Factors Associated with Delayed Inflammatory Recovery after Spinal Surgery without Surgical Site Infection: A Retrospective Study
Article Information
Hideaki Imabayashi1*, Atsushi Miyake2, Kazuhiro Chiba2
1Department of Orthopedic Surgery, Tokyo Saiseikai Central Hospital, Tokyo, Japan
2Department of Orthopedic Surgery, National Defense Medical College, Saitama, Japan
*Corresponding Author: Hideaki Imabayashi, Department of Orthopedic Surgery, Tokyo Saiseikai Central Hospital, Mita 1-4-17, Minato Ward, Tokyo 108-0073, Japan
Received: 30 January 2022; Accepted: 07 February 2022; Published: 18 February 2022
Citation:
Hideaki Imabayashi, Atsushi Miyake, Kazuhiro Chiba
View / Download Pdf Share at FacebookAbstract
Background: We have previously reported ten effective serological indicators of surgical site infection (SSI) in the perioperative period after spinal surgery. However, the false-positive fractions of these markers were 0.15-0.39 which frequencies were not negligible. These mean the delays of surgical inflammatory recoveries without SSI. This study aimed to identify and classify the factors associated with these delays.
Methods: This retrospective study enrolled 320 patients who underwent spinal surgery for causes other than infectious spondylitis without surgical site infection. Demographic data, preoperative serological data, operative times, and operative methods were examined by multivariate regression for each SSI indicator and classified into related groups.
Results: Nine significantly associated factors, age, malignancy, preoperative total protein, albumin, white blood cell count, albumin/globulin ratio, C-reactive protein, operation time, and use of spinal instrumentation were found. We classified the patients into three groups according to their preoperative nutritional status, immune-inflammation status, and surgical procedure. Malnutrition, high CRP and WBC, older age, and malignancy might be related to delays in surgical recovery without SSI; therefore, a single indicator may have limited capability in detecting SSI.
Conclusions: We have to recognize the several causes to retard surgical inflammatory recovery. Improved preoperative conditions and less invasive surgery may decrease delays and improve the accuracy of SSI indicators.
Keywords
Delayed Inflammatory Recovery, Malnutrition, Preoperative Condition, Spine Surgery, Surgical Site Infection
Delayed Inflammatory Recovery articles Delayed Inflammatory Recovery Research articles Delayed Inflammatory Recovery review articles Delayed Inflammatory Recovery PubMed articles Delayed Inflammatory Recovery PubMed Central articles Delayed Inflammatory Recovery 2023 articles Delayed Inflammatory Recovery 2024 articles Delayed Inflammatory Recovery Scopus articles Delayed Inflammatory Recovery impact factor journals Delayed Inflammatory Recovery Scopus journals Delayed Inflammatory Recovery PubMed journals Delayed Inflammatory Recovery medical journals Delayed Inflammatory Recovery free journals Delayed Inflammatory Recovery best journals Delayed Inflammatory Recovery top journals Delayed Inflammatory Recovery free medical journals Delayed Inflammatory Recovery famous journals Delayed Inflammatory Recovery Google Scholar indexed journals Malnutrition articles Malnutrition Research articles Malnutrition review articles Malnutrition PubMed articles Malnutrition PubMed Central articles Malnutrition 2023 articles Malnutrition 2024 articles Malnutrition Scopus articles Malnutrition impact factor journals Malnutrition Scopus journals Malnutrition PubMed journals Malnutrition medical journals Malnutrition free journals Malnutrition best journals Malnutrition top journals Malnutrition free medical journals Malnutrition famous journals Malnutrition Google Scholar indexed journals Preoperative Condition articles Preoperative Condition Research articles Preoperative Condition review articles Preoperative Condition PubMed articles Preoperative Condition PubMed Central articles Preoperative Condition 2023 articles Preoperative Condition 2024 articles Preoperative Condition Scopus articles Preoperative Condition impact factor journals Preoperative