Squeeze and Freeze, A Periodic Consecutive Oocyte Retrieval Method for Poor Ovarian Responders, Increases the Chance of Childbearing
Article Information
Yasutaka Murata*, Fumika Oda, Miki Shirai, Nanako Sato, Atsuko Sugita, Tomoko Murata
ART Clinic Mirai, 2-2-2, Daijyuuji, Okazaki-city, Aichi, 444-2134, Japan
*Corresponding author: Yasutaka Murata, ART Clinic Mirai, 2-2-2, Daijyuuji, Okazaki-city, Aichi, 444-2134, Japan
Received: 05 January 2022; Accepted: 12 January 2022; Published: 17 January 2022
Citation:
Yasutaka Murata, Fumika Oda, Miki Shirai, Nanako Sato, Atsuko Sugita, Tomoko Murata. Squeeze and Freeze, A Periodic Consecutive Oocyte Retrieval Method for Poor Ovarian Responders, Increases the Chance of Childbearing. Journal of Women’s Health and Development 5 (2022): 035-049.
View / Download Pdf Share at FacebookAbstract
Purpose: To assess if consecutive oocyte retrieval (OR) of more than three times for poor ovarian responders could be an effective method to obtain competent embryos becoming babies.
Methods: To maximize the chances of pregnancy for refractory infertile women, we designed a new treatment course, “Squeeze and freeze(SF).” In this method, ovarian stimulation was initiated whenever the antral follicle was visible using ultrasound, regardless of the menstrual cycle stage for 3 months, aiming to get highest number of oocytes and competent embryos for subsequent transfer. Eighty-eight patients underwent 101 treatment courses, in which retrievals at an average of 4.8 times were performed for 3 months. The clinical results and obstetric outcomes were retrospectively reviewed.
Results: The ratio of matured oocytes, 2PN embryos, available embryos, fair blastocysts per oocyte, and number of available embryos or fair blastocysts per retrieval did not differ between the two initial and later attempts. Similarly, no significant differences were observed in these parameters between the follicular- and luteal-phase retrievals. After subse-quent embryo transfer, 62 patients achieved clinical pregnancy, 49 had an ongoing pregnancy, 47 deliv-ered healthy babies.
Conclusion: Periodic consecutive OR, more than three consecutive ORs, including luteal-phase ORs, is an efficient infertility treatment for refractory women.
Keywords
Advanced Maternal Age, Consecutive Oocyte Retrievals, Luteal-Phase Stimulation, Poor Responder, Squeeze and Freeze
Advanced Maternal Age articles Advanced Maternal Age Research articles Advanced Maternal Age review articles Advanced Maternal Age PubMed articles Advanced Maternal Age PubMed Central articles Advanced Maternal Age 2023 articles Advanced Maternal Age 2024 articles Advanced Maternal Age Scopus articles Advanced Maternal Age impact factor journals Advanced Maternal Age Scopus journals Advanced Maternal Age PubMed journals Advanced Maternal Age medical journals Advanced Maternal Age free journals Advanced Maternal Age best journals Advanced Maternal Age top journals Advanced Maternal Age free medical journals Advanced Maternal Age famous journals Advanced Maternal Age Google Scholar indexed journals Consecutive Oocyte Retrievals articles Consecutive Oocyte Retrievals Research articles Consecutive Oocyte Retrievals review articles Consecutive Oocyte Retrievals PubMed articles Consecutive Oocyte Retrievals PubMed Central articles Consecutive Oocyte Retrievals 2023 articles Consecutive Oocyte Retrievals 2024 articles Consecutive Oocyte Retrievals Scopus articles Consecutive Oocyte Retrievals impact factor journals Consecutive Oocyte Retrievals Scopus journals Consecutive Oocyte Retrievals PubMed journals Consecutive Oocyte Retrievals medical journals Consecutive Oocyte Retrievals free journals Consecutive Oocyte Retrievals best journals Consecutive Oocyte Retrievals top journals Consecutive Oocyte Retrievals free medical journals Consecutive Oocyte Retrievals famous journals Consecutive Oocyte Retrievals Google Scholar indexed journals Luteal-Phase Stimulation articles Luteal-Phase Stimulation Research articles Luteal-Phase Stimulation review articles Luteal-Phase Stimulation PubMed articles Luteal-Phase Stimulation PubMed Central articles Luteal-Phase Stimulation 2023 articles Luteal-Phase Stimulation 2024 articles Luteal-Phase Stimulation Scopus articles Luteal-Phase Stimulation impact factor journals Luteal-Phase Stimulation Scopus journals Luteal-Phase Stimulation PubMed journals Luteal-Phase Stimulation medical journals Luteal-Phase Stimulation free journals Luteal-Phase Stimulation best journals Luteal-Phase Stimulation top journals Luteal-Phase Stimulation free medical journals Luteal-Phase Stimulation famous journals Luteal-Phase Stimulation Google Scholar indexed journals Poor Responder articles Poor Responder Research articles Poor Responder review articles Poor Responder PubMed articles Poor Responder PubMed Central articles Poor Responder 2023 articles Poor Responder 2024 articles Poor Responder Scopus articles Poor Responder impact factor journals Poor Responder Scopus journals Poor Responder PubMed journals Poor Responder medical journals Poor Responder free journals Poor Responder best journals Poor Responder top journals Poor Responder free medical journals Poor Responder famous journals Poor Responder Google Scholar indexed journals Squeeze and Freeze articles Squeeze and Freeze Research articles Squeeze and Freeze review articles Squeeze and Freeze PubMed articles Squeeze and Freeze PubMed Central articles Squeeze and Freeze 2023 