Cost Saving with Home-Based Chemotherapy Approach in Multiple Myeloma The ADHOMY Trial
Article Information
Schulmann S1, Achit H2, Filliatre-Clément L1, Di Santolo C3, Ferry M3, Vigneron J 4, Barbier-Lider P4, Morice S4, Campidelli A1, Schirmer L1, Keddar F1, Ikhlef S1, Moulin C1, Augé H1, Ziegler C1, Feugier P1, Saunier V5, Guillemin F2, Perrot A6
1Hematology Department, Nancy University Hospital, Vandœuvre-lès-Nancy
2Inserm CIC 1433 épidémiologie clinique, Nancy University Hospital, Vandœuvre-lès-Nancy
3Nancy Hospital at Home services HADAN, Vandœuvre-lès-Nancy
4Pharmacy Department, Nancy University Hospital, Vandoeuvre-les-Nancy
5Direction de la Recherche et de l’Innovation, Nancy University Hospital, Vandœuvre-lès-Nancy
6Hematology Department, University Cancer Institute of Toulouse Oncopole, Toulouse
*Corresponding author: Perrot Aurore, Hematology Department, University Cancer Institute of Toulouse Oncopole, Toulouse.
Received: 02 February 2024; Accepted: 13 February 2024; Published: 17 April 2024
Citation: Schulmann S, Achit H, Filliatre-Clément L, Di Santolo C, Ferry M, Vigneron J, Barbier-Lider P, Morice S, Campidelli A, Schirmer L, Keddar F, Ikhlef S, Moulin C, Augé H, Ziegler C, Feugier P, Saunier V, Guillemin F, Perrot A. Cost Saving with Home-Based Chemotherapy Approach in Multiple Myeloma the ADHOMY Trial. Journal of Pharmacy and Pharmacology Research. 8 (2024): 28-34.
View / Download Pdf Share at FacebookAbstract
Background: Bortezomib is a standard first-line therapy for multiple myeloma and also recommended in many associations for relapsing disease. When treatment is delivered in Outpatient Hospital (OH), patients should visit at least once a week for several months. Hospital-at-Home (HaH) is an attractive and suitable alternative to in-hospital treatment. The purpose of the study is to compare costs and patient-reported outcomes of two different strategies: exclusive OH-based bortezomib administration or combined administration in both OH and HaH.
Patients and Methods: A prospective non-randomized trial was conducted in Nancy University Hospital. The main objective was to compare patients’ quality of life (QoL) and costs incurred depending on two different ways of bortezomib administration: in OH only and in rotation between OH (the first cycle and every first day of each cycle) and HaH (the following days). QoL was measured using the EuroQol 5D (EQ-5D) and the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire Core 30 (QLQ-C30). The analysis was conducted from the National Health Insurance System (NHIS) perspective. Adverse events, unplanned hospitalizations, consultations and emergency unit visits were also collected.
Results: A total of 42 patients with a median age of 71 years (range 52-90) were enrolled in the study. The majority had IgG myeloma (52.4 %), 2 patients had t(4;14) and 1 patient had del(17p). Twenty patients received all bortezomib injections in OH (median of 24 injections) and 22 patients received bortezomib in OH alternately with HaH (median of 28.5 injections, 10.5 in OH and 18.0 in HaH respectively). The average cost per injection was 602.63 € in the OH group versus 479.52 € in the OH/HaH group. This represented a cost saving of 20.4% per injection to the NHIS in the combined strategy, with no difference in QoL.
Conclusion: Combined administration of bortezomib in OH and HaH was associated with a substantial cost-saving for NHIS and no difference in QoL at the end of treatment. This trial responds to French governmental and chronic patients’ wish to assess innovative ways of care and provide home treatment for as many patients as possible.