Condition Scopus journals Preoperative Condition PubMed journals Preoperative Condition medical journals Preoperative Condition free journals Preoperative Condition best journals Preoperative Condition top journals Preoperative Condition free medical journals Preoperative Condition famous journals Preoperative Condition Google Scholar indexed journals Spine Surgery articles Spine Surgery Research articles Spine Surgery review articles Spine Surgery PubMed articles Spine Surgery PubMed Central articles Spine Surgery 2023 articles Spine Surgery 2024 articles Spine Surgery Scopus articles Spine Surgery impact factor journals Spine Surgery Scopus journals Spine Surgery PubMed journals Spine Surgery medical journals Spine Surgery free journals Spine Surgery best journals Spine Surgery top journals Spine Surgery free medical journals Spine Surgery famous journals Spine Surgery Google Scholar indexed journals Surgical Site Infection articles Surgical Site Infection Research articles Surgical Site Infection review articles Surgical Site Infection PubMed articles Surgical Site Infection PubMed Central articles Surgical Site Infection 2023 articles Surgical Site Infection 2024 articles Surgical Site Infection Scopus articles Surgical Site Infection impact factor journals Surgical Site Infection Scopus journals Surgical Site Infection PubMed journals Surgical Site Infection medical journals Surgical Site Infection free journals Surgical Site Infection best journals Surgical Site Infection top journals Surgical Site Infection free medical journals Surgical Site Infection famous journals Surgical Site Infection Google Scholar indexed journals Neutrophil articles Neutrophil Research articles Neutrophil review articles Neutrophil PubMed articles Neutrophil PubMed Central articles Neutrophil 2023 articles Neutrophil 2024 articles Neutrophil Scopus articles Neutrophil impact factor journals Neutrophil Scopus journals Neutrophil PubMed journals Neutrophil medical journals Neutrophil free journals Neutrophil best journals Neutrophil top journals Neutrophil free medical journals Neutrophil famous journals Neutrophil Google Scholar indexed journals C-reactive protein articles C-reactive protein Research articles C-reactive protein review articles C-reactive protein PubMed articles C-reactive protein PubMed Central articles C-reactive protein 2023 articles C-reactive protein 2024 articles C-reactive protein Scopus articles C-reactive protein impact factor journals C-reactive protein Scopus journals C-reactive protein PubMed journals C-reactive protein medical journals C-reactive protein free journals C-reactive protein best journals C-reactive protein top journals C-reactive protein free medical journals C-reactive protein famous journals C-reactive protein Google Scholar indexed journals spinal surgery articles spinal surgery Research articles spinal surgery review articles spinal surgery PubMed articles spinal surgery PubMed Central articles spinal surgery 2023 articles spinal surgery 2024 articles spinal surgery Scopus articles spinal surgery impact factor journals spinal surgery Scopus journals spinal surgery PubMed journals spinal surgery medical journals spinal surgery free journals spinal surgery best journals spinal surgery top journals spinal surgery free medical journals spinal surgery famous journals spinal surgery Google Scholar indexed journals operative bleeding articles operative bleeding Research articles operative bleeding review articles operative bleeding PubMed articles operative bleeding PubMed Central articles operative bleeding 2023 articles operative bleeding 2024 articles operative bleeding Scopus articles operative bleeding impact factor journals operative bleeding Scopus journals operative bleeding PubMed journals operative bleeding medical journals operative bleeding free journals operative bleeding best journals operative bleeding top journals operative bleeding free medical journals operative bleeding famous journals operative bleeding Google Scholar indexed journals Orthopedic Surgery articles Orthopedic Surgery Research articles Orthopedic Surgery review articles Orthopedic Surgery PubMed articles Orthopedic Surgery PubMed Central articles Orthopedic Surgery 2023 articles Orthopedic Surgery 2024 articles Orthopedic Surgery Scopus articles Orthopedic Surgery impact factor journals Orthopedic Surgery Scopus journals Orthopedic Surgery PubMed journals Orthopedic Surgery medical journals Orthopedic Surgery free journals Orthopedic Surgery best journals Orthopedic Surgery top journals Orthopedic Surgery free medical journals Orthopedic Surgery famous journals Orthopedic Surgery Google Scholar indexed journals
