Green Copper Nano-Pesticide Synthesized by Using Annona Squamosa L., Seed and their Efficacy on Insect Pest as well as Non-Target Species
Article Information
Perumal Vivekanandhan1, Kannan Swathy2, Adelina Thomas3, Patcharin Krutmuang*4, 5 Eliningaya J Kweka*6, 7
1Department of Biotechnology, AVS Arts and Science College, Omalur, Tamil Nadu, India
2Molecular Entomology Laboratory, Department of Biotechnology, School of Biosciences, Periyar University, Salem- 636 011, Tamil Nadu, India
3School of Pharmacy, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
4Innovative Agriculture Research Center, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
5Research Center of Microbial Diversity and Sustainable Utilization, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
6Division of Livestock and Human Diseases Vector Control, Tropical Pesticides Research Institute, Arusha, Tanzania
7Department of Medical Parasitology and Entomology, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
*Corresponding Author: Patcharin Krutmuang, Eliningaya J Kweka, Research Center of Microbial Diversity and Sustainable Utilization, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
Received: 9 July 2021; Accepted: 20 July 2021; Published: 30 July 2021
Citation: Perumal Vivekanandhan, Kannan Swathy, Adelina Thomas, Patcharin Krutmuang, Eliningaya J Kweka. Green Copper Nano-Pesticide Synthesized by Using Annona Squamosa L., Seed and their Efficacy on Insect Pest as well as Non-Target Species. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 456-473.
View / Download Pdf Share at FacebookAbstract
Insect pest resistance challenge to existing classes of insecticides is jeopardizing the currently attained achievements in pests control for the public health and economy worldwide. Alternative pesticides with different mode of action are of paramount importance. The pesticidal efficacy of Annona squamosa L. seed derived green copper nanoparticles (CuNPs) against Anopheles stephensi, Tenebrio molitor and non-targeting organism Artemia nauplii at 24h post treatment at laboratory condition was conducted. A. squamosa L., seed derived cupper nanoparticles were characterized through, UV-vis spectrophotometer, FT-IR, XRD, and EDaX, HR-SEM, TEM and AFM analysis. Insect larvae were exposed to discriminating dosage of 170μg/ml deliver significant mortality rate comparable to other concentrations against An. stephensi and T. molitor. The synthesis CuNPs lower toxicity was observed on non-targeting organism A. nauplii. The present study suggests this bio-rational green copper nano-pesticide from A. squamosa L., seed displays a significant effect to reduce the larvae of insect pest and lower toxicity to beneficial organism. These results suggest that, use of A. squamosa L., seed derived CuNPs is a rapid, environmentally safer, and greener approach for insect control. This could lead to a new avenue in pest control strategy.
Keywords
Annona squamosa L; Anopheles stephensi; Tenebrio molitor; CuNPs; Green synthesis; Artemia nauplii
Article Details
1. Introduction
Pest control is one of the most important concepts in worldwide due to the public health and economic impact caused[1, 2]. Different classes of chemical insecticides commonly used in vector control programs such as organochlorines, organophosphorus, carbamates, and pyrethroids mainly used for control of medical and agricultural pest control have shown to lose the efficacy [3-11]. The continued usage different type of chemical insecticides that shows significant drawbacks and challenges, like, pollution, pesticide accumulation and development of insecticide resistance in insect pest [2], so researcher to find and alternative, cost–effective and green pesticide led to resurgences in pest populations.
Annona squamosa L., is also known as master-apple or sweetsop is a species in the Annonaceae family plant that is native to the tropical region of the world and extensively cultivated for fragrant, juicy and its fruits which contains huge amounts of vitamin C than citrus fruits. A. squamosa L., seeds are toxic in natures, also seeds are used for insecticidal activities against insect pest. Seeds contain high oil content, that can be used in several pharmacological industries like, soap manufacture industries, drug industries and pesticidal industries. The fresh leaves, unripe fruits, bark and root extracts have been used for treat fevers, rheumatism, diarrhea, dysentery, and other ailments. A. squamosa has been found to possess antidiabetic, abortificant, antimicrobial, antitumor, antioxidant, larvicidal properties [12-19].