Keywords
multiple myeloma, bortezomib, outpatient, home care, cost-utility analysis
multiple myeloma articles, bortezomib articles, outpatient articles, home care articles, cost-utility analysis articles
Article Details
1. Introduction
Cancer prevalence is constantly increasing with 385000 new cases in 2015 in France but improved management reduced associated mortality [1]. Multiple myeloma (MM) accounts for 1% of all cancers and 12% of malignant blood diseases, being the second most frequent hematologic malignancy with a median age at diagnosis of 70 years old. Its rising frequency is linked to the ageing of the population (incidence) and the effectiveness of new molecules on the overall survival (prevalence) [2, 3]. Bortezomib is a proteasome inhibitor targeting the chymotrypsin-like activity of proteasome 26S which induces cell death by blocking activation cascades in the cancerous cells. The initial Marketing Authorization has been delivered in 2004 in France. The tolerance profile is mainly marked by hematological toxicity, peripheral (mainly sensitive) neuropathies, arterial hypotension and gastrointestinal disorders. Bortezomib is administered subcutaneously since 2012, after Moreau et al revealed no inferiority compared to intravenous route and even a decrease in the incidence of peripheral neuropathy [4]. Bortezomib is a standard first-line therapy and is also recommended in many associations for relapsing disease. For young and fit patients, the combination of bortezomib, thalidomide and dexamethasone showed superiority compared to thalidomide and dexamethasone alone in induction therapy prior to autograft in a randomized phase 3 trial [5]. In patient’s ineligible for autograft, the addition of bortezomib to the melphalan-prednisone regimen, in first line treatment, increased progression-free survival [6]. Novel agents as anti CD38 monoclonal antibody has also been granted marketing authorization, in combination with lenalidomide and dexamethasone, or with bortezomib and dexamethasone, for the treatment of adults having received at least one prior treatment [7, 8].
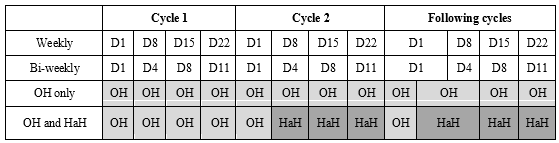
Bortezomib-based treatments are usually prescribed for 4 to 9 cycles with an assessment of myeloma clinical and biological response criteria at the beginning of each cycle. Since September 2016, Nancy Hospital at Home services (HADAN) has handled home administrations bortezomib for patients who lived in their area of activity (up to 25 miles away) and met the National Agency for Accreditation and Health Assessment (ANAES) criteria for chemotherapy at home and [9]: absence of severe adaptive or psychological disorders, ability to understand the protocol, absence of cognitive impairment, availability and agreement of the attending physician, home safety and hygiene. The first cycle of injections is delivered in Outpatient Hospital (OH), as well as the first day of each following cycle to identify bortezomib adverse events while the remaining injections of each cycle are delivered at home by Hospital at Home (HaH). Patients who did not meet the ANAES criteria or who lived outside the HADAN area of activity got all their injections in OH. HaH may be an interesting alternative to OH exclusive care in order to improve patients comfort during chemotherapy and to avoid overwhelming OH, which carrying capacities are limited. Here we report the results of acost and QoL analysis comparing hospital and home-based administration of bortezomib in patients with MM.
2. Patients and Methods
2.1 Inclusion criteria
Patients were considered eligible if they met the following criteria: adults aged 18 years or older, enrolled in a social security scheme, who were to receive a treatment with bortezomib for MM in Nancy University Hospital. The treatment had previously been approved by a multidisciplinary local team. Patients who were already participating in another trial, had a follow-up and/or treatment for another condition requiring a particular care during the bortezomib treatment period were excluded. In this prospective non-randomized trial, the method of care (i. e. OH or OH/HaH) was based on a routine manner, according to patient’s eligibility criteria for HaH (including patient consent, general practitioner consent, home located less than 40 kilometers from Nancy University Hospital). The study was approved by the ethics committee of Angers II, West of France. Patients gave oral informed consent including for associated drugs prescribed with bortezomib and were able to declare their method of transport from home to hospital. The ADHOMY trial was registered as NCT03493737 in clinicaltrials.gov.
