Development of Okra (Abelmoschus esculentus L.) Inulin extruded Snacks and Evaluation of their Prebiotic and Safety Potentials
Article Information
Uchenna Umeonuoraha*, Gibson Arueyab, Temitope Ogunbanwoc, Yetunde Ogunremid
a,bDepartment of Food Technology, Faculty of Technology, University of Ibadan, Nigeria.
cDepartment of Microbiology, Faculty of Science, University of Ibadan, Nigeria.
dDepartment of Clinical Pharmacy and Pharmacy Administration, University of Ibadan, Nigeria.
*Corresponding Author: Uchenna Umeonuorah, Department of Food Technology, Faculty of Technology, University of Ibadan, Nigeria.
Received: 28 January 2022; Accepted: 07 February 2022; Published: 15 March 2022
Citation: Uchenna Umeonuorah, Gibson Arueya, Temitope Ogunbanwo, Yetunde Ogunremi. Development of okra (Abelmoschus esculentus L.) Inulin extruded snacks and evaluation of their prebiotics and safety potentials. Journal of Food Science and Nutrition Research 5 (2022): 358-370.
View / Download Pdf Share at FacebookAbstract
Food industries are currently facing the challenge of meeting consumers’ demand for functional foods that provide additional health benefits. Development of appropriate extruded food snacks with inulin inclusion and its associated prebiotic benefits has not be fully exploited. This work is therefore aimed at bridging this gap. Okra pod (Abelmoschus esculentus L) inulin was blended with rice flour and other raw material inputs (sugar, salt, fat and water). These were subjected to extrusion conditions (feed rate: 300g/min, barrel temperature: 125oC and internal pressure: 20.5x10-5N/M2) for 30min. Thereafter, the extrudates were air cooled at room temperature. The resulting extrudates were subjected to bifidogenic invitro analysis using pure probiotic organisms (Bifidobacterium bifidum and Lactobacillus bulgaricus) and safety analyses using animal studies (wistar rats n=50). There were no significant differences (P<0.05) on the percentage weight gain of the experimental animals. Wistar rats fed with experimental snack samples showed progressive weight gain compared with the ones fed with control sample. Mean haemoglobin values were between 15.57mg/dl and 16.93mg/dl indicating that the snack samples are safe for human consumption. Generally, the developed snack samples were proved to have prebiotic potentials as they enhanced the growth of Bifidobacterial bifidum and Lactobacillus bulgaricus bacteria thrice more than the control sample.
Keywords
Okra pod, Inulin, Prebiotics, Probiotics, Extrusion
Okra pod articles, Inulin articles, Prebiotics articles, Probiotics articles, Extrusion articles
Okra pod articles Okra pod Research articles Okra pod review articles Okra pod PubMed articles Okra pod PubMed Central articles Okra pod 2023 articles Okra pod 2024 articles Okra pod Scopus articles Okra pod impact factor journals Okra pod Scopus journals Okra pod PubMed journals Okra pod medical journals Okra pod free journals Okra pod best journals Okra pod top journals Okra pod free medical journals Okra pod famous journals Okra pod Google Scholar indexed journals Inulin articles Inulin Research articles Inulin review articles Inulin PubMed articles Inulin PubMed Central articles Inulin 2023 articles Inulin 2024 articles Inulin Scopus articles Inulin impact factor journals Inulin Scopus journals Inulin PubMed journals Inulin medical journals Inulin free journals Inulin best journals Inulin top journals Inulin free medical journals Inulin famous journals Inulin Google Scholar indexed journals Prebiotics articles Prebiotics Research articles Prebiotics review articles Prebiotics PubMed articles Prebiotics PubMed Central articles Prebiotics 2023 articles Prebiotics 2024 articles Prebiotics Scopus articles Prebiotics impact factor journals Prebiotics Scopus journals Prebiotics PubMed journals Prebiotics medical journals Prebiotics free journals Prebiotics best journals Prebiotics top journals Prebiotics free medical journals Prebiotics famous journals Prebiotics Google Scholar indexed journals Probiotics articles Probiotics Research articles Probiotics review articles Probiotics PubMed articles Probiotics PubMed Central articles Probiotics 2023 articles Probiotics 2024 articles Probiotics Scopus articles Probiotics impact factor journals Probiotics Scopus journals Probiotics PubMed journals Probiotics medical journals