HPTLC Fingerprinting and Cytotoxicity of Secondary Metabolites of Equisetum Diffusum D. Don Extracts
Article Information
Bich-Loan Thi NGUYEN1,2*, Claudio PALMIERI1, Kim-Thanh Thi NGUYEN2, Diep Hong LE2, Trang Thi NGO2, Chung Kim TRAN2, Huy Quang NGUYEN2, Hai The PHAM2, Marc MULLER3, Pierre DUEZ1, Amandine NACHTERGAEL1
1Unit of Therapeutic Chemistry and Pharmacognosy, University of Mons (UMONS), Mons, Belgium
2Faculty of Biology, VNU University of Science, Hanoi, Vietnam
3GIGA I3 - Laboratory for Organogenesis and Regeneration, University of Liège, Liège, Belgium
*Corresponding Author: Bich-Loan Thi NGUYEN, Faculty of Biology, VNU University of Science, Hanoi, Vietnam
Received: 13 September 2021; Accepted: 21 September 2021; Published: 21 October 2021
Citation:
Bich-Loan Thi NGUYEN, Claudio PALMIERI, Kim-Thanh Thi NGUYEN, Diep Hong LE, Trang Thi NGO, Chung Kim TRAN, Huy Quang NGUYEN, Hai The PHAM, Marc MULLER, Pierre DUEZ, Amandine NACHTERGAEL. HPTLC Fingerprinting and Cytotoxicity of Secondary Metabolites of Equisetum Diffusum D. Don Extracts. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 596-613.
View / Download Pdf Share at FacebookAbstract
The aerial parts of Equisetum diffusum D.Don (Equisetaceae ED), a Vietnamese folk medicine used for hypertensive, anti-inflammatory, diuretic and hemostatic properties, were collected in Northern Vietnam. The secondary metabolites of the sequential n-hexane, ethyl acetate and methanol extracts were profiled by HPTLC with chromatographic conditions and derivatization reagents characteristic for flavonoids, polyphenols and terpenoids/steroids. All these metabolite classes were present in the methanol extract whereas the ethyl acetate extract comprised some polyphenols and flavonoids; no characteristic compound class could be identified in the n-hexane extract. HPLC allowed to determine the concentration of isoquercitroside, the major flavonoid of the methanol extract (1.60 ± 0.04 mg/g dry weight; n=3). In a preliminary assessment of Equisetum diffusum aerial parts safety, the cytotoxicity of these 3 ED extracts was investigated on the FHs 74 Int human intestinal epithelial cell line, a non-cancerous, non-transformed normal cell line. The ethyl acetate extract was shown to be the most toxic with an IC50 of 8.7 ± 2.3 µg/mL, followed by the n-hexane and methanol extracts, with an IC50 of 46.1 ± 3.6 µg/mL and 124.7 ± 23.0 µg/mL, respectively.
Keywords
Equisetum diffusum D.Don; Equisetum arvense L.; Fingerprint; HPTLC; HPLC; Secondary metabolites; Isoquercitroside
Equisetum diffusum D.Don articles; Equisetum arvense L. articles; Fingerprint articles; HPTLC articles; HPLC articles; Secondary metabolites articles; Isoquercitroside articles
Article Details
1. Introduction
Medicinal herbs have a long history of development in the world. Their traditional use is documented in many handbooks of phytotherapy and is more and more popular in the field of alternative and complementary medicine [1]. The medicinal herbs market grows every year and will reach a CAGR (Compound annual growth rate) of 5.34 % from 2021 to 2028 to reach 426.43 billion US dollars by 2028 [2]. Ethnopharmacological inquiries provide us with important information about traditional medicinal plants and countless papers study their various effects, properties and characteristics. Nevertheless, our knowledge of the field is still largely imperfect and many questions remain on the safety of traditional remedies [3].
The demand for medicinal herbs and complementary medicines is increasing worldwide and, consequently, the number of low-quality herbs on the market is steadily increasing. Therefore, methods for authentication of herbal material and standardization of their active ingredients are very important and necessary. Until recently, medicinal herbs were often identified according to the morphology of used parts, such as the roots, leaves, flowers, or stems [4, 5]. However, medicinal herbs on the market are often chopped or even powdered for sale, making such identification difficult. Nowadays, DNA barcoding technology can be applied to define plants in general and medicinal plants in particular [5-7].
This method gives accurate results, sometimes at the level of species or even variety, but has not been widely applied because of the high cost and the requirement for specific techniques and instruments. Also, DNA analyses give no clues on the quality of the identified material, notably its content in active compounds. Fingerprinting methods using High-Performance Thin-Layer Chromatography (HPTLC) represent an elegant alternative to both identify species and assess the quality of medicinal plants and extracts. This method begins with the extraction of the plant and continues with the chromatography of the extract. The results are presented in the form of a "fingerprint", i.e. a specific sequence of peaks or zones due to the migration of known or unknown components of the extract in the selected chromatographic conditions.
The fingerprint of botanically authenticated raw material serves as a primary reference to all unknown material that needs to be characterized. Identification can also be performed on the basis of chemical reference compounds whenever the sample is expected to contain those. One of the advantages of HPTLC fingerprints is that it mostly relies on a visual impression, yielding a rapid and easy interpretation. A broad spectrum of constituents can be detected and described without the need to know the chemical nature of each of the zones visualized on the chromatogram [8].
