Experimental Compatibility of Bulinus truncatus Populations with Schistosoma haematobium in Cameroon
Article Information
Mohamed Bagayan1, Alvine Christelle Kengne-Fokam2,3, Hugues Clotaire Nana-Djeunga4, Hermann Sorgho5, Dramane Zongo5, Flobert Njiokou2,3*
1Animal Biology and Ecology Laboratory, University of Joseph Ki-Zerbo, PO Box 7021, Ouagadougou, Burkina Faso
2Parasitology and Ecology Laboratory, Faculty of Science, University of Yaoundé 1, PO Box 812, Yaoundé, Cameroon
3Centre for Research in Infectious Diseases (CRID), PO Box 13591, Yaoundé, Cameroon
4Centre for Research on Filariasis and other Tropical Diseases (CRFilMT), PO Box 5797, Yaoundé, Cameroon;
5Health Sciences Research Institute (IRSS) PO Box 7192, Ouagadougou, Burkina Faso
*Corresponding Author: Flobert Njiokou, Parasitology and Ecology Laboratory, Faculty of Science, University of Yaoundé 1, PO Box 812, Yaoundé, Cameroon
Received: 03 May 2022; Accepted: 10 May 2022; Published: 23 June 2022
Citation: Mohamed Bagayan, Alvine Christelle Kengne-Fokam, Hugues Clotaire Nana-Djeunga, Hermann Sorgho, Dramane Zongo, Flobert Njiokou. Experimental Compatibility of Bulinus truncatus Populations with Schistosoma haematobium in Cameroon. Journal of Environmental Science and Public Health 6 (2022): 243-256.
View / Download Pdf Share at FacebookAbstract
Purpose: The present study was initiated to estimate the risk of Schistosoma haematobium expansion by assessing the susceptibility of Bulinus truncatus populations to Schistosoma haematobium.
Methods: Bulinus truncatus snails were harvested from three Cameroonian localities (Loum, Mbafam, Kekem) and one situated in Burkina Faso (Yamtenga). Once G1 samples constituted, they were divided into exposed and control groups and infested with two S. haematobium strains (Loum and Barombi-Kotto). The prepatent period, infection success rate and cercarial chronobiology were studied. Effects of S. haematobium infection on snail oviposition and mortality rates were also investigated.
Results: Results showed that all Cameroonian Bulinus truncatus populations were susceptible to S. haematobium. Their infection success rates varied from 75.5% to 6.3% between sympatric and allopatric couples (p<0.05). The shortest prepatent period was found in an allopatric couple (26.90 days). The mortality rate varied among populations, it was different between exposed and control snail groups in Loum (p=0.001) while it was similar in Mbafam (p=0.423).
Conclusion: Epidemiologically, these susceptible Bulinus populations could induce a reappearance and/or an increase of the urinary schistosomiasis prevalence in Cameroon.
Keywords
Bulinus truncates; Schistosoma haematobium; Compatibility; Cameroon
Bulinus truncates Articles; Schistosoma haematobium Articles; Compatibility Articles; Cameroon Articles
Bulinus truncates articles Bulinus truncates Research articles Bulinus truncates review articles Bulinus truncates PubMed articles Bulinus truncates PubMed Central articles Bulinus truncates 2023 articles Bulinus truncates 2024 articles Bulinus truncates Scopus articles Bulinus truncates impact factor journals Bulinus truncates Scopus journals Bulinus truncates PubMed journals Bulinus truncates medical journals Bulinus truncates free journals Bulinus truncates best journals Bulinus truncates top journals Bulinus truncates free medical journals Bulinus truncates famous journals Bulinus truncates Google Scholar indexed journals Schistosoma haematobium articles Schistosoma haematobium Research articles Schistosoma haematobium review articles Schistosoma haematobium PubMed articles Schistosoma haematobium PubMed Central articles Schistosoma haematobium 2023 articles Schistosoma haematobium 2024 articles Schistosoma haematobium Scopus articles Schistosoma haematobium impact factor journals Schistosoma haematobium Scopus journals Schistosoma haematobium PubMed journals Schistosoma haematobium medical journals Schistosoma haematobium free journals Schistosoma haematobium best journals Schistosoma haematobium top journals Schistosoma haematobium free medical journals Schistosoma haematobium famous journals Schistosoma haematobium Google Scholar indexed journals Compatibility articles Compatibility Research articles Compatibility review articles Compatibility PubMed articles Compatibility PubMed Central articles Compatibility 2023 articles Compatibility 2024 articles Compatibility Scopus articles Compatibility impact factor journals Compatibility Scopus journals Compatibility PubMed journals Compatibility medical journals Compatibility free journals Compatibility best journals Compatibility top journals Compatibility free medical journals Compatibility famous journals Compatibility Google Scholar indexed journals malaria articles malaria Research articles malaria review articles malaria PubMed articles malaria PubMed Central articles malaria 2023 articles malaria 2024 articles malaria Scopus articles malaria impact factor journals malaria Scopus journals malaria PubMed journals malaria medical journals malaria free journals malaria best journals malaria top journals malaria free medical journals malaria famous journals malaria Google Scholar indexed journals parasitic disease articles parasitic disease Research articles parasitic disease review articles parasitic disease PubMed articles parasitic disease PubMed Central articles parasitic disease 2023 articles parasitic disease 2024 articles parasitic disease Scopus articles parasitic disease impact