2.2 OH Treatment
In OH, patients are welcomed by a nurse who carries out a quick interrogation to identify any objection to the treatment (infectious signs, hemorrhagic syndrome, neuropathy or digestive disorders). Blood pressure, heart and respiratory rates, oxygen saturation and temperature are checked. Before the 1st and 3rd injections, blood tests are performed and patients are given a medical consultation, while the 2nd and 4th sessions are only handled by a nurse, who notifies the medical staff in case of abnormalities. If there is no objection to chemotherapy, the authorized hospital pharmacy is instructed to reconstitute bortezomib injection according to the medical prescription. The chemotherapy protocol is edited on the first day of each cycle for the whole cure.
2.3 Combined administration in OH and HaH
Chemotherapy at home is requested by the referring hospital hematologist on the first cycle of bortezomib. The HaH coordinating physician and nurse call and/or visit the patient to collect information about his home and social environment. The general practitioner (GP) is informed of the treatment plan as he will be requested to check the patient before the 3rd injection of each cycle, while the coordinating nurse will be in charge of clinical examination before the 2nd and 4th injections. The chemotherapy protocol is edited and sent to HaH on day 1 (D1) for the whole cure. Bortezomib is collected by HaH nurses on the day of the injection, given that the maximum duration between reconstitution and injection must not exceed 8 hours. Chemotherapy at home is administered by registered nurses trained in the care of oncology patients. They are responsible for the proper management of toxic waste, in accordance with the Official 2006 Regulation report [10].
Outcomes
The primary endpoint was the cost per injection in each group and patients’ QoL based on the EQ-5D generic and the QLQ-C30 oncology-specific questionnaires. They included 5 items with 5 possible answers and 30 items scored from 1 to 4, respectively. Patients were asked to fill the questionnaires on D1 of the first, second and final cycle of chemotherapy. The secondary endpoint was reporting of unscheduled hospital admissions, general practitioner and emergency care unit consultations, infections, cytopenia, digestives and neurological disorders related to myeloma and/or the treatment.
Cost assessment
Cost were investigated from the French National Health Insurance System (NHIS) perspective and expressed in Euro (€). In France, cancer medical expenses are fully covered by the NHIS, so patients have no extra charge to pay for their treatment - Direct medical costsincluded bortezomib, hospitalizations, nurses, nursing assistants, physiotherapist, psychologist and social worker costs. These data were collected from hospital electronic records and HaH patient’s files. Drugs and health care costs during hospitalization were considered included in the Diagnosis Related Group (DRG) fixed rates (OH and HaH), for homogeneous group of patients, except for cost of expensive drugs like Bortezomib which were directly paid by the NHIS. The DRG related to bortezomib administration is labelled “Chemotherapy for tumor, in sessions” and received the following code 28Z07Z. In 2018, the tariff set for this DRG was 382.77€ per patient in OH in public hospitals.
HaH structures are also funded by the NHIS. The daily cost is set according to 3 items: main purpose of care (here chemotherapy), associated care and Karnofsky Index for functional impairment. Each patient’s stay was classified in the corresponding Homogeneous Group of Tariffs (HGT) which included the costs of physician visit (clinical examination and checking biological results), nurses care (delivering and monitoring chemotherapy), biological tests, treatments (except for expensive ones as explained above), medical devices and patient’s transportation.
- Non-medical direct costs were mainly related to patient’s transportation costs to OH. In 2018, patients were reimbursed up to 0.30 € per kilometer for the use of their personal car, a fixed amount between 11.97 € and 13.85 € plus 0.89 cents per kilometer for a Light Medical Vehicle and a fixed amount between 51.30 € to 57.37 € plus 2.19 € per kilometer for the use of an ambulance [11, 12]. Cost of nurses’ travels from HaH to the hospital pharmacy and to patients’ home were included in the HaH daily cost.
