Advances in Extracellular Vesicles in the Treatment of Osteosarcoma
Article Information
Zhefei Du1† , Lixin Xie1† , Daihan Xie1† , Kesheng Wang2 , Qiuxia Peng2 , Chao Fang2*
1Tongji University School of Medicine, Tongji University, No. 500 Zhen-nan Road, Shanghai 200092, China
2Central Laboratory of Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Tongji University, No. 301 Yan-chang-zhong Road, Shanghai 200072, China
†These authors contributed equally to this work
*Corresponding Author: Chao Fang, Central Laboratory of Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Tongji University, No. 301 Yan-chang-zhong Road, Shanghai 200072, China.
Received: 25 September 2023; Accepted: 03 October 2023; Published: 09 October 2023
Citation: Zhefei Du, Lixin Xie, Daihan Xie, Kesheng Wang, Qiuxia Peng, Chao Fang. Advances in extracellular vesicles in the treatment of osteosarcoma. Journal of Spine Research and Surgery. 5 (2023): 88-95.
View / Download Pdf Share at FacebookAbstract
Osteosarcoma, a prevalent primary malignant bone tumor, presents a pressing challenge in terms of improving patient prognosis effectively. Extracellular vesicles, encompassing vesicular structure with a bilayered biomembrane released by cells, have been shown to play a significant role in the pathogenesis and progression of osteosarcoma. This mini-review provides an overview of the fundamental biological characteristics of extracellular vesicles and their therapeutic implications in osteosarcoma. Furthermore, it presents an outlook on the current limitations of extracellular vesicles and their potential future applications.
Keywords
Cancer therapy, Extracelluar vesicles, Invasion and metastasis, Mechanism, Osteosarcoma
Cancer therapy articles Cancer therapy Research articles Cancer therapy review articles Cancer therapy PubMed articles Cancer therapy PubMed Central articles Cancer therapy 2023 articles Cancer therapy 2024 articles Cancer therapy Scopus articles Cancer therapy impact factor journals Cancer therapy Scopus journals Cancer therapy PubMed journals Cancer therapy medical journals Cancer therapy free journals Cancer therapy best journals Cancer therapy top journals Cancer therapy free medical journals Cancer therapy famous journals Cancer therapy Google Scholar indexed journals Extracelluar vesicles articles Extracelluar vesicles Research articles Extracelluar vesicles review articles Extracelluar vesicles PubMed articles Extracelluar vesicles PubMed Central articles Extracelluar vesicles 2023 articles Extracelluar vesicles 2024 articles Extracelluar vesicles Scopus articles Extracelluar vesicles impact factor journals Extracelluar vesicles Scopus journals Extracelluar vesicles PubMed journals Extracelluar vesicles medical journals Extracelluar vesicles free journals Extracelluar vesicles best journals Extracelluar vesicles top journals Extracelluar vesicles free medical journals Extracelluar vesicles famous journals Extracelluar vesicles Google Scholar indexed journals Invasion and metastasis articles Invasion and metastasis Research articles Invasion and metastasis review articles Invasion and metastasis PubMed articles Invasion and metastasis PubMed Central articles Invasion and metastasis 2023 articles Invasion and metastasis 2024 articles Invasion and metastasis Scopus articles Invasion and metastasis impact factor journals Invasion and metastasis Scopus journals Invasion and metastasis PubMed journals Invasion and metastasis medical journals Invasion and metastasis free journals Invasion and metastasis best journals Invasion and metastasis top journals Invasion and metastasis free medical journals Invasion and metastasis famous journals Invasion and metastasis Google Scholar indexed journals Mechanism articles Mechanism Research articles Mechanism review articles Mechanism PubMed articles Mechanism PubMed Central articles Mechanism 2023 articles Mechanism 2024 articles Mechanism Scopus articles Mechanism impact factor journals Mechanism Scopus journals Mechanism PubMed journals Mechanism medical journals Mechanism free journals Mechanism best journals Mechanism top journals Mechanism free medical journals Mechanism famous journals Mechanism Google Scholar indexed journals Osteosarcoma articles Osteosarcoma Research articles Osteosarcoma review articles Osteosarcoma PubMed articles Osteosarcoma PubMed Central articles Osteosarcoma 2023 articles Osteosarcoma 2024 articles Osteosarcoma Scopus articles Osteosarcoma impact factor journals Osteosarcoma Scopus journals Osteosarcoma PubMed journals Osteosarcoma medical journals Osteosarcoma free journals Osteosarcoma best