Antibacterial Screening of Selected Plants from Southwest USA in Search of Potential Natural Alternatives for Antibacterial Application
Article Information
Merhavy ZI1,2, Varkey TC1,4*, Courtois EC3, Varkey JA1,5, De Bruyn G1, Huls CE1,2
1Center for Novel Antimicrobial Products, College of Science, Engineering, and Technology, Grand Canyon University, Phoenix, AZ, USA
2Ross University School of Medicine, Bridgetown, Barbados, USA
3The University of Texas at Austin, Austin, TX, USA
4Dell Medical School at The University of Texas at Austin, Austin, TX, USA
5University of Notre Dame – Notre Dame, IN, USA
*Corresponding Author: Varkey TC. Center for Novel Antimicrobial Products, College of Science, Engineering, and Technology, Grand Canyon University, Phoenix, AZ, USA
Received: 13 April 2021; Accepted: 22 April 2021; Published: 28 April 2021
Citation: Merhavy ZI, Varkey TC, Courtois EC, Varkey JA, De Bruyn G, Huls CE. Antibacterial Screening of Selected Plants from Southwest USA in Search of Potential Natural Alternatives for Antibacterial Application. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 251-261.
View / Download Pdf Share at FacebookAbstract
The emergence of drug resistant microorganisms has posed important public health issues. The annual cost of treating antibiotic resistant infections in the United States alone has been estimated to be as high as $30 billion. This has led to an urgent need for new antimicrobial drugs, particularly from natural resources. Phytochemicals obtained from medicinal plants have been used widely in the development of novel therapeutics including antimicrobial agents. Therefore, it is imperative to detect substances which have inhibitory effects on the growth of bacterial species. This paper explores the efficacy of ethanol (80%) extracts of leaves of several plant species from southern Arizona that were screened for their antimicrobial efficacy against Staphylococcus epidermidis, Mycobacterium smegmatis, and Streptococcus mutans. Extracts were prepared by a maceration process and the antibacterial activity of different plants were evaluated and compared by measuring their zones of inhibition. Most notably, Lagerstroemia, Mahonia aquifolium, and Punica granatum expressed the highest antibacterial activity of the thirty-three (33) plant extracts tested.
Keywords
Antimicrobial; Antibacterial and Plants
Article Details
1. Introduction
The use of medicinal plants as a source for medical relief can be traced back over five millennia to higher-evolved plants in many cultures globally [1, 2]. Because the vast majority of these plants have not been evaluated for medicinal uses, it is imperative to detect substances which have an inhibitory effect on the growth of bacterial species. Angiospermic plants possess a reservoir of these effective therapeutic compounds and constitute an inexhaustible source of harmless protectants against detrimental bioflora [3-5]. Many of these southwestern native plant species are multi-purpose herbal plants which have been used as human food as well as an alternative for medicinal purposes worldwide. Many of these plants have species that are naturally occurring in the Sonoran Desert, and because of the center’s location, these species were chosen to be used for experimentation.
With the ultimate goal of offering appropriate and effective antibacterial drugs to patients, plants have proven to be a valuable source of natural products for maintaining human health [6]. The historical use of plant compounds for pharmaceutical purposes has shown considerable support for the role of medicinal plants to become a promising source in creating a variety of drugs [6] as roughly 80% of individuals from developed countries use medicinal compounds derived from medicinal plants [1, 6-8]. These plants’ introduction into traditional medicine as antibiotics have become one of many developed countries’ most valuable weapon in fighting bacterial infections and have greatly benefitted the health-related quality of human life ever since their discovery [9].
In addition to the rising costs of antibiotic resistant infection treatment, the core of the underlying problem remains that these detrimental floras are becoming increasingly antibiotic resistant to many of the current treatments [6, 9, 10]. In combination with this reality and the growing consumer desire for natural, effective products [1], the experimentation done by this research group has been seeking out potential alternatives for antibacterial application. Current methods of assessment of antibacterial and antimicrobial properties can be often seen through methods such as Kirby-Bauer, minimum inhibitory concentration (MIC), thin layer chromatography (TLC), and various other tests that commonly utilize microbial culture techniques [11]. Positive test results to these various methods have often expressed high correlations to effective means of antibacterial agents. Of these tests, the Kirby-Bauer test was chosen and used as the primary method for experimentation in this research.
