Abbreviated MRI for Preoperative Assessment of Breast Cancer: is Maximal Intensity Projection (MIP) of the First Post Contrast Acquisition Subtracted (Fast) Sequence Sufficient for Disease Evaluation?
Article Information
Rami Hajri1, Alexandre Ponti1, Jean-Yves Meuwly1, Sylvain Eminian1, Jean-Baptiste Ledoux1,2, Estelle Tenisch1, Leonor Alamo-Maestre1, Clarisse Dromain1, Naïk Vietti Violi1*
Affiliation:
1Department of Radiology, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland
2Centre for Biomedical Imaging (CIBM), Lausanne, Switzerland
Rami Hajri and Alexandre Ponti contributed equally to this paper.
Corresponding author:
Naïk Vietti Violi, Department of Radiology, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland.
Received: October 17, 2022; Accepted: November 22, 2022; Published: December 21, 2022
Citation: Rami Hajri, Alexandre Ponti, Jean-Yves Meuwly, Sylvain Eminian, Jean-Baptiste Ledoux, Estelle Tenisch, Leonor Alamo-Maestre, Clarisse Dromain, Naïk Vietti Violi. Abbreviated MRI for Preoperative Assessment of Breast Cancer: is Maximal Intensity Projection (MIP) of the First Post Contrast Acquisition Subtracted (Fast) Sequence Sufficient for Disease Evaluation?. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 452-459.
View / Download Pdf Share at FacebookAbstract
Objectives: The aim of this study was to assess the diagnostic performance of abbreviated MRI (AMRI) using the maximal intensity projection (MIP) reconstruction of the first post-contrast acquisition subtracted (FAST) compared with MIP+FAST and full-protocol MRI (fpMRI) for the preoperative assessment of breast cancer (BC) in a biopsy-proven cancer population.
Methods: In this monocentric retrospective study, two readers consensually assessed two AMRI protocols consisting of MIP reconstruction of the FAST (MIP) and MIP+FAST. 228 patients were included with a breast MRI performed between 2013 and 2014, 207 of them (90.8%) had biopsyproven cancer with 256 lesions. Data of MIP and MIP+FAST were compared to full-protocol MRI (fpMRI) reading and to the reference standard including 6-month follow-up imaging and pathology as the reference.
Results: MIP, MIP+FAST and fpMRI demonstrated a per-lesion sensitivity for BC detection of 87.5% (224/256, 95%CI: 82.9-91.3%) and 97.7% (250/256, 95-99.1%) and 98.4% (252/256, 96.1-99.6%), respectively with a statistical difference between MIP compared to MIP+FAST and fpMRI when considering confidence intervals. Per-lesion specificity was not different [MIP: 47.6% (10/21, 25.7-70.2%), MIP+FAST: 52.4% (11/21,29.8-74.3%, fpMRI: 66.7% (14/21, 43-85.4%)].
Conclusion: AMRI using only MIP is not accurate for the pre-operative assessment of BC due to lower sensitivity when compared to MIP+FAST and fpMRI. AMRI using the MIP+FAST acquisition in the preoperative setting seems promising as it could be used as the same protocol for both screening and staging in case of positive cases, without need for a recall fpMRI. This needs confirmation with cohort including higher rate of negative cases in order to evaluate the specificity.
Keywords
Abbreviated MRI; Breast Neoplasms; Magnetic Resonance Imaging; Preoperative assessment
Abbreviated MRI articles; Breast Neoplasms articles; Magnetic Resonance Imaging articles; Preoperative assessment articles
Abbreviated MRI articles Abbreviated MRI Research articles Abbreviated MRI review articles Abbreviated MRI PubMed articles Abbreviated MRI PubMed Central articles Abbreviated MRI 2023 articles Abbreviated MRI 2024 articles Abbreviated MRI Scopus articles Abbreviated MRI impact factor journals Abbreviated MRI Scopus journals Abbreviated MRI PubMed journals Abbreviated MRI medical journals Abbreviated MRI free journals Abbreviated MRI best journals Abbreviated MRI top journals Abbreviated MRI free medical journals Abbreviated MRI famous journals Abbreviated MRI Google Scholar indexed journals Breast Neoplasms articles Breast Neoplasms Research articles Breast Neoplasms review articles Breast Neoplasms PubMed articles Breast Neoplasms PubMed Central articles Breast Neoplasms 2023 articles Breast Neoplasms 2024 articles Breast Neoplasms Scopus articles Breast Neoplasms impact factor journals Breast Neoplasms Scopus journals Breast Neoplasms PubMed journals Breast Neoplasms medical journals Breast Neoplasms free journals Breast Neoplasms best journals Breast Neoplasms top journals Breast Neoplasms free medical journals Breast Neoplasms famous journals Breast Neoplasms Google Scholar indexed journals Magnetic Resonance Imaging articles Magnetic Resonance Imaging Research articles Magnetic Resonance Imaging review articles Magnetic Resonance Imaging PubMed articles Magnetic Resonance Imaging PubMed Central articles Magnetic Resonance Imaging 2023 articles Magnetic Resonance Imaging 2024 articles Magnetic Resonance Imaging Scopus articles Magnetic Resonance Imaging impact factor journals Magnetic Resonance Imaging Scopus journals Magnetic Resonance Imaging PubMed journals Magnetic Resonance Imaging medical journals Magnetic Resonance Imaging free journals Magnetic