Equisetum diffusum D.Don (ED) [synonyms, Equisetum arvense subsp. diffusum (D.Don) Fraser-Jenk., Equisetum diffusum var. caespitosum Milde, Equisetum diffusum var. nudum Milde, Equisetum diffusum var. paucidentatum C.N.Page, Equisetum diffusum var. polystachyum Milde, Equisetum diffusum var. ramosum Milde, Equisetum mekongense C.N.Page, Equisetum wallichianum C.N.Page] [9] is known in Vietnamese as “Moc tac trãi”; this Equisetaceae is distributed in Vietnam, Bangladesh, China, Himalaya, India, Laos, Myanmar, Nepal, Pakistan, Thailand and Tibet. In Vietnam, it is used in traditional medicine to treat low blood pressure and inflammation, and as a diuretic and hemostatic [10]. So far, very few studies have been published on Equisetum diffusum but another species, Equisetum arvense L. (EA), commonly known as "horsetail", has long been used in European and Chinese traditional medicines to treat different disorders, including bone pathologies [11], based on its alleged content in silica and supposedly important organo-silicon compounds [12].
Hydroalcoholic extracts of EA stems demonstrated antioxidant [13, 14], antinociceptive and anti-inflammatory properties [15, 16]. Other activities such as sedative, anticonvulsant [17], antiproliferative [18], antibacterial [18, 19], anticancer [20], and antidiabetic [21] have also been demonstrated for aerial parts of EA.
It has been shown that TLC fingerprinting allows distinguishing EA from other Equisetum species, including ED [7]. Therefore, in this study, we aim to further develop fingerprinting techniques, using both HPTLC and HPLC. The sequential n-hexane, ethyl acetate, and methanol extracts of aerial parts of ED will be subjected to HPTLC with different mobile phases and detection reagents to separate and visualize the bioactive compounds flavonoids, polyphenols, and terpenoids; in addition, the major flavonoid, isoquercitroside, will be quantified by High Performance Liquid Chromatography (HPLC).
In a preliminary assessment of Equisetum diffusum aerial parts safety, the cytotoxicity of the 3 ED extracts will be investigated on the FHs 74 Int human intestinal epithelial cell line, a non-cancerous, non-transformed normal cell line.
2. Material and Methods
2.1 Plant material
Aerial parts of ED were collected in October 2018 from Sa Pa (22°21'05.8"N 103°48'56.5"E), Lao Cai province, Northern Vietnam. A voucher specimen was deposited in the Museum of Biology, Faculty of Biology, VNU-University of Science, Hanoi, Vietnam, where it was identified by Dr Kim-Thanh Thi NGUYEN.
2.2 Chemicals
n-Hexane, ethyl acetate (EtOAc), methanol (MeOH), acetonitrile, and formic acid were purchased from VWR (Radnor, PA, USA); the Hybri-Care® from ATCC (Rockville, MD, USA); FBS (fetal bovine serum), EGF (human recombinant growth factor) and PBS from Gibco (Thermo Scientific, Waltham, MA, USA); and accutase from Corning (Mediatech, Manassas, VA, USA). HPTLC analyses were performed on silica gel 60 F254 HPTLC plates (Merck, Germany). Sodium bicarbonate, p-anisaldehyde, 2-aminoethyl diphenylborinate, polyethylene glycol 400 (macrogol), bismuth nitrate, potassium iodide, myricetin, rutin, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and o-dianisidine bis(diazotized) zinc double salt (fast blue salt B) were purchased from Sigma (St. Louis, MO, USA); isoquercitroside, quercetin, caffeine, noscapine, vanillin, and ferulic acid from the European Directorate for the Quality of Medicines (EDQM, Strasbourg, France); sinapic acid, oleanoic acid, maslinic acid, betulinic acid, geniposide from Extrasynthese (Genay, France); Folin-Ciocalteu reagent from Merck (Merck, Germany).
The p-anisaldehyde-sulfuric acid reagent was prepared by mixing in the following order, 0.5 mL of p-anisaldehyde, 10 mL of glacial acetic acid, 85 mL of methanol and 5 mL sulfuric acid. The Dragendorff’s reagent was prepared by mixing 5 mL Solution A (0.17 g of bismuth nitrate in 10 mL of a 20 % acetic acid aqueous solution), 5 mL Solution B (4 g potassium iodide in 10 mL of water), 20 mL acetic acid and 70 mL water. The FHs 74 Int cells (ATCC: CCL-241) were obtained from ATCC (Rockville, MD, USA) and cultured in Hybri-Care® medium, supplemented with 10 % FBS, 1.5 g/L sodium bicarbonate, and 0.0025 % EGF, at 37°C under 5 % CO2.
2.3 Plant extraction
The aerial parts of ED were collected, cleaned, and washed with water and then with distilled water, dried in a 40°C oven and ground to powder (size smaller than 0.5 mm). The extraction protocol follows a previously described procedure [22] with some modifications. Dried powder of ED (50 g) was grinded with sand and n-hexane, sonicated for 15 min and extracted successively (for each solvent: 500 mL, 3 times, 20 h, agitation 360 rpm at room temperature (RT) with n-hexane, ethyl acetate, and methanol.