journals Osteosarcoma top journals Osteosarcoma free medical journals Osteosarcoma famous journals Osteosarcoma Google Scholar indexed journals osteoblasts articles osteoblasts Research articles osteoblasts review articles osteoblasts PubMed articles osteoblasts PubMed Central articles osteoblasts 2023 articles osteoblasts 2024 articles osteoblasts Scopus articles osteoblasts impact factor journals osteoblasts Scopus journals osteoblasts PubMed journals osteoblasts medical journals osteoblasts free journals osteoblasts best journals osteoblasts top journals osteoblasts free medical journals osteoblasts famous journals osteoblasts Google Scholar indexed journals Spine Tumor articles Spine Tumor Research articles Spine Tumor review articles Spine Tumor PubMed articles Spine Tumor PubMed Central articles Spine Tumor 2023 articles Spine Tumor 2024 articles Spine Tumor Scopus articles Spine Tumor impact factor journals Spine Tumor Scopus journals Spine Tumor PubMed journals Spine Tumor medical journals Spine Tumor free journals Spine Tumor best journals Spine Tumor top journals Spine Tumor free medical journals Spine Tumor famous journals Spine Tumor Google Scholar indexed journals nanoscale vesicles articles nanoscale vesicles Research articles nanoscale vesicles review articles nanoscale vesicles PubMed articles nanoscale vesicles PubMed Central articles nanoscale vesicles 2023 articles nanoscale vesicles 2024 articles nanoscale vesicles Scopus articles nanoscale vesicles impact factor journals nanoscale vesicles Scopus journals nanoscale vesicles PubMed journals nanoscale vesicles medical journals nanoscale vesicles free journals nanoscale vesicles best journals nanoscale vesicles top journals nanoscale vesicles free medical journals nanoscale vesicles famous journals nanoscale vesicles Google Scholar indexed journals plasma membrane articles plasma membrane Research articles plasma membrane review articles plasma membrane PubMed articles plasma membrane PubMed Central articles plasma membrane 2023 articles plasma membrane 2024 articles plasma membrane Scopus articles plasma membrane impact factor journals plasma membrane Scopus journals plasma membrane PubMed journals plasma membrane medical journals plasma membrane free journals plasma membrane best journals plasma membrane top journals plasma membrane free medical journals plasma membrane famous journals plasma membrane Google Scholar indexed journals apoptosis articles apoptosis Research articles apoptosis review articles apoptosis PubMed articles apoptosis PubMed Central articles apoptosis 2023 articles apoptosis 2024 articles apoptosis Scopus articles apoptosis impact factor journals apoptosis Scopus journals apoptosis PubMed journals apoptosis medical journals apoptosis free journals apoptosis best journals apoptosis top journals apoptosis free medical journals apoptosis famous journals apoptosis Google Scholar indexed journals
Article Details
INTRODUCTION
Osteosarcoma (OS) is a prevalent primary malignant bone tumor characterized by clinical manifestations such as pain, swelling, and reduced mobility. It has the highest incidence among adolescents and individuals over 60 years old [1]. Since 1980, surgical resection and chemotherapy have been the primary clinical approaches for treating OS [2]. The 5-year event-free survival rate for non-metastatic OS is approximately 60%. However, in individuals with multiple lung nodules or radiographically detected tumor metastasis in other locations, the 5-year event-free survival rate drops below 20% [2]. Improving the prognosis of OS patients effectively is currently an urgent issue.
The major pathological feature of OS is the presence of osteolytic tumor cells capable of producing osteoid-like matrix [3]. In addition to chromosomal complexity, alterations in copy number, and mutations in tumor suppressor genes (e.g., TP53), key molecular changes occur at the molecular level during the occurrence and development of OS [4]. These changes primarily include: (1) the enhanced osteolytic activity of osteoclasts due to cytokines produced by tumor cells (such as IL-1, IL-6, TNF-α), disrupting the balance between osteoblasts and osteoclasts; (2) the release of bone matrix factors, including IGF-1 and TGF-β, during the process of bone resorption, promoting tumor cell proliferation and establishing a malignant cycle in the development of OS; (3) other factors, such as extracellular vesicles (EVs) in the tumor microenvironment (TME) and bone marrow mesenchymal stem cells (MSCs), have been demonstrated to be involved in the occurrence and development of OS [5-8].