Nanoscience is one of the active research areas in modern material science. Based upon their specific characteristics as size, distribution and morphology of the nanoparticles have distinct properties with the bulk form of the same material. Nanotechnology is a branch of science that embodies biological, chemical, physical and electrical and electronics engineering. The field offers a promising way to improve the properties of metals by transforming them into nanoparticles within a size range of 1-99nm [20]. Nanotechnology has gained massive applications in the field of pesticides [21]. The metal and metal oxide derived nanoparticles is effective promising pesticidal candidates in recent years in the pesticide industries. The green synthesis of nanoparticles is nontoxic, economical and more stable in environmental conditions for few months [22]. Recent studies have been reported that silver, gold, or Zinc oxide nanoparticles raved good antimicrobial and larvicidal activity against human pathogens and medical and agricultural pest [23]. The development of copper and copper oxide nanopesticides has received considerable attention in recent years in pharmacological and pesticidal industries [24]. Moreover, copper nanoparticles may be incorporated into the polymer matrix and used as medical equipment, such as anti-inflammatory devices [25]. Several physical, chemical methods used in the synthesis of copper nanoparticles raise several problems like high temperature and pressure, reducing agents and organic solvents and hazardous chemicals they obtained rifampicin copper nanoparticles may be applied as additives in health care products, like bandages, wound dress or powders to improve antibiotics activity against human pathogens.
In the present study, the green synthesis copper nanoparticles (CuNPs) from Annona squamosa L., seed has been made to screen for the pesticidal activity against larvae of Anopheles stephensi, Tenebrio molitor and non-targeting organism.
2. Materials and Methods
2.1 Collection of plant material
Annona squamosa L., seed, were collected from Sanarappatti village, Dharmapuri, District, Tamil Nadu, India (12.1211° N, 78.1582° E). Collected A. squamosa L., seed were washed with running tap water for removing unwanted dust particles. The seeds were cut into small pieces with help of knife and then fruits were dried at room temperature (28 ± 2oC) for 20 days in laboratory condition. The dehydrated seed was crushed by electronically stainless blender [1, 26].
2.2 Preparation of aqueous fruits extract
The 15gm of A. squamosa L., seed powder was boiled it at 65°C for 25 min. The seed extract was filtered through Whatman no. 1 filter paper and filtered seed extract was stored at 4°C and then used for nanoparticles preparation.
2.3 Synthesis of CuNPs
Copper nanoparticles were synthesized by treating 75ml of 1mM copper oxide metal solution with 25 ml of seed extract and the mixture of solution were incubated at room temperature at dark condition (28 ± 2oC) for 24h and after 24h incubation time solution colour was changed into brown colour indicating the synthesized of CuNPs (Figure. 1).
2.4 Characterization of CuNPs
The aromatic plant-mediated reduction of CuNPs was monitored by sampling the reaction mixture at normal intervals times and the CuNPs absorption were analysed using, UV-Vis spectram wavelength range is 200-700nm. The CuNPs solution mixture was subjected to centrifugation at 25, 000 rpm for 13min, the centrifuged final pellet was stored for further experiments. X-ray diffraction (XRD) analysis was carried out in PAN analytical q X-Pert PRO. Diffractometer operating range at 40kV with a current of 30A using Cu Ka for the purpose of purity and crystalline size of the CuNPs. Transmission electron microscopy (TEM) used for evaluation of CuNPs nature using JEM-1200 with operating range at 80-90 kV.
The size and surface morphology feature of CuNPs was evaluated by (Scanning Electron Microscope-JEOL JSM-6390), operated at 15kV. Energy Dispersive X-ray (EDaX) analysis, the particles were dried on a carbon coated copper grid and performed on SEM instrument with thermo EDaX attachment. The powder sample of green synthesis CuNPs was analyzed by Fourier transform infrared spectroscopy (FT-IR) using a Shimadzu infrared spectrometer (Model- 400) with KBr as a background over the range is 400-4000 cm_1.
2.5 Mosquito Rearing
An. stephensi mosquito larvae were obtained from IVCZ, Hosur, Tamil Nadu, India, which have been successfully maintaining in laboratory condition. All the experiments were carried out at (28 ± 2 °C; 70-80%) relative humidity and 14:10 light and dark photoperiod. Larvae were fed with millet powder [27].
2.6 Stored product pest
Tenebrio molitor culture was held in the laboratory at (28 ± 2°C, 70-80% RH) with a 14:10 light-dark photoperiod. The larvae were reared in a plastic container (55cm long × 38cm wide × 10cm) and fed with wheat bran containing a rich source of macro and micro nutrition. The healthy 3rd instar was used for bioassays in the laboratory.
2.7 Artemia nauplii culturing
A.nauplii larvae were incubated in 500 mL of seawater with a salinity of 30 parts per thousand in a conical flask under laboratory condition (pH 8.4; light 16:8 h; temperature 26 ± 2 °C) were maintained. Aeration was maintain using a fish tank oxygen air pump.