Resonance Imaging best journals Magnetic Resonance Imaging top journals Magnetic Resonance Imaging free medical journals Magnetic Resonance Imaging famous journals Magnetic Resonance Imaging Google Scholar indexed journals Preoperative assessment articles Preoperative assessment Research articles Preoperative assessment review articles Preoperative assessment PubMed articles Preoperative assessment PubMed Central articles Preoperative assessment 2023 articles Preoperative assessment 2024 articles Preoperative assessment Scopus articles Preoperative assessment impact factor journals Preoperative assessment Scopus journals Preoperative assessment PubMed journals Preoperative assessment medical journals Preoperative assessment free journals Preoperative assessment best journals Preoperative assessment top journals Preoperative assessment free medical journals Preoperative assessment famous journals Preoperative assessment Google Scholar indexed journals Breast Cancer articles Breast Cancer Research articles Breast Cancer review articles Breast Cancer PubMed articles Breast Cancer PubMed Central articles Breast Cancer 2023 articles Breast Cancer 2024 articles Breast Cancer Scopus articles Breast Cancer impact factor journals Breast Cancer Scopus journals Breast Cancer PubMed journals Breast Cancer medical journals Breast Cancer free journals Breast Cancer best journals Breast Cancer top journals Breast Cancer free medical journals Breast Cancer famous journals Breast Cancer Google Scholar indexed journals Diffusion Weighted Images articles Diffusion Weighted Images Research articles Diffusion Weighted Images review articles Diffusion Weighted Images PubMed articles Diffusion Weighted Images PubMed Central articles Diffusion Weighted Images 2023 articles Diffusion Weighted Images 2024 articles Diffusion Weighted Images Scopus articles Diffusion Weighted Images impact factor journals Diffusion Weighted Images Scopus journals Diffusion Weighted Images PubMed journals Diffusion Weighted Images medical journals Diffusion Weighted Images free journals Diffusion Weighted Images best journals Diffusion Weighted Images top journals Diffusion Weighted Images free medical journals Diffusion Weighted Images famous journals Diffusion Weighted Images Google Scholar indexed journals First Post Contrast Subtracted articles First Post Contrast Subtracted Research articles First Post Contrast Subtracted review articles First Post Contrast Subtracted PubMed articles First Post Contrast Subtracted PubMed Central articles First Post Contrast Subtracted 2023 articles First Post Contrast Subtracted 2024 articles First Post Contrast Subtracted Scopus articles First Post Contrast Subtracted impact factor journals First Post Contrast Subtracted Scopus journals First Post Contrast Subtracted PubMed journals First Post Contrast Subtracted medical journals First Post Contrast Subtracted free journals First Post Contrast Subtracted best journals First Post Contrast Subtracted top journals First Post Contrast Subtracted free medical journals First Post Contrast Subtracted famous journals First Post Contrast Subtracted Google Scholar indexed journals Maximal Intensity Projection articles Maximal Intensity Projection Research articles Maximal Intensity Projection review articles Maximal Intensity Projection PubMed articles Maximal Intensity Projection PubMed Central articles Maximal Intensity Projection 2023 articles Maximal Intensity Projection 2024 articles Maximal Intensity Projection Scopus articles Maximal Intensity Projection impact factor journals Maximal Intensity Projection Scopus journals Maximal Intensity Projection PubMed journals Maximal Intensity Projection medical journals Maximal Intensity Projection free journals Maximal Intensity Projection best journals Maximal Intensity Projection top journals Maximal Intensity Projection free medical journals Maximal Intensity Projection famous journals Maximal Intensity Projection Google Scholar indexed journals Negative Predictive Value articles Negative Predictive Value Research articles Negative Predictive Value review articles Negative Predictive Value PubMed articles Negative Predictive Value PubMed Central articles Negative Predictive Value 2023 articles Negative Predictive Value 2024 articles Negative Predictive Value Scopus articles Negative Predictive Value impact factor journals Negative Predictive Value Scopus journals Negative Predictive Value PubMed journals Negative Predictive Value medical journals Negative Predictive Value free journals Negative Predictive Value best journals Negative Predictive Value top journals Negative Predictive Value free medical journals Negative Predictive Value famous journals Negative Predictive Value Google Scholar indexed journals Turbo Spin Echo articles Turbo Spin Echo Research articles Turbo Spin Echo review articles Turbo Spin Echo PubMed articles Turbo Spin Echo PubMed Central articles Turbo Spin Echo 2023 articles Turbo Spin Echo 2024 articles Turbo Spin Echo Scopus articles Turbo Spin Echo impact factor journals Turbo Spin Echo Scopus journals Turbo Spin Echo PubMed journals Turbo Spin Echo medical journals Turbo Spin Echo free journals Turbo Spin Echo best journals Turbo Spin Echo top journals Turbo Spin Echo free medical journals Turbo Spin Echo famous journals Turbo Spin Echo Google Scholar indexed journals