articles Squeeze and Freeze 2024 articles Squeeze and Freeze Scopus articles Squeeze and Freeze impact factor journals Squeeze and Freeze Scopus journals Squeeze and Freeze PubMed journals Squeeze and Freeze medical journals Squeeze and Freeze free journals Squeeze and Freeze best journals Squeeze and Freeze top journals Squeeze and Freeze free medical journals Squeeze and Freeze famous journals Squeeze and Freeze Google Scholar indexed journals Follicular-phase articles Follicular-phase Research articles Follicular-phase review articles Follicular-phase PubMed articles Follicular-phase PubMed Central articles Follicular-phase 2023 articles Follicular-phase 2024 articles Follicular-phase Scopus articles Follicular-phase impact factor journals Follicular-phase Scopus journals Follicular-phase PubMed journals Follicular-phase medical journals Follicular-phase free journals Follicular-phase best journals Follicular-phase top journals Follicular-phase free medical journals Follicular-phase famous journals Follicular-phase Google Scholar indexed journals in vitro fertilization articles in vitro fertilization Research articles in vitro fertilization review articles in vitro fertilization PubMed articles in vitro fertilization PubMed Central articles in vitro fertilization 2023 articles in vitro fertilization 2024 articles in vitro fertilization Scopus articles in vitro fertilization impact factor journals in vitro fertilization Scopus journals in vitro fertilization PubMed journals in vitro fertilization medical journals in vitro fertilization free journals in vitro fertilization best journals in vitro fertilization top journals in vitro fertilization free medical journals in vitro fertilization famous journals in vitro fertilization Google Scholar indexed journals fertility articles fertility Research articles fertility review articles fertility PubMed articles fertility PubMed Central articles fertility 2023 articles fertility 2024 articles fertility Scopus articles fertility impact factor journals fertility Scopus journals fertility PubMed journals fertility medical journals fertility free journals fertility best journals fertility top journals fertility free medical journals fertility famous journals fertility Google Scholar indexed journals embryos articles embryos Research articles embryos review articles embryos PubMed articles embryos PubMed Central articles embryos 2023 articles embryos 2024 articles embryos Scopus articles embryos impact factor journals embryos Scopus journals embryos PubMed journals embryos medical journals embryos free journals embryos best journals embryos top journals embryos free medical journals embryos famous journals embryos Google Scholar indexed journals achieve pregnancy articles achieve pregnancy Research articles achieve pregnancy review articles achieve pregnancy PubMed articles achieve pregnancy PubMed Central articles achieve pregnancy 2023 articles achieve pregnancy 2024 articles achieve pregnancy Scopus articles achieve pregnancy impact factor journals achieve pregnancy Scopus journals achieve pregnancy PubMed journals achieve pregnancy medical journals achieve pregnancy free journals achieve pregnancy best journals achieve pregnancy top journals achieve pregnancy free medical journals achieve pregnancy famous journals achieve pregnancy Google Scholar indexed journals
Article Details
Abbreviations:
FPS: Follicular-phase stimulation; LPS: Luteal-phase stimulation; OR: Oocyte retrieval; DOR: Decreased ovarian reserve; SF: Squeeze and freeze
1. Introduction
Time is an important factor in infertility treatment, especially for women of advanced age or decreased ovarian reserve (DOR) [1-3]. In addition, as time progresses, the situation worsens as a result of mater-nal aging, further DOR, diminished number of oocyt-es retrieved, increased aneuploidy rate and miscarry-iage rate, and so on [4-7]. Among current in vitro fertilization (IVF) techniques, no therapy can restore the embryo’s intrinsic competence, and the final infertility treatment outcomes depend on acquiring a potential embryo that will develop into a baby. To achieve this goal, the clinician should tailor the ovari-an stimulation process to use the maximum ovarian reserve and retrieve the highest possible number of oocytes as early as possible in the patient’s life.
Traditionally, ovarian stimulation was initiated in the early follicular phase because fresh transfer and a receptive endometrium are essential. However, with advanced vitrification techniques [8, 9], ovarian stimulation can be disconnected completely from the menstrual cycle, with no impact on the implantation rate, under the freeze-all strategy [10]. Recently, random-start ovarian stimulation has been proposed for cancer patients who desire emergency fertility preservation before beginning cancer treatment. In this process, ovarian stimulation begins randomly, at any phase in the menstrual cycle, and oocytes or embryos are preserved [11, 12]. Double ovarian stimulation (DuoStim) has also been proposed as method of harvesting more oocytes efficiently within the limited time available, providing double the opportunity for oocyte retrieval (OR) within a cycle [13-15].
Studies on these techniques have reported similar developmental potential of the oocytes retrieved from both follicularphase stimulation (FPS) and luteal-phase stimulation (LPS), and subsequent frozen embryo transfer provides optimal pregnancy out-comes [16-19].