2.8 CuNPs larval toxicity
Target toxicity bioassay was carried out according to WHO protocol [28]. Twenty-five (25) third instar larvae of An. stephensi were taken to the larval bioassay container containing 249 ml tap water, we select different testing concentration (50, 75, 100,150 and 175 μg/ml), each concentration has three replicates, and each replicate has 25 larvae. Control was set up with dechlorinated tap water. After 24 h of exposure, the numbers of dead larvae were counted for probit analysis.
- squamosa L., seed- derived CuNPs were determined by calculating the larval mortality and lethal concentrations (LC50 and LC90) were evaluated with five different concentrations (50, 75, 100, 150 and 175 μg/ml). Each concentration had three replicates and each replicate had 25, 3rd instar larvae of T. molitor. Five concentrations of A. squamosa L., seed- derived CuNPs were applied in a 1µl solution in the diet of the larva. The dead T. molitor larvae were counted 24h post-treatment.
2.9 CuNPs toxicity on Artemia nauplii
Newly hatched A. nauplii were collected with micropipette and used for toxicity assays under different test concentrations (50, 75, 100, 150 and 175 μg/ml), of A. squamosa L., seed- derived CuNPs. Sterile seawater was used as a control. The experiment was investigated. A. nauplii probit analysis LC50 and LC90 values were observed after 24 h post-treatment and the experiment was replicated 3 times for each concentration.
2.10 Statistical analysis
The mosquito, stored grain pest larvae and adult A. nauplii mortality were calculated by the probity analysis. To predict LC50 and LC90 and other statistics at 95% of confidential limits of upper confidence limit (UCL) and 50% of lower confidence limit (LCL) values and chi-square tests were calculated through SPSS- 26.0.
3. Results
3.1 Formation and conformation of CuNPs
The synthesized copper nanoparticles show dark brown colour, which represented the reduction of 1 Mm of the copper nanoparticles (CuNPs) (Figure 1).
3.2 UV-Visible Spectrophotometer
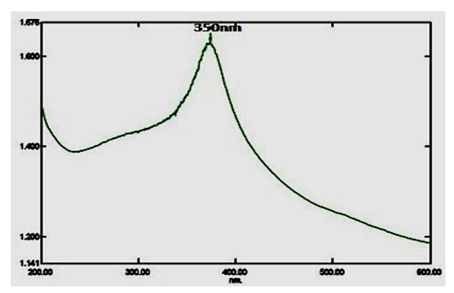
Copper nanoparticles (CuNPs) were characterized via UV-visible spectrophotometer analysis, and it is most widely used as an initial confirmation technique of nanoparticles synthesis. A strong absorption peak range is 350nm (Figure. 2).
3.3 EDaX (Energy dispersive X-Ray) Analysis
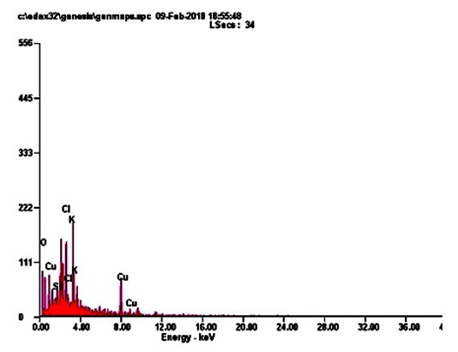
Energy-dispersive microanalysis to gain further insight into the CuNPs analysis of the sample was performed using EDaX techniques. It is giving an exact detail, about the metal elements with an appropriate composition in a dry powder of CuNPs. The peaks around range from 22.0. The result indicates that the reaction product presenting in the CuNPs (Figure. 3; Table 3). CuNPs present in elements following such as, OK, SIK, CLK, KK and CUK indicate that the extracellular organic moieties are absorbed on the surface of the metallic nanoparticles.
3.4 HR-SEM (Scanning Electron Microscope)
The SEM analysis result clearly shows an external morphology of CuNPs and also reveals an exact size ranging from 10.47nm to 25.58nm (Figure. 4).
3.5 TEM (Transmission Electron Microscopy)
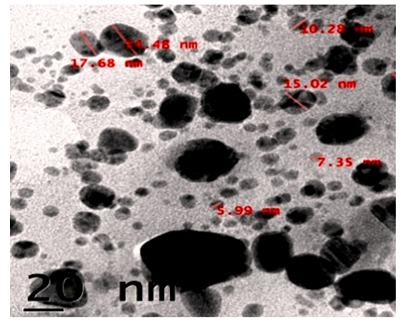
TEM analysis results showed the poly-dispersed roughly spherical shape of CuNPs. The size ranges from 5.99nm to 24.48nm (Figure. 5).