Article Details
Abbreviations:
AMRI- Abbreviated MRI; BC- Breast Cancer; CI- Confidence Intervals; DWI- Diffusion Weighted Images; FAST- First Post Contrast Subtracted; MIP- Maximal Intensity Projection; NPV- Negative Predictive Value; PPV- Positive Predictive Value; TSE- Turbo Spin Echo; WI- Weighted Images
Introduction
Breast cancer (BC) is the most frequently diagnosed neoplasm and the leading cause of cancer related death in women [1]. Magnetic resonance imaging (MRI) has a higher sensitivity compared to conventional mammography and ultrasound for BC detection [2–4] and to depict additional areas of malignancy such as multifocal, multicentric or contralateral cancer that could change the patient’s management [5]. In particular cases, it is also more accurate than mammography and ultrasound for determining true tumor size [4] and extension for tumors involving the chest wall [6,7]. Also it can maximize tumor excision by a more precise definition of its extent, reducing the rate of re-excision, local recurrence and development of new contralateral cancer [8,9]. However, the systematic use of MRI in BC patients remains controversial as preoperative breast MRI has the potential for personalized surgical treatment but the added surgical or outcome benefits still need to be demonstrated [10,11]. The limited specificity of the technique is also a disadvantage as it can lead to additional costly and unnecessary biopsies and delays in regional and systemic treatments [12, 13]. Therefore preoperative MRI is not routinely performed in clinical setting but in some accepted indications such as women with dense breast, women with high risk for contralateral disease, invasive lobular histology, and tumors contiguous to chest well, locally advanced breast cancer who will benefit from neoadjuvant systemic therapy [14]. According to the guidelines of the European Society of Breast Cancer Specialists, the standard full-protocol MRI (fpMRI) takes around 20 to 30 minutes to perform, with additional substantial time to analyze all sequences beside of thousands of images that need to be archived [15]. Motivated to provide optimized methods for BC screening, novel abbreviated breast MRI (AMRI) protocols have been described. The concept initially developed by Kuhl et al in 2014 [16], consists in selecting only one or few MRI sequences for targeted questions such as BC detection for instance. In the screening setting, AMRI equals fpMRI in terms of sensitivity and specificity [17] and could maximizes cost-effectiveness and increase access to breast MRI thanks to shorter acquisition and reading times [30]. Since then, multiple abbreviated protocols have been investigated integrating T2 weighted images (WI), diffusion weighted images (DWI), pre-contrast T1WI, post-contrast T1WI with or without subtraction and maximal intensity projection (MIP) of the first post contrast acquisition subtracted (FAST), alone or in combination. However, the most accurate AMRI protocol for BC detection still needs to be validated. Several studies have demonstrated the promising role of abbreviated MRI in the screening setting but little studies have investigated the role of AMRI in the pre-operative setting [18]. To date, AMRI using only the MIP of the FAST was never studied in the preoperative context.
Nevertheless, it seems important to determine if AMRI can be used for both screening and preoperative assessment in case of positive cases or if a complete MRI is required before surgery. On this basis, the aim of the present study was to assess the diagnostic performance of AMRI using MIP alone compared to MIP+FAST and fpMRI for the preoperative assessment of BC in a population with non-conclusive standard imaging using pathology or 6-month imaging follow-up as the reference.
Material and Methods
Study Population
This retrospective single center study was approved by our Institutional Review Board, which waived the requirement for informed consent. According to the policy of our tertiary referral center for breast diagnosis, all women with histological diagnosis of BC and with diagnostic problems not solved by mammography or ultrasound (US) were referred for breast MRI. We retrospectively searched for all women who underwent breast MRI for the aforementioned indications between January 2013 and December 2014. Amongst the 247 initially eligible patients, 19 patients were excluded for the following reasons: start of neo-adjuvant therapy before the MRI (n=11), significant post-biopsy changes (voluminous seroma or hematoma) affecting MRI quality (n=3) and breast lesion without definite diagnosis (n=5). In patients that had more than one MRI, only the first one was selected for the analysis. The final study population included 228 women (mean age: 59 ±13 years, range: 26-88).