factor journals parasitic disease Scopus journals parasitic disease PubMed journals parasitic disease medical journals parasitic disease free journals parasitic disease best journals parasitic disease top journals parasitic disease free medical journals parasitic disease famous journals parasitic disease Google Scholar indexed journals health articles health Research articles health review articles health PubMed articles health PubMed Central articles health 2023 articles health 2024 articles health Scopus articles health impact factor journals health Scopus journals health PubMed journals health medical journals health free journals health best journals health top journals health free medical journals health famous journals health Google Scholar indexed journals human migration articles human migration Research articles human migration review articles human migration PubMed articles human migration PubMed Central articles human migration 2023 articles human migration 2024 articles human migration Scopus articles human migration impact factor journals human migration Scopus journals human migration PubMed journals human migration medical journals human migration free journals human migration best journals human migration top journals human migration free medical journals human migration famous journals human migration Google Scholar indexed journals population articles population Research articles population review articles population PubMed articles population PubMed Central articles population 2023 articles population 2024 articles population Scopus articles population impact factor journals population Scopus journals population PubMed journals population medical journals population free journals population best journals population top journals population free medical journals population famous journals population Google Scholar indexed journals urinary schistosomiasis articles urinary schistosomiasis Research articles urinary schistosomiasis review articles urinary schistosomiasis PubMed articles urinary schistosomiasis PubMed Central articles urinary schistosomiasis 2023 articles urinary schistosomiasis 2024 articles urinary schistosomiasis Scopus articles urinary schistosomiasis impact factor journals urinary schistosomiasis Scopus journals urinary schistosomiasis PubMed journals urinary schistosomiasis medical journals urinary schistosomiasis free journals urinary schistosomiasis best journals urinary schistosomiasis top journals urinary schistosomiasis free medical journals urinary schistosomiasis famous journals urinary schistosomiasis Google Scholar indexed journals
Article Details
1. Introduction
Schistosomiasis is the second most important parasitic disease after malaria in tropical and sub-tropical regions [1]. It is caused by a trematode worm of the genus Schistosoma. Eight species of schistosomes have anthropophilic affinity, among which Schistosoma haematobium, S. mansoni and S. guineensis are found in Cameroon [2-4]. These species are transmitted by seven (07) Intermediate snail host species, including Bulinus truncatus (B. truncatus) which is the main S. haematobium vector [2, 5, 6]. Bulinus truncatus is distributed throughout the country, and it is involved in the transmission of urinary schistosomiasis in several foci [2, 7, 8]. It has been described in the Littoral and Western regions along with the species B. globosus and B. forskalii [5].
In Littoral and Western regions of Cameroon, a decrease in schistosomiasis prevalence was observed in several transmission foci after implementation of the control program [9, 10]. Among these transmission foci, Loum, Kékem and Mbafam are hotspots where mass treatments with Praziquantel have reduced S. haematobium prevalence [10, 11]. Before starting mass drug treatments, the prevalence in Loum was 62.9% among school-age children [12]. A larger study in the Littoral showed further, a prevalence of 1.8% among school-age children [10]. In these regions, agriculture and market gardening are so prevalent that these localities are attractive for populations wishing to develop such activities. B. truncatus populations are ubiquitous in these areas where urinary schistosomiasis is present in isolated foci and where it seems to be controlled. An emergence of Schistosomiasis haematobium occurred in France on the island of Corsica [13] where B. truncatus snails were teemed. The parasite was a hybrid between human (S. haematobium) and livestock (S. bovis) parasites [14] originating from Senegal [15]. Indeed, the presence of snails and human migration may constitute a risk for the spread of schistosomiasis [16].
It then appears important to know the susceptibility of B. truncatus hosts to allopatric parasite strains in a context where the rate of human migration had raised in Cameroon especially due to localized armed conflicts and developmental project implementation. The study of snails/schistosome compatibility has shown varying degrees of susceptibility of B. truncatus to S. haematobium infection depending on the origin of the parasite [2, 17, 18]. This susceptibility of Bulinus sp. to S. haematobium has consequences on snail biology [19, 20]. Thus, infested snails may experience a decrease in fecundity up to and including parasitic castration, disrupted growth and increased mortality [21, 22]. The present study aimed to determine the susceptibility of some Bulinus truncatus populations to S. haematobium and the infection effect on their survival and reproduction.