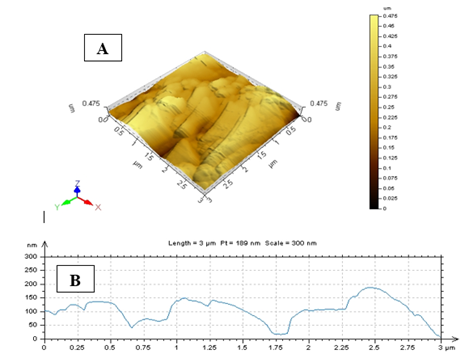
3.6 AFM (Atomic Force Microscope Analysis)
AFM was performed to know the topological map of the surface of the synthesis nanoparticles. Our results deal with the exact values AFM image of A. squamosa L., seed extract derived CuNPs. AFM imaging technique that reveals X value 3.0µm and Y value is 0.49nm and Z value is in the golden image was 0.49nm (Figure. 6).
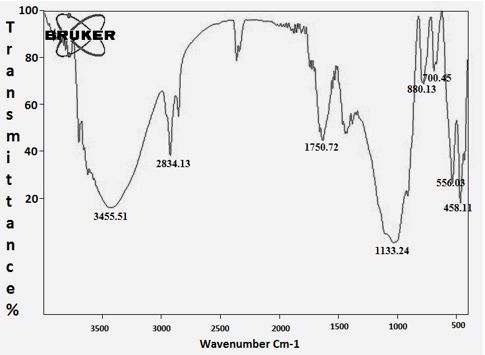
3.7 FT-IR analysis
FT-IR characterizations were done for identification of major functional groups present in the CuNPs synthesized from A. squamosa L., seed extract. The 3455.51cm-1 assigned to ArO-H H bonded, the 2834.13 assigned with -CH2-and 1750.72cm-1 assigned to Ar-CH=CHR. The weaker band at 556.03cm-1 corresponds to C-Br stretching in Misc group (Figure. 7; Table. 2).
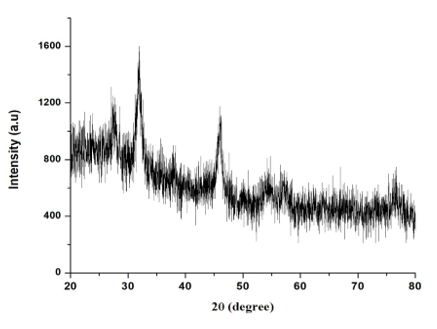
3.8 XRD (X-Ray different meter)
The CuNPs crystalline nature of the CuNPS were confirmed by XRD analysis. The botanical synthesized CuNPs by employing A. squamosa L., seed extract was further demonstrated and confirmed by the characteristic peaks observed in the XRD image (Figure. 8). The broadening of Bragg’s peaks indicates the formation of nanoparticles.
The XRD clearly determined and evaluated that, the crystalline structure of CuNPs, it also reveals that, the CuNPs have high purity. It has showed 3 strongest peak value at 5.73202, 4.84293 and 3.14685 which is corresponding to the intense counts (IC) as 500, 1250,750 in its values multiply of peak (Figure. 8).
3.9 Larval bioassay
The (A. squamosa L., seed) extract derived CuNPs as evaluated for larvicidal activity against A. stephensi larvae. Result shows that CuNPs have strong insecticidal activity of An. stephensi mosquito larvae. The 170µg/ml produced 93% mortality on A. stephensi mosquito larvae at 24h post treatment. The LC50 and LC90 values were in An. stephensi, 97.301-111.758μg/ml. (Table. 1).
squamosa L., seed derived CuNPs have strong insecticidal activity against T.molitor larvae. The 170µg/ml concentration produced 93% mortality on T.molitor larvae at 24h post treatment. The LC50 and LC90 values were in T.molitor larvae, 239.430-338.680μg/ml. (Table. 1).
3.10 CuNPs toxicity on Artemia nauplii
squamosa L., seed derived CuNPs as evaluated for compare the toxicity with aquatic bio indicator A. nauplii. Our results clearly show that CuNPs produced less amount of toxicity was observed than insect pest. The mortality rate range is 36%. LC50 and LC90 values were in A. nauplii, 204.541-342.241μg/ml (Table 1).