Statistics
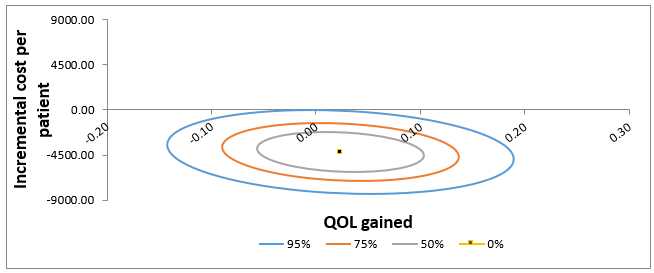
Patient’s EQ-5d scores were calculated by using the EQ-5d crosswalk index value tool with French weighting (version 2.0). For QLQ-C30, we used the PROscorer R package for calculation. Descriptive analysis was reported with means and standard deviations, medians and ranges. Student t test and chi-squared test were used to test for differences between OH and combined OH/HaH care, at the 0.05 type I risk level. Statistical analysis was performed with SAS 9.4 software. Sensitivity analysis was represented as confidence ellipses on the cost-effectiveness plane. These ellipses represent the different credible region for the cost-effectiveness coordinates (at 95%, 75%, and 50%).
RESULTS
Patients
Between April and October 2018, 42 patients from Nancy University Hospital were enrolled in the study. They were all about to start a treatment involving bortezomib for a newly diagnosed or relapse/refractory MM. Patients were mostly female (57.1%) and the median age was 71 years (range 52-90). The majority of patients had IgG myeloma (52.4%), 2 patients had t(4;14) and 1 patient had del(17p). Bortezomib was most often prescribed with thalidomide and dexamethasone in the OH group (N = 5/20) and with cyclophosphamide and dexamethasone in the OH/HaH group (N = 11/22). Twenty patients received all their bortezomib injections in OH and 22 were treated in combination between OH and HaH. Six patients were unable to fill the final QoL questionnaires: 2 patients died during the study, 1 patient was switched to a palliative care and 3 patients couldn’t be assessed at the right time.
Table 1: Baseline characteristics
|
OH group |
OH/HaH group |
Total |
|
|
N=20 |
N=22 |
N=42 |
|
|
Age |
|||
|
Median - yr |
66.5 |
77 |
71 |
|
Range - yr |
52-90 |
55-90 |
52-90 |
|
Distribution - no. of patients (%) |
|||
|
<65 yr |
7 (35) |
4 (18.2) |
11 (26.2) |
|
65-74 yr |
7 (35) |
5 (22.7) |
12 (28.6) |
|
≥75 yr |
6 (30) |
13 (59.1) |
19 (45.2) |
|
Male/female - no. of patients |
9/11 |
9/13 |
18/24 |
|
ECOG performans status - no. of patients (%) |
|||
|
0 |
1 (5) |
1 (4.5) |
2 (4.8) |
|
1 |
11 (55) |
16 (72.7) |
27 (64.3) |
|
2 |
6 (30) |
2 (9.1) |
8 (19) |
|
3 |
2 (10) |
3 (13.6) |
5 (11.9) |
|
R-ISS score - no. of patients (%) |
|||
|
1 |
6 (30) |
3 (13.6) |
9 (21.4) |
|
2 |
6 (30) |
5 (22.7) |
11 (26.2) |
|
3 |
2 (10) |
1 (4.5) |
3 (7.1) |
|
Unknown |
6 (30) |
13 (59.1) |
19 (45.2) |
|
Serum ß2-microglobulin - no. of patients (%) |
|||
|
<3.5 mg/L |
13 (65) |
9 (41) |
22 (52.4) |
|
≥3.5 mg/L |
5 (25) |
11 (50) |
16 (38.1) |
|
Unknown |
2 (10) |
2 (9) |
4 (9.5) |
|
Creatinine clearance (mL/min)– no. of patients (%) |
|||
|
<30 |
3 (15) |
2 (9.1) |
5 (11.9) |
|
30-50 |
1 (5) |
1 (4.5) |
2 (4.8) |
|
50-80 |
4 (20) |
16 (72.2) |
20 (47.6) |
|
≥80 |
12 (60) |
3 (13.6) |
15 (35.7) |
|
Bone lesions – no. of patients/no. of patients investigated |
17/18 |
Oct-16 |
27/34 |
|
Previous regimen |
|||
|
Median - no. |
0 |
0 |
0 |
|
Range - no. |
0-4 |
0-4 |
0-4 |
|
Distribution - no. of patients (%) |
|||
|
0 regimen |
14 (70) |
13 (59.1) |
27 (64.3) |
|
1 regimen |
1 (5) |
4 (18.2) |
5 (11.9) |
|
2, 3 or 4 regimens |
5 (25) |
5 (22.7) |
10 (23.8) |
|
Previous therapies - no. of patients (%) |
