TSH and GH Producing Macroadenoma. Escape to Control with Long-Acting Somatostatin Analogues (SSA) After 12 Years Follow-Up. About A Case
Article Information
Jose Atencia Goñi1*, Rogelio García-Centeno1, María Picallo Pérez1, Pascual Elvira Ruiz2, Laura González Fernández1, Olga González Albarrán1
1Division of Endocrinology and Nutrition, Hospital General Universitario Gregorio Marañón, Madrid, Spain
2Department of Radiology, Hospital General Universitario Gregorio Marañón, Madrid, Spain
*Corresponding Authors: Dr. Jose Atencia Goñi, Division of Endocrinology and Nutrition, Hospital General Universitario Gregorio Marañón, Madrid, Spain
Received: 27 November 2020; Accepted: 21 December 2020; Published: 25 January 2021
Citation: TSH and GH Producing Macroadenoma. Escape to Control with Long-Acting Somatostatin Analogues (SSA) After 12 Years Follow-Up. About A Case. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 114-123.
View / Download Pdf Share at FacebookAbstract
Tyroid-stimulatin hormone (TSH) - producing pituitary adenomas known as thyrotropinomas or TSHomas account for a small percentage of pituitary tumors [1]. Approximately 30% of them co-secrete growth hormone (GH), Prolactin, or αGSU of TSH [2]. We introduce a patient with a 12 years follow-up at our center with medical treatment by long-acting somatostatin analogues (SSA) due to rejected surgery. Along the treatment there is a scape with a secondary production of GH without image changes at the MRIs. We suggest a progressively acquired resistance to the medical treatment.
Keywords
Macroadenoma; TSH; GH; Somatostatin analogues; Escape control
Macroadenoma articles; TSH articles; GH articles; Somatostatin analogues articles; Escape control articles
Macroadenoma articles Macroadenoma Research articles Macroadenoma review articles Macroadenoma PubMed articles Macroadenoma PubMed Central articles Macroadenoma 2023 articles Macroadenoma 2024 articles Macroadenoma Scopus articles Macroadenoma impact factor journals Macroadenoma Scopus journals Macroadenoma PubMed journals Macroadenoma medical journals Macroadenoma free journals Macroadenoma best journals Macroadenoma top journals Macroadenoma free medical journals Macroadenoma famous journals Macroadenoma Google Scholar indexed journals TSH articles TSH Research articles TSH review articles TSH PubMed articles TSH PubMed Central articles TSH 2023 articles TSH 2024 articles TSH Scopus articles TSH impact factor journals TSH Scopus journals TSH PubMed journals TSH medical journals TSH free journals TSH best journals TSH top journals TSH free medical journals TSH famous journals TSH Google Scholar indexed journals GH articles GH Research articles GH review articles GH PubMed articles GH PubMed Central articles GH 2023 articles GH 2024 articles GH Scopus articles GH impact factor journals GH Scopus journals GH PubMed journals GH medical journals GH free journals GH best journals GH top journals GH free medical journals GH famous journals GH Google Scholar indexed journals Somatostatin articles Somatostatin Research articles Somatostatin review articles Somatostatin PubMed articles Somatostatin PubMed Central articles Somatostatin 2023 articles Somatostatin 2024 articles Somatostatin Scopus articles Somatostatin impact factor journals Somatostatin Scopus journals Somatostatin PubMed journals Somatostatin medical journals Somatostatin free journals Somatostatin best journals Somatostatin top journals Somatostatin free medical journals Somatostatin famous journals Somatostatin Google Scholar indexed journals analogues articles analogues Research articles analogues review articles analogues PubMed articles analogues PubMed Central articles analogues 2023 articles analogues 2024 articles analogues Scopus articles analogues impact factor journals analogues Scopus journals analogues PubMed journals analogues medical journals analogues free journals analogues best journals analogues top journals analogues free medical journals analogues famous journals analogues Google Scholar indexed journals Escape control articles Escape control Research articles Escape control review articles Escape control PubMed articles Escape control PubMed Central articles Escape control 2023 articles Escape control 2024 articles Escape control Scopus articles Escape control impact factor journals Escape control Scopus journals Escape control PubMed journals Escape control medical journals Escape control free journals Escape control best journals Escape control top journals Escape control free medical journals Escape control famous journals Escape control Google Scholar