The extracts were filtered and the solvents were evaporated under reduced pressure in a Rotavapor to obtain the respective crude extracts (n-hexane, ethyl acetate and methanol extract). These extracts were stored at -20°C until use. After sequential extraction, the yields of the n-hexane, ethyl acetate, and methanol extracts were 1.96, 1.78 and 3.35 expressed in % of dry weight, respectively.
2.4 Qualitative and quantitative phytochemical analysis
Secondary metabolites of plants have been classified into major chemical groups such as alkaloids, flavonoids, terpenoids, polyphenols, or steroids. In the first step, a qualitative analysis was performed to identify the presence of these groups in the extracts of ED, following standard methods [23]. Polyphenols were researched from their coloration with FeCl3; flavonoids from their coloration change according to pH; terpenoids and steroids from their reaction with CHCl3 and concentrated sulfuric acid; alkaloids from their precipitation by the Dragen-dorff’s reagent.
The total polyphenol content was estimated by mixing 1 mL of a 1 mg/mL solution of extract in methanol with 2.5 mL of Folin-Ciocalteu reagent and 2.5 mL of 2 % Na2CO3, heating at 45oC for 45 min and measuring the absorbance at 765 nm; a blank was prepared using 1 mL of MeOH instead of the extract. The total polyphenol content was expressed as Gallic Acid Equivalent (GAE, mg/g dry weight) using the following equation based on the calibration curve: y = 0.0039x - 0.0197 (R² = 0.9911) where y was the absorbance and x the gallic acid concentration (µg/mL). The total flavonoid content was estimated by mixing 1 mL of a 1 mg/mL solution of extract in methanol with 1 mL of 2 % AlCl3, incubating 60 min at room temperature and measuring the absorbance at 415 nm; a blank was prepared using 1 mL of MeOH instead of the extract. The total flavonoid content of the extract was expressed as Quercetin Equivalent (QE, mg/g dry weight) using the following equation based on the calibration curve: y = 0.012x + 0.4965 (R² = 0.9541) where y was the absorbance and x the quercetin concentration (µg/mL).
2.5 MTT assay
The MTT assay was performed following a previously described protocol [24] with some modifications. Firstly, cells were seeded into 96-well plates at a density of 4000 cells/well. After 24 h of incubation, the extracts were added. After 48 h, 200 µL of a 0.5 mg/mL MTT solution were added into each well and incubated for 4 h at 37oC, 5 % CO2.
The MTT solution was then carefully eliminated, 100 µL of DMSO were added to dissolve the formed formazan and, the plate was measured at 570 nm and 690 nm. The cell viability (%) was calculated using the following equation:
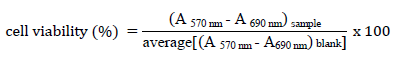
The experiment was performed in triplicate and expressed as mean +/- standard deviation; IC50, the concentration of drug or extract required for 50 % cell viability reduction, was determined by fitting a curve to experimental points, using the GraphPad 5.0 software. The fitted curves “% of cell viability” versus “log (catalyst concentration)”.
2.6 High-performance thin-layer chromatography (HPTLC) profiling
HPTLC was performed according to the procedure of the European Pharmacopeia 10 [25], using Camag Automatic TLC Sampler (ATS 4), Automatic Developing Chamber 2 (ADC 2), Derivatizer and TLC Visualizer 2. The Camag systems were driven by the software visionCATS version 2.5.
The HPTLC was performed on silica gel 60 F254 HPTLC plates (Merck, Germany); 10 µL of samples were applied in 8-mm wide bands, the plates were activated on MgCl2 (~ 33 % RH) and the tank saturated for 20 min; the solvent systems and derivatizing solutions [23] are described in Table 1. All images of the plates were recorded under white light, UV254 and UV365 illumination.
|
Compound classes |
Mobile phase |
Spray reagent |
Heat |
Visible light |
UV (365 nm) |
|
Alkaloids |
Mb1 = chloroform-methanol (30 : 13, v/v) |
Dragendorff’s reagent |
RT for 10 min |
Yellow |
Dark blue, dark violet |
|
Flavonoids |
Mb2 = formic acid - water - methyl ethyl ketone - ethyl acetate (1 : 1 : 3 : 5, v/v/v/v) |
NP(a) and PEG(b) |
100°C for 3 min |
Yellow, red |
Yellow, green |
|
Polyphenols |
Mb3 = chloroform - ethyl acetate - methanol – water (15 : 50 : 22 : 10, v/v/v/v) |
Fast blue salt B(c) |
RT for max 10min |
Purple, orange, brown |
Dark violet |
|
Terpenoids |
Mb4 = toluene - ethyl acetate - methanol - formic acid - acetic acid (100 : 15 : 10 : 2 : 1, v/v/v/v/v) Mb5 = chloroform - ethyl acetate - methanol - water (15 : 50 : 22 : 10, v/v/v/v) |
10 % sulfuric acid (v/v) in methanol |
100°C for 3 min |
Violet, purple |
Violet, purple |
|
Antioxidant |
Mb2 and Mb4 |
DPPH(d) reagent |
No |
White band on a purple background |
No |
(a) NP reagent: 1 g of 2-aminoethyl diphenylborinate in 100 mL of methanol
(b) PEG reagent: 5 g of polyethylene glycol 400 (macrogol) in 100 mL of ethanol (96 % v/v).