|||
|
Bortezomib |
5 (25) |
4 (18.2) |
9 (21.4) |
|
Lenalidomide |
5 (25) |
7 (31.8) |
12 (28.6) |
|
Thalidomide |
0 |
1 (4.5) |
1 (2.4) |
|
Bortezomib administration sequence - no. of patients (%) |
|||
|
Weekly |
11 (55) |
18 (81.8) |
29 (69) |
|
Bi-weekly |
9 (45) |
4 (18.2) |
13 (31) |
yr, years; no., number; %, percentage; R-ISS, Revised International Staging System; VCD, bortezomib
On the 36 patients who completed the study, 18 were treated in the OH group and 18 in the OH/HaH group. A total of 661 bortezomib injections were performed in OH (492 for OH group and 169 for OH/HaH group) and 287 at home. Patients in the OH group received an average of 27 injections and patients of the OH/HaH group received an average of 25 injections (9 in OH, 16 at home). The median duration of care was 8 months with a median of 7 cycles of bortezomib in both groups. The average number of unscheduled hospital admissions, general practitioner and emergency care unit consultations related to myeloma or the treatment was 1.6 per patient (1.4 for OH patients and 1.7 for OH/HaH patients).
Quality of life
OH group patients underwent a median of 6 cycles of bortezomib versus 7.5 cycles for the combined group patients. QoL questionnaires filled at the end of the treatment line revealed no significant difference. Physical and functional scales from QLQ-C30 neither differed at the end of treatment between the two groups (Tables 2 & 3).
Cost analysis
Direct medical and non-medical costs collected are detailed in Table 2. A median of 27.3 bortezomib injections in OH group and 25.3 in OH/HaH group (respectively 9.4 in OH and 15.9 in HaH) were recorded. Patients transportation cost was driven by the distance from home to hospital (median of 50 miles for OH patients versus 5 miles for OH/HaH patients) and the type of vehicle used (personal car for 2 vs 6, light sanitary vehicle for 12 vs 11 patients, ambulance for 4 vs 1 patients). Cost analysis resulted in an average cost per injection of 602.63 € in OH versus 479.52 € in OH/HaH. This differential represents an average cost saving of 123.11 € per injection for the NHIS.
Table 2: QoL at the end of treatment and cost analysis
|
OH group |
OH/HaH group |
OH/HaH - OH |
|
|
N=18 |
N=18 |
||
|
Quality of life |
|||
|
Final EQ5D |
0.729 |
0.753 |
0.024 |
|
QLQ-C30 |
72.037 |
72.079 |
0.042 |
|
Cost - € |
|||
|
Average cost of hospital stays |
10462.38 |
11546.24 |
1083.86 |
|
Average cost of transportation |
5832.91 |
531.69 |
-5301.22 |
|
Average total cost per patient |
16295.29 |
12077.93 |
-4217.36 |
|
Average cost per injection (SD) |
602.63 (157.31) |
479.52 (32.77) |
-123.11 |
Δ, difference between OH/HaH group and OH group; SD, standard deviation; €, euros; EQ5D and QLQC30 range from 0 to 1.
Statistics showed no significant difference in QOL at baseline, as measured by EQ-5D and QLQ-C30 questionnaires. Patients in OH/HaH group have slightly but non significant higher score of QoL compared to OH group at baseline. After the first cycle of treatment, patients in OH/HaH group showed also better improvement in QoL compared to OH group. However, at the end of treatment, the gap in QoL between the two groups was narrowed as a consequence of higher improvement in QoL in the OH group.