The three microbes utilized in this study were Staphylococcus epidermidis, Mycobacterium smegmatis, and Streptococcus mutans. S. epidermidis is a bacterium that is typically associated with human skin, and less associated with human mucus, exemplifying anaerobic activity for its function and survival [12]. M. smegmatis is a mycobacterium that is considered to be a non-pathogenic microorganism as it exhibits quick regeneration time, and therefore, it is commonly used in laboratories for conducting bacterial experiments such as with this study [12]. Additionally, S. mutans is an anaerobic bacterium that is typically found in the oral cavity of humans. It is known to be a key contributor in tooth decay and has been shown to be a contributor to endocarditis [12, 13]. The three microbes were chosen for this study due to their prevalence and universal usage in assessing antimicrobial effectiveness.
Deserts are commonly known to be home to a wide variety of plant species that are incredibly resilient and impervious to many of the harsh conditions in which their environments pose. Due to these severe conditions, desert plants have evolved to become resistant to many bacterial and fungal species [8, 14], which has become the basis for the initial intrigue of this research. The deserts located in southwestern United States houses many available plants that have historical medicinal and healing properties [1, 15, 16], but have seldom been chemically explored. Many medicinal applications of common desert plants such as treating burns, lowering blood sugar in diabetics, reducing symptoms of allergies, improving immune system, preventing wound infection, etc. [1, 17] have already been identified in certain desert plants such as Prickly Pear, Mesquite, and Ephedra. Industries predominantly interested in exploring similar plants in recent times have been observed by evaluating a diverse array of plant extracts for their antimicrobial properties and possible applications. With the expansive variety and supply of underexplored desert plants nearby, the objective of the experiment was to provide preliminary data to express antibacterial properties that many of these plants possess for potential antibacterial applications in medicine.
2. Materials and Methods
2.1 Plant material
Taking small excursions into the desert outside of the metro-city area of Phoenix, Arizona, 33 samples were collected of plant leaves, seeds, and stems to run extractions on. Eleven separate plant species were collected during this period. Once collected, the plant parts were set aside to dry for a 15-day period following the protocol by Ahmad et al. [18] in the room temperature laboratory to ensure that the extraction would be easy. Following the drying, the plant parts were ground up using a blender, mortar, and pestle. 100 grams of each plant were collected from the grindings that were created using the blender, mortar, and pestle.
2.2 Extract preparations
All 33 extracts were created using the 80/20 mixture of Ethanol to water and Methanol to water (tinctures). The extractions were carried out with slight modification from the methods laid down by Ahmad et al. [18] who used a 70/30 mixture. The use of the 80/20 mixture was to increase the amount of non-polar plant compounds that were extracted. The tinctures were created by taking plant samples from the capped flasks and were then mixed with the 80/20 mixture of either Ethanol or Methanol. These were then allowed to sit, capped, at 37°C for a 7-day period with periodic shaking from the researchers. These were then filtered using Whatman No. 1 filter paper. This again was done following the protocol described by Ahmad et al [18].
2.3 Commercial antibiotics
Based on the commercial uses, commonality, and prescription uses to combat bacterial diseases in patients within and surrounding the Phoenix area, one commercially available antibiotic medication, Gentamicin, was used in this trial as a control on the effectiveness of the plant extracts. Gentamicin is widely known for its ability to inhibit protein biosynthesis by irreversibly binding the aminoglycoside to the 30S bacterial ribosome subunit.
2.4 Microorganisms
Three bacterial strains were chosen to provide proof of action against the different major groups of bacterial types which cause disease in humans: S. epidermidis and S. mutans, Gram-Positive bacteria, and Mycobacterium smegmatis, an acid-fast bacterium. In analyzing these plants, it was decided that they should be tested against fairly common bacteria that can be easily cultured in a laboratory setting. The laboratory resources were dedicated to these bacteria as the laboratory budget was low and these were the most readily available at the time.
2.5 Inoculum preparation
The microorganisms were cultured in a shaker at room temperature for 2 hours in Lysogeny Broth (LB) before streaking on a nutrient rich LB agar plate. This was chosen because of its nutrient richness ensuring that the experiment would not be confounded by lack of nutrients. To ensure sterility of the experiment, these streaks were performed under a sterile hood with disposable inoculating loops. This was done similarly to the methodology of Grainge and Alvarez [4].