Article Details
1. Introduction
Surgical site infection (SSI) is a major complication of spinal surgery, and its prevention and early detec- tion are of utmost importance [1-3]. After spinal surgery in patients without SSI, lymphocyte counts rapidly decreased to less than 1000/µL on posto- perative days 1 and 2, then gradually increased to over 1000/µL by postoperative day 4 and returned to preoperative levels in 3 weeks. Neutrophil counts rapidly increase, peaking on postoperative days 1 and 2, then gradually decreasing on day 4 and norma- lizing in 2–3 weeks [3-5]. We previously reported ten effective serological indicators, four indicators were white blood cell (WBC) count, neutrophil count,
neutrophil-to-lymphocyte ratio (NLR), and C-reac- tive protein (CRP) levels on postoperative day 7 [6]. For the ratio of serological cell counts between perio- perative periods, significant differences were obser- ved among six items, namely lymphocyte ratio from preoperative day to postoperative day 7 (LRp7), NLR ratio from preoperative day to postoperative day 7 (NLRRp7), neutrophil ratio from postoperative days 2 to 7 (NR27), lymphocyte ratio from postoperative days 2 to 7 (LR27), NLR ratio from postoperative days 2 to 7 (NLRR27), and CRP ratio from posto- perative days 2 to 7 (CR27) [6]. In terms of accuracy, the area under the curve (AUC) for these indicators was 0.711 to 0.825, specificities were 0.61 to 0.89, which indicates relatively high accuracy. The false- positive fractions, calculated as “1-specificity”, were 0.11 to 0.39, indicating delayed surgical inflamm- atory recovery without SSI. In actual clinical settings, false-positive cases are commonly encounered after surgery, presenting a diagnostic conundrum [6, 7]. The present study aimed to identify factors associated with delayed surgical inflammatory recovery and to utilize these results in accurately diagnosing SSI.
2. Methods
2.1 Study design and subjects
This retrospective study included 419 consecutive patients who underwent spinal surgery at a university hospital between 2012 and 2016. We excluded all patients that were administered unplanned antimicr- obial agents, except for prophylactic use, on the day of surgery and subsequently on day 1 after surgery, based on the recommendations of the Centers for Disease Control and Prevention [8]. Further, we excluded all cases with missing serological data. SSI was defined according to the criteria of the Centers for Disease Control and Prevention. The diagnosis was made by the attending surgeon based on the need for debridement, blood cultures that tested positive for infectious agents, or drainage of the surgical wou- nd within 4 weeks [8]. Initially, 419 cases were regis- tered, of which nine cases were excluded for the use of unplanned antimicrobial agents without SSI and 81 cases were excluded for missing values. Finally, 329 patients were included in the study. The research was performed in accordance with the ethical standards of our institute (Approval No. 3042).
2.2 Leucocyte data
White blood cell and differential counts were measured preoperatively and on postoperative days 1, 2, and 7. A lymphocyte count < 1000/µL was defined as lymphopenia. An automated cell counter (XN- 3100; Sysmex Corporation, Kobe, Japan) was used to determine the absolute white blood cell count and the percentage of neutrophils and lymphocytes. The neutrophil/lymphocyte ratio was also calculated.
2.3 Calculating the number for positive SSI indicators
If positive SSI indicators were met, 1 point was assigned. We calculated the total number of positive indicators in the SSI and non-SSI groups.