indexed journals thyrotropinomas articles thyrotropinomas Research articles thyrotropinomas review articles thyrotropinomas PubMed articles thyrotropinomas PubMed Central articles thyrotropinomas 2023 articles thyrotropinomas 2024 articles thyrotropinomas Scopus articles thyrotropinomas impact factor journals thyrotropinomas Scopus journals thyrotropinomas PubMed journals thyrotropinomas medical journals thyrotropinomas free journals thyrotropinomas best journals thyrotropinomas top journals thyrotropinomas free medical journals thyrotropinomas famous journals thyrotropinomas Google Scholar indexed journals TSHomas articles TSHomas Research articles TSHomas review articles TSHomas PubMed articles TSHomas PubMed Central articles TSHomas 2023 articles TSHomas 2024 articles TSHomas Scopus articles TSHomas impact factor journals TSHomas Scopus journals TSHomas PubMed journals TSHomas medical journals TSHomas free journals TSHomas best journals TSHomas top journals TSHomas free medical journals TSHomas famous journals TSHomas Google Scholar indexed journals hyperthyroidism articles hyperthyroidism Research articles hyperthyroidism review articles hyperthyroidism PubMed articles hyperthyroidism PubMed Central articles hyperthyroidism 2023 articles hyperthyroidism 2024 articles hyperthyroidism Scopus articles hyperthyroidism impact factor journals hyperthyroidism Scopus journals hyperthyroidism PubMed journals hyperthyroidism medical journals hyperthyroidism free journals hyperthyroidism best journals hyperthyroidism top journals hyperthyroidism free medical journals hyperthyroidism famous journals hyperthyroidism Google Scholar indexed journals macroadenomas articles macroadenomas Research articles macroadenomas review articles macroadenomas PubMed articles macroadenomas PubMed Central articles macroadenomas 2023 articles macroadenomas 2024 articles macroadenomas Scopus articles macroadenomas impact factor journals macroadenomas Scopus journals macroadenomas PubMed journals macroadenomas medical journals macroadenomas free journals macroadenomas best journals macroadenomas top journals macroadenomas free medical journals macroadenomas famous journals macroadenomas Google Scholar indexed journals
Article Details
1. Introduction
Tyroid-stimulatin hormone (TSH) - producing pituitary adenomas known as thyrotropinomas or TSHomas account for a small percentage of pituitary tumors [1]. Approximately 30% of them co-secrete growth hormone (GH), Prolactin, or aGSU of TSH [2]. According to the Swedish national registry of TSH-producing tumors, its incidence has increased to 2.8 cases per million inhabitants [3]. The diagnosis of these tumors can be complex and their analytical manifestations may be confused with a resistance to thyroid hormones. They usually present with signs and symptoms generated by hyperthyroidism together with goiter [2] and symptoms due to the mass effect of the tumor. Although most are macroadenomas at diagnosis, it seems that the better sensitivity of current laboratory and imaging tests could have improved their early diagnosis[4].
Regarding treatment, the guidelines of the European Thyroid Association recommend surgery as the first step in the management of these tumors. Medical treatment consists of the use of long-acting somatostatin analogues (SSA) together with the possibility of using dopamine agonists [5].
We present the case of a TSHoma with 12 years of follow-up at our center.
2. Case Report
This is a male patient referred to our consultation in 2008 at the age of 73 years. The main reason was the loss of 8 kg of weight and palpitations together with the analytical appearance of TSH of 13.5 mIU / L (0.35-4.94) and T4L in the normal range (0.7-1, 5). Initially, treatment was started with Levothyroxine 25 mcg, which was increased up to 175 mcg daily. As a consequence, TSH remained elevated with T4L, which reached a value of 3 ng / dL. The patient started with a weight of 65 kg which dropped to 58 kg before consultation.
In the absence of analytical control, a TRH test and a SHBG value were requested, showing a basal TSH value of 26.3 and a stimulated value of 26.4 mUI/L. The SHBG value was 120 nmol/L Because of them, a magnetic resonance imaging (MRI) of the turkish chair was requested, showing the presence of a pituitary macroadenoma of 25 × 19 × 16 mm (LAT, CC and AP) with infiltration of the left cavernous sinus and walls of the right cavernous sinus contacting the optic chiasm without displacing it (Figure 1 and 2). To complete the study, an Octreoscan (Figure 3) was performed, which was positive for somatostatin receptors.