In this study, we report a combination technique using random-start and DuoStim. We further extend these ideas to establish a new treatment strategy called “SF.” In this new scheme, consecutive ORs are planned within a specified time period (in this study, we selected 3 months) as far as the antral follicle is identified at any phase of the menstrual cycle. We squeeze out all possible oocytes and freeze all available embryos in the period. After all oocytes are retrieved, embryo transfers are planned. This unprec-edented treatment course aims to maximize patient outcomes refractory to infertility treatment by obtain-ing the greatest number of eggs in a limited time at the earliest stage of their lives. We termed this technique “squeeze and freeze” (SF course) and began using this method in our clinic for difficult outpatients in June 2017. Because of our institution’s capacity, we invited a limited number of patients to participate, although many more hoped to adopt this course of treatment. Thus, this study represents a proof-of-concept investigation of the SF course of treatment.
2. Materials and Methods
2.1 Study patients
To maximize the chance of refractory infertile patients to achieve pregnancy, we designed the time-sensitive SF course to consist of 3 months of consecutive OR. The aim of this treatment course is to acquire as many oocytes as possible in a limited period of time as early as possible in the patient’s life. After fertilization and embryo culture, the acquired competent embryos, blastocysts in general, were vitrified for subsequent transfer. This process was performed quickly to minimize the amount of time lost as a result of futile embryo transfer or miscarriage. To participate in the SF course, patients had to meet at least one of the three following criteria: (1) maternal age ≥40 years, (2) three or fewer oocytes retrieved after previous stimulation, and (3) reduced ovarian reserve (anti-Müllerian hormone [AMH] <1.0 ng/mL). Between June 2017 and October 2018, we implemented 101 treatment courses and performed 485 OR cycles.
This was a retrospective cohort study conducted in a private fertility center in Japan. The study was approved by our clinic’s Institutional Review Board and carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All the candidates provided informed consent after receiving individual counseling about the time significance and merit and demerit of multiple consecutive stim-ulations.
This study’s main objective was to confirm the efficacy of the SF strategy for patients who are refractory to treatment and who fulfill the Bologna criteria [20]. We also examined whether more than three consecutive ORs and luteal-phase ORs could obtain competent embryos more efficiently, ultima-tely increasing the chance of childbearing.
2.2 Ovarian stimulation protocol
Ovarian stimulation was considered during any phase of the menstrual cycle, when one or more antral follicles was recognized by transvaginal ultrasound, to squeeze out all oocytes. In the first attempt, ovarian stimulation began on cycle day 2 to 4, as is the normal convention, and a short, antagonist, or mild stimulation protocol was selected for each patient. Gonadotropin-releasing hormone (GnRH) agonist nasal spray (Buserelin acetate; Buserecur®, Fuji Pharm, Japan) and/or clomiphene citrate (CC; Clomid®, Fuji Pharm, Japan)/aromatase inhibitor (AI; Femara®, Novartis, Basel, Switzerland) and/or gonadotropin injection were administered.
Follicular monitoring was performed at the beginning, 5-7 days after the start and then as need-ed, continuing every 2-3 days until the follicle reach-ed approximately 18 mm in diameter. Serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, and progesterone levels were measu-red on ultrasound days using the analyzer Cobas e 411 plus (Roche, Basel, Switzerland), which uses electrochemilumin-escence technology for immune-assay analysis. A GnRH antagonist was administered when the serum LH level was elevated or the leading follicle reached 16mm in diameter. final oocyte maturation was triggered by a GnRH agonist nasal spray and/or human chorionic gonadotropin (rec-hCG 250 μg; Ovidrel®, Merck Biopharma, Darmstadt, Germany). Thirty-six hours after the trigger, ORs were per-formed via transvaginal ultrasound, in which all the follicles with a diameter greater than 10 mm were aspirated.
The next trial began when the preceding trial resulted in a smaller number of retrieved oocytes (e.g., fewer than five), and at least one antral follicle was recognized by transvaginal ultrasound examination. In general, CC began the day after retrieval, when there were one or two visible antral follicles. When more than three antral follicles were visible, daily administration of 150 IU human menopausal gona-dotropin (Ferring®, Ferring Pharma, Saint-Prex, Switzerland) was started following the same timing. After one week or longer, we attempted to retrieve the newly matured follicles in the luteal phase after administering the rec-hCG maturation trigger. Folli-cle monitoring was sometimes more difficult in the luteal phase because of the presence of corpora lutea and hormones; thus, we used serial ultrasound exam-inations and serum hormone measurements to help determine the timing.
The same was true for the luteal-phase ORs, and careful examination helped distinguish the new follicles. When no follicle growth was detected after the seventh day, ovarian stimulation was stopped until the next menstruation. Thus, we attempted to repeat the OR as many times as possible for 3 months, regardless of the menstrual cycle.
2.3 Embryo culture and assessment, and cryo-preservation
Sperm was collected after liquefaction and centri-fugation and swim-up and then provided for conventional in vitro fertilization (IVF) or intra-cytoplasmic sperm injection (ICSI), depending on the semen condition. IVF was performed by inseminating each oocyte with 105 motile spermatozoa/mL, and ICSI was performed after 2-4 h of incubation, after the cumulus and corona radiata cells were removed by hyaluronidase and pipetting. Embryos were cultured in the medium NX (Continuous Single Culture NX Complete Medium: IS Japan, Co. Ltd.) or S1 (SAGE 1-Step Medium: Origio Japan, Co. Ltd.) at 37°C in 6% carbon dioxide and 5% oxygen tension in a time-lapse incubator (Embryo Scope plus; Vitrolife, Co. Ltd., Göteborg, Sweden). Embr-yos that developed to the blastocyst stage were graded according to Gardner’s criteria. A blastocyst with a score better than grade 3BB was considered fair. Embryos that scored greater than grade 3CC on day 4 to 7 were vitrified.