2. Materials and Methods
2.1 Snail harvesting and rearing
Snails were collected using a stainless steel sieve mounted on a 1.5 m wooden handle to comb aquatic vegetation, or by hand-picking from the mud [4]. They were found in Loum (4°42'58'' N; 9°44'11" E) (Littoral region, Cameroon); in Kékem (5°09'47'' N, 10°0'37'' E) and Mbafam (5°7'7'' N 10°1'25'' O) (West region, Cameroon) and in Yamtenga (12°20’39.984’’ N 1°27’012’’ O) (Centre region, Burkina Faso). All the snails collected were brought alive to the snail rearing room of the Faculty of Science at Yaoundé 1 University, and maintained at 26 ± 1 °C throughout the experiment, under a 12 L/12 D photoperiod. They were identified based on their shell morphology using [23] and [24] identification criteria and fed ad libitum with dried lettuce. Water and lettuce were changed every day in very young snails rearing boxes, and every two days in those with juveniles and adults. Small floating pieces of polystyrene were introduced in these rearing boxes for egg capsules laying. For each sample, 20 wild parents (G0) were isolated, two egg capsules were taken per parent and placed in separate boxes for incubation and hatching. One week after hatching, five offspring of each G0 parent were taken at random to constitute a sample of about 100 G1 juveniles per population. These G1 juveniles were placed individually in 125 ml boxes and fed with dried lettuce. For each snail sample, G1 juveniles were divided into two groups, one to be exposed to the parasite, and the other as control.
2.2 Urine sample collection and parasite eggs acquisition
A parasitological survey was performed in Loum (4°42'58'' N, 9°44'11" E) and Barombi-Kotto (4°28'4" N and 9°15'2" E), a village located in the South West Cameroon region. Urine samples were collected from children aged between 5 and 15 in primary schools. Each child received a sterile urine collection container to be filled with urine, and these urine pots were placed in a cooler before being transported to the laboratory. Once in the laboratory, they were analyzed according to the protocol used by [2]. Positive urine samples were used for parasite acquisition. Eggs were obtained after a serie of washing and sedimentation of urine using saline solution, then spring water. Isolated eggs hatched under the action of both osmotic and thermal shocks.
Indeed, the pellet obtained after sedimentation was exposed to an artificial source of light for 30 min. After hatching, these eggs released miracidia necessary for juvenile snail infection.
2.3 Snail infection and follow-up of compatibility metrics
Snails to be exposed (2–4 mm) were placed individually in the well of a microtiter plate containing spring water. Under a binocular dissecting microscope, 5 miracidia were collected using a micropipette and transferred to each well. After five hours, each potentially infected snail was returned to its rearing box. Between 24 and 60 days post-infection, infected snails were followed up daily for cercarial shedding. Snails were individually placed in petri-dishes containing spring water and exposed to an artificial source of light (60 watt lamp) for 1 hour. This exposure water was then observed under a binocular magnifying glass. The prepatent period (duration of the larval development up to the first cercarial shedding) and the number of snails surviving at day 60 post-infection were recorded. The infection rate of each population was calculated using the formula proposed by Tian-Bi [16].
2.4 Chronobiology of the cercarial emission
For the study of this parameter, ten positive snails, having emitted cercariae, from Loum and Mbafam and eight from Kekem were used. The rate of cercarial emission was determined every hour using a protocol based on the combined methods of [18] and [25]. Snails were individually placed in pots containing 5 ml of spring water and kept from 7 am to 6 pm under light source. After each hour, snails were transferred to new pots and the water containing cercariae was spilled in a gridded cup where cercariae were counted using a binocular magnifying glass.
2.5 Influence of Schistosoma haematobium infection on Bulinus truncatus reproduction
For each population two groups of juveniles were constituted; one group made of S. haematobium exposed individuals and the other made of healthy individuals. For each snail and in each group, the age at sexual maturity (age at first egg-laying) was recorded, and the number of egg capsules, as well as eggs laid over 30 days, were counted twice a week using a binocular magnifying glass.
2.6 Statistical analysis
Data were entered, processed in Excel 2010 and analyzed with SPSS 20.0 (IBM version 20.0). The success infection rate and the mortality rate were calculated with 95% confidence intervals. The Chi-square test was used to compare the relationships between infection success rates, mortality rates and snail survival rates. Non-parametric tests (Mann-Whitney and Kruskal-Wallis tests) were carried out to compare the mean prepatent period, age at oviposition, number of clutches and number of eggs laid by exposed and control snails. The significance level was set at 5%.
3. Results
3.1 Susceptibility of three B. truncatus populations with Loum strain of Schistosoma haematobium
The results of susceptibility of B. truncatus from Loum, Mbafam and Kekem to Schistosoma haematobium of Loum are presented in Table 1. The success infection rates varied significatively between exposed samples. The highest rate was observed in the allopatric snail population of Mbafam (75.5%, CI 95%: 62.4% – 85.1%). The sympatric snails of Loum displayed a moderate infection success rate (39.1%, CI 95%: 30.5%– 48.4%) higher than in Kékem allopatric snails (12.1%) with the differences being statistically significant (p<0.0001). Regarding the prepatent period, our results showed that cercarial shedding started between the 26th and 30th day post-infection. The prepatent period is longer in the sympatric population than in the allopatric population of Mbafam (p < 0.0001).