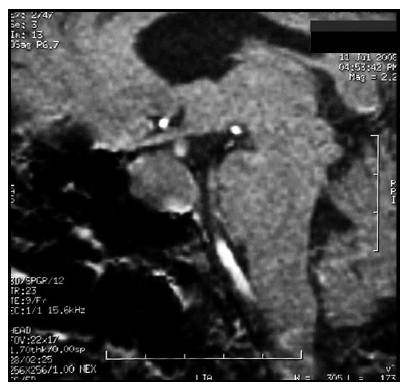
Figure 1: MRI 2008. T1 sequence in sagittal orientation with presence of pituitary macroadenoma 25 × 19 × 16 mm (volume 3.9748 cc) that infiltrates the left cavernous sinus.
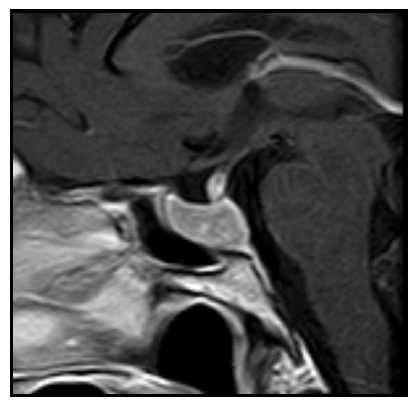
Figure 2: 2015 MRI. T1-weighted sagittal image with c.i.v.: 18 × 10 × 18 mm intraselar mass (volume = 1.69452 cc).
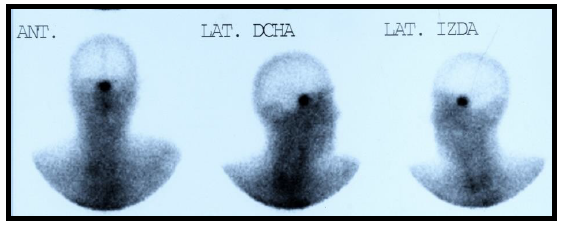
Figure 3: Octreoscan 2009: positive uptake in the pituitary region.
After confirming the clinical diagnosis of TSH-producing macroadenoma (TSHoma), treatment with Octreotide LAR 20 mg was started every 28 days in 2009 with good tolerance and rapid analytical normalization and disappearance of the symptoms described, including the gain of 10 kg of weight. The 2009 MRI control showed radiological stability, so it was decided to increase the dose to 30 mg per day with a 50.3% volume reduction observed the following year. Neurosurgery evaluation was requested, but given the good clinical and radiological evolution, we agreed with the patient on follow-up and medical treatment.
The patient has been following up at our center since then. Table 1 shows the evolution of laboratory values over the years. As seen in the subsequent MRI controls, the size of the adenoma decreases discreetly over time (Table 2). In 2016 an increase in size is reported, which is why we requested two controls in 2017 showing smaller sizes. Since then stability or discreet growth of the lesion has been observed.
Table 1: Evolution of analytical values during follow-up.
|
Date (M/Y) |
Size in mm (TR/CC/AP) on reports |
Volume (cc) |
|
2008 |
25 × 19 × 16 |
39,748 |
|
2009 |
25 × 19 × 16 |
39,748 |
|
2010 |
21 × 12 × 15 |
19,769 |
|
01/2012 |
22 × 13 × 19 |
28,419 |
|
05/2014 |
21 × 9 × 19 |
18,780 |
|
05/2015 |
18x 10 × 18 |
16,945 |
|
04/2016 |
25 × 13 × 21 |
35,694 |
|
03/2017 |
19 × 11 × 21 |
22,954 |
|
09/2017 |
20 × 11 × 15 |
17,259 |
|
06/2018 |
19 × 13 × 21 |
27,128 |
|
04/2019 |
19,8 × 12,9 × 20 |
26,716 |
Table 2: Evolution of adenoma size on MRI. Volume calculated with the formula TRxCCxAPx0,523 (synthesized ellipsoid volume formula).
|
Time |
Glucose (mg/dL) |
GH (µg/L) |
|
-15 min |
128 |
1,96 |
|
Basal |
128 |
1,98 |
|
30 min |
166 |
1,51 |
|
60 min |
275 |
1,49 |
|
90 min |
302 |
1,37 |
|
120 min |
332 |
1,14 |
Table 3: Oral glucose overload test.
