Histopathological Effect of Varying Dose of Acetylsalicylic Acid (Aspirin) on Liver of Adult Wistar Rats
Article Information
Idehen I Charles1*, Bankole J Kayode1, Airhomwanbor Kingsley1, Dic-Ijiewere Ebenezer2, Okparaku Sunday1, Ehimara Raphael2, OsaroboEseiwi3, Aigbiremolen Emmanuel1
1Department of Medical Laboratory Science, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria.
2Department of Chemical Pathology, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria.
3Department of Heamatology, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria.
*Corresponding Author: Idehen I Charles, Basic Medical Sciences, Department of Medical Laboratory Science, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria
Received: 28 November 2018; Accepted: 10 December 2018; Published: 20 December 2018
Citation:
Idehen I Charles, Bankole J Kayode, Airhomwanbor Kingsley, Dic-Ijiewere Ebenezer, Okparaku Sunday, Ehimara Raphael, Osarobo Eseiwi, Aigbiremolen Emmanuel. Histopathological Effect of Varying Dose of Acetylsalicylic Acid (Aspirin) on Liver of Adult Wistar Rats. Journal of Biotechnology and Biomedicine 1 (2018): 028-033.
View / Download Pdf Share at FacebookAbstract
Aspirin is one of the widely-used, cheap and over-the-counter available non-steroidal anti-inflammatory drugs. It has anti-platelet, analgesic and anti-pyretic effects which can result in its indiscriminate ingestion. The liver is the organ of drug metabolism, bio-regulation and immuno-modulation. Thus, this study investigates the effects of varying aspirin doses on the gross and cellular architecture of the liver in Adult Wistar rat model. Thirty rats of comparable weights were divided into 5 groups; consisting of a control group and 4 tests. Group A served as control and was not treated while groups B, C, D and E served as the tests and were treated daily with 35, 70, 105 and 140 mg/kg body weight of aspirin respectively for 30 days. At the end of the experiment, the rats were anesthetized, and the liver dissected out for gross and histological studies. There was a slight dose-dependent increase in body weight but no noticeable gross liver change. However, there were dose dependent histo-pathological changes including sinusoidal congestion and increased cell basophilia from the 70 mg/kg to 140 mg/kg dose. Conclusively, aspirins at doses 70 mg/kg body weight and higher are associated with hepatic pathologies and care should be taken when these doses are administered for long durations.
Keywords
Aspirin, Anti-inflammatory drugs, Liver, Histology, Hepatotoxic
Aspirin articles Aspirin Research articles Aspirin review articles Aspirin PubMed articles Aspirin PubMed Central articles Aspirin 2023 articles Aspirin 2024 articles Aspirin Scopus articles Aspirin impact factor journals Aspirin Scopus journals Aspirin PubMed journals Aspirin medical journals Aspirin free journals Aspirin best journals Aspirin top journals Aspirin free medical journals Aspirin famous journals Aspirin Google Scholar indexed journals Anti-inflammatory drugs articles Anti-inflammatory drugs Research articles Anti-inflammatory drugs review articles Anti-inflammatory drugs PubMed articles Anti-inflammatory drugs PubMed Central articles Anti-inflammatory drugs 2023 articles Anti-inflammatory drugs 2024 articles Anti-inflammatory drugs Scopus articles Anti-inflammatory drugs impact factor journals Anti-inflammatory drugs Scopus journals Anti-inflammatory drugs PubMed journals Anti-inflammatory drugs medical journals Anti-inflammatory drugs free journals Anti-inflammatory drugs best journals Anti-inflammatory drugs top journals Anti-inflammatory drugs free medical journals Anti-inflammatory drugs famous journals Anti-inflammatory drugs Google Scholar indexed journals Liver articles Liver Research articles Liver review articles Liver PubMed articles Liver PubMed Central articles Liver 2023 articles Liver 2024 articles Liver Scopus articles Liver impact factor journals Liver Scopus journals Liver PubMed journals Liver medical journals Liver free journals Liver best journals Liver top journals Liver free medical journals Liver famous journals Liver Google Scholar indexed journals Histology articles Histology Research articles Histology review articles Histology PubMed articles Histology PubMed Central articles Histology 2023 articles Histology 2024 articles Histology Scopus articles Histology impact factor journals Histology Scopus journals Histology PubMed journals Histology medical journals Histology free journals Histology best journals Histology top journals Histology free medical journals Histology famous journals Histology Google Scholar indexed journals Hepatotoxic articles