|
Infestation parameters |
Snail samples |
||
|
Kekem |
Loum |
Mbafam |
|
|
No. exposed snails |
66 |
110 |
53 |
|
No. infested snails |
8 |
43 |
40 |
|
Infection success rate ( with 95% CI) |
12.1% (6.3 %- 22.1%) |
39.1% (30.5 %– 48.4%) |
75.5%* (62.4 %– 85.1%) |
|
Mean prepatent period (days: m±se) |
30±0.0 |
28.47 ± 0.146 |
26.9 ± 0.277* |
No: Number of; * p<0.05; 95% CI: Confidence interval at 95%; m±se: mean (m) and standard error (se)
Table 1: Susceptibility of Bulinus truncatus from Kekem, Mbafam and Loum to Schistosoma haematobium of Loum.
3.2 Susceptibility of Bulinus truncatus from Kekem and Yamtenga (Burkina Faso) to Schistosoma haematobium of Barombi-Kotto
The results of the snail exposition to Barombi-Kotto parasite strain are presented in Table 2. This second experimentation was made for comparison purpose. Infection success rates of exposed samples were very low but significantly (p = 0.0059) higher for kekem snails (6.3 %) than for Yamtenga snails (0.64%). Post-exposed mortality rate was significantly (p = 0.00243) higher for Kekem than for Yamtenga snails.
|
Experimental parameters |
B. truncatus |
populations |
P value |
|
Kekem |
Ouagadougou |
||
|
No. of snails |
158 |
157 |
|
|
No. of infected snails |
10 |
1 |
|
|
Infection success rate ( 95% CI) |
6.3% (3.5% - 11.26%) |
0.64% (0.1% - 3.5%) |
0.0059 |
|
Prepatent period |
30 ±0.00 |
30±0.00 |
|
|
No. of snails dead |
108 |
88 |
|
|
Mortality rate ( 95% CI) |
68.4% (60.7% – 75.1%) |
56.1 (48.2% - 63.6%)* |
0.00243 |
*: p <0.05; 95% CI: Confidence interval at 95%
Table 2: Kekem and Yamtenga Bulinus truncatus susceptibilty to Schistosoma haematobium of Barombi-Kotto.
3.3 Cercarial emission chronobiology
Cercarial emission peaks were observed between 8 am and 11 am in different B. truncatus populations (Figure 1). In sympatric snails, the first peak was observed between 8-9 am and the second peak between 10-11 am. The emission peak in Kékem B. truncatus population was observed between 10 and 11 am while in Mbafam snails, it happened between 09-10 am. Additionally, the peak of Kékem was the highest with 4972 cercariae emitted, followed by Mbafam with 3207 cercariae and finally Loum with 2094 cercariae between 8 am and 11 am.
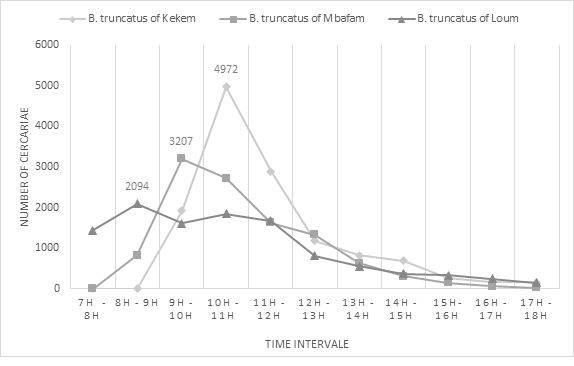
Figure 1: Chronobiology of Schistosoma haematobium cercariae in infested B.truncatus individuals.
3.4 Influence of infection on reproduction and survival of Bulinus truncatus
Table 3 shows the number of egg capsules and egg embryos laid by snails in each population. In the sympatric population of Loum, the number of egg capsules and egg embryos laid by control individuals were respectively similar to those laid by exposed individuals (p = 0.573; p = 0.622 ). In the allopatric population of Mbafam however, control individuals laid respectively more egg capsules and egg embryos than exposed snails (p ≤0.001). The age of first egg-laying was similar between control and exposed snails from Loum (p = 0.072). On the other hand, it was higher for control than for exposed snails from Mbafam (p = 0.001). Analyses showed that the oviposition period was longer in exposed than in control snails from the sympatric population of Loum (p < 0.0001). In comparison, the oviposition period did not differ significantly (p = 0.552) between exposed and control snails in Mbafam.