To preserve the value of the concept, a robust cryopreservation program is mandatory. The vitrify-cation and warming procedures were performed according to the Cryotop Safety Kit manual (Kitazato Co, Japan) at room temperature. In the vitrification procedures, blastocysts were equilibrated for 12-15 min in an equilibration solution and then washed and floated for 45-60 s in a vitrification solution (VS). Subsequently, blastocysts were placed on the Cryotop sheet with a minimum volume of VS. Immediately, the Cryotop sheets were submerged into liquid nitrogen. In the warming procedures, the Cryotop sheets were placed into a thawing solution and incubated at 37°C within 1 s. After 1 min, the blastocysts were placed in a diluent solution for 3 min. finally, the blastocysts were placed in a washing solution for 5 min. The blastocysts were cultured at 37°C for at least 3 h before ET.
2.4 Endometrial preparation and frozen embryo transfer
Thawed embryo transfers were performed in hormone replacement cycles. Endometrial prepara-tion was undertaken by incremental doses of transdermal estradiol (ESTRANA® Tapes 0.72 mg; Hisamitsu Pharmaceutical Co., Inc., Tokyo, Japan). After confirming that an endometrial thickness grea-ter than 8 mm by ultrasonography, oral progestin (chlormadinone acetate; Lutoral®, Fuji Pharm, Tokyo, Japan) and transvaginal natural progesterone (LUTINUS®, Ferring Pharma; or OneCrinone®, Merck Biopharm) were administered daily. Blast-ocyst transfer was performed on day 5 or 6 of progesterone administration.
2.5 Pregnancy diagnosis
Clinical pregnancy was defined as a pregnancy when a gestational sac was observed in the uterus by ultrasound. Ongoing pregnancy was defined as a pregnancy in which fetal cardiac activity was detec-ted by ultrasound beyond 9 weeks.
2.6 Statistical analysis
Chi-square test and t tests were performed to compare the differences between the two groups. A statistically significant difference was defined as P<0.05.
3. Results
A total of 101 SF courses were initiated in 88 patients, and 485 OR cycles were performed between June 2017 and October 2018. Patient characteristics were as follows: average age at the start of treatment, 39.5 ± 3.8 years (range, 27-46 years); average number of previous IVF attempts, 3.6 ± 4.2 (range, 0-23); and average AMH level, 0.81 ± 0.71 ng/mL (range, 0.03-5.62 ng/mL), which was obtained at the patient’s first outpatient examination, on average 1.1 years before the start of the SF course. All the course attendants could not achieve ongoing pregnancy in the preceding conventional treatment at our clinic or at other institutions, including very severe patients with repeated unsuccessful treatments who were close to considering treatment termination. Data are presented as mean±SD. An average 4.8 ± 1.0 (range, 1-8; median, 5) cycles of OR were performed during the 90-day treatment period, resulting in an average of 12.6 ± 6.2 (range, 1-34, median, 11) total harvested oocytes, 8.1 ± 4.5 (range, 1-23; median, 6) 2PN embryos, 4.8 ± 3.4 (range, 0-17; median, 4) vitrified embryos, and 2.7 ± 2.8 (range, 0-12, median, 2) fair-grade blastocysts (Table 1).
To examine the efficacy of more than three continuous trials, 485 OR cycles were divided into two groups. The first group included the first or second retrievals of the SF course (201 cycles, 529 oocytes), and the second group included attempts after the patient’s third retrieval (284 cycles, 740 oocytes). The ratio of matured oocytes, 2PN embryos, available embryos, fair-grade blastocysts per total oocytes harvested, number of available embryos, and fair blastocysts per OR cycle were reviewed and compared. In the first and second attempts, the maturation rate was 81.1% (429/529), the 2PN rate was 64.1% (339/529), the rate of available embryos was 35.9% (190/529), and the rate of fair-grade embryos was 19.8% (105/529). The number of available embryos per OR cycle was 0.95 (191/201), and the number of fair-grade blastocysts was 0.52 (105/201). On the other hand, after the third OR attempt, these parameters were as follows: maturation rate 81.6% (604/740), 2PN rate 64.7% (479/740), rate of available embryos 39.3% (291/740), and rate of fair-grade embryos 22.7% (168/740). The number of available embryos per OR cycle was 1.02 (289/284), and the number of fair-grade blastocysts was 0.59 (168/284). There were no statistically significant differences in any parameter between the two groups, although latter trials in the SF course tend to result in an increased rate of viable embryos and fair blastocysts (Table 2).
We next examined the efficacy of luteal-phase ORs. For this analysis, the group was divided into two groups: the FPS and LPS groups. FPS includes the cycles in which ovarian stimulation began in the early follicular phase (343 cycles, 973 oocytes), and LPS includes cycles in which ovarian stimulation began in the luteal phase (142 cycles, 296 oocytes). The ratio of matured oocytes in both groups, FPS and LPS, was 80.7% (785/973) and 83.8% (248/296), respectively. The 2PN rate was 63.1% (614/973) and 68.9% (204/296), the rate of available embryos was 36.5% (355/973) and 42.6% (126/296), and the rate of fair-grade blastocysts was 19.6% (191/973) and 27.7% (82/296), respectively. The number of available embryos per OR cycle was 1.03 (353/343) and 0.89 (126/142), and the number of fair-grade blastocysts was 0.56 (192/343) and 0.58 (82/142), respectively. The rate of fair-grade blastocysts was significantly higher in the LPS group than in the FPS group (p < 0.01). Although the number of retrieved oocytes in the LPS group was smaller than in the FPS group, the number of fair-grade blastocysts was not reduced. Thus, treatment efficacy in LPS is not diminished, and OR in the LPS is worth attempting for patients with DOR (Table 3).