2.4 Data collection
We collected data regarding the age and sex, preoperative serologic parameters such as albumin (ALB pre), total protein (TP pre), globulin (GLB pre), albumin/globulin ratio (AGR pre), C-reactive protein (CRP pre), operation time (Op time), and
operative bleeding (Op bleeding). We also collected data on malignancies, malignant tumor surgery, emergency procedures, and spinal instrumentation. Surgical intervention was classified into three ordinal scales as follows: scale 1 included operations without spinal instrumentation, such as laminoplasty and laminectomy; scale 2, included one level of spinal fusion with instrumentation; and scale 3, comprised further invasive spinal fusions with instrumentation at more than one level.
2.5 Statistical analysis
Data are presented as numbers for categorical variables, and median and range for continuous varia- bles. Correlation was assessed using the correlation ratio (η2) for interval and ratio variables and Cramér’s measure of association (V) for nominal variables. Factors with a high η2 (>0.15) and high Cramér’s V (> 0.15) were selected for multivariate logistic regression analysis to identify the association with each serological indicator. The areas under the (AUC) receiver operating characteristic (ROC) curve analyses were calculated for each factor, and the cut- off value for each associated factor was also calculated. To summarize the significant factors associated with SSI indicators, we classified the associated factors. Statistical analyses were performed using SPSS for Windows (version 22.0; IBM Corp., Armonk, NY, USA), with statistical significance set at a two-tailed p-value of < 0.05.
3. Results
3.1 Patient characteristics
Among the 329 patients, 320 had no SSI (non-SSI group) and 9 developed SSI (SSI group). The non-
SSI group comprised 167 men and 153 women, with a median age of 68 years (range, 11-90 years). The median operative time was 166 min (range, 38-515 min), and the median intraoperative blood loss was 103 mL (range, 3-2475 mL). The surgical scale was as follows: Scale 1 was 147; Scale 2, 57; Scale 3, 116 cases; and spinal instrumentation, 174 cases. Figure 1 shows a histogram of the total number of positive indicators in the SSI and non-SSI groups. There were 50 cases in the non-SSI group. The median values were 2 points (range: 0-10) in the non-SSI group and 8 points (range: 4-10) in the SSI group. The cut-off value for SSI was 6 points (p < 0.001, specificity: 0.87, sensitivity: 0.89).
3.2 Factors associated with SSI indicators
The factors showing a strong correlation (η2 > 0.15 or Cramér’s V > 0.15) with SSI indicators are shown in Table 1 and further assessed using multivariate logistic regression (Table 2). Totally, nine associated factors were identified. The preoperative CRP (CRP pre: p = 0.004. Odds: 1.41) and operation time (Op time: p < 0.001, Odds: 1.005) for SSI indicator of in the relation to WBC cell counts at postoperative day 7, using spinal instrumentation (Spinal instru- mentation: p = 0.004, Odds: 3.63), preoperative AGR (AGR pre: p = 0.001, Odds: 0.167) and operation time (Op time: p = 0.002, Odds: 1.005) for SSI indicator in relation to Neutrophil cell counts at day 7 postoperatively, preoperative total protein (TP pre: p
= 0.007,Odds: 0.552), preoperative AGR (AGR pre: p = 0.002, Odds: 0.259) and operation time (Op time: p < 0.001, Odds: 1.007) for NLR at day 7 postoperatively, using spinal instrumentation (Spinal instrumentation: p = 0.001, Odds: 3.179), preopera-tive WBC (WBC pre: p = 0.010, Odds: 1.0001) and preoperative albumin (ALB pre: p = 0.045, Odds: 0.581) for CRP at postoperative day 7. Operation time (Op time: p<0.001, odds: 1.006) for SSI indi- cator in the relation to NLR ratio from preoperative day to postoperative day 7 (NLRRp7), preoperative WBC count (WBC pre: p = 0.015, odds: 1.0001), and preoperative CRP (CRP pre: p = 0.040, odds: 1.223) for neutrophil cell ratio from postoperative days 2 to 7 (NR27). Preoperative albumin (ALB pre: p = 0.011, odds: 0.542) for lymphocyte cell ratio from postop- erative days 2 to 7 (LR27), preoperative total protein (TP pre: p < 0.001, odds: 0.442), and preoperative CRP (CRP pre: p = 0.009, odds: 1.299) for NLR ratio from postoperative days 2 to 7 (NLRR27), age (p < 0.001, odds: 1.035), and malignancy (p = 0.010,
odds: 2.581) on postoperative days 2 to 7 (CR27).