Probiotics free journals Probiotics best journals Probiotics top journals Probiotics free medical journals Probiotics famous journals Probiotics Google Scholar indexed journals Extrusion articles Extrusion Research articles Extrusion review articles Extrusion PubMed articles Extrusion PubMed Central articles Extrusion 2023 articles Extrusion 2024 articles Extrusion Scopus articles Extrusion impact factor journals Extrusion Scopus journals Extrusion PubMed journals Extrusion medical journals Extrusion free journals Extrusion best journals Extrusion top journals Extrusion free medical journals Extrusion famous journals Extrusion Google Scholar indexed journals food products articles food products Research articles food products review articles food products PubMed articles food products PubMed Central articles food products 2023 articles food products 2024 articles food products Scopus articles food products impact factor journals food products Scopus journals food products PubMed journals food products medical journals food products free journals food products best journals food products top journals food products free medical journals food products famous journals food products Google Scholar indexed journals cholesterol level articles cholesterol level Research articles cholesterol level review articles cholesterol level PubMed articles cholesterol level PubMed Central articles cholesterol level 2023 articles cholesterol level 2024 articles cholesterol level Scopus articles cholesterol level impact factor journals cholesterol level Scopus journals cholesterol level PubMed journals cholesterol level medical journals cholesterol level free journals cholesterol level best journals cholesterol level top journals cholesterol level free medical journals cholesterol level famous journals cholesterol level Google Scholar indexed journals nutritional requirements articles nutritional requirements Research articles nutritional requirements review articles nutritional requirements PubMed articles nutritional requirements PubMed Central articles nutritional requirements 2023 articles nutritional requirements 2024 articles nutritional requirements Scopus articles nutritional requirements impact factor journals nutritional requirements Scopus journals nutritional requirements PubMed journals nutritional requirements medical journals nutritional requirements free journals nutritional requirements best journals nutritional requirements top journals nutritional requirements free medical journals nutritional requirements famous journals nutritional requirements Google Scholar indexed journals chocolate manufacturing articles chocolate manufacturing Research articles chocolate manufacturing review articles chocolate manufacturing PubMed articles chocolate manufacturing PubMed Central articles chocolate manufacturing 2023 articles chocolate manufacturing 2024 articles chocolate manufacturing Scopus articles chocolate manufacturing impact factor journals chocolate manufacturing Scopus journals chocolate manufacturing PubMed journals chocolate manufacturing medical journals chocolate manufacturing free journals chocolate manufacturing best journals chocolate manufacturing top journals chocolate manufacturing free medical journals chocolate manufacturing famous journals chocolate manufacturing Google Scholar indexed journals
Article Details
1. Introduction
The World Health Organization (WHO) and food industries placed a high premium on food products that meet consumers’ demand for a healthy lifestyle, with the aim of reducing the risk of chronic illness [1]. Currently, food industries are facing a serious challenge of meeting consumers’ demand for foods that provide additional health benefits such as lowering of cholesterol level, aid in digestion and at the same time meet nutritional requirements [2]. Some of the reasons for the increased in demand include the influence of modern lifestyles, rising costs of health care due to higher life expectancy, and older people’s natural interest in increasing their quality of lives [3]. Today, foods are not only intended to satisfy hunger and provide necessary nutrients for humans but also helps to prevent nutrition-related diseases and improve physical and mental well-being of the consumers [4]. However, consumers’ preference towards natural products or ingredients has