Next, we present an impressive clinical course in which the patient underwent eight OR trials over 90 days, which was the highest number of trials in this study. At the beginning of the course, she was 40 years old with DOR and an AMH value of 0.79 ng/mL (which was checked 4 months before the course started). Ten oocytes, including nine MII oocytes, were collected during the course, and six blastocysts were vitrified following fertilization and embryo culture. She experienced four consecutive retrievals following LPS, the corpus luteum continued to exist, and she had no menstruation during the period. We did not attempt a fifth consecutive LPS because of her personal reasons and re-started stimulation for 6th OR after the next menstruation. Follicle maturation was observed at approximate 10-day intervals, resulting in eight OR trials. She became pregnant after her first embryo transfer with the embryo derived from the fourth consecutive and luteal-phase retrieval, and she delivered a healthy baby (Table 4).
At the time of writing (March 2021), the clinical outcomes of the SF course after subsequent embryo transfers were reviewed. Thirteen patients have tried the course twice, and 88 women have received thawed embryo transfers. Transferred embryos were selected based on morphology, irrespective of timing of ORs and FPS or LPS. Sixty-two women have achieved clinical pregnancy, and 49 women have achieved an ongoing pregnancy. Forty-seven women have delivered healthy babies. Twenty-six of these babies were derived from the oocyte in the latter ORs of the course, and thirteen were derived from LPS cycles.
In addition, eight women have conceived second babies derived from the oocytes collected during the course. Eighteen women have terminated fertility treatment without ongoing pregnancy. There are 15 women who are still undergoing treatment and have not achieved an ongoing pregnancy, but these women still have reserved embryos (figure 1).
|
Results per treatment course (n = 101) |
Average (mean±SD) |
Range (min-max) |
Median |
|
Attempted number of oocyte retrievals |
4.8 ± 1.0 |
1-8 |
5 |
|
Number of retrieved oocytes |
12.6 ± 6.2 |
1-34 |
11 |
|
Number of 2PN embryos |
8.1 ± 4.5 |
1-23 |
6 |
|
Number of vitrified embryos |
4.8 ± 3.4 |
0-17 |
4 |
|
Number of fair-grade blastocysts (Gardner score > 3BB) |
2.7 ± 2.8 |
0-12 |
2 |
An average of 4.8 cycles of OR were performed during the 90-day treatment period, resulting in an average of 12.6 total harvested oocytes, 8.1 2PN embryos, 4.8 vitrified embryos, and 2.7 fair-grade blastocysts.
Table 1: Clinical outcomes of the 3-month treatment course.
|
OR at first and second trial (201 cycles) |
OR at third and latter trial (284 cycles) |
P value |
|
|
Total number of oocytes and number per OR (average; min-max) |
529 (2.63; 0-11) |
740 (2.61; 0-12) |
— |
|
Rate of matured oocytes (No. of MII oocyte/total No. of oocytes) |
81.1% (429/529) |
81.6% (604/740) |
n.s. |
|
Rate of 2PN embryos (No. of 2PN embryos/total No. of oocytes) |
64.1% (339/529) |
64.7% (479/740) |
n.s. |
|
Rate of available embryos (No. of vitrified embryos/total No. of oocytes) |
35.9% (190/529) |
39.3% (291/740) |
n.s. |
|
Rate of fair-grade blastocysts (No. of fair-grade embryos/total No. of oocytes) |
19.8% (105/529) |
22.7% (168/740) |
n.s. |
|
No. of available embryos per OR |
0.95 (191/201) |
1.02 (289/284) |
n.s. |
|
No. of fair-grade blastocysts per OR |
0.52 (105/201) |
0.59 (168/284) |
n.s. |
OR, oocyte retrieval; n.s, not significant. No statistically significant differences were found between the initial two attempts and latter attempts. The consecutive treatments did not deteriorate the clinical result.
Table 2: Comparison of embryo parameters between the initial two attempts and the latter attempts.
|
FPS (343 cycles) |
LPS (142 cycles) |
P value |
|
|
Total number of oocytes and number per OR (average; min-max) |
973 (2.84; 0-12) |
296 (2.08; 0-11) |
— |
|
Rate of matured oocytes |
80.7% (785/973) |
83.8% (248/296) |
n.s. |
|
(No. of MII oocytes/total no. of oocytes) |
|||
|
Rate of 2PN embryos |
63.1% (614/973) |
68.9% (204/296) |
n.s. |
|
(No. of 2PN embryos/total no. of oocytes) |
|||
|
Rate of available embryos |
36.5% (355/973) |
42.6% (126/296) |
n.s. |
|
(No. of vitrified embryos/total no. of oocytes) |
|||
|
Rate of fair-grade blastocyst (No. of fair-grade embryo/total no. of oocytes) |
19.6% (191/973) |
27.7% (82/296) |
P < 0.01 |
|
|
|||
|
No. of available embryos per OR |
1.03 (355/343) |
0.89 (126/142) |
n.s. |
|
No. of fair-grade blastocysts per OR |
0.56 (191/343) |
0.58 (82/142) |
n.s. |
FPS, follicular-phase stimulation; LPS, luteal-phase stimulation; PN, pronuclear; MII, metaphase II; OR, oocyte retrieval
The treatment efficacy was not diminished with LPS, and the OR with LPS is worth attempting for patients with DOR.