|
Mosquito Larvae(N=450) |
Treatments |
Concentration (µg/ml) |
% Mortality |
LC50 (LCL-UCL) (μg/ml) |
LC90 (LCL-UCL) (μg/ml) |
χ2 df (3) |
|
An. stephensi |
Aqueous |
Control |
1.33 |
189.13 |
321.01 |
1.61 |
|
50 |
6.66 |
(167.74-227.16) |
(269.72-421.31) |
|||
|
75 |
13.33 |
|||||
|
100 |
24 |
|||||
|
150 |
33.33 |
|||||
|
175 |
44 |
|||||
|
CuNPs |
Control |
1.33 |
53.97 |
170.18 |
3.44 |
|
|
50 |
46.66 |
(29.83-69.48) |
(150.40-203.49) |
|||
|
75 |
58.66 |
|||||
|
100 |
74.66 |
|||||
|
150 |
80 |
|||||
|
175 |
93.33 |
|||||
|
T. molitor |
Aqueous |
Control |
1.33 |
239.43 |
338.68 |
1.44 |
|
50 |
1.33 |
(207.74-311.95) |
(279.32-480.33) |
|||
|
75 |
1.33 |
|||||
|
100 |
5.33 |
|||||
|
150 |
13.33 |
|||||
|
175 |
18.66 |
|||||
|
CuNPs |
Control |
1.33 |
92.88 |
216.38 |
3.09 |
|
|
50 |
26.66 |
(77.59-105.96) |
(189.59-262.49) |
|||
|
75 |
48 |
|||||
|
100 |
57.33 |
|||||
|
150 |
69.33 |
|||||
|
175 |
80 |
|||||
|
A. nauplii |
Aqueous |
Control |
1.33 |
292.632 |
417.19 |
1.73 |
|
50 |
1.33 |
(233.75- |
(313.94- |
|||
|
|
505.56) |
800.34) |
||||
|
75 |
2.33 |
|||||
|
100 |
4 |
|||||
|
150 |
5.33 |
|||||
|
175 |
12 |
|||||
|
CuNPs |
Control |
1.33 |
204.54 |
342.24 |
2.65 |
|
|
50 |
4 |
(178.88-254.14) |
(282.84-465.86) |
|||
|
75 |
13.33 |
|||||
|
100 |
20 |
|||||
|
150 |
32 |
|||||
|
175 |
36 |
Table. 1: A. squamosa L., seed extract derived copper nanoparticles against larvae of A. stephensi, T. molitor and non-targeting species A. nauplii at 24h post treatment.
Note: na = total number of larvae used per each organism, 25 per replicate, three replicates were carried out, five concentrations were tested, LC50 = Lethal concentration killing 50% of exposed organisms, LC90 = Lethal concentration killing 90% of exposed organisms, LCL = 95% lower confidence limits, UCL = 95% upper confidence limits, χ2 = chi square (n.s.=not significant, p > 0.05); df = degrees of freedom.
|
S. no |
Vibrational assignment |
Observed wavenumber (cm-1) |
Functional group |
Visible intensity peak |
|
1 |
ArO-H H bonded |
3455.51 |
Phenols |
Broad |
|
2 |
-CH2- |
2834.13 |
Alkanes |
Medium sharp |
|
3 |
Ar-CH=CHR |
1750.72 |
Alkenes |
Medium |
|
4 |
S-O Sulfone 2 |
1133.24 |
Misc |
Small broad |
|
5 |
S-OR ester |
880.13 |
Misc |
Medium |
|
6 |
=CH out of plane |
700.45 |
Alkenes |
Medium |
|
7 |
C-Br stretch |
556.03 |
Misc |
Medium sharp |
|
8 |
S-S disulfide asym |
458.11 |
Misc |
Medium sharp |
Table 2: FT-IRanalysis of A. squamosa L., seed extract derived copper nanoparticles.
|
Element |
Weight% |
Atomic% |
|
K |
48 |
70.65 |
|
SI |
2.47 |
3.44 |
|
CL |
14 |
6.41 |
|
K |
13.53 |
9 |
|
CU |
22 |
8 |
|
Total |
100 |
Table. 3: EDaX image give an exact elementary composition of CuNPs. It shows major elements such as, OK, SIK, CLK and KK.
4. Discussion
This study has shown that, the green nanoparticles synthesis has high response impact on mortality of targeted and less impact on non-targeted insects. The synthetic pesticides have caused remarkable toxic effect against medical and agricultural pests on previous time which have led to the development of insecticides resistance among disease pests but the current days insect pest can got chemical insecticide resistance capability [2, 29]. In this present time the green nanoparticles synthesis is defeat by aromatic plant derived chemical constituents for biological activities and these nanoparticles attract attention as alternative pesticides due to its biological efficacy in small concentrations [25].