Despite the gradual elucidation of the underlying mechanisms involved in the proliferation and metastasis of OS, the current clinical treatment strategies for OS remain limited. On one hand, tumor cells exhibit strong invasiveness, often leading to metastasis in the lungs and distal ends of long bones, posing challenges for surgical treatment [1]. On the other hand, tumor cells demonstrate resistance to various chemotherapy drugs [9]. To overcome these difficulties, there is a need to develop an effective and safe drug delivery system to improve the 5-year survival rate of OS patients and minimize the side effects associated with chemotherapy. Studies have shown that in the OS microenvironment, there is close crosstalk between tumor cells, bone matrix cells, and immune cells, mediated by cytokines, direct cell-to-cell contact, and EVs [10] (Figure 1).
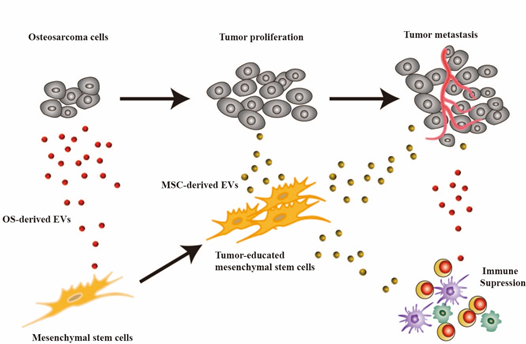
Figure 1. EVs mediate cellular communication in the TME of OS: Tumor cells secrete EVs that induce phenotypic changes in MSCs. These altered MSCs, through the secretion of EVs, participate in tumor cell proliferation and metastasis, as well as immune cell suppression.
EVs are a collective term for various vesicular structures released by cells, characterized by a double-layered biomembrane. EVs can be divided into small extracellular vesicles (sEVs), also known as exosomes, microvesicles (MVs) and larger apoptotic bodies based on the diameter of the vesicles [11]. EVs contain nucleic acids, proteins, lipids, and even mitochondria, playing a crucial role in cell communication within the TME and participating in various biological processes such as tumor development, angiogenesis, and immune response [12,13]. Due to their unique characteristics, EVs are considered a potential therapeutic strategy for OS: (1) nanoscale size; (2) cell targeting ability; (3) high stability to counteract enzymatic degradation; (4) capability to deliver cargo [14,15].
This mini-review begins by providing an overview of the generation and structural patterns of EVs in OS, followed by a review of the significant roles of EVs derived from the TME in the occurrence, development, and metastasis of OS. Furthermore, the current strategies for preclinical treatment utilizing EVs are discussed, along with prospects for future research and clinical applications.
Properties of Extracellular Vesicles
EVs are diminutive vesicular structures generated intracellularly and subsequently released into the extracellular milieu, exhibiting a distinctive lipid bilayer enveloping the cytoplasmic content [16]. EVs, serving as endogenous nanocarriers, enable the transportation of vital cellular components such as proteins, nucleic acids, lipids, and therapeutic agents, thereby fulfilling their role as a cellular-based nanoscale drug delivery system [17,18]. Categorized based on their dimensions and cellular origins, EVs can be broadly classified into three principal subtypes: exosomes, MVs, and apoptotic bodies [19]. Notably, these EVs secreted by cells present within the intricate milieu of the TME act as mediators, profoundly affecting intercellular communications within the tumor stroma by facilitating the transfer of non-coding RNA and requisite proteins, thereby expediently promoting cellular proliferation, metastasis, as well as augmenting tumor immune evasion and dissemination [20-22].
Classification and Characteristics of Extracellular Vesicles
EVs, derived from cellular origins, are a class of nanoscale vesicular entities that have emerged as a subject of substantial research interest. Among them, exosomes, as the smallest EV subtype, typically exhibit diameters ranging from 30 to 150 nanometers, and have demonstrated significant potential in OS therapeutics [23]. Exosomes are generated during the intricate process involving inward budding of the plasma membrane and the formation of intraluminal vesicles (ILVs) within the intracellular multivesicular bodies (MVBs), while engaging in crosstalk with other intracellular vesicles and organelles, thereby resulting in the ultimate composition of exosomes [24]. The bi-layered vesicular structures formed are specifically recognized and recruited by the polymeric exosomal sorting complex required for transport (ESCRT) machinery, culminating in the fusion of vesicles with the cytoplasmic membrane and their subsequent release [25]. As natural nanocarriers, exosomes harbor a diverse array of constituents encompassing nucleic acids, proteins, lipids, amino acids, and metabolites, which propagate through bodily fluids, facilitating intercellular communication and the modulation of cellular functionality and metabolic processes [26,27]. MVs, representing a slightly larger category of EVs, exhibit diameters ranging from 40 to 1000 nanometers, and originate from distinct microdomains on the cellular membrane. They primarily transport membrane-associated proteins and lipids, forming through processes such as membrane budding or shedding [28]. Apoptotic bodies, the largest structures among EVs, typically ranging from 1 to 5 micrometers in diameter, predominantly emerge during cellular apoptosis. These entities carry cellular debris and organelles, and are typically recognized and cleared by immune surveillance mechanisms [29] (Figure 2).