Table 3: Comparison of embryo parameters between FPS and LPS cycles.
OR, oocyte retrieval; LP, luteal phase; FP, follicular phase; LH, luteinizing hormone; CC, clomiphene citrate; hMG, human menopausal gonadotropin
Impressive clinical course with eight OR trials over 90 days, the highest number of trials in this study.
Table 4: Clinical course of the women who experienced eight trials of OR during the SF course.
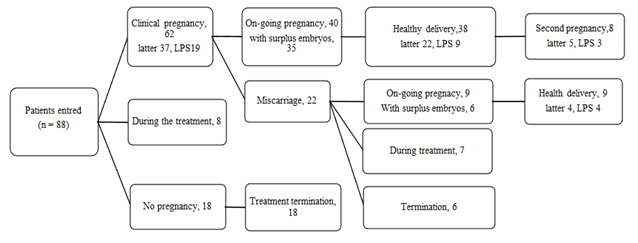
figure 1: Clinical outcomes after the SF course (March 2021).
Later, derived from the embryos collected from the latter trial of the course (later than the third trial); LPS, derived from the embryos collected from the LPS (luteal-phase stimulation). Among the 88 women who have received embryo transfers, 62 women have achieved clinical pregnancy and 49 women have achieved an ongoing pregnancy. Forty-seven women have delivered healthy babies. Twenty-six of these babies were derived from the oocyte in the latter ORs of the course, and thirteen were derived from LPS cycles. In addition, eight women have conceived second babies derived from the oocytes collected during the course.
4. Discussion
In this study, we extended the concept of random-start and DuoStim and furthermore intended to acquire an increasing number of oocytes to maximize the chance of obtaining a potential embryo. It is estimated that, in order to obtain at least one euploid embryo, the collection of 5, 7, 10, and 20 oocytes is required in women aged 35-37, 38-40, 41-42, and >42 years, respectively [21]. In our clinical setting, which includes many refractory patients, collecting enough oocytes to achieve a pregnancy within two or three trials of OR is a difficult task. In Japan, many women with advanced maternal age or DOR have been undergoing ORs and embryo transfers for many years. They are suffering from both physical and mental stress and feel as though they are in a dark tunnel with no visible exit. This treatment environment is particularly severe in Japan for several reasons, including that oocyte donation is not permitted, preimplantation genetic testing for aneuploidy (PGT-A) is regulated and not available in our clinic, and single-embryo transfer is strongly recommended. Success in this restricted situation is a most challenging task for clinicians. Therapeutic efficacy is low, no matter what efforts we undertake. When patients experience miscarriage, months of time has been lost until the next trial. This is a heavy blow to all women, and the resulting physical and psychological damages can be endless. Thus, the SF technique can help save precious time and decrease the time lost after miscarriage.
Another problem arising from this treatment might be an increased physical burden, mental stress, and economic load. Although it depends on the clinic’s policy, this course may be more cost effective. We set a low fixed treatment cost for 3 months so that most patients could repeat the OR without worrying about the cost. Looking back over the SF course, 3 months of treatment might be appropriate. Because one of the stressors patients face is an uncertain future, setting one periodic goal helps them to retain positive feelings and relieve the stress. Treatment discontinuation in poor responders is a crucial issue, but none of the patients dropped out during the period owing to a failed attempt. The accessibility of patients is one of the main advantages of this limited time-frame consecutive strategy. Some patients could not obtain a successful result and thus terminated their infertility treatment after the SF course and subsequent transfer. They did not have feelings of regret because they believed they did everything they could. On the other hand, 13 women could not end their treatment and hoped to start a second trial of the SF course.
Three follicle recruitment theories have been postulated to date. One in the classical “single recruitment” theory, and the others include the “wave” theory and “continuous recruitment” theory [22]. In humans, the single recruitment theory has traditionally supported that only a single major wave of follicle development occurs during the intra-ovulatory period. However, previous studies have demonstrated the appearance of more than one wave of follicular growth within a cycle, suggesting the presence of obtainable follicles during the luteal phase [23]. In fact, some recent studies using random ovarian stimulation for women with cancer [24-26] or luteal-phase ovarian stimulation for poor responders (DuoStim) demonstrated that LPS is an adequate method for obtaining a sufficient number of competent oocytes [27]. In addition, we experienced a thought-provoking case of folliculogenesis. This patient underwent eight ORs over 90 days, including four consecutive retrievals after LPS, during which no menstruation occurred. In this course, we commonly experienced two to three serial luteal-phase ORs in many other patients. It is thought that follicle maturation could occur at approximate 10-day intervals and that consecutive luteal-phase follicle maturation could possibly be endless. Although the biological process for folliculogenesis is not yet completely understood, this intriguing evidence add some clues in favor of the “continuous recruitment” theory, in that recruitable antral follicles are continuously present in the ovaries [20].