MRI Protocol
Breast MRI examinations were performed with different systems, either 1.5-T: GE Optima (n=121) (GE Healthcare), Siemens Aera (n=64) (Siemens Healthineers), Philips Achieva (n=19) (Philips Medical System), Siemens Avanto (n=7), Philips Ingenia (n=5), Siemens Espree (n=3), GE Signa (n=2), Siemens Symphony (n=2), Philips Intera (n=1) or 3-T: Siemens Skyra (n=7). Four MRI were performed at 1.0-T on a Philips HFO machine. A bilateral dedicated 16-channel or 4- channel coil was used. The fpMRI settings evidenced slight variations among the different machines used but all included a flash gradient-echo (GRE) 3DT1 WI or turbo spin echo (TSE) T1 WI and a TSE T2 WI or turbo spin STIR/SPARE T2 WI. Dynamic contrast-enhanced images were acquired using a transverse 3D fast spoiled GRE sequence T1 WI with fat suppression or 3D T1 flash GRE with fat-sat suppression WI (Siemens Avanto and Siemens Skyra machines) with a voxel size between 0.3 x 0.3 x 1.2 mm and 0.9 x 0.9 x 2.0 mm, 1.2-mm section with no gap, a FOV ranging between 240 and 380, a matrix size between 288 x 248 and 512 x 512 before and after intravenous injection of Gadoteric acid
– Gadoterate meglumine (Dotarem, Guerbet) at 2mL/s with total dose of 0.2 mmol/kg.. Image post-processing included the subtraction of the first acquired contrast-enhanced images to the non-contrast-enhanced series as well as maximum intensity projection (MIP).
Image Analysis
Two virtual AMRI protocols were extracted from the full breast MRI: (1) the MIP reconstruction alone, entitled MIP and (2) the MIP + FAST. No other sequence was reviewed by reader. Breast MRI examinations were interpreted on a picture archiving and communication system (PACS). Two radiologists (a fellow and an experienced radiologist in breast imaging with 25 years of expertise), blinded of all patient data (including medical history, clinical and imaging data) read in consensus two AMRI protocols. Readers initially analyzed the MIP and classified each examination as positive or negative for suspicious enhancement based only on the MIP image. All enhancing lesions > 5 mm were considered suspicious, according to the clinical recommendations [19,20], with exception of lesions presenting classical characteristic of benign intra mammary lymph node (< 1cm, oval or lobulated shaped, well defined margins, fatty hilum) [21]. Enhancement < 5 mm were considered benign and interpreted as focus of background enhancement. In case of multiple suspicious lesions, up to four lesions were recorded based on size and suspicious aspect. For each lesion, readers were asked to report the lesion size (in mm) and location (breast side and internal/external quadrant), as the analysis was based only on the transverse MIP, readers were not able to statute on the cranio-caudal location. They distinguished between multifocal (lesion in the same quadrant than the index lesion), multicentric (lesion in a different quadrant than the index lesion) or bilateral (lesion in another breast than the index lesion) extension. After the MIP analysis, the readers were then asked to review the MIP+FAST and to report any change in interpretation as well as the quadrant location. Time for each AMRI interpretation was recorded. AMRI acquisition time was approximately 5 minutes including a localizer followed by pre and post contrast sequences acquisition and processing of the FAST series. Acquisition time of both AMRI protocols is similar as FAST is needed to perform the MIP reconstruction.
Reference Standard
The reference standard was based on the result of the pathological analysis after surgery for all BC cases, after surgery or biopsy for all benign lesions and on the fpMRI and clinical data including all follow-up information (with a minimum of 6-month imaging follow-up) for the negative MRIs. Based on pathology, BC type, grade, location, size (in mm) and presence of multifocal or multicentric lesions were recorded by an independent radiologist who was not involved in AMRI reading. Patients were categorized in 2 different groups: [1] positive for BC, [2] negative for BC. Additionally, result of the MRI report from the clinical reading was recorded for correlation with the AMRI classification. A correlation was performed between pathological and MRI location in order to confirm the concordance between tumor and lesion at MRI.
Statistical Analysis
Data of the AMRI reading (MIP and MIP+FAST) were correlated and compared to the results of the fpMRI reading and to the reference standard in order to assess each AMRI protocol diagnostic performance for detection of BC and to compare with the fpMRI. A comparative analysis between the results using the MIP and the MIP+FAST data was also performed. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnosis accuracy of AMRI protocols and fpMRI, including 95% confidence intervals (95% CI) were calculated using the reference standard classification as the reference, based on McNemar test. Analysis was performed on a per-lesion basis. Lesion was considered true positive when BC was found at pathology and was considered positive at MRI, with the condition that BC pathological location was consistent with MRI interpretation. Lesion was considered true negative when no BC cancer was found at pathology and no lesion was found at MRI in the same location, including at 6-month follow-up. Lesion was considered false positive when no BC was found at pathology but a lesion was depicted at MRI in the corresponding location. Lesion was considered false negative when BC was found at pathology but not lesion was depicted at MRI in the corresponding location. Analyses were performed with a commercially available system (MedCalc Software version 18.0), using a reference alpha value of 0.05.