Table 3: Effect of Bortezomib mode of administration on QOL based on EQ-5D and QLQ-C30
|
Baseline QOL |
After 1 cycle QOL change from baseline |
At the end of treatment QOL change from baseline |
|
|
Based on EQ-5D |
|||
|
OH group (control)…………………………….a |
0.602 |
+0,047 (0.649) |
+0.127 (0.729) |
|
OH/HaH group (intervention)...………….b |
0.695 |
+0,071 (0.766) |
+0.058 (0.753) |
|
Difference intervention-control……….b-a |
0.093 |
+0.024 (+0.117) |
-0,069 (+0.024) |
|
P-Value |
0.18 |
0.069 |
0.73 |
|
Based on QLQ-C30 |
|||
|
OH group (control) ……………………………a |
62.2 |
+6.5 (68.7) |
+9.8 (72.0) |
|
OH/HaH group (intervention)…………….b |
65.6 |
+7.5 (73.2) |
+6.4 (72.0) |
|
Difference intervention-control……….a-b |
3.4 |
+1.05 (+4.5) |
-3.4 (0.04) |
|
P-value |
0.56 |
0.33 |
0.99 |
Figure 2 illustrates the sensitivity analysis for the estimated difference in cost and QoL gain. The 95% confidence ellipse is mainly located in the cheaper quadrants on the cost-effectiveness plan.
Discussion
This trial aimed to examine how myeloma patients receiving home-based bortezomib treatment assessed their QoL compared to exclusive hospital-based administration. Results could not evidence any significant difference in QoL at the end of treatment. However, we have to emphasize that patient in OH group showed better QoL improvement upon the completion of treatment (+0.127) compared to patient in OH/HaH group (+0.058). Unbalance in certain characteristics between compared groups at baseline could explain difference in clinical gain. Moreover, patients in OH/HAH were older than those included in OH group (table1).
This study also showed that a potential cost saving of 20.4 % per injection can be achieved by NHIS through outsourcing bortezomib administration at home alternatively with OH administration. Transportation costs differ greatly between the two groups because patients treated at home use fewer sanitary transportation and also because transportation costs of HaH nurses are already included in the HGT, unlike the hospital DRG. The cost assessment did not take into account costs related to emergency consultations and hospitalizations or additional blood tests at home as they represented only minimal costs for the NHIS. It did not include also indirect costs related to productivity loss as the ADHOMY population was mostly retired. However, patients’ sick leaves and caregivers’ time off paid work due to the disease can easily be valued using the human capital approach [13]. Petrucci et al. investigated the cost of illness of MM in Italy during one year of disease management, including working days and hours lost by patients and caregivers from a societal perspective [14]. The highest work-related productivity losses were reported by patients who underwent allogenic stem cell transplant. In this study, housewives’ productivity loss had not been included.
From the hospital perspective, outsourcing chemotherapy might induce a decrease in activity for OH as it involves a decrease in the number of patients treated in OH. The creation of an outsourcing package to reward the hospital effort for organizing chemotherapy at home instead of OH administration could counterbalance this loss for hospital activity.
The HaH organization in Nancy involves admitting the patient for two days in HaH (the day before for pre-chemotherapy evaluation and the day of the injection) instead of only one day for the injection in OH. In HaH, patients cannot be discharged if the period between two hospitalizations is less than 4 days, so if chemotherapy is planned twice a week (on D1, 4, 8 and 11), the patient stays in HaH for the whole cycle and the cost of care might be higher. Discussions are currently being held regarding a specific cost for pre-chemotherapy assessment days in order to value more accurately each day in HaH. In previous French studies regarding to cost-savings of home bortezomib injection, costs didn’t include physician visit and biological tests for one [15] and only direct costs were recorded for the other [16]. For both, no QoL evaluation was assessed.