Hepatotoxic Research articles Hepatotoxic review articles Hepatotoxic PubMed articles Hepatotoxic PubMed Central articles Hepatotoxic 2023 articles Hepatotoxic 2024 articles Hepatotoxic Scopus articles Hepatotoxic impact factor journals Hepatotoxic Scopus journals Hepatotoxic PubMed journals Hepatotoxic medical journals Hepatotoxic free journals Hepatotoxic best journals Hepatotoxic top journals Hepatotoxic free medical journals Hepatotoxic famous journals Hepatotoxic Google Scholar indexed journals albino articles albino Research articles albino review articles albino PubMed articles albino PubMed Central articles albino 2023 articles albino 2024 articles albino Scopus articles albino impact factor journals albino Scopus journals albino PubMed journals albino medical journals albino free journals albino best journals albino top journals albino free medical journals albino famous journals albino Google Scholar indexed journals hypertrophied articles hypertrophied Research articles hypertrophied review articles hypertrophied PubMed articles hypertrophied PubMed Central articles hypertrophied 2023 articles hypertrophied 2024 articles hypertrophied Scopus articles hypertrophied impact factor journals hypertrophied Scopus journals hypertrophied PubMed journals hypertrophied medical journals hypertrophied free journals hypertrophied best journals hypertrophied top journals hypertrophied free medical journals hypertrophied famous journals hypertrophied Google Scholar indexed journals Drug articles Drug Research articles Drug review articles Drug PubMed articles Drug PubMed Central articles Drug 2023 articles Drug 2024 articles Drug Scopus articles Drug impact factor journals Drug Scopus journals Drug PubMed journals Drug medical journals Drug free journals Drug best journals Drug top journals Drug free medical journals Drug famous journals Drug Google Scholar indexed journals liver articles liver Research articles liver review articles liver PubMed articles liver PubMed Central articles liver 2023 articles liver 2024 articles liver Scopus articles liver impact factor journals liver Scopus journals liver PubMed journals liver medical journals liver free journals liver best journals liver top journals liver free medical journals liver famous journals liver Google Scholar indexed journals immuno-modulation articles immuno-modulation Research articles immuno-modulation review articles immuno-modulation PubMed articles immuno-modulation PubMed Central articles immuno-modulation 2023 articles immuno-modulation 2024 articles immuno-modulation Scopus articles immuno-modulation impact factor journals immuno-modulation Scopus journals immuno-modulation PubMed journals immuno-modulation medical journals immuno-modulation free journals immuno-modulation best journals immuno-modulation top journals immuno-modulation free medical journals immuno-modulation famous journals immuno-modulation Google Scholar indexed journals
Article Details
1. Introduction
Aspirin, an acetylated salicylate (acetylsalicylic acid- ASA), is classified among the non-steroidal anti-inflammatory drugs (NSAIDs) [1]. It is one of the well-known, cheap, easily available and widely used Non-Steroidal Anti Inflammatory Drug (NSAID). It is used in versatile purposes which include anti-inflammatory (in joint diseases), anti-platelets (in cardiovascular disease), analgesic and antipyretic [2]. Aspirin is a safe drug at low doses but also has life-threatening side effects when administered at high doses. It has also been reported to cause adverse effects in pregnancy [3]. In-vitro and in-vivo studies showed that aspirin at high doses caused necrosis of the blood vessel tissues [4]. Long-term therapeutic use of aspirin has been associated with nephrotoxicity, hepatotoxicity, gastrointestinal ulcerations, and renal cell cancer and adverse effects to multiple organ systems [5, 6]. Administration over a period of time has been documented to cause significant histopathological changes in the liver [7] that is confirmed to the cellular level [8].
ASA is metabolized via conjugation in the liver to form salicyic acid and several other metabolites which may have justified its hepatotoxic potentials. ASA disperse throughout the body after ingestion, with the highest concentrations found in the blood plasma, liver, renal cortex, heart and lungs [9]. These claims that ASA is toxic to the liver were documented over 30 years ago and thus necessary to validate these claims and possible outcomes of varying doses. It is therefore the aim of this study to investigate the effect of varying doses of aspirin on the liver histological architecture using albino Wister rats as model.