encouraged the continual search for viable natural sources for prebiotics [5]. Inulin belongs to the natural fructans molecules in which fructose monomers connected by β-(2 1) glycoside bond. It is naturally found in plants such as chicory roots, Jerusalem Artichoke, agave, cereals, fruits and vegetables such as banana, onions, asparagus, okra and others [6]. Generally, inulin is high in fibre and has the potential benefits to aid in digestion, reduce body cholesterol level [7,8]. It is gaining rapid recognition due to its application in both food and non-food uses [9]. In food and beverage industries, it is widely used to produce food and drinks with health benefiting properties. It has long been accepted and recognized by US Food and Drug Administration (FDA) and other regulatory authorities as generally recognized as safe (GRAS) to be used in food production. The Global inulin market demand has been projected to USD 1.5 billion by 2023 according to inulin market size and share report for 2020. The dairy sector is one of the areas where inulin has been widely used, because of its prebiotic properties as well as the texture it imparts, comparable to fat creaminess [10]. Chicory inulin has successfully been utilized in chocolate manufacturing [11]. At present, there is no documented literature regarding snack foods-based chicory root inulin and indeed from any other source including okra pod. Admittedly, the production of these snack food incorporating alternate inulin source such as okra pod would generate novel functional foods while also reducing pressure on inulin sourced from chicory. Snacks constitute an important part of a vital part of daily nutrient and calorie intake for many consumers [12]. This work is therefore aimed at utilizing okra (Abelmoschus esculentus L.) pod inulin along with other raw material inputs (salt, sugar, fat and water) in the development of safe and acceptable snack foods exihibiting significant prebiotic potentials. This constitutes the main objective of this study.
2. Materials and Methods
2.1 Raw Material Sourcing and Pre-processing
Rice (Oryza glaberrima) flour was sourced from Abakiliki rice mill, Ebonyi State. The pure microorganism cultures (Bifidobacterium bifidum (ATCC-11863) and Lactobacillus bulgaricus (BAA-365) as well as commercial inulin were purchased from ThermoFisher Scientific, USA. Snack ingredients such as salt, sugar (Dangote brand) and blue band butter were purchased at Foodco supermarket Bodija, Ibadan, Oyo state, Nigeria. Okra (Abelmoschus esculentus L) pods inulin (% purity: 95.00-96.05%) was used. All chemicals used for the laboratory analysis were of analytical grade.
2.2 Snack samples
The inulin was formulated with rice flour and other raw material inputs (sugar, fat, salt and water) to form dough. That was extruded using a single-screw extruder (NASOD Ltd, China) set at optimum parameters (feed rate: 300g/min, barrel temperature: 125oC and internal pressure: 20.5x10-5N/M2 for 30min). (Table 1 and Figure 1) [13]. The extrudate was allowed to air-cool and subsequently packed in a Ziploc packaging material prior to analysis (Plate 1).
|
Samples |
||||
|
Ingredients |
CSS |
CISS |
SISS |
OISS |
|
Inulin Powder (g) |
0 |
25 |
25 |
25 |
|
Rice Flour (g) |
500 |
500 |
500 |
500 |
|
Sugar (g) |
50 |
50 |
50 |
50 |
|
Salt (g) |
7 |
7 |
7 |
7 |
|
Fat (g) |
15 |
15 |
15 |
15 |
|
Water (ml) |
300 |
300 |
300 |
300 |
CSS = Snack food produced without Inulin (Control)
CISS = Snack food made with commercial Inulin inclusion
SISS = Snack food made from sundried okra pod-Inulin Inclusion
OISS = Snack food made from oven dried okra pod-Inulin Inclusion
Table 1: Recipe for the extruded snack food samples
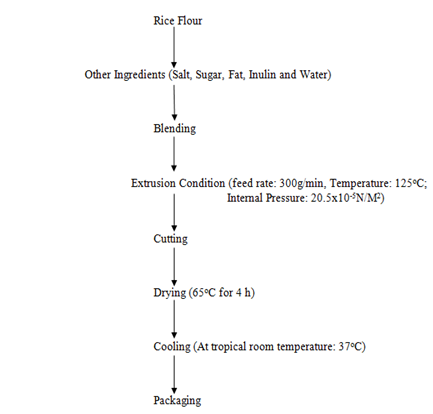
Figure 1: Production of extruded snack food from okra pod Inulin and other ingredients
3. Results
All glass wares were washed thoroughly with water and detergent, rinsed and thereafter allowed to air dry before sterilizing in an oven for one hour at 180°C. Inoculating loops were sterilized by flaming across Bunsen flame at intervals. All chemicals and stains were of analytical grade. The media used were prepared according to manufacturers' standard prior to sterilization at 121°C for 15 min [14]. The work bench was cleaned with disinfectant before and after each experimentation to ensure an aseptic working condition.