The cut-off values for the associated factors are shown in Table 3. The maximum cut-off values were as follows, CRP pre was 0.5 µg/dL, Op time was 240 mins, WBC pre was 7500 /µL. The minimum cut-off values were: AGR pre, 1.4; ALB pre, 3.7 g/dL; and TP pre, 6.4 g/dL. The cut-off age was 73 years. We categorized the nine associated factors into three groups: the first group included preoperative factors such as nutritional status, including TP pre and ALB pre. The second group included preoperative immu- ne-inflammation status including age, malignancy, WBC pre, CRP pre, and AGR pre. The first and second groups were preoperative patient-associated factors. The third group was operative procedure, which included operative time and spinal instrum- entation.
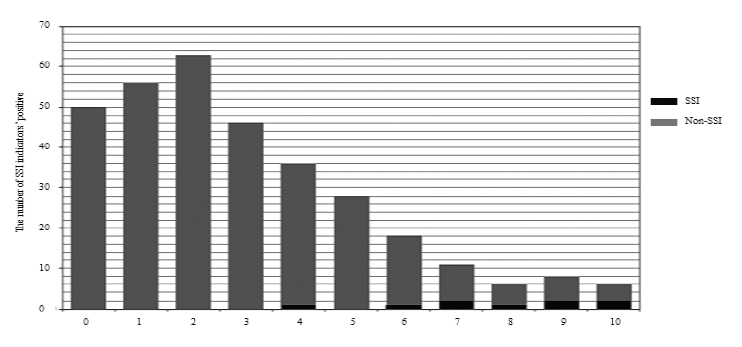
Figure 1: Histogram of non-SSI and SSI groups for the number of positive SSI indicators.
The values over 0.15 are described. The grey labels are exceptions due to the direct relationship between associated factors and SSI indicators. WBC pre: preoperative white blood cell count; NLR pre: preoperative neutrophil to lymphocyte ratio; Neutrophil pre: preoperative neutrophil count; Lymphocyte pre: preoperative lymphocyte count; PLT pre: preoperative platelet counts; CRP pre: preoperative C-reactive protein; ALB pre: preoperative albumin value; TP pre: preoperative total protein value; GLB pre: preoperative globulin value; AGR pre: preoperative albumin/globulin ratio; Op time: operation time; Op bleeding: operation bleeding.
Table 1: Correlation using the correlation ratio (η2) and Cramér’s measure of association (V).
WBC, white blood cells; NLR, neutrophil/lymphocyte ratio; CRP, C-reactive protein; LRp7, lymphocyte ratio from preoperative day to postoperative day 7; NLRRp7: NLR ratio from preoperative day to postoperative day 7; NR27: neutrophil ratio from postoperative days 2 to 7; LR27: lymphocyte ratio from postoperative days 2 to 7; NLRR27: NLR ratio from postoperative days 2 to 7, CR27: CRP ratio from postoperative days 2 to 7; AUC: area under the curve. WBC pre: preoperative white blood cell count, CRP pre: preoperative C-reactive protein, ALB pre: preoperative albumin value, TP pre: preoperative total protein value, AGR pre: preoperative albumin/globulin ratio, Op time: operation time.
Table 2: Multivariate logistic regression analysis for each SSI indicator.