In the SF course, an average of 4.9 trials of ORs were performed on average (range, 1-8) over the 90-day period among 88 women with severe conditions. This translates to an OR chance 1.6 times higher than a conventional monthly trial, resulting in more oocytes (average 12.6 ± 6.2 oocytes) over the duration of the course. Similar to previous reports on DuoStim and LPS [28], we found no significant difference between FPS and LPS in all parameters, but we noted a superior rate of fair-grade blastocysts per retrieved oocyte in the LPS group. In addition, three or more, or even eight, consecutive trials did not diminish treatment efficacy or embryo competence. All of these results verify that ovarian stimulation can begin at any phase of the menstrual cycle and can be repeated any number of times, irrespective of menstrual cycle. Thus, the SF course can be used to maximize the potency of refractory patients by obtaining more chances for OR, accumulating the highest number of oocytes and competent embryos in a limited period. Although this was a retrospective clinical study in a heterogenous patient population, the delivery of healthy babies, and also siblings, proves the strategy’s clinical utility.
Without new technology to restore intrinsic embryo competence, clinicians can only improve on the currently available methods, among which are the selection of proper ovarian stimulation and time management. One proposed strategy in patients with poor response is embryo accumulation and vitrification [29-31] with double stimulation in the follicular and luteal phase of the same ovarian cycle (i.e., DuoStim) [32-35]. This method offers the possi-bility of the maximum attempts at oocyte collection to obtain the highest number of eggs in a single menstrual cycle. Our strategy, the “SF,” further extends the treatment period from a single menstrual cycle to 3 months, without regard for the menstrual cycle. The availability of PGT-A in Japan would also contribute to reducing the treatment period by avoiding futile embryo transfer or miscarriage [36]. The treatment period could be reconsidered in each patient according to the number of euploid embryos acquired or their background, such as maternal age and family planning. A result of no euploid embryos provides patients the next chance of OR at in earlier stage of their lives or allows them to decide on treatment termination and pursue a new stage in their life. This course might hold not only medical, but also social, meaning. The most suitable target popu-lation for the SF course might include very severe patients with DOR, with shortened menstrual cycles, and a low level of luteal-phase hormone, in which lutealphase oocyte maturation could occur easily and early because of constantly high concen-trations of FSH [37]. Most of these patients could experience more ORs (e.g., five to eight) than average, but some of these patients with further severe DOR, who are perimenopausal, or who rarely ovulate during the period could achieve only one or two OR trials; thus, the treatment would not be effective, and they were not retrospectively eligible. On the other hand, some of these patients might decide to stop treatment after two or three ORs (i.e., before the duration of the course is over), because they have acquired a satisfa-ctory number of embryos. Further examination is required to find the most suitable target for this treat-ment and the most suitable period for each patient with the aim of maximizing this strategy’s utility.
In conclusion, this study demonstrated that embryos derived from LPS or from consecutive OR over a 3-month duration have similar development potential as conventional stimulation. Periodic consecutive ORs has been proven to enhance the possibility of child-bearing by increasing the chance of OR and increasing the number of oocytes and competent embryos within the limited time. Our experience provides new insight into folliculogenesis, in which oocyte maturation could occur every 10 days, and continuous luteal-phase follicle maturation could occur repeatedly, during a period in which no mens-truation occurs. This new concept provides a new and effective choice for infertility treatment, especially for time-sensitive women with advanced maternal age or DOR. This trial certainly demands further investigation to define the proper target and period and to assess the safety and actual clinical efficiency.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to thank Enago (www. enago.jp) for the English language review.
Author Declarations
Competing interests
The authors declare that they have no competing inte-rests.
Ethics approval
The study was approved by the clinic’s Institutional Review Board (The title of the clinical trials registry: Squeeze and freeze, three months consecutive oocyte retrieval, for poor ovarian responders. The reception number: 2017_001) and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent to participate
All participants provided informed consents after receiving counseling for this infertility treatment.
Consent for publication
Not applicable.
Availability of data and material
The data supporting the findings of this study are available within the article and its supplementary materials and from the corresponding author on request.
Code availability
Not applicable
References
- Liu K, Case A. Reproductive Endocrinology and Infertility Committee. Advanced reprod-uctive age and fertility. J Obstet Gynaecol Can 33 (2011): 1165-1175.
- Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril 103 (2015): 1136-1143.
- Ubaldi FM, Cimadomo D, Vaiarelli A, et al.
Advanced maternal age in IVF: Still a chall-
enge? The present and the future of its treatment. Front Endocrinol. (Lausanne) 10 (2019): 94.
- Cimadomo D, Fabozzi G, Vaiarelli A, et al. Impact of maternal age on oocyte and embryo competence. Front Endocrinol. (Lausanne) 29 (2018a): 327.
- Franasiak JM, Forman EJ, Hong KH, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 101 (2014): 656-663.
- Goldman MB, Thornton KL, Ryley D, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T). Fertil Steril 101 (2014): 1574-1581.
- Wennberg AL, Opdahl S, Bergh C, et al. Effect of maternal age on maternal and neon-atal outcomes after assisted reproductive tech-nology. Fertil Steril 106 (2016): 1142-1149.
- Kuwayama M, Vajta G, Kato O, et al. Highly efficient vitrification method for cryopreserva-tion of human oocytes. Reprod Biomed Online 11 (2005): 300-308.