(c) Fast blue salt B reagent: 0.5 g of fast blue salt B in 100 mL of water
(d) DPPH reagent: 0.5 g of diphenyl-1-picrylhydrazyl (DPPH) in 100 mL of methanol (100 % v/v)
Table 1: Secondary metabolites classes, mobile phases, spray reagents used for HPTLC profiling of Equisetum diffusum.
2.7 High-performance liquid chromatography (HPLC)
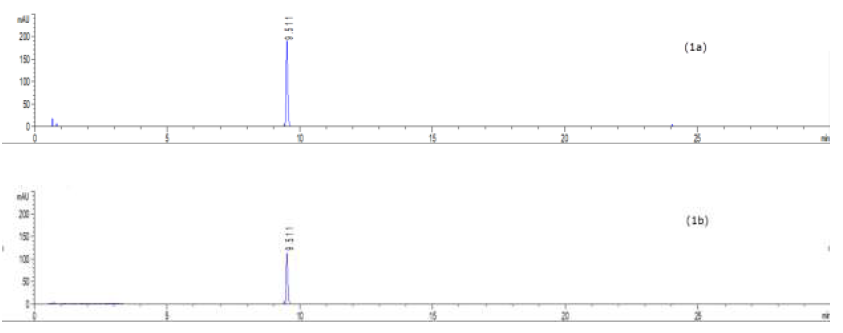
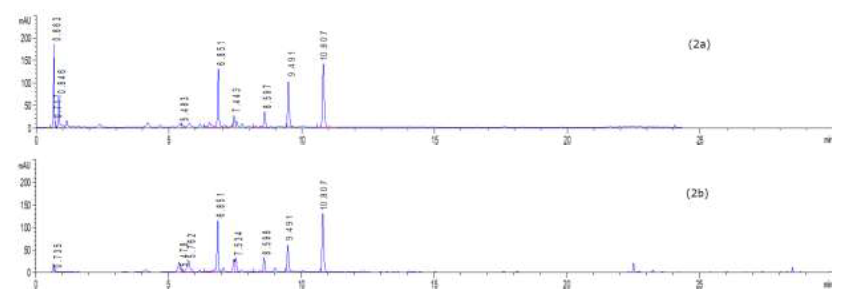
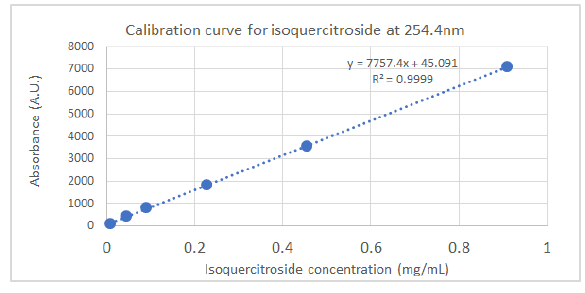
HPLC analyses were carried out on an HPLC Agilent (model 1260 Infinity II, Agilent Technologies, Santa Clara, CA, USA) system equipped with a diode array detector, using a C18 column Infinity Lab Poroshell 100 × 4.6 mm I.D., 4 µm (Agilent Technologies, Santa Clara, CA, USA). The injection volume was 5 µL and the detection wavelengths were set at 254.4 nm and 326.4 nm. The separation of compounds was achieved at a flow rate of 1.5 mL/min using 1.0 % v/v formic acid in water (solvent A) and acetonitrile (solvent B) as mobile phases with an elution gradient as follows: 0–2 min, 5 % B; 2–6 min, 5–15 % B; 6–20 min, 15-36 % B; 20–27 min, 35–95 % B; 27–29 min, 95–100 % B; 29–35 min, 100 % B. The isoquercitroside was determined from the calibration curve: y = 7757.4x + 45.091 (R² = 0.9999), where y was the area of isoquercitroside peak and x the isoquercitroside concentration (mg/mL).
3. Results
3.1 Qualitative analysis
Table 2 shows the results of the phytochemical screening detecting the presence of secondary metabolites classes. Most of tested metabolite classes were present in the methanol extract whereas the ethyl acetate extract comprised mainly polyphenols and flavonoids; no characteristic compound class could be identified in the n-hexane extract.
|
Bioactive compounds classes |
n-Hexane extract |
Ethyl-acetate extract |
Methanol extract |
|
Alkaloids |
- |
- |
- |
|
Flavonoids |
- |
+/- |
+++ |
|
Polyphenols/tannins |
- |
+/- |
+++ |
|
Terpenoids |
- |
- |
+ |
|
Steroids |
- |
- |
+ |
Table 2: Qualitative analysis of the extracts of ED.