The current result shows that copper nanoparticles synthesized from A. squamosa L., seed extract and nanoparticles synthesized were preliminarily confirmed by using spectrophotometer in absorption ranges from 200-700nm. A. squamosa L., seed extract derived nanoparticles absorption peak range at 350nm, that indicating the synthesized of copper nanoparticles. Similarly, Vivekanandhan and others reported that entomopathogenic fungi derived nanoparticles absorption range at 350nm [26]. The FT-IR results show strong functional groups such as, Phenols, Alkanes and Alkenes these functional groups may be involved in insecticidal activities. Similarly, Pseudomonas fluorescence derived silver nanoparticles contains functional groups such as Alkanes and Alkenes in previous reports [30]. Similarly, flavonoids, triterpenoids and polyphenols functional groups were reported in entomopathogenic fungi derived silver nanoparticles against three major mosquito species [31]. Therefore, the aromatic plants contain a huge amount of terpenoids that shows amazing activity to convert the aldehydes groups to carboxylic acids in the metal ions.
The findings of this study shows that EDaX peak range around 22.0% to the binding energies of synthesized copper ions (Figure. 3). The EDaX record for CuNPs synthesized extracts showed signal of copper from 2keV. The x-ray emission from the macro or microchemical constituents presents within the seeds of A. squamosa L. Ramyadevi and colleagues reported that botanical derived copper nanoparticles binding energy maximum range is 21.23% [24]. The XRD results shows the occurrence of sharp bands this might be due to the stabilization of the synthesized nanoparticles by the seed extract of A. squamosa L., seed, that can be involved for reduction properties, and thus confirm the Crystallization of the biological mediated organic phase occurs on the surface of the CuNPs.
The HR-SEM investigation of green synthesized CuNPs was discernible due to their size difference. HR-SEM image of CuNPs observed from the electron microscopic view, that shows majority is spherical with a tiny amount of stretched out of particles range from 10.47nm-25.58nm. The uniformly distributed copper nanoparticles on the surface of the slides are viewed. The identical range of CuNPs suggests that tiny particles are observed. HR-SEM analysis of CuNPs was discernible due to their size difference of synthesized CuNPs. Transmission Electron Microscopy (TEM) analysis CuNPs shows that the particles are poly-dispersed more or less spherical in shape range is 5.99-24.48nm. The AFM analysis results deal with the exact values of A. squamosa L., seed extract derived CuNPs reveals that X value 3.0µm and Y value is 0.49nm and Z value 0.49nm.
The plant contains huge amounts of secondary metabolites that serve as protection from environmental condition, insects, plant pathogens etc. The macro/micro chemical constituents like phenolics, terpenoids, alkaloids, steroids and essential oils shows remarkable insecticidal activities against medical and agricultural insect pest [26, 32, 33]. The bioreductor cofactor involved a significant function in the reduction process of metal nanoparticles. The plant containing phenols plays an important part in the reduction of metal-ions to CuNPs. So, in the arrangement with mosquito nets with other mosquito control tools like, plant synthesized CuNPs produced a significant impact on adult of An. stephensi, A. aegypti and C. quinquefasciatus mosquitoes. The aromatic plant mediated synthesized of CuNPs, being readily obtainable and their application way is simple and effective against disease causing mosquito species.
The results show that A. squamosa L., seed derived green pesticides (CuNPs) has good larvicidal activities with low LC50 and LC90 values were An. stephensi, 189.128-321.004μg/ml. Similarly, entomopathogenic fungi F. oxysporum mediated silver nanoparticles produced remarkable toxic efficacy against larvae of three major mosquito species under laboratory condition [31], also Kalaimurugan and others reported that Pseudomonas fluorescence derived silver nanoparticles cause remarkable mortality rate on three major mosquito species larvae [30]. Nasrollahzadeh and colleagues reported that green synthesis of palladium nanoparticles using H. rhamnoides L., plant extract produced remarkable catalytic activity [34]. The A. squamosa L., seed-derived CuNPs have strong insecticidal activity against T.molitor larvae which showed that, at the 170µg/ml concentration produced 93% mortality at 24h post-treatment. The synthesis CuNPs had lower toxicity in non-targeting aquatic beneficial aquatic shrimp, A. nauplii. Similarly, Suganya and others reported that the plant-mediated
synthesis NPs from L. aspera showed strong mosquitocidal activity on targeted pest Ae.aegypti [35]. The metal nanoparticle conjugated with botanical extracts is extremely effective against insects with a lower toxic to non-target species [36]. The previous studies reported that S. rubra and
P.daemia mediated AgNPs do not cause any efficacy
against freshwater fishes after 48h post-treatment [37]. The B. cristata mediated synthesized AgNPs did not cause any toxic efficacy on nontarget species [38]. Similar, studies reported that the biosynthesized nanoparticles using leaf extract of L. aspera showed potential mosquito larvicidal efficacy against Ae. aegypti [35]. Hence, the insecticidal activities of synthesized AgNPs force the denaturation of the sulfur-containing proteins or phosphorous-containing compounds like nucleic acids that, leads to the denaturation of proteins and enzymes also reduces the cellular membrane activities and stop the ATP synthesis. In the final stage of the process the cellular functions and insect cell will death [39]. Today, environmental safety is considered to be most necessary for the application of CuNPs with botanical extract and highly appreciable and practical. The green synthesized insecticide is eco-friendly, effective, cheaper, pollution-free and target specific to the insect pest.