3.1.1 Assessment of prebiotic potential of the inulin and its food snack derivatives: The determination of Bifidogenic potential of the inulin and its snack derivative were conducted following the method of Huebner [15]. Bifidobacterium bifidum and Lactobacillus bulgaricus were the microbes of probiotic interest. These were cultured on 2% glucose, 2% inulin and on the media without inulin. Their growth rates were determined using single beam spectrophotometer (752 N BOSCH). The isolates from the organism were sub cultured on Mann Rogosa Sharpe (MRS) agar medium and was incubated micro-aerophillically at 37oC for 48 h for further analysis. Bifidobacterium bifidum and Lactobacillus bulgaricus were obtained and homogenized in sterile peptone. They were next cultured unto MRS broth at 37oC for 24 hours each. Thereafter, a loopful of the broth were sub cultured by streaking unto MRS agar and inoculated micro-aerophillically at 37oC for 24 48 and 72 h in an anaerobic chamber (SNR-0004621 China). Distinct colonies of Bifidobacterium bifidum and Lactobacillus bulgaricus were further sub cultured on MRS agar media plates separately. Repeated streaking was done to obtain pure cultures and thereafter morphologically characterized. The pure isolates were next stored in MRS agar slants at 4oC.
3.1.2 Evaluation of the growth culture pattern: A single colony of Bifidobacterium bifidum and Lactobacillus bulgaricus were inoculated in 10ml sterilized MRS broth and incubated in an anaerobic chamber at 37oC for 24, 48 and 72 h. Thereafter, it was centrifuged at 300 rpm for 10 min and the supernatant decanted. Overnight cultures were diluted by one tenth using basal MRS medium. The Inulin and the snacks were prepared by taking 2 g each of the samples of the inulin and the snacks samples. Ten milliliters of liquid media were added to the sample (basal MRS for probiotics) and transferred by pipette. The prebiotics was diluted through the addition of various amount based on the inulin and the snack food samples to form colony units per gram (Cfu/g). With the dilutions made, 10 µl was placed on an MRS agar plate and spread. Final dilution plated for the 0 h time were 10-4 and 10-5. The sample were then incubated at 37oC for 24, 48 and 72 h. After 24 h, the samples were diluted and plated for final dilutions of 10-7 and 10-8. Plates were incubated at 37oC for 24, 48 and 72 h. Plates were also counted in the dilution with the colonies and recorded. The colony forming unit per gram (Cfu/g) were computed.
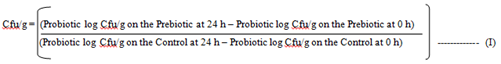
Cfu/g = Colony forming unit per gram.
Control = Control product without prebiotic (it was tested and used in place of glucose)
3.2 Animal Studies
3.2.1 Safety considerations: Twenty-five (25) male wistar rats (4 weeks old) with mean body weight of 98 - 102 g were used for this assessment following ethical clearance from the Animal Care and Use Research Ethics Committee (ACUREC) of University of Ibadan, Nigeria. The animals were obtained and ascertained healthy from Animal House of Veterinary medicine, of the same University. The animals were housed in well ventilated house (RH: 65%) at the Department of Clinical Pharmacy University of Ibadan, Nigeria. The rats were kept in animal cages, in five groups of five rats each and they were randomly assigned to dietary treatments. The first group of the five served as control. The animal study lasted for four weeks after 10 days of acclimatization to the diets. The feeding dose procedure of Organization for Cooperation and Development (OECD) as described by Botham [16] was adopted. Five hundred grams of the samples and potable water were served to the animals daily at 8:00am. The animals were inspected after every 24 hours for appearance of signs of toxicity and possible deaths [16]. The weight of each animal in the diet groups was taken on weekly basis, with a weighing balance. The difference between the initial and the final weight was taken to be the weight gained by the rat. The average weight and percentage weight gained by the rats in each diet group was determined.