3.2 Quantitative analysis
Table 3 present the total phenolics and flavonoids content of ED methanolic extract. Humidity of plant’s powder is 4.04 %. There were no measurable polyphenols and flavonoids in the n-hexane and ethyl acetate extracts, despite a faint colorimetric detection in the ethyl acetate extract (Table 2).
|
Extraction yield (%) |
Total phenolics content (GAE mg/g DW plant) |
Total phenolics content (GAE mg/g DW extract) |
Flavonoids content (QE mg/g DW plant) |
Flavonoids content (QE mg/g DW extract) |
|
3.49 |
1.09 ± 0.08 |
32.7 ± 2.3 |
1.58 ± 0.12 |
47.2 ± 3.6 |
Table 3: Total phenolic and flavonoid content of ED methanolic extract.
3.3 HPTLC profiling of antioxidant compounds
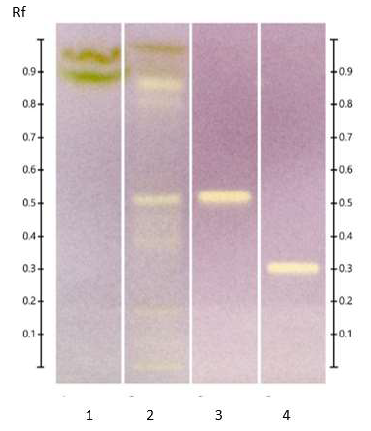
The yellowish bands on a blue DPPH background (Figures 2 and 3) indicate the presence of antioxidants in the methanol and n-hexane extracts of ED aerial parts.
Figure 2: HPTLC antioxidant profile of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: formic acid - water - methyl ethyl ketone - ethyl acetate (1:1:3:5 v/v/v/v); the plate was sprayed with DPPH and visualized under visible light. Tracks: EtOAc extract (1); MeOH extract (2); isoquercitroside (3); and rutin (4).
3.4 HPTLC profiling
3.4.1 Flavonoids: Flavonoids are an important class of natural products widely distributed in the plant kingdom that correspond to a class of low-molecular-weight phenolic compounds. They have favorable biochemical and antioxidant effects associated with a protective effect against several diseases such as cancer, Alzheimer's disease, atherosclerosis, etc. They constitute one of the most characteristic classes of compounds in higher plants [26].
3.4.2 Terpenoids: Terpenoids are the largest and most diverse group of secondary metabolites from natural sources. Many terpenoids are biologically active and are used worldwide for the treatment of diseases such as cancer, malaria, etc.
An interesting example of this group are taxol and its derivatives [27].
Figure 3: HPTLC antioxidant profile of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: toluene - ethyl acetate - methanol - formic acid - acetic acid) (100:15:10:2:1 v/v/v/v/v); the plate was sprayed with DPPH and visualized under visible light. Tracks: n-hexane extract (1); EtOAc extract (2); MeOH extract (3); and rutin (4).
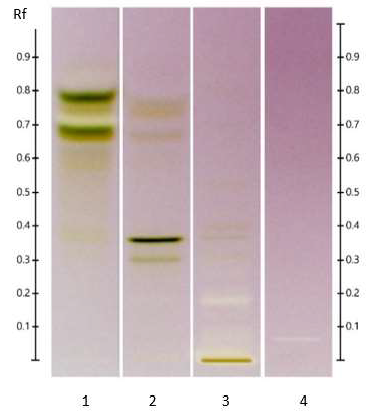
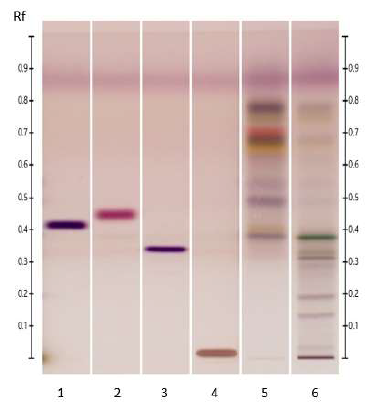
Figure 4: HPTLC profiles of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: formic acid - water - methyl ethyl ketone - ethyl acetate (1:1:3:5 v/v/v/v); the plate was heated at 100°C for 3 min, sprayed with NP and PEG reagents and visualized under UV365 (tracks 1, 3, 4, 5) and visible (tracks 2, 6, 7, 8) light. Tracks: methanolic extract (1 and 2); isoquercitroside (3 and 6); myricetin (4 and 7) and rutin (5 and 8).
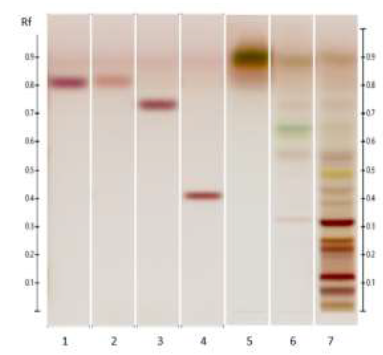
Figure 5: HPTLC profile of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: toluene – ethyl acetate – methanol – formic acid – glacial acetic acid (10:1.5:1:0.2:0.1 v/v/v/v/v), the plate was heated at 100°C for 3 min, sprayed with 10 % acid sulfuric in methanol and visualized under visible light. Tracks: oleanoic acid (1); betulinic acid (2); maslinic acid (3); geniposide (4); n-hexane extract (5); ethyl acetate extract (6).