2. Materials and Methods
Aspirin (containing 300 mg/tablet acetylsalicylic acid) with trade name Emzor pharmaceutical was purchased from a pharmacy in the study area. This experimental study was conducted in the histological laboratory of Ambrose Alli University, Ekpoma- Nigeria. The rats were procured from the Animal Farm, College of Medicine, Ambrose Alli University, Ekpoma and transferred to the site of experiment where they were allowed two (2) weeks of acclimatization. A total of 30 albino Wistar rats of comparable weights ranging from 165-200 g were used. They were kept in wire mesh cages with tripod that separates the animal from its feces to prevent contamination. During the period of acclimatization, the rats were maintained in accordance with the standard guide for the care and use of Laboratory animals and fed rat chow and clean water ad libitum.
The albino Wistar rats were divided into 5 groups. Group A served as the control and groups B, C, D and E rats were administered daily dose of 35 mg/kg, 70 mg/kg , 105 mg/kg and 140 mg/kg body weight respectively. The aspirin solution was prepared daily by grinding the aspirin tablets and diluting the powder in clean water. Using 1 ml syringe, the corresponding dose was given to each rat based on body weight. The treatment was carried out daily for 30 consecutive days. At the end of 30 days treatment, the rats were sacrificed, and the liver harvested and clean-dried with blotting paper for gross examination. Thereafter, the liver was fixed in 10% Formal saline for histological processing. The classical paraffin sectioning (3-5 µ thick) was cut, stained with Haematoxylin and Eosin staining technique and observed under light microscopy for histopathological changes as previously documented in Idehen et al. [10] and micrographic pictures taken.
3. Result
Figure 1 shows the mean body weight gain of rats after varying dose of aspirin treatment for 30 days.
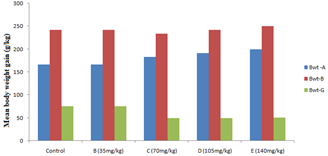
Figure 1: Mean body weight gain after varying dose of aspirin treatment for 30 days.
Key: Bwt-A-weight before experiment; Bwt-B-weight after experiment; Bwt-G-body weight gain; values are mean ± standard deviation.
Except for the group treated with 35 mg/kg aspirin, there were decrease in body weight of the rats in group C, D and E treated with 70 mg/kg, 105 mg/kg and 140 mg/kg aspirin respectively compared to the control. Gross examination of the liver showed no observable alteration (Table 1).
|
Group |
Dosage of aspirin (mg) |
Gross findings |
Microscopic examinations |
||
|
Sinusoidal Congestion |
Increased cell Basophilia |
Vacoulation |
|||
|
A-Control |
- |
Normal |
0/6 |
0/6 |
0/6 |
|
B |
35 |
Normal |
0/6 |
0/6 |
0/6 |
|
C |
70 |
Normal |
0/6 |
1/6 |
2/6 |
|
D |
105 |
Normal |
0/6 |
2/6 |
3/6 |
|
E |
140 |
Normal |
2/6 |
5/6 |
4/6 |
Table 1: Gross and histological observations in the liver of rats treated with varying doses of aspirin.
However, histological observations showed normal histology of liver in group A with no observable alteration in hepatocytes (A), sinusoids (S) and central vein (V). Group B (test group treated with 35 mg/kg of aspirin) also showed no sign of any form of pathology. There were increased cell Basophilia in group C (test group treated with 70 mg of aspirin) and D (test group treated with 105 mg of aspirin) and the liver section of group E (test group treated with 140 mg of aspirin) showed severely increased amount of cell basophilia and moderate sinusoidal congestion (Figure 2).
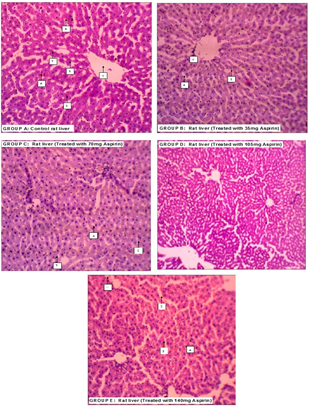
Figure 2: Histological presentations of the livers of rats treated with varying doses of aspirin compared to control (H and E × 100).
4. Discussion
In the present study, aspirin ingestions at a dose greater that 70 mg/kg resulted in decrease body weight gain, no gross alteration in the appearance of the liver but increased cell basophilia and at a dose of 140 mg/kg causes moderate sinusoidal congestion. These findings suggest that aspirin at high dose of 140 mg/kg may be hepatotoxic. In line with these findings, Mitchell et al. [7] has previously documented aspirin to cause significant histopathological changes in the liver and Rau et al. [8] reported aspirin treated rat to present liver with hepatocytes degenerative and pycknotic changes, vacuolization, clear sinusoids dilations and hypertrophied hepatocytes. In the present study, except for group B treated with 35 mg/kg aspirin that showed comparable hepatocytes histology with the control, doses from 70 mg/kg cause changes in liver histology. The non observable changes with 35 mg/kg aspirin support the fact that this dose is well tolerated by the liver. Although there has been no published work on the effect of aspirin on the liver using a dose as low as 35 mg/kg, however, Kawar et al. [11], has reported doses as low as 35 mg of soluble aspirin to cause cardio-vascular disorders especially in the Elderly.