3.2.2 Sub-Acute Toxicity: Following days of acclimatization, the intubate feeding continued daily for four weeks. At the end of the four weeks, the animals were sacrificed and blood samples and the organs (heart, liver, kidney, spleen and lungs) were taken for biochemical, histopathological and haematological analysis. The blood samples were obtained through induced bleeding (from the orbital sinus) into heparinized and ethylene diaminetetraacetic (EDTA) coated bottles.
3.2.3 Biochemical Analysis: Roche diagnostic kits was used to determine the bio-chemical parameters - Total protein, Albumin Globulin, Packed cell volume, Aspartate aminotransferase, Alanine aminotransferase, Alkaline phosphatase, Blood urea nitrogen and Creatinine according to the method of Burtis et al [17]. Four milliliters of blood sample were collected by ocular puncture from each of the experimental animal using capillary tube and was dispensed into commercially prepared heparin containers. Blood was centrifuged at 2000 g for 15 min. The supernatant was stored at -18 °C and used for all the assays.
3.2.4 Histopathological Analysis: The method of Adesiji [18] was adopted in the Histopathological Analysis. The rats from each group (I-V) were sacrificed through vascular dislocation. Five organs - heart, liver, kidney, spleen and lungs were removed from the animals. The organs were weighed and subjected to histopathological analysis (Toxicity sign) after fixation in slides through the following steps: fixation, dehydration, clearing, infiltration, embedding, cutting, sectioning, mounting and staining.
3.2.5 Haematological Analysis: The following parameters were evaluated: Packed cell volumes, Haemoglobin concentration, Red blood cell counts, White blood cell counts, Platelets, Lymphocytes, Heterophiles, Monocytes and Eosinophils. They were evaluated using sysmex automated haematology Anayzer KX-21 (Sysmex Corporation, Kobe, Japan).
3.3 Statistical analysis of the data
All the analyses were done in triplicates and the data obtained were statistically analyzed using a one-way analysis of variance (ANOVA) and means were separated by Duncan’s Multiple Range Test (DMRT) using the Statistical package for social science (SPSS) IBM version 26.0 package. Least significant difference was considered at 0.05 level [19].
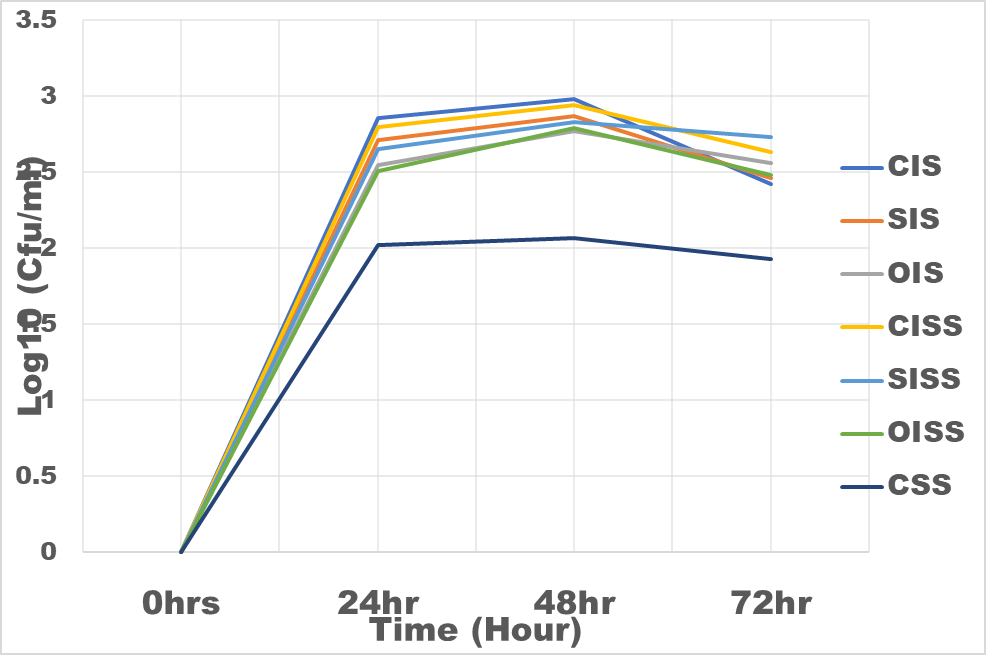
Figure 2: The growth rate of Bifidobacteria bifidum in inulin and snack samples
CIS = Commercial Inulin Sample
CSS = Control Snacks Sample
SIS = Sundried Inulin Sample
OIS = Oven dried Inulin Sample
CISS = Commercial Inulin Snacks Sample
SISS = Sundried Inulin Snacks Sample
OISS = Oven dried Inulin Snacks Sample
4. Results and Discussion
4.1 Prebiotic potential of the inulin and its food snack derivatives