Figure 6: HPTLC profile of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: chloroform – ethyl acetate – methanol – water (15: 50: 22:10 v/v/v/v), the plate was heated at 100°C for 3 min, sprayed with 10 % acid sulfuric in methanol and visualized under visible light. Tracks: oleanoic acid (1); betulinic acid (2); maslinic acid (3); geniposide (4); n-hexane extract (5); ethyl acetate extract (6); methanolic extract (7).
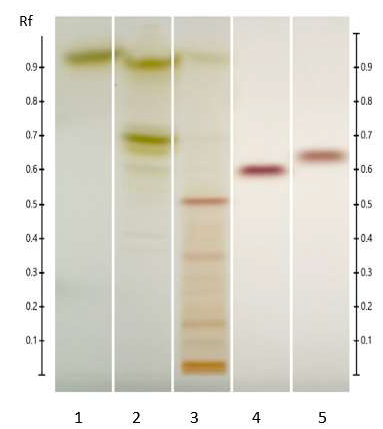
Figure 7: HPTLC profile of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: chloroform – ethyl acetate – methanol – water (15: 50: 22:10 v/v/v/v); the plate was sprayed with fast blue salt B reagent and visualized under visible light. Tracks: n-hexane extract (1); ethyl acetate extract (2); methanolic extract (3); sinapic acid (4); ferulic acid (5).
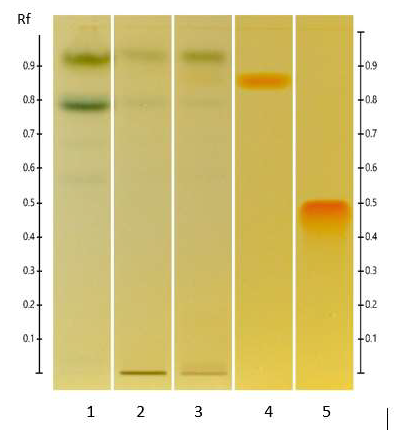
Figure 8: HPTLC profile of ED aerial parts extracts; silica gel 60 F254 plate; mobile phase: chloroform – methanol (30: 13 v/v); the plate was sprayed with Dragendorff’s reagent and visualized under visible light. Tracks: n-hexane extract (1); ethyl acetate extract (2); methanolic extract (3); quinine sulfate (4); theophylline (5).
3.4.3 Phenolic compounds: Phenolic compounds are a heterogeneous group of phytochemicals comprising phenol rings bearing one or more hydroxyl groups and can be divided into several classes. The main groups of phenolic compounds include flavonoids, phenolic acids, tannins, stilbenes, and lignans. Therefore, they are the most abundant secondary metabolites in plants [28].
3.4.4 Alkaloids: Consistently with the results obtained in the phytochemical screening, no yellow or orange bands were detected in the different ED extracts upon spraying the plate with Dragendorff’s reagent (Figure 8) confirming the absence of alkaloids in all three extracts.
3.5 High-performance liquid chromatography (HPLC)
To profile flavonoids and evaluate the concentration of isoquercitroside in ED aerial parts, the methanolic extract was analyzed by HPLC (Figures 9-10). From an isoquercitroside standard curve, its concentration was calculated in the methanol extract (1.60 ± 0.04 mg/g dry weight plant, i.e. 47.9 ± 1.2 mg Isoque-rcitroside/g dry extract weight).
3.6 Cytotoxicity
The effect of ED extracts on FHs 74 Int cells viability was evaluated with the MTT assay over 48 h contact. The EtOAc extract appears to be the most toxic with an IC50 of 8.66 ± 2.31µg/mL, followed by the n-hexane and methanol extracts, with IC50 of 46.13 ± 3.63 µg/mL and 124.73 ± 22.97 µg/mL, respectively.
4. Discussion
Some 33,400 plants are used for traditional medicine around the world [29]. The first and major step when using a herb resides in the authentification of the material. In a recent research, Lagoudakis et al. present a method to identify common horsetail by using DNA barcoding, focusing on the distinction between Equisetum arvense and other Equisetum species. According to these authors, the genus Equisetum is resolved in 2 major clades, each comprising seven species and corresponding (i) to the two subgenera Equisetum and Hippochaete; and (ii) in a sister species, Equisetum bogotense Kunth. Except for E. diffusum D. Don and E. sylvaticum, all species were recovered as monophyletic, including E. arvense and E. palustre [7].
TLC chemical profiles (according to Identification C of the European Pharmacopoeia monograph for E. arvense) positively identify E. arvense (all marker bands were detected) and discriminate it from all tested species; the ED chromatogram shows additional bands in both high and low Rf zones [7]. In our study, the HPTLC fingerprint of ED methanol extract (system Mb2; Figure 4) is consistent with this previously described profile.
Our phytochemical screening of ED revealed the presence of a series of secondary metabolites. Our analyses indicate that the methanol extract (3.35 %) is the extract containing the most phytochemical compounds, with a noticeable content in total phenolic compounds (1.09 ± 0.08 mg GAE/g dry weight), flavonoids (1.58 ± 0.12 mg QE/g dry weight) and isoquercitroside (1.60 ± 0.04 mg/g dry weight).