- Toner JP, Coddington CC, Doody K, et al. Society for assisted reproductive technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril 106 (2016): 541-546.
- Mizrachi Y, Horowitz E, Farhi J, et al. Ovarian stimulation for freeze-all IVF cycles: a syste-matic review. Hum Reprod Update 26 (2020): 118-135.
- Cakmak H, Kat Z, Cedars MI, et al. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril 100 (2013): 1673-1680.
- Rashidi BH, Tehrani ES, Ghaffari F. Ovarian stimulation for emergency fertility preserva-tion in cancer patients: a case series study. Gynecol Oncol Rep 10 (2014): 19-21.
- Labarta E DuoStim. A new strategy proposed for women with poor ovarian response. Fertil Steril 113 (2020): 76-77.
- Vaiarelli A, Cimadomo D, Petriglia C, et al. DuoStim-a reproducible strategy to obtain more oocytes and competent embryos in a short time-frame aimed at fertility preservation and IVF purposes. A systematic review. Ups J Med Sci 125 (2020): 121-130.
- Vaiarelli A, Cimadomo D, Conforti A, et al. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfil-ling the Bologna criteria: a case series. Fertil Steril 113 (2020): 121-130.
- Vaiarelli A, Cimadomo D, Alviggi E, et al. The euploid blastocysts obtained after luteal phase stimulation show the same clinical, obs-tetric and perinatal outcomes as follicular phase stimulation-derived ones: a multicenter study. Hum Reprod 35 (2020): 2598-2608.
- Li Y, Yang W, Chen X, et al. Comparison between follicular stimulation and luteal stimulation protocols with clomiphene and HMG in women with poor ovarian response. Gynecol Endocrinol 32 (2016): 74-77.
- Qin N, Chen Q, Hong Q, et al. Flexibility in starting ovarian stimulation at different phases of the menstrual cycle for treatment of infertile women with the use of in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril 106 (2016): 334-341.
- Wang N, Wang Y, Chen Q, et al. Luteal-phase ovarian stimulation versus conventional ovar-ian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol 84 (2016): 720-728.
- Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimu-lation for in vitro fertilization: the Bologna criteria. Hum Reprod 26 (2011): 1616-1624.
- Vaiarelli A, Cimadomo D, Ubaldi N, et al. What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol 30 (2018b): 155-162.
- Baerwald AR, Adams GP, Pierson RA. Ovar-ian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 18 (2012): 73-91.
- Baerwald A, Adams G, Pierson R. Chara-cteristics of ovarian follicular wave dynamics in women. Biol Reprod 69 (2003): 1023-1031.
- Anderson RA, Kinniburgh D, Baird DT. Preliminary experience of the use of a gona-dotrophin-releasing hormone antagonist in ovulation induction/in-vitro fertilization prior to cancer treatment. Hum Reprod 14 (1999): 2665-2668.
- de Melo Gagliato D, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32 (2014): 735-744.
- von Wolff M, Thaler CJ, Frambach T, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril 92 (2009): 1360-1365.
- Kuang Y, Chen Q, Hong Q, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI prog-rammes (Shanghai protocol). Reprod Biomed Online 29 (2014a): 684-691.
- Ubaldi FM, Capalbo A, Vaiarelli A, et al. Follicular versus luteal phase ovarian stimula-tion during the same menstrual cycle (DuoS-tim) in a reduced ovarian reserve population results in a similar euploid blastocyst for-mation rate: new insight in ovarian reserve exploitation. Fertil Steril 105 (2016): 1488-1495.
- Chatziparasidou A, Nijs M, Moisidou M, et al. Accumulation of oocytes and/or embryos by vitrification, a new strategy for managing poor responder patients undergoing pre implant-ation diagnosis. F1000 Res 2 (2013): 240.
- Cobo A, Garrido N, Crespo J, et al. Accum-ulation of oocytes: a new strategy for mana-ging low-responder patients. Reprod Biomed Online 24 (2012): 424-432.
- Martinez F, Barbed C, Parriego M, et al. Use-fulness of oocyte accumulation in low ovarian response for PGS. Gynecol Endocrinol 32 (2016): 577-580.
- Cimadomo D, Vaiarelli A, Colamaria S, et al. Luteal phase anovulatory follicles result in the production of competent oocytes: intra-patient paired case-control study comparing follicular versus luteal phase stimulations in the same ovarian cycle. Hum Reprod 33 (2018b): 1442-1448.
- Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril 101 (2014b): 105-111.
- Vaiarelli A, Venturella R, Vizziello D, et al. Dual ovarian stimulation and random start in assisted reproductive technologies: from ovarian biology to clinical application. Curr Opin Obstet Gynecol 29 (2017): 153-159.
- Vaiarelli A, Cimadomo D, Trabucco E, et al. Double stimulation in the same ovarian cycle (DuoStim) to maximize the number of oocytes retrieved from poor prognosis patients: a multicenter experience and SWOT analysis. Front Endocrinol 9 (2018a): 317.
- Sato T, Sugiura-Ogasawara M, Ozawa F, et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod 34 (2019): 2340-2348.
- Hale GE, Zhao X, Hughes CL, et al. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the staging of reproductive ageing workshop (STRAW) staging system J Clin Endocrinol Metab 92 (2007): 3060-3067.


 Impact Factor: * 1.1
Impact Factor: * 1.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 