2.6 Chemicals
All chemicals were of analytical grade. Ethanol was from Fisher Scientific (Massachusetts, USA) and the water was from the Deionizer at Grand Canyon University (Arizona, USA).
2.7 Kirby-Bauer test
Using nutrient-rich agar plates, Kirby-Bauer tests were run to determine if the plant extracts had any effects on the various bacterial species that were selected as test subjects. The Kirby-Bauer test method requires that a small disk is submerged in the solution and then allowed to dry in a sterile environment. Following this, the disks were applied to the surface of an agar plate with bacteria covering the surface of the plate and allowed to incubate for 48 hours to allow for enough bacterial growth that the results could be seen with the naked eye. The Kirby-Bauer tests were run according to the protocol described by Digrak et al. [7]. If the compounds in the plants were effective, it was easy to observe a small dead space without bacterial growth around the disks, or the zone of inhibition. These zones’ diameters were then measured and recorded. This information is included in the data section of this document. This method was chosen as a simple test run as it is an effective and efficient test run before continuing to a Thin Layer Chromatography to isolate specific compounds unique to these Sonoran Desert plants for further testing.
3. Results
Staphylococcus epidermidis, Mycobacterium smegmatis, and Streptococcus mutans were found to be resistant against various plant extracts with only 5 extracts showing moderate to high activity on each. Each of the extracts, with few exceptions due to limitations in availability, were measured out to 6 grams of dry weight in order to maintain a consistent concentration throughout each extract. Mycobacterium smegmatis was found to be the least resistant, overall, against many of these extracts, having only 5 that had not been able to produce any visible zone of inhibition. In contrast, Streptococcus mutans had expressed the highest level of resistance against the extracts, only having 9 of the 33 plants producing a zone of inhibition of any kind. Staphylococcus epidermidis expressed slightly less resistance to these extracts, having 13 plants produce zones of inhibitions (ZOI) of any size.
Notable results were found in five extracts that exhibited moderate to high antimicrobial activity against the three bacterial strains in comparison to the control of Gentamicin. In order of Staphylococcus epidermidis, Mycobacterium smegmatis, and Streptococcus mutans, each sample exhibited a mean average of Zone of Inhibition (ZOI) measured in millimeters (mm); Lagerstroemia (5, 6, 5.5), Mahonia aquifolium (6, 6, 5), Punica granatum (6.5, 8, 7.5), Myrtus communis (3, 4, 4), Prosopis glandulosa (5, 1, 3). These results can be located in Table 1, which also exhibits the mean average of Gentamicin for Zone of Inhibition against the three bacterial strain.
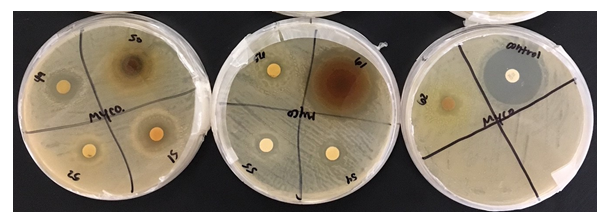
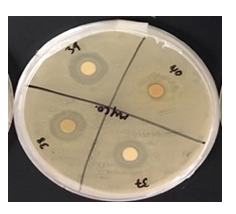
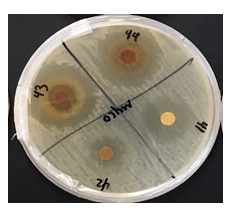
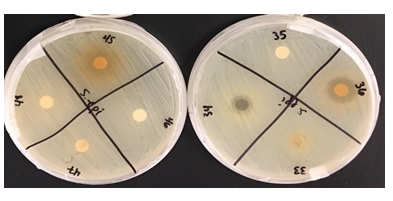
Figure 1: This project aimed to investigate the effect of identified plant extracts against three common target bacterial pathogens, to establish antimicrobial response through detection of inhibition zones to further in the hunt for new natural methods for antimicrobial resistance in reducing microbial and fungal activity.
Figure 3: Looking at the bacterial culture of Mycobacterium smegmatis, one can see that the Gaura, C. californica, Lagerstroemia, and M. aquifolium, respectively, had a relatively large effect on the bacteria with antibacterial activity in the range of a 3 as it is comparable to those of smaller zones expressed.
Table 1: Mean zone of inhibition (mm) of all extracts through 3 trials for each bacterium.