Results
Reference Standard
Amongst the 228 study patients, 207 patients (90.8%) presented pathology-proven BC for a total of 256 lesions (mean lesion size: 22 ± 20 mm, range: 5-120 mm). Four patients had bilateral cancers. Amongst the 21 remaining patients, 16 patients (7%) had benign lesions while 5 patients (5%) had no lesion, including at least 6-month clinical and imaging follow-up. Detailed pathologic findings are presented in Table 1.
Breast Cancer Detection
Comparison of each AMRI protocol diagnostic performance (MIP and MIP+FAST) to full protocol MRI presented in Table 2. The per-lesion sensitivity for BC detection was 87.5% for the MIP (224/256 BC, 95%CI: 82.9- 91.3%), 97.7% for MIP+FAST (250/256 BC, 95-99.1%)
and 98.4% for fpMRI (252/256 BC, 96.1-99.6%), with a
Table 1: Pathologic diagnosis of the study population.
statistical difference between MIP compared to MIP+FAST and fpMRI as evidenced by not overlapping 95% CI. The per-lesion specificity was 47.6% for the MIP (10/21 non BC, 25.7-70.2%), 52.4% for the MIP+FAST (11/21 non BC, 29.8-
|
Pathologic diagnosis |
n |
% |
|
|
Malignant |
207 |
90.8 |
|
|
Histologic Type |
|||
|
Ductal carcinoma in situ |
17 |
||
|
Invasive ductal carcinoma |
153 |
||
|
Invasive lobular carcinoma |
34 |
||
|
Invasive neuro-endocrine carcinoma |
2 |
||
|
Invasive cribriform carcinoma |
1 |
||
|
Histologic grade |
|||
|
Carcinoma in situ |
17 |
||
|
G1 |
87 |
||
|
G2 |
88 |
||
|
G3 |
1 |
||
|
Benign |
16 |
7 |
|
|
Atypical ductal hyperplasia |
3 |
||
|
Intraductal papilloma |
2 |
||
|
Adenosis |
1 |
||
|
Angiomyolipoma |
1 |
||
|
Cystic cavity |
1 |
||
|
Fibrosis |
1 |
||
|
Pseudoangiomatous stromal hyperplasia |
1 |
||
|
Sclerosing lesion |
1 |
||
|
Diabetic mastopathy |
1 |
||
|
Fibrocystic modifications |
1 |
||
|
Fibroadenoma |
1 |
||
|
Fibroepithelial lesion |
1 |
||
|
Focus of hyperplasia |
1 |
||
|
Normal |
5 |
2.2 |
|
74.3%) and 66.7% for the fpMRI (14/21 non BC, 43-85.4%), without statistical difference when considering the 95% CI. Accuracy was statistically different for MIP (84.5%, 79.7- 88.5%) when compared to MIP+FAST (94.2%, 64.7-81.7%) and fpMRI (96%, 93-98%). Examples of lesion interpretation are presented in Figure 1. Time for interpretation and radiologist’s consensus was <10 seconds for MIP and <30 seconds for MIP + FAST in all cases.
False Positives
All false positives at MIP (n=11) and MIP+FAST (n=10), correlated with benign findings: atypical ductal hyperplasia (n=2), intraductal papilloma (n=2), adenosis (n=1), angiomyolipoma (n=1), pseudoangiomatous stromal hyperplasia (n=1), sclerosing lesion (n=1), fibrocystic modifications (n=1), fibroepithelial lesion (n=1), focus of hyperplasia (n=1) (Figure 2). Seven of these lesions were also false positives at fpMRI: atypical ductal hyperplasia (n=2), intraductal papilloma (n=1), adenosis (n=1), pseudoangiomatous stromal hyperplasia (n=1), sclerosing lesion (n=1), fibrocystic modifications (n=1).
False Negatives
Amongst the 32 false negatives cases with MIP, 23 were carcinoma in situ and the 9 others were T1a tumors, including 7 invasive ductal carcinomas and 2 invasive lobular carcinoma (mean size: 9 ± 2 mm, range: 6-12 mm, all G1) (Figure 2). Amongst the 6 false negatives with MIP + FAST, 4 were carcinoma in situ and the 2 others were T1a invasive ductal carcinomas (mean size: 8 ± 2 mm, range: 6-10 mm, all G1). All of them were also false negatives at MIP reading. Four of these lesions were also missed at fpMRI reading (two carcinoma in situ and two invasive ducal carcinomas). All patients with no lesion (n=5) were correctly categorized as negative at both AMRI.