Results of the evaluation of prebiotic properties of the inulin and snack samples were as shown in figure 3 and 4. With respect to snack samples with inulin, the snack food supported the growth of the Bifidobacterium bifidum and Lactobacillus bulgaricus 30% higher than the control (snack sample without inulin). Commercial inulin enhanced growth better (35%). This could be as a result of higher degree of polymerization (DP 17.5) of the chicory inulin used. According to [20], inulin DP has a great influence on its mechanism of regulating intestinal microbial flora; higher DP are more resistant to saccharolytic fermentation so that metabolism takes place more distally in the column. These results are in the agreement with that of [21] who observed that inulin can boost the population of Bifidobacterium and Lactobacillus in the colon and hence better intestinal health [22]. The fermentation of inulin and fructo-oligosaccharide in the posterior part of the intestine produced short-chain fatty acid such as butyric acid, propionic acid and acetic acid thus reducing the pH of the intestine and thereby stimulating proliferation of beneficial bacteria [23].
4.2 Safety
Results of an acute toxicity test showed that there was no such sign as there was no death of the experimental animals (wistar rat) during the daily observation after oral administration of the snack samples. The animals has a significant body weight increase (Figure 4), indicating that the snack samples doesn’t have any retardation effect on the growth of the animals. The percentage weight gain of the experimental animals fed with snack food ranged from 243.8 g-249.4 g. There was a significant weight gain (35%) across all the test group and the control group. The increase in weight gain indicated that the snack samples supported the growth of the experimental animals (wistar rats) and this is in agreement with the previous research by [24]. There was no recorded death among the experimental animal throughout the feeding duration. The animals looked healthy throughout the period of feeding duration. This also assured that the snack samples are safe for human consumption.
4.3 Biochemical and histopathological parameters in wistar rats
Table 2 showed effects of snack samples on some mean values of biochemical parameters in wistar rats. The alkaline phosphatase (ALP) index for test snack samples were not significantly different from control snacks samples. Experimental results for alanine aminotransferase (ALT) across all the grouping were also not significantly different (p<0.05) from the control. The total protein indicator in the animal group fed with snacks samples compared favorably with that of the control group. In terms of histopathological analysis, there were no necrosis in the tissues (liver, kidney, heart, spleen) of experimental animals in both control and test groups. This is an indication that the snack food samples are safe for consumption.