4. Discussion
As there are many different pathogens that become prevalent and more antibacterial- or antimicrobial-resistant, there has been research done in various fields to find new methods for combating these pathogens and the new strains that arise. Based on numerous other research projects and studies, desert plants have expressed high potentials for new antibacterial properties that have yet to be fully discovered [15]. expressed many of the potential antimicrobial properties of desert plants in the southwest United States [15], however, minimal research studies have since explored these potentials as well as many Middle Eastern and African desert plants [18-26]. There are hundreds of known desert plants in existence, many which have not yet been comprehensively explored, assessing the potential effectiveness of many of these plants as means for natural methods of antibacterial products must be investigated. Antibacterial efficacy is often determined by methods of Kirby-Bauer plate diffusion techniques [11]. In the present study, nutrient-rich agar was used to perform these tests, offering several advantages to identifying potential antibacterial properties each plant may possess.
In the current study, 33 unique plants that had been acquired from various regions of the Arizona state had been tested against 3 common bacterial pathogens, Staphylococcus epidermidis, Mycobacterium smegmatis, and Streptococcus mutans, had been grown on nutrient-rich agar plates. These tinctures were then placed in the agar by method of Kirby-Bauer and monitored over a 48-hour period. Once Kirby-Bauer for each tincture had been completed, each zone of inhibition (ZOI) was inspected and recorded. After, averaging and compiling the data into one comprehensive table, it was made clear that certain extracts had performed better than others as their ZOI were larger. The zones were assessed on both relative activity as well as by measurement in mm. Upon inspection of the data, only 5 extracts had expressed a zone of moderate to high activity on all 3 bacterial cultures: M. communis, Gaura, Lagerstroemia, P. granatum, and P. glandulosa. It has been made clear that M. smegmatis showed the least antibacterial-resistant activity as 27 of the 33 extracts averaged to express a clear ZOI. This was highly comparable to that of Staphylococcus epidermidis and Streptococcus mutans, which had 13 and 9 extracts which expressed clear ZOI, respectively. Based on these findings, many of these plant extracts, particularly the 5 which expressed ZOI in all 3 bacterial cultures, have potential to be utilized as natural methods for antibacterial application.
In looking ahead to future directions, the group plans to further explore the composition of the more successful plant species from the experiment by performing MIC, TLC, and NMR tests in an attempt to identify any specific antimicrobial compounds. Additionally, these tests would be performed in hopes to identify any common compounds shared between the various plant species to aid in further understanding antimicrobial resistance.
5. Conclusion
communis, Gaura, Lagerstroemia, P. granatum, and P. glandulosa extract were the only samples to show moderate to high antibacterial activity against Staphylococcus epidermidis, Mycobacterium smegmatis, and Streptococcus mutans. Overall, this project has yielded results helpful to the search of antimicrobial compounds. Further screening and characterization of antimicrobial principles are in progress.
Acknowledgements
This research is in compliance with federal regulations and institutional policies relating to infectious agents and did not receive any sources of funding or assistance outside of Grand Canyon University.
The authors of this paper are incredibly thankful to the Dean, Dr. Mark Wooden, and Assistant Dean, Dr. Jon Valla, of the College of Science, Engineering, and Technology at Grand Canyon University for the development and continuing support of the Research and Design Program at Grand Canyon University to provide the research facility, equipment, funding, publicity, and opportunities to continuously support this research. The authors would also like to acknowledge Faculty Research Mentor, Dr. Ramesh Velupillaimani for the continuous support and mentorship throughout the duration of this project. Additionally, the research group would like to thank the Glendale Xeriscape Garden and their staff of Glendale, AZ for graciously donating larger quantities of the specified plant material to make up the initial samples used for this research.
Disclosures
The research team had not received any additional outside funding from corporations or any other entities other than Grand Canyon University. Concurrently, the research team does not hold positions which could compromise the integrity of the research project.
References
- Kemper KJ, Chiou V. Aloe vera. The Center for Holistic and Pediatric Education and Research (1999).
- Thomson WAR. Medicines from the Earth: A guide to healing plants. New York, NY: McGraw-Hill (1978).
- Dube S, Shukla HS, Tripathi SC. In vitro evaluation of some natural products for their fungitoxicity. Pesticides 12 (1988): 11-12.