|
Experimental variables |
Exposed and control snail groups |
|||||
|
Loum |
Mbafam |
|||||
|
Exposed |
Control |
P |
Exposed |
Control |
p |
|
|
No. snails |
110 |
100 |
53 |
53 |
||
|
No. egg-laying snails |
32 |
37 |
41 |
25 |
||
|
(%) |
29.09 |
33.6 |
0.862 |
77.3 |
47.1 |
0.12 |
|
No. egg capsules (m±se) |
3.08±0.783 |
1.87±0.581 |
0.573 |
4.51±1.22 |
11.09±1.705 |
0.0001 |
|
No. egg embryos (m±se) |
5.90±1.745 |
3.60±1.315 |
0.622 |
10.2±3.069 |
22.15±3.581 |
0.0001 |
|
Laying period (m±se) (days) |
44.2±0.999 |
36.2±1.246 |
0.0001 |
38.9±0.968 |
37.6±1.211 |
0.552 |
|
Age at first egg-laying (m±se) (days) |
49.7±0.844 |
55.6±2.034 |
0.072 |
45.5±0.719 |
49.6±0.934 |
0.001 |
No.: Number of; %: percentage; (m±se) : mean (m) and standard error
Table 3: Reproduction of Bulinus truncatus snails according to their infested status.
|
Experimental variables |
Exposed and control snail groups |
||||
|
Loum |
Mbafam |
||||
|
Exposed |
Control |
Exposed |
Control |
||
|
No. of snails |
110 |
100 |
53 |
53 |
|
|
No. of snails dead before cercarial shedding |
4 |
0 |
1 |
0 |
|
|
Mortality rate before cercarial release (%) |
3,6% (CI :1.4% - |
0% (CI : 0% – 3.7%) |
1,9% (CI : 0.3% - 9.9%) |
0% (CI : 0% - 6.7%) |
|
|
No. of snails dead during experiment |
61 |
78 |
18 |
22 |
|
|
Mortality rate at the end of the experiment (CI 95% ) |
55.5% (46.1% - 64.4%) |
78% (68.9% - 85%) |
34% (CI : 22.7% - 47.4%) |
41.5% (29.2% - 45.9%) |
|
|
Test |
p = 0.001 |
p = 0.423 |
|||
|
No. of snails alive at the end of the experiment |
49 |
22 |
35 |
31 |
|
|
Survival rate (CI 95% ) |
44.5% (CI : 35.6% - 53.8%) |
22% (CI : 15% - 31.1%) |
66% (CI : 52.6% - 77.3%) |
58.5% (CI : 45.1% - 70.7%) |
|
|
Test |
p < 0.0001 |
p = 0.423 |
|||
|
Mean survival time (days) (m±es) |
51.6 ± 1.04 |
43.4 ±1.30 |
55.9 ±1.3 |
54.62 ±0.67 |
|
|
Test |
p < 0.0001 |
p = 0.523 |
|||
95% CI: Confidence interval at 95%; (m±es): mean (m) and standard error (es)
Table 4: Evolution of post infestation survival of Loum and Mbafam snails.
3.5 Survival of Bulinus truncates snails from Mbafam and Loum
Survival rates in allopatric and sympatric populations are displayed in Table 4. These survival rates significantly differed between control and exposed snails from Loum (p < 0.0001) while they were almost similar between control and exposed snails from Mbafam (p = 0.423). Mortality at the end of the study was significantly higher in control than in exposed snails in Loum (p = 0.001), whereas it was almost similar between exposed and control snails from Mbafam (p = 0.423).
3. Discussion
All B. truncatus populations from Loum, Kekem and Mbafam were susceptible to Loum strain of S. haematobium whereas snails from Yamtenga and Kekem were rather resistant to Barombi-Kotto strain of S. haematobium. Infection success rates varied among snail populations. In the present study, the highest success rate was found in the allopatric pair (Loum parasite/Mbafam snails). Our results are different from those commonly found in the literature according to which the highest infection success rates are often observed in sympatric pairs [2, 16, 26, 27], the main explanation being a better adaptation of S. haematobium to its local intermediate host [28].
But this hypothesis is questioned by other results [26 29, 30]. Indeed, [29] found that the susceptibility rate of Bulinus truncatus from Saudi Arabia was higher than those from Egypt exposed to a strain of S. haematobium from Egypt. Further, after conducting a meta-analysis on the compatibility of S. mansoni with Biomphalaria alexandrina in Egypt, [30] revealed no evidence of local adaptation for the sympatric parasite which is in line with our results. Finally, schistosome/snail compatibility may not only depend on the level of parasite adaptation to its host, but also to the host genetic susceptibility that may vary among snail populations. In this study, Mbafam snails appeared more susceptible to schistosome than Loum and Kekem snails. This result underlined the risk of expansion of urinary schistosomiasis in Mbafam knowing that human migrations are high between regions. The sympatric pair had the second-highest level of infection success rate. This help to understand the persistence of urinary schistosomiasis in Loum and confirm previous studies that have pointed B. truncates as the major intermediate host of S. haematobium in this locality [7, 31]. The allopatric population of Kékem had the lowest infection success rate. This snail population appears to be more adapted to S. haematobium from the same locality, as suggested by [2] after observing a very high infection success rate for Kekem sympatric pair. This observation is confirmed by the lowest infection success rate obtained in Kekem snails infected with the Barombi-Kotto parasite strain. The geographic distance between Barombi-Kotto parasite strain (Central Africa) and Yamtenga snails (West Africa) could account for their poor adaptation.The rate at which cercariae are released is linked to both genetic factors of the parasite, behaviour of the definitive hosts [32, 33] and environment of the snail host [34]. In line with these thoughts, our results on cercarial chronobiology of S. haematobium from Loum showed all cercarial peaks situated in the morning between 8 am and 11 am.