MIP versus MIP+FAST Acquisition Reading on a
Patient Basis
When comparing the MIP and the MIP+FAST analysis on a patient basis, the MIP+FAST was useful to define the
Table 2: Per-lesion diagnostic performance of AMRI protocols and fpMRI for breast cancer detection with 95% confidence intervals.
|
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
|
|
MIP (ratio, 95% CI) |
87.5% (224/256, 82.9-91.3%) |
47.6% (10/21, 25.7-70.2%) |
95.3% (93.1-96.9%) |
23.8% (15.2-35.2%) |
84.5% (79.7-88.5%) |
|
MIP + FAST (ratio, 95% CI) |
97.7% (250/256, 95-99.1%) |
52.4% (11/21, 29.8-74.3%) |
96.1% (94.1-97.5%) |
64.7% (43-81.7%) |
94.2% (90.8-96.7%) |
|
Full protocol MRI reading (ratio, 95% CI) |
98.4% (252/256, 96.1-99.6%) |
66.7% (14/21, 43-85.4%) |
97.3% (95.2-98.5%) |
77.8% (55.8-90.6%) |
96% (93-98%) |
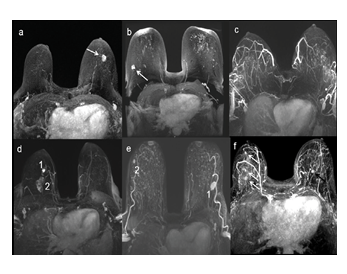
Figure 1: MIP reconstruction of the FAST presenting different examples of examinations : (a) a unique mass in LB EQ (arrow), (b) a unique mass in RB EQ (arrow) with bilateral foci (non-suspicious enhancements), (c) no suspicious lesion, (d) multifocal cancer with an index lesion in RB IQ (1) and a second lesion (2) in the same quadrant, (e) bilateral cancer with an index lesion in LB EQ (1) associated with a contralateral lesion in RB EQ (2), (f) bilateral cancer with multicentric disease in RB EQ (white arrows) and a contralateral mass in LB IQ (black arrow). MIP: maximal intensity projection, FAST: first post contrast subtracted, LB: left breast, RB: right breast, EQ: external quadrant, IQ: internal quadrant.
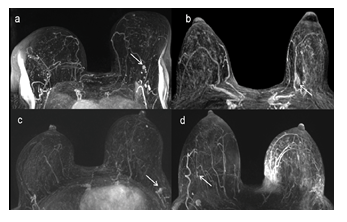
Figure 2: MIP reconstruction of the FAST presenting examples of false positives (a and b) and false negatives (c and d) cases: (a) A unique mass in LB EQ (arrow), biopsy proven as a sclerosing lesion, considered as positive for BC at both AMRI interpretation. (b) A unique non-mass enhancement in LB IQ (arrow) biopsy-proven atypical ductal hyperplasia, considered as positive for BC at both AMRI interpretation. (c) A unique mass in LB EQ, confounded with a lymph node at MIP interpretation and considered as positive at MIP
+ FAST interpretation, related to invasive ductal carcinoma stage T1a at biopsy. (d) A subtle mass in RB EQ (arrow), not considered as suspicious at both AMRI interpretation that was related to DCIS at biopsy. MIP: maximal intensity projection, FAST: first post- contrast subtracted, AMRI: abbreviated MRI, LB: left breast, RB: right breast, EQ: external quadrants, IQ: internal quadrants, DCIS: ductal carcinoma in situ.
location of the enhancement in the cranio-caudal orientation and to evaluate disease extension in case of multiple enhancements. In our study, the MIP alone under-estimated the extent of disease in a total of 27 patients (13% of patients with BC) with 24 patients considered unifocal instead of multifocal and 3 patients considered unicentric instead of multicentric (Figure 3).
Discussion
In this retrospective study with a large number of BC included, we demonstrated the limited added-value of AMRI using the MIP alone for BC preoperative assessment due to its lower sensitivity for lesion detection (87.5%) when compared to the fpMRI (98.4%), resulting in a lower accuracy (84.5% for MIP and 96% for fpMRI). Conversely, AMRI using MIP+FAST evidenced no difference in terms in sensitivity (97.7%) and accuracy (94.2%) when compared to fpMRI. Moreover, as the MIP alone is limited for the lesion location evaluation, it is associated with a high rate of disease underestimation (13%), which is not acceptable in the preoperative setting. Regarding specificity, we found no statistical difference between both AMRI and fpMRI. However, this is probably due to the small number of negative cases in the present study as the specificities of both AMRI seems to be in the lower range (MIP: 47.6%, MIP+FAST: 52.4%) compared to fpMRI reading (66.7%). This study is the first work evaluating MIP alone for preoperative assessment of BC. Our results in terms of sensitivity of MIP for BC detection on a lesion-basis (87.5%) is in the lower range compared to a meta-analysis from Geach et al. that evidenced 94.8% sensitivity for BC detection, on a patient-basis[22]. This highlights that MIP alone is highly sensitive on a patient-basis, but the sensitivity is lower on a lesion-basis, which is crucial for the evaluation of disease extension (e.g., presence of additional disease). Moreover, the MIP lacks information regarding cranio-caudal location of the lesions and limits the evaluation of tumor burden (by superposition of lesions on the MIP reconstruction). These data are essential in the preoperative setting as the
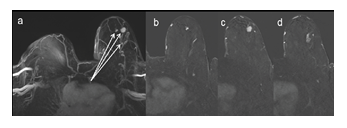
Figure 3: 67 year-old woman with biopsy proven invasive ductal cancer in left breast. MIP reconstruction of the FAST evidenced multiple masses in LB EQ. The analysis of MIP + FAST allowed the cranio-caudal location of the lesions and thus the differentiation between multifocal and multicentric extent, in the present case, multicentric. MIP: maximal intensity projection, FAST: first post contrast subtracted, LB: left breast, EQ: external quadrant.