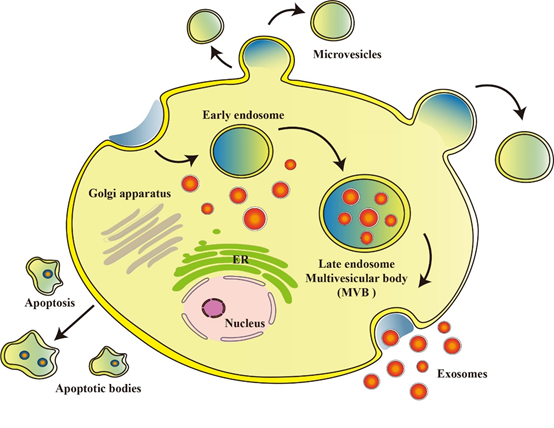
Fig.2 Formation and release of EVs: EVs are nanoscale vesicles derived from cells. Exosomes are generated through a process involving inward budding of the plasma membrane and formation of MVB within the cell. MVs are mainly formed by shedding or release of the cell membrane. Apoptotic bodies are formed during cell apoptosis, carrying cellular debris and organelles.
Origin and Structure of Extracellular Vesicles
EVs originating from diverse cellular sources possess distinct biological activities and are increasingly employed in OS therapy. These EVs can derive from various entities, including OS tumor cells, MSCs, osteoblasts, macrophages, tissue-engineered implants, and even artificial nanovesicles. Notably, a multitude of cell types, such as epithelial cells, hematopoietic cells, tumor cells, and MSCs, have been shown to secrete EVs, among which MSCs have gained significant attention as an ideal cell source for large-scale production due to their ease of isolation, ethical neutrality, and expandability [30]. Lin et al. employed an exosome isolation kit to isolate EVs (MSC-EVs) from bone marrow-derived MSCs (BM-MSCs). Subsequently, they harnessed these EVs for the efficient targeting OS cells by engineering them as carriers for doxorubicin (Exo-Dox) through a process involving the combination of MSC-EVs with Dox-HCl, followed by desalting using triethylamine and overnight dialysis using phosphate-buffered saline (18). Furthermore, research has evidenced the presence of miRNAs associated with cell adhesion and apoptosis within EVs derived from OS cell lines, rendering them potential therapeutic targets for addressing OS metastasis [31]. Ji et al. identified sEVs of OS cell line origin as vehicles for delivering miR-19a-3p, which, via the modulation of intercellular communication, promotes osteoclast differentiation and bone destruction, thus offering novel prospects for targeted OS therapy [32]. Conversely, EVs derived from human OS cells have been shown to exert effects on bone cells, including osteoblasts [33]. Rucci et al. conducted comprehensive investigations on the impact of osteoblast-derived EVs (OB-EVs) on the tumor phenotype, mitochondrial energy metabolism, and oxidative damage of MNNG/HOS cells. Intriguingly, their findings unveiled that OB-EVs can abate the invasive behavior and vitality of OS cells via redox-dependent signaling pathways, without perturbing mitochondrial dynamics and energy metabolism, thereby elucidating previously unexplored facets of their functionality [34]. Additionally, Yin et al. provided compelling evidence that EVs sourced from M1-polarized macrophages (M1EVs) can serve as immunomodulators within the TME for OS therapy [35].
Moreover, extensive endeavors are currently underway to develop exosome mimetics (EMs) exhibiting enhanced carrier capabilities to address the formidable challenges associated with the purification and large-scale production of exosomes [36, 37]. Wang et al. devised a sequential cell extrusion approach to fabricate EMs that not only retain the functional attributes of exosomes but also facilitate high-yield provision of chemotherapeutic agents [38].