A previous screening by Subba et al. indicates the presence, in ED aerial parts methanol extract, of alkaloids, flavonoids, tannins, phenols, steroids, phlorotannins, saponins, glucosides, and carbohydrates and the absence of terpenoids, anthraquinones, and proteins [30]; Still in the study of Subba et al, the methanol extract of ED has investigated the total tannins (37.80 ± 0.05 mg GAE/g dry extract wt), phenols (17.40 ± 0.06 mg GAE/g dry extract wt), flavonoids (~ 17 mg rutin equivalent/g dry extract wt) and flavonols contents (~ 47 mg rutin equivalents/g) [30].
The stems of E. arvense are also described to contain minerals, potassium and calcium, various flavonoids, phenolic, triterpenoids, phytosterols, and low amounts of essential oil [19]. In a study by Grundemann et al. [31], the amount of isoquercitroside was 43.1 ± 0.7 µg/mL methanol extract. Radulovic et al. (2006) identified 25 volatile compounds in EA essential oil, accounting for 54.23 % of peak areas [19]. A further study identified 52 constituents in the ED essential oil; the major compounds were phytol (35.63 %), hexacosane (8.04 %), cadin-4-ene-7-ol (cis) (5.32 %), n-decane (3.31 %), heptacosane (2.92 %), phytone (2.95 %), 4-heptanone-2-methyl (2.24 %) and n-nonane (2.24 %), accounting for 55.27 % of peaks areas [32].
The extracts of EA aerial parts have been shown to present anticancer, antioxidant, anti-inflammatory and antibacterial activity [13-16], [18]. Carsten et al. have shown that EA extract inhibits T cells in a dose-depending manner without the induction of apoptosis or necrosis [31]. In a study by Dukic et al., the EA antioxidant activity and phenolic compounds were determined in 3 extracts (n-BuOH, EtOAc, and water); the highest and lowest antioxidant activities were expressed by the EtOAc and the water extract, respectively. Whereas the major flavonoid of both the EA EtOAc and n-BuOH extracts was also isoquercitroside, a high proportion of phenolic acids and a low percentage of flavonoids were detected in the water extract [14]. This major flavonoid of EA and ED, isoquercitroside, presents properties of cardio- angio- and neuro-protection, is anti-inflammatory and was shown to prevent diabetic complications [31, 33].
Our cytotoxicity study of ED extracts on small intestine cells (FHs 74 Int cell line) indicates that the ethyl acetate extract presents the highest toxicity, followed by the n-hexane extract and finally the methanol extract. From IC50 of extracts, the n-hexane and EtOAc extracts were classified as moderately cytotoxic (2 < IC50 < 90 µg/mL) whereas the MeOH extract is not cytotoxic (IC50 > 90 µg/mL) [34, 35].
5. Conclusions
Medicinal herbs have been used for a very long time but are still often identified according to the morphology of whole parts of a plant. HPTLC fingerprinting has the advantages of a low-cost method that gives a quasi-immediate image of the characteristic phytochemicals in a plant and, therefore, is more and more applied. From the present study, chromatographic profiles (HPTLC and HPLC) of ED using different chromatographic conditions allow clear profiles for identification of the plant, also conforming to a previous method shown to be species-specific. Except for alkaloids, several groups of secondary metabolites were found, especially in the methanol extract of ED, isoquercitroside being the major flavonoid (1.60 ± 0.04 mg/g). In a preliminary assessment of Equisetum diffusum aerial parts safety, the cytotoxicity of these 3 ED extracts was investigated on the FHs 74 Int human intestinal epithelial cell line, a non-cancerous, non-transformed normal cell line. The ethyl acetate extract was shown to be the most toxic with an IC50 of 8.7 ± 2.3 µg/mL, followed by the n-hexane and methanol extracts, with an IC50 of 46.1 ± 3.6 µg/mL and 124.7 ± 23.0 µg/mL, respectively.
Acknowledgements
This research was funded by ARES (Académie de Recherche et d’Enseignement Supérieur) and the Ministry of Cooperation DGD (Direction Générale de la Coopération au Développement) in Belgium and by VNU University of Science, Vietnam National University, Hanoi under project number TN.20.08.
References
- Palit P. Bioactivity-Guided Phytofractions in Natural Products and Drug Discovery, Elsevier (2018): 57-71.
- Global Medicinal Herbs Market Insights Report 2021 Worldwide Market Trends and Opportunities to 2028 (2021).
- Shaw D, Graeme L, Pierre D, er al. Pharmacovigilance of herbal medicine. Journal of Ethno pharmacology 140 (2012): 513-518.
- Simpson MG. Evolution and Diversity of Vascular Plants in Plant Systematics, Elsevier (2010): 73-128.
- Kress WJ, Wurdack KJ, Zimmer EA, et al. Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences 102 (2005): 8369-8374.
- Hollingsworth PM, Graham SW, Little DP. Choosing and Using a Plant DNA Barcode. PLoS ONE 6 (2011): e19254.
- Saslis-Lagoudakis CH. et al. Identification of common horsetail (Equisetum arvense; Equisetaceae) using Thin Layer Chromatography versus DNA barcoding. Sci Rep 5 (2015): 11942.