presence of multifocal or multicentric disease will change treatment planning. In the present study, tumor burden was underestimated in 13% of patients, confirming the weakness of the MIP for the preoperative assessment of BC. Conversely, per-patient sensitivity and accuracy of MIP+FAST evidenced to be higher when compared to MIP and not different from fpMRI, which is promising in the preoperative setting. With the MIP+FAST, the cranio-caudal orientation of the lesions can be assessed, avoiding disease staging underestimation. These results are in line with previous data published by Girometti et al who evidenced that AMRI with MIP+FAST is comparable to fpMRI for BC staging [18]. When considering specificity, our results for both AMRI protocols (47.6% for MIP and 52.3% for MIP+FAST are in the lower range than previous works performed on BC screening (94.6% specificity in a recent meta-analysis)[22]. To note, specificity of fpMRI in the present study is also in the lower range (66.7%) than previous published data [22]. Nevertheless, our specificity results are in line with a comparative work including selected patients with BC and benign lesions: Grimm et al. included 25% of benign lesions in their study cohort and presented between 45 and 52% of specificity [23]. To note, all patients with no lesion (n=5) were correctly categorized as negative at both AMRI. Even if the specificities seem lower with AMRI compared with fpMRI, we found no statistical difference. This is probably due to the small number of negative cases in our cohort. As specificity is of crucial importance in the preoperative setting in order to avoid unnecessary interventions, further investigations are mandatory to evaluate the specificity of the AMRI for BC detection in the preoperative setting including larger cohorts with higher rate of negative cases. The false negatives (n=32 with MIP and n=6 with MIP + FAST) were early stage and/or small sized lesions (either carcinoma in situ or T1a cancers) and four of them were also missed on fpMRI reading. It was not possible to determine if those false negatives might have changed the surgical planification compared with fpMRI because of the retrospective design of the study. In this study, a large list of different machine brands and types were used, evidencing that MIP+FAST has a good sensitivity independently on the machine used. Although in this setting, it remains primordial to achieve a high image quality of the pre- and post-contrast sequences in order to allow appropriate subtraction and MIP reconstruction images [24]. Timewise, radiologists spend < 10s to find a consensus based on the MIP and < 30s based on the MIP+FAST. It is a substantial reduction in interpretation time when compared to an average of 6 minutes for fpMRI protocol according to previous published study [25]. AMRI is also associated to a shorter examination time (4 minutes for AMRI versus 23 minutes for fpMRI according to previous studies [25]), which means less discomfort for patients and increased access to breast MRI. As the promising use of AMRI in BC screening programs is growing [16,26,28,29], our results suggest that AMRI using FAST + MIP could be sufficient for concomitant accurate preoperative staging for positive cases, avoiding the need for a recall fpMRI before surgery. This management will be associated to a shortened time between BC diagnostic and treatment and a reduction of discomfort and anxiety in patients with a newly diagnosed BC. Further works are required to investigate this clinically relevant question. The present study has several limitations such as its retrospective design in a selected population, allowing the rate of positive cases. The study was based on a population with a majority of cancer biopsy-proven as reflected by the low rate of negative exams included (only 5 negative MRIs and 16 MRIs with benign lesions), which limits the analysis in terms of specificity. The high number of positive cases may overestimate the diagnostic performance. As a matter of fact, the negative predictive value of both abbreviated protocols is lower than one would expect as our population had a much higher breast cancer incidence than expected in a general screening population. Second, the diagnostic performance of the fpMRI was based on the clinical reading, performed by one reader. While previous studies showed high inter-reader agreement when comparing AMRI readings [30-32], in the present study we focused on BC evaluation in the pre-operative setting. As our primary outcome was the evaluation of MIP for the staging of BC, both AMRI protocols were assessed during the same reading session and in a consensus and not independently from each other, making the inter-reader agreement not assessable. Third, breast enhancements smaller than 5 mm after ruling out their possible intra-mammary lymph nodal origin, were considered benign and interpreted as focus of background enhancement. No kinetics evaluation was done for these small lesions. Finally, we compared AMRI protocols diagnostic performance to the fpMRI, which included access to comparative imaging and patient data, conversely to AMRI readings.
Conclusion
AMRI using only MIP is not accurate for the pre-operative assessment of BC due to lower sensitivity for lesion detection when compared to fpMRI. AMRI using the MIP+FAST acquisition in the preoperative setting seems promising with no difference in diagnostic performance compared to fpMRI. This needs confirmation in large cohort including higher rate of negative cases. If confirmed, AMRI using MIP+FAST could be used for both screening and BC staging in case of positive cases without need for a recall fpMRI.