5. Conclusion
The findings of this study have indicated that, green synthesized copper nanoparticles (CuNPs) had remarkable toxicity against larvae of A. stephensi, T. molitor after 24h -post-treatment, also less toxic efficacy was against the non-targeted organism, A. nauplii.
Acknowledgement
The authors also thank the IVCZ, Hosur for the disease mosquito larvae. We acknowledge the Karunya University for, FE-SEM/EDaX and AFM investigation nanoparticles. also thank STIC, Cochin, for their help in the XRD and FT-IR characterization of nanoparticles. This research was partially supported by Chiang Mai University, Thailand.
References
- P Vivekanandhan, K Swathy, D Kalaimurugan, et al. Larvicidal toxicity of Metarhizium anisopliae metabolites against three mosquito species and non-targeting organisms, Plos one 15 (2020): e0232172.
- P Vivekanandhan, A Thendralmanikandan, E Kweka, et al. Resistance to temephos in Anopheles stephensi larvae is associated with increased cytochrome P450 and α-esterase genes overexpression, International Journal of Tropical Insect Science (2021): 1-6.
- CL Wanjala, EJ Kweka. Malaria Vectors Insecticides Resistance in Different Agroecosystems in Western Kenya, Frontiers in Public Health 6 (2018).
- S Mbepera, G Nkwengulila, R Peter, et al. The influence of age on insecticide susceptibility of Anopheles arabiensis during dry and rainy seasons in rice irrigation schemes of Northern Tanzania, Malaria Journal 16 (2017) 364.
- EJ Nnko, C Kihamia, F Tenu, et al. Insecticide use pattern and phenotypic susceptibility of Anopheles gambiae sensu lato to commonly used insecticides in Lower Moshi, northern Tanzania, BMC Research Notes 10 (2017) 443.
- EJ Kweka, HD Mazigo, LJ Lyaruu, et al. Anopheline Mosquito Species Composition, Kdr Mutation Frequency, and Parasite Infectivity Status in Northern Tanzania, Journal of Medical Entomology 57 (2020): 933-938.
- MA Kulkarni, M Rowland, M Alifrangis, et al. Occurrence of the leucine-to-phenylalanine knockdown resistance (kdr) mutation in Anopheles arabiensis populations in Tanzania, detected by a simplified high-throughput SSOP-ELISA method, Malaria Journal 5 (2006): 56.
- A Mahande, I Dusfour, J Matias, et al. Knockdown resistance, Rdl alleles, and the annual entomological Inoculation rate of wild mosquito populations from Lower Moshi, Northern Tanzania, Journal of Global Infectious Diseases 4 (2012): 114-119.
- I Ullah, S Wazir, N Abbas, et al. Monitoring of field-evolved resistance to flonicamid, neonicotinoid, and conventional insecticides in the Oxycarenus hyalinipennis costa, Environmental Monitoring and Assessment 193 (2021): 382.
- TE Nkya, FW Mosha, SM Magesa, et al. Increased tolerance of Anopheles gambiae ss to chemical insecticides after exposure to agrochemical mixture, Tanzania journal of health research 16 (2014).
- TE Nkya, R Poupardin, F Laporte, et al. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions, Parasites & Vectors 7 (2014): 480.
- G Jain, V Dixit, Effect of Annona squamosa EtOH extract and testicular functin of dogs (Canis indicus Linn.), II Annual Session of Science, Abstr-15 Bhavnagar (1982): 22.
- A Mishra, J Dogra, J Singh, et al. Post–coital antifertility activity of Annona squamosa and Ipomoea fistulosa, Planta Medica 35 (1979): 283-285.
- B Pardhasaradhi, M Reddy, AM Ali, et al. Antitumour activity of Annona squamosa seed extracts is through the generation of free radicals and induction of apoptosis (2004).
- V Kothari, S Seshadri, Antioxidant activity of seed extracts of Annona squamosa and Carica papaya, Nutrition & food science (2010).