The different peak slice of hours observed between B. truncatus populations could reflect genetic differences among these populations. Cercarial emission peaks are adapted to the behaviour of the definitive host in order to increase the chance of infection of this definitive host and to allow the continuation of the parasite cycle [25, 35, 36, 37]. Thus, schistosomes infesting humans have diurnal cercarial emission peaks whereas schistosomes infesting antelopes and rodents displayed nightly cercariae emission concurring with the periods when these animals frequent aquatic environments [25]. Results obtained in other studies [37, 38, 39] on S. haematobium and S. mansoni [40] shows cercarial emission peaks located between 11 am and 3 pm. Our results could be explained by differences in applied protocols to stimulate cercarial emission [41, 42], or in the probably genetic differences between S. haematobium strains studied.
The influence of S. haematobium on B. truncatus reproduction was determined by assessing the number of egg capsules and egg embryos laid, the age at sexual maturity, the duration of the egg-laying period and the survival period of snail populations. Egg-laying was observed in all exposed and control B. truncatus individuals showing that infection with S. haematobium from Loum, though altering host fecundity does not forestall its reproduction. The results are comparable to those of [43] who found that B. truncatus was not castrated when exposed to S. haematobium from Barombi-Kotto. In opposition to our results, [38] observed a castration of infected B. truncatus, with a total cessation of oviposition. Our results suggest a good adaptation of Loum strain to these snail populations and a risk of expansion of schistosomiasis in localities where these snail populations are present. Several studies revealed that infection of B. globosus by S. haematobium reduces its oviposition [19, 44]. During our study, this observation was verified in the B. truncatus population of Mbafam where the number of eggs and egg embryos laid were higher in the controls than in the exposed snails. In addition, infection seems to induce early reproduction in B. truncatus from Mbafam since the age of sexual maturity is lower in exposed than in control snails.
The mortality rate is a very difficult parameter to analyze [8]. It could be related to the pathogenicity of parasites [8, 44, 45], to the intrinsic conditions of snails or to experimental conditions [2]. In the present study, the mortality rate was similar in exposed and control snails in the Mbanfam population. In contrast, in the sympatric population from Loum, mortality was higher in the controls than in the exposed snails. This latter result suggests that the parasite is not associated with the mortality of Loum snails. The lesser adaptation of this Loum population to the laboratory conditions could account for this higher mortality. In fact, this population was collected in the Littoral region where temperature is higher than in our snail rearing room. The variability observed in mortality rates between different populations at the end of the experiment could also reflect genetic differences between these snail populations, implying different individual fitness.
This compatibility study between B. truncatus and S. haematobium is in line with the 5th WHO guideline for the elimination of schistosomiasis which promote snail surveillance [46]. It delivers probant data of schistosomiasis threat in studied localities. It can be used to reinforce sensitization of community and their commitment in schistosomiasis control; especially through behavioural change and snail control interventions.
5. Conclusions
The results obtained during the present study showed that despite their geographical distribution, the different populations of Bulinus truncatus are all compatible with Loum strain of S. haematobium. They showed a better adaptation of Loum S. haematobium to Bulinus truncatus of Mbafam through a higher infection rate and a shorter prepatent period. This snail population remains thus a potential host for the expansion and re-emergence of urinary schistosomiasis in this locality. Infection did not substantially affect the reproduction of B. truncatus populations confirming the compatibility of Loum S. haematobium to these snail host populations.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and investigation were performed by Mohamed BAGAYAN and Alvine Christelle KENGNE-FOKAM, Hermann SORGHO and Dramane ZONGO. Statistical analyses were realized by Mohamed BAGAYAN and Hugues Clotaire NANA-DJEUNGA. The first draft of the manuscript was written by Mohamed BAGAYAN, Alvine Christelle KENGNE-FOKAM and Hugues Clotaire NANA-DJEUNGA. The study supervision was done by Flobert NJIOKOU. All authors read and approved the final manuscript.
Ethics Approval
All procedures performed in the part of this study involving human participants were in accordance with ethical standards. The study was approved by the Cameroon National Ethic Committee for Research in Human Health (CNERSH) and an ethical clearance was issued (N°2016/02/716/CE/CNERSH/SP). Verbal informed consent was obtained from all legal guardian participants included in this study.
Conflict of Interests
We manifest that there is no financial or another conflict of interest.
References
- Hotez PJ, Kamath A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Negl Trop Dis 3.8 (2009): e412
- Njiokou F, Teukeng F, Bilong Bilong CF, et al. Étude expérimentale de la compatibilité entre Schistosoma haematobium et deux espèces de bulins au Cameroun. Bull Soc Pathol Exot 97.1 (2004): 43-46.