During follow-up, a progressive increase in the IGF-1 value was identified reaching +4.53 SD in 2018. However, the patient did not present acral enlargement or any symptoms related with acromegaly. A GH test was requested after oral glucose overload (Table 3), which was pathological, confirming the co-secretion of TSH and GH. At this time we decided to associate Cabergoline at an ascendant dose of 2 mg per week, achieving a progressive decrease in IGF-1 levels in subsequent controls. Simultaneously, screening tests were requested with the finding of a subcentimetric multinodular goiter, 2 subcentimetric polyps at colonoscopy and a benign prostatic hyperplasia. The patient was also diagnosed with diabetes mellitus, which was kept under control with Metformin 850 mg 1 tablet daily and asymptomatic cholelithiasis. An echocardiogram was requested. Hypertrophy was discarded, but it showed moderate mitral and tricuspid regurgitation with preserved ejection fraction.
Nowadays, the patient remains on Octreotide LAR 30 mg every 28 days together with Cabergoline. The last control MRI is from March 2019 showing radiological stability (Figure 5) with a size of 19 × 13 × 21 mm (Volume = 2,712 cc), similar to 2017 control (Figure 4). No signal changes were observed in T2 that could condition different response to SSA [6]. The blood test shows controlled thyroid function with TSH of 2.55 mUI / L and T4L 1 ng / dL together with IGF1 of 258 μg / L (+3.25 SD adjusted for age). The patient remains stable without new symptoms. He has not required surgery.
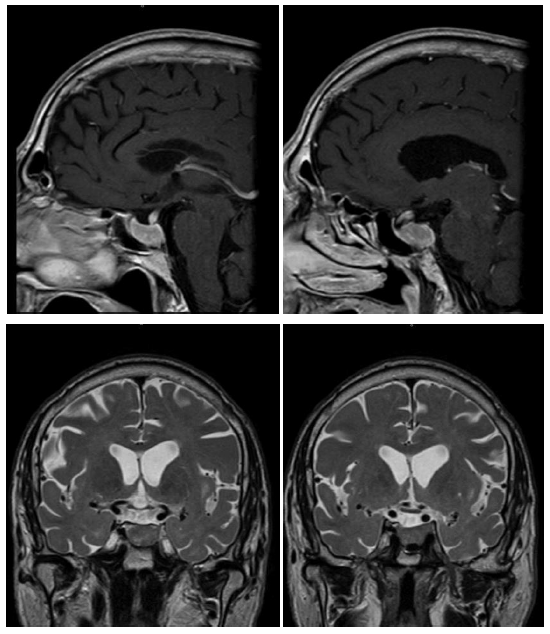
Figure 4: MRI T1 sequences with gadolinium and T2 sequences of 2017.
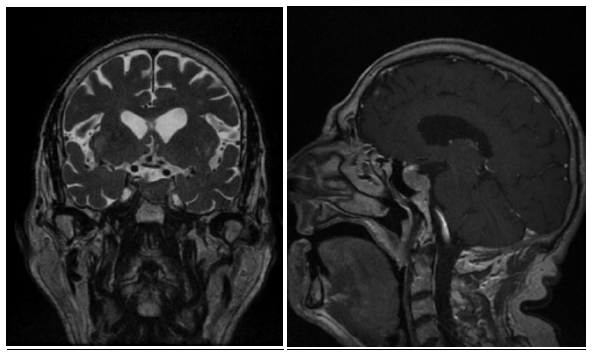
Figure 5: MRI T1 sequence with gadolinium and T2 sequence of 2019.
3. Discussion
This case report presented a TSH-producing macroadenoma with 12 years of follow-up, showing co-secretion with GH in the last years. Approximately 70% of TSHomas only secrete TSH with a 30% mixed secretion with Prolactin or GH (16%) [1]. Furthermore, these tumors usually appear in the form of macroadenomas that invade the periselar structures [7]. Regarding clinical symptoms, a percentage may be asymptomatic, although the most frequent symptoms will be derived from hyperthyroidism, chiasmatic compression, or hormonal involvement of the rest of the pituitary [1]. Up to 65% may associate an increase in thyroid size [8]. Our patient presented the symptoms of weight loss and the presence of a multinodular goiter on neck ultrasound without any changes related to the mass effect and without involvement of other pituitary axes.