The role of extracellular vesicles in osteosarcoma
In previous studies, EVs were found to be able to excrete metabolic wastes from cells, perform cellular communication, and act as nano-delivery carriers for nucleic acids and drugs [39-41]. EVs are highly stable, small in size, and low in immunogenicity, and therefore have potential for disease treatment [39,42].
Various healthy and cancer cells can produce EVs, which regulate TME, affect OS cell proliferation, migration, invasion and angiogenesis, and promote OS cell immune escape through cellular communication and cell signaling [43,44]. Han et al. found that the exosome miR-1307 secreted in OS cells inhibited AGAP1 expression, thus enhancing the growth, invasion and migration of OS cells. Therefore, the miR-1307-AGAP1 axis may provide a new target for future treatment of osteosarcoma [45]. Li et al. demonstrated that OS cells generate exosome lnc-OIP5-AS1 and act on other OS cells to regulate angiogenesis in OS through miR-153 and ATG5 [46]. EVs secreted by cancer cells can deliver tumor-associated antigens (TAAs) and generate anti-tumor immune responses. However, they also inhibit immune cells, thereby promoting the escape of tumor cells [47,48]. Kerri et al. showed that metastatic OS cells produce exosomal TGFb2 that promotes the M2 phenotype, which promotes immunosuppression and facilitates tumorigenesis [49]. In addition, EVs are also associated with the immune microenvironment within OS cells, and second-generation sequencing has demonstrated that specific miRNAs, such as miR-21-5p and miR-148a, have a significant impact on the TME [50]. EVs secreted by OS cells may also affect bone development. An OS-derived exosome called miR-501-3p, promotes osteoclastogenesis and worsens bone loss through the PTEN/ PI3K/Akt signaling pathway [51].
EVs derived from other cells also have effects on OS cells. Exosomal-miR-206 from bone marrow mesenchymal stem cells (BMSCs) inhibited OS cell proliferation, migration and invasion by targeting TRA2B [52]. Li et al. found that BMSC-EVs promoted the proliferation, invasion and migration of OS cells through the MALAT1/ miR-143/NRSN2/Wnt/b-catenin axis [53]. The macrophage-derived exosome lnc-LIFR-AS1 promotes the proliferation, invasion and apoptosis of OS cells through the miR-29a/NFIA axis [54]. Moreover, Tao et al. showed that EWSAT1 acts in concert with EVs to induce an increase in the secretion of angiogenic factors, which affects tumor angiogenesis [55]. Ge et al. proved that exosomal LCP1 secreted by BMSCs could promote bone proliferation and metastasis via the JAK2/STAT3 pathway [56].
The application of extracellular vesicles in osteosarcoma treatment
OS is highly heterogeneous and genetically complex, which makes its treatment challenging. Kyung et al. demonstrated that EVs activate OS cell apoptosis and have anti-tumor effects [35]. In addition, the properties of EVs allow them to specifically target OS cells as drug carriers for treatment [57,58]. Conventional chemotherapeutic agents used for OS treatment include Methotrexate, Doxorubicin (Dox), Ifosfamide, Cyclophosphamide, Carboplatin or Cisplatin, Gemcitabine and/or Etoposide, etc. EVs loaded with chemotherapeutic drugs can significantly reduce the dose-dependent side effects of chemotherapeutic drugs and improve their efficacy in cancer treatment [59]. Wei et al. prepared exosomes loaded with Doxorubicin and demonstrated its advantages in chemotherapy targeting OS [18]. It is noteworthy that EVs also affect cellular resistance to chemotherapy. Pan et al. have shown that exosomes from cisplatin-resistant cells (CDDP) increases the expression levels of multidrug resistance-associated protein 1 and p -glycoprotein, and promotes chemoresistance to CDDP in MG63 and U2OS cells [60]. Multidrug-resistant OS cells can enhance drug resistance by secreting exosomes carrying MDR-1 mRNA and its p -glycoprotein product [61].
miRNA therapeutics are receiving a significant amount of attention, but the lack of good and safe target cell vectors limits their application, while EVs provide a direction for the safe and effective delivery of miRNAs to OS cells. OS-derived EVs binding miR-21 were able to act on fibroblasts, immune cells and endothelial cells to affect angiogenesis, metastasis and immune escape of OS cells [62]. Shimbo et al. found that exosomes binding artificial miR-143, acting on BMSCs, were able to have a therapeutic effect on OS cells and significantly reduce metastasis [63]. Furthermore, EVs carrying miR-150 can reduce the proliferation and migration of OS cells by targeting IGF2BP1 (insulin-like growth factor-2 mRNA-binding protein 1) [64]. EVs-derived circRNAs can be delivered to OS cells, affecting their gene expression and protein function and thus tumor metastasis(65). Studies have demonstrated that circRNA overexpression can inhibit the proliferation, migration and invasion of OS cells, and circRNA-rich exosomes may be a good therapeutic target for OS [66] (Figure 3). In addition, Jiang et al. found that exosomes loaded with miR-144-3p and ZEB1, could regulate the iron death mechanism to modulate OS development, demonstrating that ferritin-associated miR-144-3p could be used as an anticancer therapeutic target [67].