- Grainge MD, Alvarez AM. Antibacterial and antifungal activity of Artabotrys hexapetalus leaf extracts. International Journal of Tropical Plant Diseases 5 (1987): 173-179.
- Renu D, Amrita S, Rudra PO, et al. Possible therapeutic potential of Helicteres isora (L.) and it’s mechanism of action in diseases. Journal of Medicinal Plants Studies 3 (2015): 95-100.
- Nascimento GGF, Locatelli J, Freitas PC, et al. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian Journal of Microbiology 31 (2000): 247-256.
- Digrak M, Alma MH, Ilçim A, et al. Antibacterial and antifungal effects of various commercial plant extracts. Pharmaceutical Biology 37 (2008): 216-220.
- Tenango MP, Miguel-Chávez RS, Soto-Hernandez, M. et al. Aromatic and medicinal plants in Mexico. In H. El-Shemy (Eds.), Aromatic and medicinal plants – back to nature. London, U.K, IntechOpen (2017): 29-45.
- Bhalodia NR, Shukla VJ. Antibacterial and antifungal activities from leaf extracts of Cassia fistula I.: An ethnomedicinal plant. Journal of Advanced Pharmaceutical Technology & Research 2 (2011): 104-109.
- Ventola CL. The antibiotic resistance crisis: Part 1: Cause and treats. Pharmacy and Therapeutics, 40 (2015): 277-283.
- Shetty S, Mahin-Syed-Ismail P, Varghese S, et al. Antimicrobial effects of Citrus sinensis peel extracts against dental caries bacteria: An in vitro study. Journal of Clinical and Experimental Dentistry 8 (2016): 71-77.
- Watson R. General and medical microbiology (2021).
- Varkey TC, Lanier B, Merhavy ZI. Case of Complex Endocarditis with Associated Pericardial Effusion. Cardiol 3 (2020): 1016.
- Salazar SFM, Verdugo AE, López CG, et al. Activity of medicinal plants, used by native populations from Sonora, Mexico, against enteropathogenic bacteria. Pharmaceutical Biology 46 (2008): 732-737.
- Hoffmann JJ, Timmermann BN, McLaughlin SP, et al. Potential antimicrobial activity of plants from the southwestern United States. International Journal of Pharmacognosy 31 (1991): 101-115.
- Maruzzella JC, Marx H. Antimicrobial activity of desert plants. Nature 202 (1964): 825-826.
- Ríos JL, Recio MC. Medicinal plants, and antimicrobial activity. Journal of Ethnopharmacology 100 (2005): 80-84.
- Ahmad M, Ghafoor N, Aamir MN. Antibacterial activity of mother tinctures of Cholistan desert plants in Pakistan. Indian Journal of Pharmaceutical Sciences 74 (2012): 465-468.
- Anyanwu MU, Okoye RC. Antimicrobial activity of Nigerian medicinal plants. Journal of Intercultural Ethnopharmacology 6 (2017): 240-259.
- Dabur R, Gupta A, Mandal TK, et al. Antimicrobial activity of some Indian medicinal plants. African Journal of Traditional, Complementary and Alternative Medicines: AJTCAM 4 (2007): 313-318.
- Khan MF, Tang H, Lyles JT, et al. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Frontiers in Pharmacology 9 (2018): 1-17.
- Mustafa G, Ahmed S, Ahmed N, et al. Phytochemical and antibacterial activity of some unexplored medicinal plants of Cholistan desert. Journal of Botany 48 (2016): 2057-2062.
- Romha G, Admasu B, Gebrekidan TH, et al. Antibacterial activities of five medicinal plants in Ethiopia against some human and animal pathogens. Evidence-Based Complementary and Alternative Medicine (2018).
- Shahat AA, Mahmoud EA, Al-Mishari AA, et al. Antimicrobial activities of some Saudi Arabian herbal plants. African Journal of Traditional, Complementary and Alternative Medicines: AJTCAM 14 (2017): 161-165.
- Arabian herbal plants. African Journal of Traditional, Complementary and Alternative Medicines: AJTCAM 14 (2017): 161-165.
- Taye B, Giday M, Animut A, et al. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pacific Journal of Tropic Biomedicine 1 (2011): 370-375.
- Zain ME, Awaad AS, Al-Outhman MR, et al. Antimicrobial activities of Saudi Arabian desert plants. Phytopharmacology 2 (2011): 106-113.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 