Typical laboratory manifestations are elevated T3L or T4L with abnormally high TSH [1]. Analytical confirmation is based on a suppression test. Although T3 suppression is indicated, it may be contraindicated in older patients with comorbidities [9]. Stimulation with 200 µg of TRH would be the main alternative, causing TSH to not rise in 85% of patients with TSHoma [7]. After the analytical confirmation, a turkish chair MRI should be performed to look for the adenoma [5]. In our case, we did not have the initial T3L value, which was probably high, conditioning the patient's weight loss.
As previously indicated, the recommended treatment consists of removal of the adenoma [5]. According to the series [4, 9-14], surgery achieves euthyroidism in 80% of patients. The surgery has her own implicit complications and with macroadenomas, hypopituitarism can be an undesirable consequence. Regarding medical treatment, drugs can be used for the symptomatic control of hyperthyroidism [15]. The SSAs, due to the over-expression of somatostatin receptors in these tumors [16], may play a role before and after surgery in case of lack of control [17]. In some cases, it may be necessary to perform a total thyroidectomy to control hyperthyroidism [18].
In patients, such as ours, in whom surgery is rejected or contraindicated, two lines of treatment may be recommended. On the one hand, radiotherapy [5, 19] and, on the other, the SSAs that manage to restore hormonal control of the thyroid axis in 95% of cases, as well as a significant decrease in the size of the adenoma around 50% [7]. This control can be observed in the first 3 months of treatment [20]. In our case, the hormonal control was excellent from the beginning and regarding the size, a decrease of 3 mm could be seen in each axis of the adenoma that was mostly maintained during the follow-up.
Based on what we observed in the follow-up, it seems that the dose received by our patient of Octreotride LAR 30 mg every 28 days maintains hormonal control and tumor size stable after the initial reduction. Our case stands out for the progressive appearance of high IFG-1 values despite treatment with SSA, confirming non-suppression of GH with an oral glucose overload test. This co-secretion over time has been previously reported in another case treated with SSA without surgery [21] although the appearance of elevated GH was 12 months after starting treatment. Unlike our case that happened several years after and confirmed 2 years ago.
Hormonal co-secretion is explained by its dependence on the PIT-1 [22] factor. Back in 1989 the appearance of secretion granules with TSH and GH within the same adenoma cells is demonstrated [23]. The authors of the previously described case consider, however, that it could be a subpopulation of cells that are resistant to the action of SSA and, due to local changes caused by the sensitive cells, the autonomous production of GH is triggered [21]. The evolution of our case seems to go against this theory since resistant cells are unlikely to remain dormant for so many years. We neither had an excess of GH at diagnosis reason why we discarded the initial co-secretion. It could also be a tumor dedifferentiation that leads to this co-growth, but it seems unlikely in our case given the stability and appearance of the pituitary adenoma. We propose as a possible mechanism the acquisition of resistance to SSA over time. Unfortunately, we do not have a biological sample to study this phenomenon.
However, the data suggests that the leak to SSA occurs progressively and, although the diagnosis is made in 2018, it seems to have been in progress since at least 2016. Treatment with Cabergoline (as has been done in other series [20]) seems to have improved IGF-1 values to optimal levels. Given the advanced age of our patient, in addition to the limited symptoms or associated comorbidity to excess GH, the surgery could be avoided. We are tracking the IGF-1 values in order to decide dosage changes of Cabergoline or SSA.
4. Conclusions
Thyrotropinomas are rare pituitary adenomas. Hormonal co-secretion is observed with some frequency either analytically or immunohistochemically, and their surgical treatment is usually effective. The appearance of late GH co-secretions that escape the control of analogues has been observed in cases with SSA-based treatment therefore we recommend its active screening during the follow-up of thyrotropinomas. A longer-term follow-up and more cases are needed to draw firm conclusions about this phenomenon.
References
- Beck-Peccoz P, Giavoli C, Lania A. A 2019 update on TSH-secreting pituitary adenomas. J Endocrinol Invest 42 (2019): 1401-1406.
- Beck-Peccoz P, Persani L. Thyrotropinomas. Endocrinol Metab Clin North Am 37 (2008): 123-134.
- Önnestam L, Berinder K, Burman P, et al. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J Clin Endocrinol Metab 98 (2013): 626-635.
- Socin HV, Chanson P, Delemer B, et al. The changing spectrum of TSH-secreting pituitary adenomas: Diagnosis and management in 43 patients. Eur J Endocrinol 148 (2003): 433-442.