- Patel D Jayshree, K Vipin. Annona squamosa L.: Phytochemical analysis and Antimicrobial Screening, Journal of pharmacy research 1 (2008): 34-38.
- G Vidyasagar. A comparative antimicrobial activity of methanolic root, leaf, seed cotyledon extracts of Annona squamosa L, International Journal of Pharmacy and Pharmaceutical Sciences 4 (2012): 289-292.
- JL Ray, J Althammer, KS Skaar, et al. Metabarcoding and metabolome analyses of copepod grazing reveal feeding preference and linkage to metabolite classes in dynamic microbial plankton communities, Molecular Ecology 25 (2016): 5585-5602.
- RS Tomar, SS Sisodia, Antidiabetic activity of Annona squamosa L. in experimental induced diabetic rats, Int J Pharm Biol Arch 3 (2012): 1492-1495.
- S Priya, S Santhi, A review on nanoparticles in mosquito control-a green revolution in future, Int. J. Res. Appl. Sci. Eng. Technol (2014): 378-387.
- Y Wang, J Wang, W Wang, et al. A mixed-space approach to first-principles calculations of phonon frequencies for polar materials, Journal of Physics: Condensed Matter 22 (2010): 202201.
- P Azmath, S Baker, D Rakshith, et al. Mycosynthesis of silver nanoparticles bearing antibacterial activity, Saudi Pharmaceutical Journal 24 (2016): 140-146.
- S Baker, KM Kumar, P Santosh, et al. Extracellular synthesis of silver nanoparticles by novel Pseudomonas veronii AS41G inhabiting Annona squamosa L. and their bactericidal activity, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 136 (2015): 1434-1440.
- J Ramyadevi, K Jeyasubramanian, A Marikani, et al. Synthesis and antimicrobial activity of copper nanoparticles, Materials letters 71 (2012): 114-116.
- G Benelli, A Caselli, A Canale. Nanoparticles for mosquito control: challenges and constraints, Journal of King Saud University-Science 29 (2017): 424-435.
- P Vivekanandhan, T Kavitha, S Karthi et al. Toxicity of Beauveria bassiana-28 mycelial extracts on larvae of Culex quinquefasciatus mosquito (Diptera: Culicidae), International journal of environmental research and public health 15 (2018): 440.
- P Vivekanandhan, S Bedini, M Shivakumar. Isolation and identification of entomopathogenic fungus from Eastern Ghats of South Indian Forest soil and their efficacy as biopesticide for mosquito control, Parasitology international, 76 (2020): 102099.
- WHO, Guidelines for laboratory and field testing of mosquito larvicides, World Health Organization (2005).
- B Liu, JW Jiang, BC Wilson, et al. Hong, Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide, Journal of Pharmacology and Experimental Therapeutics 295 (2000): 125-132.
- D Kalaimurugan, P Vivekanandhan, P Sivasankar, et al. Larvicidal activity of silver nanoparticles synthesized by Pseudomonas fluorescens YPS3 isolated from the Eastern Ghats of India, Journal of Cluster Science 30 (2019): 225-233.
- P Vivekanandhan, S Deepa, EJ Kweka et al. Toxicity of Fusarium oxysporum-VKFO-01 derived silver nanoparticles as potential inseciticide against three mosquito vector species (Diptera: Culicidae), Journal of Cluster Science 29 (2018): 1139-1149.
- EAS Shaalan, D Canyon, MWF Younes, et al. A review of botanical phytochemicals with mosquitocidal potential, Environment international 31 (2005): 1149-1166.
- P Vivekanandhan, S Senthil-Nathan, MS Shivakumar. Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors, Physiological and Molecular Plant Pathology 101 (2018): 156-162.
- M Nasrollahzadeh, SM Sajadi, M Maham. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki–Miyaura coupling in water, Journal of Molecular Catalysis A: Chemical 396 (2015): 297-303.
- G Suganya, S Karthi, MS Shivakumar. Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti, Parasitology research 113 (2014): 875-880.
- M Govindarajan, G Benelli. α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae), Parasitology research 115 (2016): 2771-2778.
- RS Patil, MR Kokate, CL Jambhale, et al. One-pot synthesis of PVA-capped silver nanoparticles their characterization and biomedical application, Advances in natural sciences: nanoscience and nanotechnology 3 (2012): 015013.
- G Benelli. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review, Parasitology research 115 (2016): 23-34.
- SS Shankar, A Rai, A Ahmad, et al. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth, Journal of colloid and interface science 275 (2004): 496-502.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 