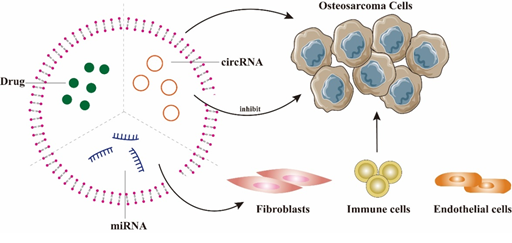
Figure 3. The main application of EVs in OS treatment: EVs can be loaded with drugs or miRNAs and target OS cells. In addition, miRNA can act on fibroblasts, immune cells and endothelial cells, thereby affecting the angiogenesis, metastasis and immune escape of OS cells. Overexpression of circRNA in EVs can inhibit the proliferation, invasion and migration of OS.
Concerns and Perspectives
To date, the precise role of EVs in OS remains elusive. While previous studies have demonstrated promising therapeutic efficacy of EVs in animal models, there is still a significant gap between EV-based treatments and OS clinical management. Several factors contribute to this disparity. Firstly, OS exhibits a relatively low incidence compared to other malignancies, with only 4.3 cases per million individuals in the 0-14 age group in the United States, making large-scale clinical trials challenging to conduct [68]. Additionally, the genomic instability of OS introduces individual variations that pose challenges for EV-based therapies [69].
Regarding EVs, the lack of established standardized methods for in vivo EV purification hinders the confirmation of certain research findings. Moreover, not all cargo carried by EVs influences the occurrence and progression of OS, necessitating further investigation to elucidate the relative importance of each cargo component in OS development. Furthermore, the therapeutic efficacy of EVs may vary depending on their specific sources. For instance, EVs derived from plasma are considered metabolic waste products of diseased tissues [70]. Existing research primarily focuses on solid OS tumors, and little attention has been given to exploring the use of EVs to target circulating tumor cells involved in OS hematogenous metastasis. Most importantly, the clinical application of EVs should be grounded in safety and efficacy. While enhancing the tumor-targeting capabilities of EVs, thorough assessment of their long-term effects on the organism in animal models is paramount.
As natural mediators of intercellular communication, EVs hold promise as potential candidates for OS drug delivery and gene therapy. In conclusion, a comprehensive understanding of EVs will contribute to the development of improved EV-based therapeutic strategies for OS.
Declarations
Ethical Approval
Not applicable
Consent for Publication
Not applicable
Data Availability
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current investigation.
Conflicts of Interest
There is no conflict to declare.
Funding
No financial support was provided.
Author Contributions
Chao Fang and Qiuxia Peng conceived the theme of this review. Kesheng Wang designed the review. Zhefei Du, Lixin Xie and Daihan Xie wrote the manuscript, conducted the literature investigation and interpreted the related literature, and prepared the figures and table. Zhefei Du critically analyzed the key knowledge in this review. Lixin Xie and Daihan Xie edited and revised the manuscript. All authors have read and approved the ultimate manuscript.
Acknowledgements
Not applicable.
Statements & Declarations
We declare that this manuscript is original, has never been published before, and has not been considered to be published elsewhere at present.
References
- Beird HC, Bielack SS, Flanagan AM, et al. Osteosarcoma. Nat Rev Dis Primers 8 (2022): 77.
- Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol 18 (2021): 609-624.
- Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol 125 (2006):555-581.
- Nirala BK, Yamamichi T, Yustein JT. Deciphering the Signaling Mechanisms of Osteosarcoma Tumorigenesis. Int J Mol Sci 24 (2023): 11367.
- Wittrant Y, Théoleyre S, Chipoy C, et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta 1704 (2004): 49-57.
- Alfranca A, Martinez-Cruzado L, Tornin J, Abarrategi A, Amaral T, de Alava E, et al. Bone microenvironment signals in osteosarcoma development. Cell Mol Life Sci 72 (2015): 3097-3113.