WBC, white blood cells; NLR, neutrophil/lymphocyte ratio; CRP, C-reactive protein; LRp7, lymphocyte ratio from preoperative day to postoperative day 7; NLRRp7: NLR ratio from preoperative day to postoperative day 7; NR27: neutrophil ratio from postoperative days 2 to 7; LR27: lymphocyte ratio from postoperative days 2 to 7; NLRR27: NLR ratio from postoperative days 2 to 7, CR27: CRP ratio from postoperative days 2 to 7; AUC: area under the curve. WBC pre: preoperative white blood cell count, CRP pre: preoperative C-reactive protein, ALB pre: preoperative albumin value, TP pre: preoperative total protein value, AGR pre: preoperative albumin/globulin ratio, Op time: operation time.
Table 3: Cut-off values for associated factors were determined using receiver operating characteristic (ROC) curve analyses.
4. Discussion
Some articles showed the original SSI indicator, but this was mainly a single criterion. Postoperative lym- phopenia (lymphocyte counts below 1000/µL) is an effective indicator of SSI [3-5]. Iwata et al. reported that the cut-off value for lymphocyte counts indi- cating SSI was 1180/µL on postoperative day 4 (sensitivity, 90.9%; specificity, 65.4%) and 1090/µL
at day 7 (sensitivity, 63.6%; specificity, 78.5%) [9, 10]. The re-descending lymphocytes (sensitivity 55%, specificity 81%) and re-ascending neutrophils (sensitivity 46%, specificity 93%) compared to the values on days 1 and 4 postoperatively were indi- cators of SSI [11]. For each criterion, there was a false position fraction (FPF) the same as “1-speci- ficity,” which meant the delay of surgical inflame- matory’ recovery without SSI: this current study is the only article to investigate these delays.
We found ten indicators and the best combination with the highest accuracy of area under the curve (AUC) for receiver operating characteristic (ROC) curve analyses [6]. In the current study (Figure 1), we found that only 50 patients had negative SSI indi- cators, 270 patients had one or more positive indi- cators without SSI, with a median value of 2 points. Most of the patients with SSI had six or more positive indicators. Although each indicator’s accur- acy was different and giving 1 point equally for each positive SSI indicator included problems, patients with less than six points might have a high possibility of delayed surgical recovery without SSI. These results indicate that a single indicator has limited capacity in detecting SSI, and preoperative status influences surgical inflammatory recovery.
4.1 Factors associated with SSI indicators
These factors were categorized using principal comp- onent (PC) analysis, but PC analysis was inadequate for classification (Kaiser-Meyer-Olkin measure of sampling adequacy: 0.489 < 0.5). It may be difficult to classify patients without surgical intervention, and we categorized the patients into two groups based on their preoperative nutritional status and immune- inflammation status. Nutritional status plays a key role in SSI and wound healing [12-14]. Malnutrition prolongs wound healing by promoting fibroblast proliferation and collagen synthesis [12]. For pre- operative albumin (ALB pre), some articles reported that the cut-off value of ALB pre for SSI was 3.5 g/dL [15, 16]. In our results, 3.7 g/dL was the cut-off value for SSI indicators, which means ALB pre < 3.7 g/dL can easily induce delays in CRP and lymphocyte recovery after surgery (Table 3). We consider that ALB pre < 3.7 g/dL might be in yellow light with caution for SSI.
The current study shows that malnutrition affects blood cell reactions during spinal surgery. T cells, which are lymphocytes, are highly influenced by nutrient uptake from the environment. In states of severe malnutrition, T-cell survival, proliferation, and inflammatory cytokine production are all decreased, as are T-cell glucose uptake and metabolism [17, 18]. Thus, immune cells themselves are affected by malnutrition and are related to delayed operative inflammatory recovery. Immune-inflammation status included factors such as age, malignancy, WBC pre-, CRP pre-, and AGR pre. Aging is associated with a state of immunosuppression related to inflammation [19]. “Inflammaging” is a state of chronic, low-level systemic inflammation in the absence of clinically defined infection and is one of the consequences of aging in humans [19]. The current study, which was conducted on participants over 73 years of age, may induce a delay in CRP recovery. Neoplastic immu- nity, which is strongly influenced by innate and adaptive immunity, acts actively with malignant tumors. The microenvironment of cancer and inflam- mation are closely related. Inflammation related to cancer can also be immunosuppressive [20-22].
