-178.
- Bellakhdar J. Médecine traditionnelle et toxicologie ouest-sahariennes. Rabat, Éditions techniques nord-africaines (1978).
- Bouderbala S, Lamri-Senhadji M, Prost J, et al. Changes in antioxydant defense status in hypercholesterolemic rats treated with Ajuga iva. Phytomedicine 15 (2008): 453-461.
- Chenni A, AitYahia D, Boukortt FO, et al. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. of Ethnopharm 109 (2007): 207-213.
- Chabane D, Saidi F, Rouibi A, et al. Activité hypoglycémique de l’extrait aqueux d’Ajuga iva L. Schreber chez les rats diabétiques induite par l’alloxane. Afrique Science: Revue Internationale des Sciences et Technologie 9 (2013): 120-127.
- El-Hilaly J, Lyoussia B, Wibob M, et al. Vasorelaxant effect of the aqueous extract of Ajuga iva in rat aorta. J. of Ethnopharm 93 (2004): 69-74.
- Chen H, Tan RX, Liu ZL, et al. Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J. of Natural Products 59 (1996): 668-670.
- Terahara N, Callebaut A, Ohba R, et al. Acylated an thocyanidins 3-sophoroside-5-glucosides from Ajuga reptans flowers and the corresponding cell cultures. Phytochemistry 58 (2001): 493-500.
- Akbay P, Calis I, Heilmann J, et al. Ionone, iridoid and phenylethanoid glycosides from Ajuga salicifolia. Z. Naturforsch 58 (2003): 177-180.
- Falleh H, Ksouri R, Boulaaba M, et al. Phenolic nature, occurrence and polymerization degree as marker of environmental adaptation in the edible halophyte Mesembryanthemum edule. South African J. of Botany 79 (2011): 117-124.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269 (1999): 337-341.
- Braca A, Tommasi ND, Bari LD, et al. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod 64 (2001): 892-895.
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food. Agric 76 (1998): 270-276.
- Dingeon B, Ferry JP, Roullet A. Automatic assay of blood sugar by Trinder's method. Ann Biol. Clin (Paris) 33 (1975): 3-13.
- Halliwell B, Gutteridge JMC, Arnoma OL. The deoxyribose method: A simple test tube assay for the determination of rate constant for reaction of hydroxyl radical. Anal Biochem 165 (1987): 215-219.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95 (1979): 351-358.
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol 37 (1988): 837-841.
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr 44 (1986): 307-315.
- Singh N, Rajini PS. Free scavenging activity of an aqueous extract of potato peel. Food Chemistry 85 (2004): 611-616.
- Göger F, Köse YB, Göger G, et al. Phytochemical characterization of phenolics by LC-MS/MS and biological evaluation of Ajuga orientalis from Turkey. Bangladesh J. Pharmacol 10(2015): 639-644.
- Belyagoubi N. Activité antioxydante des extraits des composés phénoliques de dix plantes médicinales de l’Ouest et du Sud-ouest algérien. Doctorat Thesis (2011).
- Taleb-Senouci D, Ghomari H, Krouf D, et al. Antioxidant effect of Ajuga iva aqueous extract in streptozotocin-induced diabetic rats. Phytomedicine 16 (2009): 623-631.
- Bouderbala S, Prost J, Lacaille-Dubois MA, et al. Iridoids extracts from Ajuga iva increase the antioxydant enzyme activities in red blood cell of rats fed a cholesterol-rich diet. Nutrition Res 30 (2010): 358-365.
- Boudjelal A, Siracusa L, Henchiri C, et al. Antidiabetic effects of aqueous infusions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Med 81 (2015): 696-704.
- Baghiani A, Boumerfeg S, Adjadj M, et al. Antioxidants, free radicals scavenging and xanthine oxidase inhibitory potentials of Ajuga iva extracts. Free Radicals and Antioxidants 1 (2011): 21-30.
- Derwich E, Chabir R, Taouil R, et al. In vitro antioxidant activity and GC/MS studies on the leaves of Menthapiperita (Lamiaceae) from Morocco. Int. J. Pharma. Sci. Drug Res 3 (2011): 130-136.
- Barra A, Coroneo V, Dessi S, et al. Chemical variability, antifungal and antioxidant activity of Eucalyptus camaldulensis essential oil from Sardinia. Nat. Prod. Commun 5 (2010): 329-335.
- Houghton PJ, Raman A. Laboratory hand book for the fractionation of natural extracts. First ed. ITPs, London (1998).
- Takasaki M, Yamauchi I, Haruna M, et al. New glycosides from Ajuga decumbens. J. of Natural products 61 (1998): 1105-1109.
- Akbay P, Gertsch J, Calis I, et al. Novel antileukemic sterol glycosides from Ajuga salicifolia. Helv.Chim.Acta 85 (2002): 1930-1942.
- Sadati N, Jenett-Siems K, Siems K, et al. Major constituents and cytotoxic effects of Ajuga chamaecistus ssp. tomentella. Z Naturforsch C J Biosci 67 (2012): 275-281.
- Inomata Y, Terahara N, Kitajima J, et al. Flavones and anthocyanins from the leaves and flowers of Japanese Ajuga species (Lamiaceae). Biochem. Syst. Ecol 51 (2013): 123-129.
- Syrov VN, Khushbaktova ZA, Abzalova MK, et al. Hypolipidemic and antiatherosclerotic properties of phytoecdysteroids. Dokl. Akad. Nauk USSR 9 (1983): 44-45.
- Wagner H, Lacaille-Dubois MA. Recent pharmacological results on bioflavonoids. In: Antus S, Gabor M, Vetschera K. (Eds.), Flavonoids and Bioflavonoids. Akademiai Kiado, Budapest, (1995): 53-72.
- Soto C, Recoba R, Barron H, et al. Sylimarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp. Biochem. Physiol. C Toxicol. Pharmacol 136 (2003): 205-212.
- Larkins N, Wynn S. Pharmacognosy: phytomedicines and their mechanisms. Vet. Clin. North Am. Small Anim. Pract 34 (2004): 291-327.
- Fridovich I. Biological effects of the superoxide radical. Arch. Biochem; Biophys 247 (1986): 1-11.
- Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation 80 (1989): 1115-1127.
- Badami S, Gupta MK, Suresh B. Antioxidant activity of the ethanolic extract of strigaorobanchioides. J. Ethnopharmacol 85 (2003): 227-230.
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J.Nutr 133 (2003): 3275-3284.
- Sudheesh S, Vijayalakshmi NR. Flavonoids from Punic granatum: potential antiperoxidative agents. Fito-terapia 76 (2005): 181-186.
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black tea. J. Food Lipids 2 (1995): 35-46.
- Gordon MH. The mechanism of the antioxidant action in vitro. Food antioxidants Hudson BJF (eds.) (1990): 1-18.
- Dombrecht EJ, Cos P, Van den Berghe D, et al. Selective in vitro antioxidant properties of bisphosphonates. Biochem. Biophys. Res Commun 314 (2004): 675-680.


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 