- Zheng Y, Wang G, Chen R, et al. Mesenchymal stem cells in the osteosarcoma microenvironment: their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res Ther 9 (2018): 22.
- Liao Y, Yi Q, He J, et al. Extracellular vesicles in tumorigenesis, metastasis, chemotherapy resistance and intercellular communication in osteosarcoma. Bioengineered 14 (2023):113-128.
- Baldini N, Scotlandi K, Barbanti-Bròdano G, et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med 333 (1995): 1380-1385.
- Crockett JC, Rogers MJ, Coxon FP, et al. Bone remodelling at a glance. J Cell Sci 124 (2011): 991-998.
- Görgens A, Bremer M, Ferrer-Tur R, et al. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J Extracell Vesicles 8 (2019): 1587567.
- Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6 (2015): 8472.
- Kalluri R, McAndrews KM. The role of extracellular vesicles in cancer. Cell 186 (2023):1610-1626.
- Lopez-Verrilli MA, Court FA. Exosomes: mediators of communication in eukaryotes. Biol Res. 46 (2013): 5-11.
- Walker S, Busatto S, Pham A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9 (2019): 8001-8017.
- Trams EG, Lauter CJ, Salem N Jr, et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645 (1981): 63-70.
- Shi Z, Wang K, Xing Y, et al. CircNRIP1 Encapsulated by Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Aggravates Osteosarcoma by Modulating the miR-532-3p/AKT3/PI3K/AKT Axis. Front Oncol. 11 (2021): 658139.
- Wei H, Chen J, Wang S, et al. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int J Nanomedicine 14 (2019): 8603-8610.
- Poupardin R, Wolf M, Strunk D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv Drug Deliv Rev 176 (2021):113872.
- Yang Q, Liu J, Wu B, et al. Role of extracellular vesicles in osteosarcoma. Int J Med Sci. 19 (2022): 1216-1226.
- Perut F, Roncuzzi L, Baldini N. The Emerging Roles of Extracellular Vesicles in Osteosarcoma. Front Oncol. 9 (2019):1342.
- Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer 14 (2014):722-35.
- Liang Y, Duan L, Lu J, et al. Engineering exosomes for targeted drug delivery. Theranostics 11 (2021): 3183-3195.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 367 (2020): eaau6977.
- Larios J, Mercier V, Roux A, et al. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 219 (2020): e201904113.
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 219 (2015): 396-405.
- Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. 459 (2019): 1-6.
- Kim KM, Abdelmohsen K, Mustapic M, et al. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 8 (2017).
- Zhou M, Li YJ, Tang YC, et al. Apoptotic bodies for advanced drug delivery and therapy. J Control Release 351 (2022): 394-406.
- Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65 (2013): 336-341.
- Jerez S, Araya H, Hevia D, et al. Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene 710 (2019): 246-257.
- Luo T, Zhou X, Jiang E, et al. Osteosarcoma Cell-Derived Small Extracellular Vesicles Enhance Osteoclastogenesis and Bone Resorption Through Transferring MicroRNA-19a-3p. Front Oncol 11 (2021): 618662.
- Strømme O, Psonka-Antonczyk KM, Stokke BT, et al. Myeloma-derived extracellular vesicles mediate HGF/c-Met signaling in osteoblast-like cells. Exp Cell Res 383 (2019): 111490.
- Ponzetti M, Ucci A, Puri C, et al. Effects of osteoblast-derived extracellular vesicles on aggressiveness, redox status and mitochondrial bioenergetics of MNNG/HOS osteosarcoma cells. Front Oncol 12 (2022): 983254.
- Lee KM, An JH, Yang SJ, et al. Influence of Canine Macrophage-derived Extracellular Vesicles on Apoptosis in Canine Melanoma and Osteosarcoma Cell Lines. Anticancer Res 41 (2021): 719-730.
- Shu S, Yang Y, Allen CL, et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles 9 (2020): 1692401.
- Reiner AT, Witwer KW, van Balkom BWM, et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl Med 6 (2017):1730-1739.
- Wang J, Li M, Jin L, et al. Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv 29 (2022): 3291-3303.
- Gao X, Gao B, Li S. Extracellular vesicles: A new diagnostic biomarker and targeted drug in osteosarcoma. Front Immunol 13 (2022): 1002742.
- Johnstone RM, Ahn J. A common mechanism may be involved in the selective loss of plasma membrane functions during reticulocyte maturation. Biomed Biochim Acta 49 (1990): S70-75.
- Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine 14 (2018): 195-204.
- Yang E, Wang X, Gong Z, et al. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther 5 (2020):242.
- Santos A, Domingues C, Jarak I, et al. Osteosarcoma from the unknown to the use of exosomes as a versatile and dynamic therapeutic approach. Eur J Pharm Biopharm 170 (2022): 91-111.
- Xie F, Zhou X, Fang M, et al. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv Sci (Weinh) 6 (2019): 1901779.
- Han F, Pu P, Wang C, et al. Osteosarcoma Cell-Derived Exosomal miR-1307 Promotes Tumorgenesis via Targeting AGAP1. Biomed Res Int (2021): 7358153.
- Li Y, Lin S, Xie X, et al. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am J Transl Res 13 (2021): 4211-4223.
- Jiang C, Zhang N, Hu X, et al. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol Cancer 20 (2021): 117.
- Chen H, Chengalvala V, Hu H, et al. Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B 11 (2021): 2136-2149.
- Wolf-Dennen K, Gordon N, Kleinerman ES. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology 9 (2020):1747677.
- Raimondi L, De Luca A, Gallo A, et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis 41 (2020): 666-677.
- Lin L, Wang H, Guo W, et al. Osteosarcoma-derived exosomal miR-501-3p promotes osteoclastogenesis and aggravates bone loss. Cell Signal 82 (2021): 109935.
- Zhang H, Wang J, Ren T, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett 490 (2020): 54-65.
- Li F, Chen X, Shang C, et al. Bone Marrow Mesenchymal Stem Cells-Derived Extracellular Vesicles Promote Proliferation, Invasion and Migration of Osteosarcoma Cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/β-Catenin Axis. Onco Targets Ther 14 (2021):737-749.
- Zhang H, Yu Y, Wang J, et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int 21 (2021):192.
- Tao SC, Huang JY, Wei ZY, et al. EWSAT1 Acts in Concert with Exosomes in Osteosarcoma Progression and Tumor-Induced Angiogenesis: The "Double Stacking Effect". Adv Biosyst 13 (2020): e2000152.
- Ge X, Liu W, Zhao W, et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol Ther Nucleic Acids. 21 (2020): 900-915.
- Sarhadi VK, Daddali R, Seppänen-Kaijansinkko R. Mesenchymal Stem Cells and Extracellular Vesicles in Osteosarcoma Pathogenesis and Therapy. Int J Mol Sci 22 (2021): 11035.
- Shafei A, El-Bakly W, Sobhy A, et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacother 95 (2017): 1209-1218.
- Mohammadi M, Zargartalebi H, Salahandish R, et al. Emerging technologies and commercial products in exosome-based cancer diagnosis and prognosis. Biosens Bioelectron. 183 (2021): 113176.
- Pan Y, Lin Y, Mi C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol Int 45 (2021): 858-868.
- Torreggiani E, Roncuzzi L, Perut F, et al. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol 49 (2016): 189-196.
- Wang S, Ma F, Feng Y, et al. Role of exosomal miR-21 in the tumor microenvironment and osteosarcoma tumorigenesis and progression (Review). Int J Oncol 56 (2020): 1055-1063.
- Shimbo K, Miyaki S, Ishitobi H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun 445 (2014): 381-387.
- Xu Z, Zhou X, Wu J, et al. Mesenchymal stem cell-derived exosomes carrying microRNA-150 suppresses the proliferation and migration of osteosarcoma cells via targeting IGF2BP1. Transl Cancer Res. 9 (2020): 5323-5335.
- Tu C, He J, Qi L, et al. Emerging landscape of circular RNAs as biomarkers and pivotal regulators in osteosarcoma. J Cell Physiol 235 (2020): 9037-9058.
- Li S, Pei Y, Wang W, et al. Extracellular nanovesicles-transmitted circular RNA has_circ_0000190 suppresses osteosarcoma progression. J Cell Mol Med. 24 (2020): 2202-2214.
- Jiang M, Jike Y, Liu K, et al. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol Cancer 22 (2023): 113.
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 73 (2023): 17-48.
- Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther 6 (2006): 1075-1085.
- Li S. The basic characteristics of extracellular vesicles and their potential application in bone sarcomas. J Nanobiotechnology 19 (2021): 277.


 Impact Factor: * 3.123
Impact Factor: * 3.123 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 14.90%
Acceptance Rate: 14.90%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 