Acknowledgments
None.
Funding
None.
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49 (2013): 1374-1403.
- Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast Radiology 233 (2004): 830-849.
- Hlawatsch A, Teifke A, Schmidt M, et al. Preoperative assessment of breast cancer: sonography versus MR AJR Am J Roentgenol 179 (2002): 1493-1501.
- Van Goethem M, Schelfout K, Dijckmans L, et al. MR mammography in the pre-operative staging of breast cancer in patients with dense breast tissue: comparison with mammography and Eur Radiol 14 (2004): 809-816.
- Neri A, Marrelli D, Megha T, et al. Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 BMC Surg 15 (2015): 1.
- Tsina G, Simon P. Breast magnetic resonance imaging and its impact on the surgical treatment of breast cancer. Obstet Gynecol Int 2014 (2014): 632074.
- Amin MB, Edge S, Greene F, et al. eds; American Joint Committee on Cancer. AJCC cancer staging manual. 8th New York, NY: Springer (2017): 589-636.
- Smitt MC, Nowels KW, Zdeblick MJ, et The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer 76 (1995): 259-267.
- Luini A, Rososchansky J, Gatti G, et al. The surgical margin status after breast-conserving surgery: discussion of an open issue. Breast Cancer Res Treat 113 (2009): 397-402.
- Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol 32 (2014): 392-401.
- Schnall MD, Blume J, Bluemke DA, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol 92 (2005): 32-38.
- Chandwani S, George PA, Azu M, et Role of preoperative magnetic resonance imaging in the surgical management of early-stage breast cancer. Ann Surg Oncol 21 (2014): 3473-3480.
- Xia C, Schroeder MC, Weigel RJ, et Rate of contralateral prophylactic mastectomy is influenced by preoperative MRI recommendations. Ann Surg Oncol 21 (2014): 4133-4138.
- Lehman CD, DeMartini W, Anderson BO, et Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw 7 (2009): 193-201.
- Milon A, Wahab CA, Kermarrec E, et al. Breast MRI: Is Faster Better? AJR Am J Roentgenol 214 (2020): 282-
- Kuhl CK, Schrading S, Strobel K, et Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 32 (2014): 2304-2310.
- Machida Y, Shimauchi A, Kanemaki Y, et al. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer 24 (2017): 411-419.
- Girometti R, Nitti A, Lorenzon M, et al. Comparison between an abbreviated and full MRI protocol for detecting additional disease when doing breast cancer J Magn Reson Imaging 49 (2019): e222-e30.
- Martaindale SR. Breast MR Imaging: Atlas of Anatomy, Physiology, Pathophysiology, and Breast Imaging Reporting and Data Systems Magn Reson Imaging Clin N Am 26 (2018): 179-190.
- Hirose M, Hashizume T, Seino N, et al. Atlas of breast magnetic resonance Curr Probl Diagn Radiol 36 (2007): 51-65.
- Gordon PB, Gilks B. Sonographic appearance of normal intramammary lymph nodes. J Ultrasound Med 7 (1988): 545-548.
- Geach R, Jones LI, Harding SA, et The potential utility of abbreviated breast MRI (FAST MRI) as a tool for breast cancer screening: a systematic review and meta- analysis. Clin Radiol 76 (2021): 154.e11-154.e22.
- Grimm LJ, Soo MS, Yoon S, et Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 22 (2015): 1157-1162.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening Radiology 267 (2013): 47-56.
- Harvey SC, Di Carlo PA, Lee B, et al. An Abbreviated Protocol for High-Risk Screening Breast MRI Saves Time and J Am Coll Radiol 13 (2016): 374-380.
- Kuhl Abbreviated Magnetic Resonance Imaging (MRI) for Breast Cancer Screening: Rationale, Concept, and Transfer to Clinical Practice. Annu Rev Med 70 (2019): 501-519.
- Kuhl The current status of breast MR imaging. Part Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 244 (2007): 356-378.
- Ahmadinejad N, Azhdeh S, Arian A, et Implementation of abbreviated breast MRI in diagnostic and screening settings. Acta Radiol (2022): 2841851221114434.
- Tollens F, Baltzer PAT, Dietzel M, et Economic potential of abbreviated breast MRI for screening women with dense breast tissue for breast cancer. Eur Radiol (2022).
- Petrillo A, Fusco R, Sansone M, et Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat 164 (2017): 401-410.
- Panigrahi B, Mullen L, Falomo E, et al. An Abbreviated Protocol for High-risk Screening Breast Magnetic Resonance Imaging: Impact on Performance Metrics and BI-RADS Assessment. Acad Radiol 24 (2017): 1132-1138
- Oldrini G, Derraz I, Salleron J, et al. Impact of an abbreviated protocol for breast MRI in diagnostic accuracy. Diagn Interv Radiol 24 (2018): 12-16.


 Impact Factor: * 4.1
Impact Factor: * 4.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 