- Reich E, Schibli A, Eds., High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants. Stuttgart: Georg Thieme Verlag (2007): b-002-66241.
- Royal Botanic Gardens, Kew: Medicinal Plant Names Services (2021).
- Loi Tat Do, Nhung cây thuoc và vi thuoc Viet Nam (Vietnamese medicinal plants and herbs), 8th ed. Medical Publishing House (2004).
- Costa-Rodrigues J, Carmo SC, Silva JC, et al. Inhibition of human in vitro osteoclastogenesis by Equisetum arvense. Cell Prolif 45 (2012): 566-576.
- Peggs A, Bowen H. Inability to detect organo-silicon compounds in Equisetum and Thuja. Phytochemistry 23 (1984): 1788-1789.
- Štajner D, Popovic BM, Canadanovic-Brunet J, et al. Exploring Equisetum arvense, Equisetum ramosissimum L., Equisetum telmateia L., as sources of natural antioxidants. Phytother. Res 23 (2009): 546-550.
- Mimica-Dukic N, Simin N, Cvejic J, et al. Phenolic Compounds in Field Horsetail (Equisetum arvense) as Natural Antioxidants. Molecules 13 (2008): 1455-1464.
- Do Monte FHM, dos Santos JG, Russi M, et al. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense in mice. Pharmacological Research 49 (2004): 239-243.
- Pallag A, et al. Equisetum arvense Extract Induces Antibacterial Activity and Modu-lates Oxidative Stress, Inflammation, and Apoptosis in Endothelial Vascular Cells Exposed to Hyperosmotic Stress. Oxidative Medicine and Cellular Longevity (2018): 1-14.
- Dos Santos JG, et al. Sedative and anticon-vulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia 76 (2005): 508-513.
- Bessa Pereira C, et al. Equisetum arvense hydromethanolic extracts in bone tissue regeneration: in vitro osteoblastic modulation and antibacterial activity. Cell Prolif 45 (2012): 86-396.
- Radulovic N, Stojanovic G, Palic R. Composition and antimicrobial activity of Equisetum arvense essential oil. Phytother. Res 20 (2006): 85-88.
- Mohammed HIA, Paray BA, Rather IA. Anticancer activity of EA1 extracted from Equisetum arvense. Pak. J. Pharm. Sci (2017): 4.
- Neijati Vahid SS, Fathiazar Baijani Fathllah NV, Nazemiyeh Hossine. Antidiabetic Effect of Equisetum arvense (Equisetaceae) in Streptozotocin-induced Diabetes in Male Rats. Pakistan Journal of Biological Sciences 10 (2007): 1661-1666,
- Giri DP, Rajbhandari M. Phytochemical Analysis and Constituents of Hexane Extract of Melastoma Malabathricum J. Inst. Sci. Tech 23 (2018): 18-25.
- Hadem KH, Sharan R, Kma L. Phyto-chemicals of Aristolochia tagala and Curcuma caesia exert anticancer effect by tumor necrosis factor-α-mediated decrease in nuclear factor kappaB binding activity. J Basic Clin Pharma 7 (2016): 1.
- Riss TL, Moravec RA, Niles AL, et al. Cell Viability Assays 25.
- High-performance thin-layer chromate-graphy of herbal drugs and herbal drug preparations (2.8.25) in European Pharma-copoeia, Council of Europe, Strasbourg, 10th ed., European Pharma-copoeia (Ph. Eur.) (2019).
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 5 (2016): e47.
- Isah MB, Tajuddeen N, Umar MI, et al. Terpenoids as Emerging Therapeutic Agents: Cellular Targets and Mechanisms of Action against Protozoan Parasites in Studies in Natural Products Chemistry, Elsevier 59 (2018): 227-250.
- Ndolo V, Beta T. Chapter 12. Types and Distribution of Phenolic Compounds in Grains in Food Chemistry, Function and Analysis, Beta T, Camire ME, Eds. Cambridge: Royal Society of Chemistry (2018): 235-277.
- Medicinal Plant Names Services | Kew (2021).
- Subba A, Rai S. Phytochemical screening, physico-chemical analysis and antioxidant activity of some ethnomedicinal plants from Sikkim Himalaya. Indian Journal of Natural Products and Resources 9 (2018): 235-243.
- Gründemann C, Lengen K, Sauer B, et al. Equisetum arvense (common horsetail) modulates the function of inflammatory immunocompetent cells. BMC Complement Altern Med 14 (2014): 283.
- Poonam Takuli, Kapil Khulbe PK, Charu Pant. Chemical Composition of Essential Oil of Equisetum Diffusum: A Noble Source of Phytol. IJPSR 11 (2020): 5572-5578.
- Jeremy Appleton, ND. Evaluating the Bio-availability of Isoquercetin. Natural Medi-cine Journal.
- Cytotoxicity: in vitro determination (2021).
- Kudera T, Doskocil I, Salmonova H, et al. In Vitro Selective Growth-Inhibitory Activities of Phytochemicals, Synthetic Phytochemical Analogs, and Antibiotics against Diarrhe-agenic/Probiotic Bacteria and Can-cer/Nor-mal Intestinal Cells. Pharmaceuticals 13 (2020): 233.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 