- Lawton SP, Hirai H, Ironside JE, et al. Genomes and geography: genomic insights into the evolution and phylogeography of the genusSchistosoma.Parasites Vectors 4 (2011): 131, 11.
- Kengne-Fokam AC, Nana-Djeunga HC, Djuikwo-Teukeng FF, et al. Analysis of mating system, fecundity, hatching and survival rates in two Schistosoma mansoni intermediate hosts (Biomphalaria pfeifferi and Biomphalaria camerunensis) in Cameroon. Parasites Vectors 9.1 (2016): 1-9.
- Greer GJ, Mimpfoundi R, Malek EA, et al. Human schistosomiasis in Cameroon. Distribution of the snail hosts. Am J Trop Med Hyg 42.6 (1990): 573-580.
- Ngonseu E, Greer GJ, Mimpfoundi R. Dynamique des populations et infestation de Bulinus truncatus et Bulinus forskalii par les larves de schistosomes en zone soudano-Sahélienne au Cameroun. Ann Soc Belge Méd Trop 72 (1992): 311-320.
- Southgate VR, Van Wijk HB, Wright CA. Schistosomiasis at Loum, Cameroon: Schistosoma haematobium, S. intercalatum and theit natural Hybrid Z. F. Parasitenk 49 (1976): 14-159.
- Ndassa A, Mimpfoundi R. Studies on the morphology and compatibility between Schistosoma hæmatobium and the Bulinus sp. complex (gastropoda : planorbidae) in Cameroun. Afr J Biotechnol 4.9 (2005): 1010-1016.
- Tchuem Tchuente LA, Ngassam RIK, Sumo L, et al. Mapping of schistosomiasis and soil-transmitted helminthiasis in the Regions of Centre, East and West Cameroon. PLoS Negl. Trop. Dis 6.3 (2012): e1553.
- Tchuem Tchuenté LA, Noumedem CD, Ngassam P, et al. Mapping of schistosomiasis and soil-transmitted helminthiasis in the Regions of Littoral, North-West, South and South-West Cameroon and recommendations for treatment. BMC Infect. Dis 13.1 (2013):1-12.
- Dankoni EN, Tchuem Tchuenté LA. Epidémiologie de la schistosomiase et des géohelminthiases dans l’Arrondissement de Kékem (Ouest- Cameroun). I. J. I. A. S 8 (2014): 1782-1790.
- Tchuem Tchuenté LA, Behnke JM, Gilbert FS, et al. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infectious among school children in Loum, Cameroon. Med. Int. Health 8 (2003): 975-986.
- Berry A, Moné H, Iriart X, et al. Schistosomiasis haematobium, Corsica, France. Emerging Infectious Diseases 20 (2014): 1595-1597.
- Moné H, Holtfreter MC, Allienne JF, et al. Introgressive hybridizations of Schistosoma haematobium by Schistosoma bovis at the origin of the first case report of schistosomiasis in Corsica (France, Europe). Parasitology Research 114 (2015): 4127-4133.
- Boissier J, Grech-Angelini S, Webster BL, et al. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. The Lancet. Infectious Diseases 16.8 (2016): 971-979.
- Tian-Bi TYN, N’Guessan AN, Coulibaly MY, et al. Variabilité de l’interaction Bulinus truncatus–Schistosoma haematobium à travers trois générations de mollusques: implications épidémiologiques. J Appl Biosci 4.8 (2011): 3284-3292.
- Rollinson D, Kaukas A, Johnston D, et al. Some molecular insights into schistosome evolution. J. Parasitol 27 (1997):11-28.
- Sène M, Southgate VR, Vercruysse J. Bulinus truncatus, hôte intermédiaire de Schistosoma haematobium dans le bassin du fleuve Sénégal. Soc. Pathol. Exot 97 (2004): 29-32.
- Njiokou F, Bellec C, N’Goran EKN, et al. Comparative fitness and reproductive isolation between two Bulinus globosus (Pulmonata: Planorbidae) populations. Molluscan Stud 58 (1992): 367-376.
- Zein-Eddine R, Djuikwo-Teukeng FF, Al-Jawhari M, et al. Phylogeny of seven Bulinus species originating from endemic areas in three African countries, in relation to the human blood fluke Schistosoma haematobium. BMC Evol. Biol 14.1 (2014): 1-12.
- Webster BL, Southgate VR, Tchuem Tchuenté LA. Mating interactions between Schistosoma haematobium and S. mansoni. J. Helminthol 73 (1999): 351-356.
- Webster JP, Hoffman JI, Berdoy M. Parasite infection, host resistance and mate choice: battle of the genders in a simultaneous hermaphrodite. Proc. Biol. Sci 270 (2003): 1481-485.
- Pennak E. Snail identification centre. A field guide to African freshwater snail I: West African species. Danish Bilharziasis Laboratory (1978): 5-15.
- Brown DS. Fresh water snails of Africa and their medical importance, CRC press (1994).