Another interesting result of this study is the median distance from home to hospital (50 miles for OH patients versus 5 miles for OH/HaH patients) which revealed that patients living near big cities benefit more of HaH than countryside living patients. In 2016 in France, only 4% of all chemotherapy sessions were carried out at home, the more active centers being Paris and Lyon [17]. Indeed, young and active patients would benefit more from chemotherapy in HaH, especially since they could carry on with their job. But cancer patients getting older over time, it appears essential to develop chemotherapy in HaH in more rural areas to promote home support even far from hospitals.
A large international review on chemotherapy at home was published in 2015. On the 25 studies analyzed with Drummond criteria, the authors from York and Leeds Universities found little difference for QoL, clinical and psychological effects [18]. Here, we chose to use QLQ-C30 oncology specific questionnaire instead of MY-20 myeloma specific questionnaire in order to better focus on socio-environmental factors and less on physical symptoms related to the disease. Future studies on new agents like carfilzomib or daratumumab should always use the same questionnaires to gather homogeneous and comparable results if they are administered at home. This study has some limitations. First, as it was non-randomized, there might be some bias by indication. Randomization was quite difficult because hospitalization at home was only delivered for patients living in the HADAN ranges of activity, that is 40 km around HADAN location. Thus, patient’s socio-demographic characteristics could be significantly different depending on whether they live near or far from big agglomeration. To overcome this weakness, we chose to adopt difference-in-difference approach to compare clinical outcome rather than to compare difference in outcomes at the end of the treatment. A second limitation concerns sample size, a larger sample would increase the statistical power and potentially better investigate for potential trend toward significance in QoL gain.
In the context of MM, health economic evaluation of HaH is of particular relevance. The cost analysis is accurately detailed and shows that QoL can be maintained while saving money for health insurance. These results might encourage French government to further expand home chemotherapy.
Funding:
This work was supported by a grant of the Association Française des Malades du Myélome Multiple (AF3M).
References
- Projection de l’incidence et de la mortalité par cancer en France métropolitaine en 2015 / 2015 / Maladies chroniques et traumatismes / Rapports et synthèses / Publications et outils / Accueil (2018).
- SPF - Estimation nationale de l’incidence des cancers en France entre 1980 et 2012 / 2013 / Maladies chroniques et traumatismes / Rapports et synthèses / Publications et outils / Accueil (2018).
- Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Bladé J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 118 (2011): 4519–29.
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 12 (2011): 431–40.
- Rosiñol L, Oriol A, Teruel AI, Hernández D, López-Jiménez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood 120 (2012): 1589–96.
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 359 (2008): 906–17.
- Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 375 (2016): 1319–31.
- Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med 375 (2016): 754–66.
- Critères d’éligibilité des patients à une chimiothérapie anticancéreuse à domicile. Haute Autorité de Santé (2019).
- Circulaire 13 février 2006 - Elimination des déchets (2018).
- Frais de transport: modalités de prise en charge et remboursements (2018).
- Ambulance et véhicule sanitaire léger (VSL). (2018).
- Johannesson M. The willingness to pay for health changes, the human-capital approach and the external costs. Health Policy Amst Neth 36 (1996): 231–44.
- Petrucci MT, Calabrese E, Levi A, Federico V, Ceccolini M, Rizzi R, et al. Cost of illness in patients with multiple myeloma in Italy: the CoMiM study. Tumori J (2013).
- Lasalle A, Thomaré P, Fronteau C, Mahé B, Jubé C, Blin N, et al. Home administration of bortezomib is cost-effective and is preferred by patients compared with hospital administration: results of a prospective single-center study. Ann Oncol 27 (2016): 314-8.
- Touati M, Lamarsalle L, Moreau S, Vergnenègre F, Lefort S, Brillat C et al. Cost savings of home bortezomib injection in patients with multiple myeloma treated by a combination care in Outpatient Hospital and Hospital care at Home. Support Care Cancer 24 (2016): 5007-5014.
- Statistiques HAD par mode de prise en charge | Stats ATIH (2018).
- Corbett M, Heirs M, Rose M, Smith A, Stirk L, Richardson G, et al. The delivery of chemotherapy at home: an evidence synthesis. Southampton (UK): NIHR Journals Library (2015).


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 