High WBC pre-and CRP pre- counts directly indicate preoperative inflammation. WBC > 7500/µL, CRP pre > 0.5 µg/dL are not high values, but indicate a significant possibility of delayed surgical recovery. AGR assesses levels of albumin and globulin, which constitute the principal protein comp- onents of human serum. Albumin reflects nutritional status, while globulin plays a key role in immunity and inflammation [23]. AGR is widely used as a prognostic indicator in several human cancers [24- 27]. Inflammatory conditions suppress albumin synthesis, which causes hypoalbuminemia and may increase the levels of globulin [28]. Thus, AGR is a useful biomarker of inflammation. AGR < 1.4, also induces a delay in neutrophil and NLR recovery. Surgical invasion may also affect the immune system and wound healing [29-31]. It can be assumed that a high degree of surgical invasion may cause immune- osuppression, and surgery using spinal instru- mentation is more invasive than surgery without instrumentation [31]. In summary, for preoperative patient condition, nutritional status with TP pre < 6.4 g/dL, ALB pre < 3.7 g/dL; immune-inflammation status with age >73 y/o, CRP pre > 0.5 µg/dL, WBC pre > 7500/µL, AGR pre < 1.4; mali-gnancy, and surgical invasion with operative time >240 min and using spinal instrumentation, are the nine associated factors which may cause delays in recovery from surgical inflammation. They indicate caution must be exercised for SSI. The present study was limited by its retrospective design and single-center setting. Additionally, we could not find effective factors associated with SSI indicators among the SSI and non-SSI groups, which was a direct analysis detect- ing the difference between SSI and non-SSI.
In conclusion, preoperative patient conditions such as malnutrition and high immune-inflammation status influence operative inflammatory recovery, which mimics infection even in the absence of SSI. Therefore, these factors may not always indicate SSI and, as such, must be interpreted with caution. Improving preoperative conditions such as nutritional status and immune-inflammation status may decrease the incidence of delayed recovery without SSI and may improve the accuracy of SSI indicators.
Conflict of Interest
none
References
- Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27 (1999): 97-132.
- Mok JM, Guillaume TJ, Talu U, et Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine 34 (2009): 578-583.
- Takahashi J, Ebara S, Kamimura M, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine 26 (2001): 1698-1704.
- Vulliamy PE, Perkins ZB, Brohi K, et Persistent lymphopenia is an independent predictor of mortality in critically ill emerg- ency general surgical patients. Eur J Trauma Emerg Surg 42 (2016): 755-760.
- Takahashi J, Shono Y, Hirabayashi H, et al. Usefulness of white blood cell differential for early diagnosis of surgical wound infection following spinal instrumentation Spine 31 (2006):1020-1025.
- Imabayashi H, Miyake A, Chiba K. Establis- hment of a suitable combination of serologi-cal markers to diagnose surgical site infection following spine surgery: A novel surgical site infection scoring system. J Orthop Sci 1 (2021): S0949-2658(21)00093-2.
- Imabayashi H, Miyake A, Chiba K. A novel approach for identifying serological markers indicative of surgical-site infection follow-ing spine surgery: Postoperative lymphopen-ia is a risk factor. J Orthop Sci 25 (2021): S0949- 2658(21)00095-6.
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20 (1992): 271-274.