- Ahmed AAM, Ibrahim NA, Idris MA. Laboratory studies on the prevalence and cercarial rhythms of Trematodes from Bulinus truncatus and Biomphalaria pfeifferi snails from Khartoum State, Sudan. Sultan Qaboos Univ. Med. J 6 (2006): 65-69.
- Wang CZ, Lu DB, Guo CX, et al. Compatibility of Schistosoma japonicum from the hilly region and Oncomelania hupensis hupensis from the marshland region within Anhui, China. Parasitol Res 113 (2014): 4477-4484.
- N’guessan A, Tian-Bi T, Orsot N, et al. Variabilité de la compatibilité entre Schistosoma haematobium et ses hôtes potentiels dans la zone préforestière de Côte d’Ivoire : Implications épidémiologiques. J Appl Biosci 85 (2015): 7862.
- Perrin C, Lepesant JM, Roger E, et al. Schistosoma mansoni Mucin Gene (SmPoMuc) Expression: Epigenetic control to shape adaptation to a new host. PLoS Pathog 9 (2013).
- Abou-el-naga IF. Meta-analysis indicates lack of local adaptation of Schistosoma mansoni to Biomphalaria alexandrina in Egypt. Parasitol Res 113.3 (2014): 1185-1194.
- Mostafa OMS, Bin Dajem SM, Abu El Einin HM. Susceptibility of Saudi Bulinus truncatus to infection with Egyptian Schistosoma haematobium with observations on protein electrophoretic pattern of the snails. Vet Parasitol 161 .3 (2009): 207-212.
- Tchuem Tchuenté LA, Southgate VR, Njioko F, et al. The evolution of schistosomiasis at Loum, Cameroon: replacement of Schistosoma intercalatum by S. haematobium through introgressive hybridization. R. Soc. Trop. Med. Hyg 91 (1997): 664-665.
- Mouahid A, Moné H, Chaib A, et al. Cercarial shedding patterns of Schistosoma bovis and S. haematobium from single and mixed infections of Bulinus truncatus. J Helminthol 65.1 (1991): 1-8.
- Théron A, Mouahid G, Moné H. Schistosoma mansoni: cercarial shedding patterns from a mixed infection of Biomphalaria glabrata with two (early and late) chronobiological variants. Res 83 (1997): 356-358.
- Boissier J, Mouahid G, Moné H. Schistosoma spp. Global Water Pathogen Project. Michigan State University (2019).
- Kengne-Fokam AC, Nana-Djeunga HC, Bagayan M, et al. Biomphalaria camerunensis as a viable alternative intermediate host for Schistosoma mansoni in southern Cameroon.Parasites vectors 11.1 (2018): 1-9.
- Combes C, Fournier A, Moné H, et al.. Behaviours in trematode cercariae that enhance parasite transmission: patterns and processes. Parasitology 109 (1994): 3-13.
- N’goran E, Brémond P, Sellin E, et al. Intraspecific diversity of Schistosoma haematobium in West Africa: chronobiology of cercarial emergence. Acta Trop 66 (1997): 35-44.
- Ibikounlé M, Mouahid G, Mintsa Nguema R, et al. Snail intermediate host / Schistosoma haematobium relationships from three transmission sites in Benin (West Africa ). Res 11.2 (2013): 227-233.
- Mintsa-Nguéma R, Moné H, Ibikounlé M, et al. Cercarial emergence pattern of Schistosoma haematobium from Libreville, Gabon. Parasite 21 (2014): 1-5.
- Southgate VR, Tchuem Tchuente LA, Theron A, et al. Compatibility of Schistosoma mansoni Cameroon and Biomphalaria pfeifferi Senegal. Parasitology 121 (2000): 501-504.
- Rollinson D, Stothard JR, Southgate VR. Interactions between intermediate snail hosts of the genus Bulinus and schistosomes of the Schistosoma haematobium group. Parasitology 123 (2001): 245-260.
- Ibikounlé M, Mone H, Abou Y, et al. Premier cas de chronobiologie des émissions cercariennes de type infradien chez Schistosoma mansoni dans deux foyers du sud-Bénin. Int. J. Biol. Sci 6.3 (2012): 1081-1089.
- Nkengazong L, Njiokou F, Asonganyi T. Two years impact of single praziquantel treatment on urinary schistosomiasis in the Barombi Kotto focus, South West Cameroon. J. Parasitol. Vector Biol 5.6 (2013): 83-89.
- Kalinda C, Chimbari MJ, Mukaratirwa S. Effect of temperature on the Bulinus globosus-Schistosoma haematobium system. Infect Dis Poverty 6.57 (2017): 7.
- Tchuem Tchuenté LA, Southgate VR, Théron A, et al. Compatibility of Schistosoma mansoni and Biomphalaria pfeifferi in Northern Senegal. Parasitology 118 (1999): 593-603.
- Yapi Yapi G, Toure M, Boka OM, et al. Dynamique des populations de Biomphalaria pfeifferi et de Bulinus globosus en zone d’endémie schistosomienne en Côte d ’Ivoire. Sci. J 10.17 (2014): 339-363.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 