- Beck-Peccoz P, Lania A, Beckers A, Chatterjee K, Wemeau J-L. 2013 European Thyroid Association Guidelines for the Diagnosis and Treatment of Thyrotropin-Secreting Pituitary Tumors. Eur Thyroid J 2 (2013): 76-82.
- Potorac I, Petrossians P, Daly AF, et al. T2-weighted MRI signal predicts hormone and tumor responses to somatostatin analogs in acromegaly. Endocr Relat Cancer 23 (2016): 871-881.
- Malchiodi E, Profka E, Ferrante E, et al. Thyrotropin-secreting pituitary adenomas: Outcome of pituitary surgery and irradiation. J Clin Endocrinol Metab 99 (2014): 2069-2076.
- Abs R, Stevenaert A, Beckers A. Autonomously function- ing thyroid nodules in a patient with a thyrotropin-secreting pitui- tary adenoma: possible cause-effect relationship. Eur J Endocrinol 131 (1994) :355-358.
- Tjörnstrand A, Nyström HF. Diagnostic approach to TSH-producing pituitary adenoma. Eur J Endocrinol 177 (2017): R183-R197.
- Bertholon-Grégoire M, Trouillas J, Guigard MP, Loras B, Tourniaire J. Mono- and plurihormonal thyrotropic pituitary adenomas: Pathological, hormonal and clinical studies in 12 patients. Eur J Endocrinol 140 (1999): 519-527.
- Yamada S, Fukuhara N, Horiguchi K, et al. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: A single-center study of 90 cases. J Neurosurg 121 (2014): 1462-1473.
- Van Varsseveld NC, Bisschop PHLT, Biermasz NR, Pereira AM, Fliers E, Drent ML. A long-term follow-up study of eighteen patients with thyrotrophin- secreting pituitary adenomas. Clin Endocrinol (Oxf) 80 (2014): 395-402.
- Azzalin A, Appin CL, Schniederjan MJ, et al. Comprehensive evaluation of thyrotropinomas: single-center 20-year experience. Pituitary 19 (2016): 183-193.
- Nazato DM, Abucham J. Diagnosis and treatment of TSH-secreting adenomas: review of a longtime experience in a reference center. J Endocrinol Invest 41 (2018): 447-454.
- Dyer MW, Gnagey A, Jones BT, et al. Perianesthetic management of patients with thyroid-stimulating hormone-secreting pituitary adenomas. J Neurosurg Anesthesiol 29 (2017): 341-346.
- Gatto F, Barbieri F, Gatti M, et al. Balance between somatostatin and D2 receptor expression drives TSH-secreting adenoma response to somatostatin analogues and dopastatins. Clin Endocrinol (Oxf) 76 (2012): 407-414.
- Fukuhara N, Horiguchi K, Nishioka H, et al. Short-term preoperative octreotide treatment for TSH-secreting pituitary adenoma. Endocr J 62 (2015): 21-27.
- Daousi C, Foy PM, MacFarlane IA. Ablative thyroid treatment for thyrotoxicosis due to thyrotropin-producing pituitary tumours. J Neurol Neurosurg Psychiatry 78 (2007): 93-95.
- Kuhn JM, Arlot S, Lefebvre H, et al. Evaluation of the treatment of thyrotropin-secreting pituitary adenomas with a slow release formulation of the somatostatin analog lanreotide. J Clin Endocrinol Metab 85 (2000): 1487-1491.
- Rimareix F, Grunenwald S, Vezzosi D, Rivière LD, Bennet A, Caron P. Primary Medical Treatment of Thyrotropin-Secreting Pituitary Adenomas by First-Generation Somatostatin Analogs: A Case Study of Seven Patients. Thyroid 25 (2015): 877-882.
- Glynn N, Agha A. Unexpected clinical course during treatment of a TSH-secreting pituitary adenoma. Endocr Pract 19 (2013).
- Sanno N, Teramoto A, Matsuno A, et al. Clinical and immunohistochemical studies on TSH-secreting pituitary adenoma: its multihormonality and expression of Pit-1. Mod Pathol 7 (1994): 893-899.
- Malarkey WB, Kovacs K, O’Dorisio TM. Response of a GH- and TSH-secreting pituitary adenoma to a somatostatin analogue (SMS 201-995): Evidence that GH and TSH coexist in the same cell and secretory granules. Neuroendocrinology 49 (1989): 267-274.


 Impact Factor: * 4.1
Impact Factor: * 4.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 