- Iwata E, Shigematsu H, Koizumi M, et al. Lymphocyte Count at 4 Days Postopera- tively and CRP Level at 7 Days Postopera- tively: Reliable and Useful Markers for Surgical Site Infection Following Instrum- ented Spinal Fusion. Spine 41 (2016): 1173-
- Iwata E, Shigematsu H, Yamamoto Y, et al. Lymphocyte Count at 4 Days Postopera- tively: A Reliable Screening Marker for Sur- gical Site Infection After Posterior Lumbar Decompression Spine 43 (2018): E1096-E1101.
- Yamamoto Y, Iwata E, Shigematsu H, et al. Comparison of neutrophil and lymphocyte at 1 and 4 days postoperatively: reliable and early detection markers for surgical site infec- tion following instrumented spinal fusion. Spine Surg Relat Res 2 (2018): 127-134.
- Cross MB, Yi PH, Thomas CF, et Evaluation of malnutrition in orthopaedic surgery. J Am Acad Orthop Surg 22 (2014): 193-199.
- Bohl DD, Shen MR, Mayo BC, et Malnutrition Predicts Infectious and Wound Complications Following Posterior Lumbar Spinal Fusion. Spine 41 (2016): 1693-1699.
- Ihle C, Freude T, Bahrs C, et Malnut-rition - An underestimated factor in the inpa-tient treatment of traumatology and orthope-dic patients: A prospective evaluation of 1055 patients. Injury 48 (2017): 628-636.
- Jevsevar DS, Karlin The relationship between preoperative nutritional status and complications after an operation for scoli-osis in patients who have cerebral palsy. J Bone Joint Surg Am 75 (1993): 880-884.
- Beiner JM, Grauer J, Kwon BK, et Postoperative wound infections of the spine. Neurosurg Focus. 15 (2003): E14.
- Newton R, Priyadharshini B, Turka Immunometabolism of regulatory T cells. Nat Immunol 17 (2016): 618-625.
- Cohen S, Danzaki K, MacIver NJ. Nutritional effects on T-cell immunometa-bolism. Eur J Immunol 47 (2017): 225-235.
- Franceschi C, Campisi Chronic inflame- mation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci (2014): S4-S9.
- Grivennikov SI, Greten FR, Karin Immunity, inflammation, and cancer. Cell 140 (2010): 883-899.
- Ostrand-Rosenberg Tolerance and imm- une suppression in the tumor microenviron- ment. Cell Immunol 299 (2016): 23-29.
- Shalapour S, Karin Immunity, inflame- mation, and cancer: an eternal fight between good and evil. J Clin Invest 125 (2015): 3347-3355.
- McMillan DC, Watson WS, O'Gorman P, et Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 39 (2001): 210-213.
- Azab BN, Bhatt VR, Vonfrolio S, et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mort-ality in breast cancer patients. Am J Surg 206 (2013): 764-
- Du XJ, Tang LL, Mao YP, et The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One 9 (2014): e94473.
- Duran AO, Inanc M, Karaca H, et Albumin-globulin ratio for prediction of long-term mortality in lung adenocarcinoma patients. Asian Pac J Cancer Prev 15 (2014): 6449-6453.
- Lv GY, An L, Sun XD, et al. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta- Clin Chim Acta 476 (2018): 81-91.
- Gupta D, Lis Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9 (2010): 69.
- Shen J, Liang J, Yu H, et al. Risk factors for delayed infections after spinal fusion and instrumentation in patients with Clinical article. J Neurosurg Spine 21 (2014): 648-652.
- Farshad M, Bauer DE, Wechsler C, et Risk factors for perioperative morbidity in spine surgeries of different complexities: a multivariate analysis of 1,009 consecutive patients. Spine J 18 (2018): 1625-1631.
- Yao R, Zhou H, Choma TJ, et al. Surgical Site Infection in Spine Surgery: Who Is at Risk? Global Spine 8 (2018): 5S-30S.


 Impact Factor: * 3.123
Impact Factor: * 3.123 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 14.90%
Acceptance Rate: 14.90%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 