In the present study, liver of rats treated with 70 mg and 105 mg of aspirin showed increased cell basophilia with rats treated with 140 mg/kg presenting moderate sinusoidal congestion and severely increased amount of cell basophilia. The increased cell basophilia could develop into hepatotoxicity; that is, lethal hepatocellular injury and hepatic massive micro-steatosis via mitochondrial dysfunction and lipid peroxidation mechanism resulting in marked fall of intracellular ATP and disrupt free fatty acid accumulation in liver. Sinusoidal congestion in the 140 mg/kg treated rats may be associated with accumulation of blood in the venules of the hepatocytes, which is the result of back pressure within veins. These findings are in line with Sangeetha and Krishnakumari [12]. In the liver of rats treated with aspirin, Sherifa [13] has recorded vacuolar degeneration of tubules and Talat et al. [14], reported vacuolization, clear dilations in the sinusoids and hypertrophied hepatocytes with small and degenerating nuclei, have become fused in later part of this experiment.
5. Conclusion
The administration of aspirin at high dosage for 30 days can cause hepatic problems and changes in the histological architecture of the liver. It can therefore be recommended that aspirin should not be taken at extended durations than normally prescribed by the physician. Also, individuals with liver abnormality should not take aspirin except on recommendations. Policies can also be enacted to make aspirin and other NSAIDS e.g. peroxicam and acetaminopen prescription drugs as they are highly abused due to its relative availability and lack of knowledge of its mechanism of action. More research should be carried out on the effects of aspirin on other glands and organs of the body in order to give a broader view on its mechanism of action so as to help clinicians make an informed decision on any chosen health care plan.
References
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 284 (2000): 1247-1255.
- Adnan S, Janabi Al, Ahmad M, et al. Pharmacological effects of low dose of aspirin on corpus luteum functions in mature cycling female mice. Institute of the Embryo Research and Infertility Treatment 10 (2005): 150-162.
- Collins E, Turner G. Maternal Fetal effects of regular salicylate ingestion during pregnancy. Lancet 2 (1975): 335-339.
- Smith SC, Blair SN, Bonow RO, et al. AHA/ACC Guidelines for Preventing Heart Attack and Death in Patients With Atherosclerotic Cardiovascular Disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol 38 (2001): 1581-1583.
- Gilman EA, Langman MJ, Cheng KK, et al. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ 320 (2000):1642-1646.
- Luigi DL, Laura G, Francesco R, et al. Aspirin, exercise and pituitary hormones. Official Journal of the American College of Sports Medicine 33 (2001): 2029-2035.
- Mitchell C, Garcia Rodriguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 3 (1973): 2373-2383.
- Rau Y, Farzana Y, Ghulam S. To evaluate the role of Aspirin (a NSAID) on renal parenchyma of young albino rats. Pak J Pharm Sci 21 (1989): 98-102.
- Marcia LB. Use of aspirin in children with cardiac disease. Pediatric Pharmacotherapy 13 (2007).
- Idehen IC, Bankole JK, Airhomwanbor K, et al. Spleen Histological Changes Following Monosodium Glutamate Ingestion in Adult Male Wistar Rat. Advances in Biomedical Sciences 2 (2017): 1-5.
- Kawar ME, Reham EM. Salicylate hepato-toxicity in a patient with systemic lupus erythematosus: A case report. JRMS 17 (2010): 43-45.
- Sangeetha B, Krishnakumari S. Tephrosiapurpurea (linn.) a folk medicinal plant ameliorates carbon tetrachloride induced hepatic damage in rats. Inter J Pharm Bio Sci 1 (2010): 1-10.
- Sherifa KA. Hepatic and renal biochemical responses to the toxicological interaction between acetylsalicylic acid and diazinon in albino Rats. J Egypt Soc Toxicol 35 (2006): 1-6.
- Talat Y, Farzana Y, Ghulam SQ. To evaluate the role of diclofenac sodium (a NSAID) on renal parenchyma of young albino rats. Pak J Pharm Sci 21 (2008): 98-102.


 Impact Factor: * 5.3
Impact Factor: * 5.3 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 