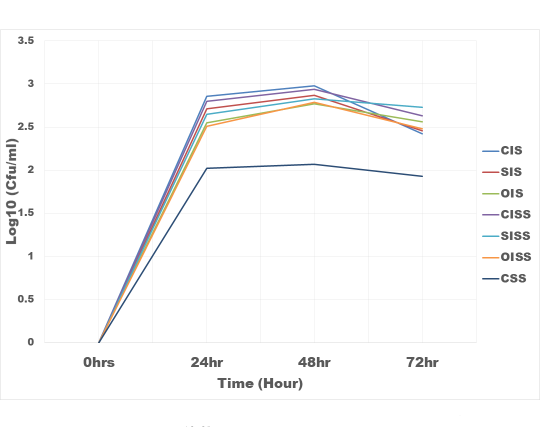
Figure 3: The growth rate Bifidobacteria bifidum in Inulin and snack samples
CIS = Commercial Inulin Sample
CSS = Control Snacks Sample
SIS = Sundried Inulin Sample
OIS = Oven dried Inulin Sample
CISS = Commercial Inulin Snacks Sample
SISS = Sundried Inulin Snacks Sample
OISS = Oven dried Inulin Snacks Sample
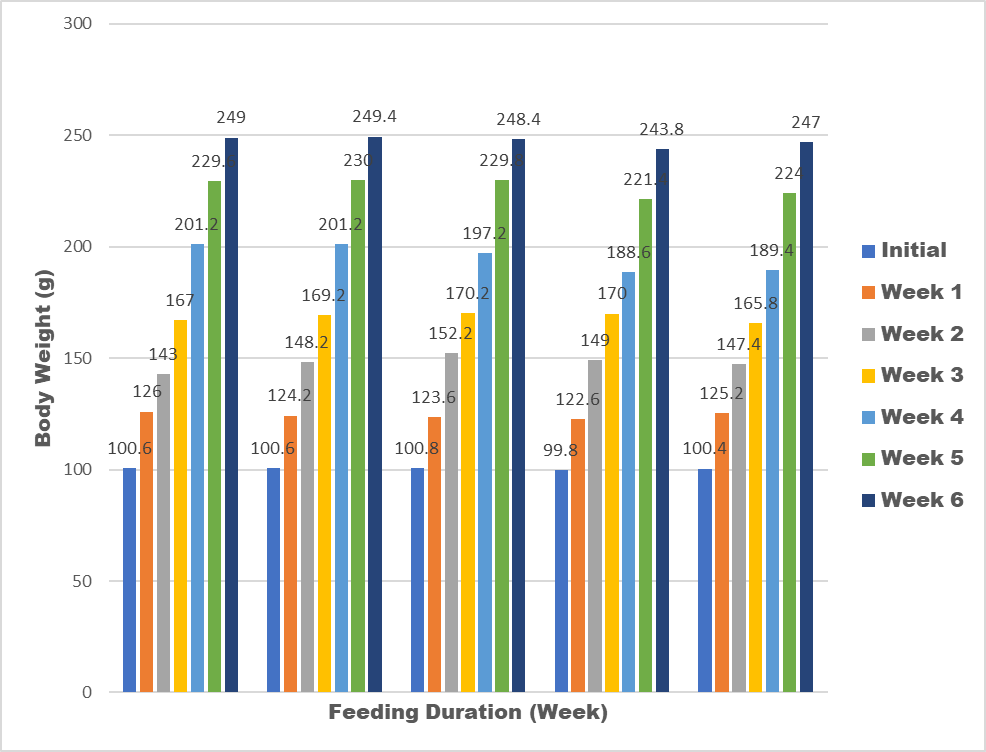
Figure 4: The growth rate Lactobacillus bulgaricus in inulin and snack samples
CRF = Rats fed on standard rat diet (Ladokun Feed) (Control I)
CSS = Rats fed on control snacks sample without Inulin (Control II)
CISS = Rats fed on snacks samples made from commercial Inulin
SISS = Rats fed on snacks samples made from sundried okra pod-Inulin
OISS = Rats fed on snacks samples made from oven dried okra pod-Inulin
|
Animals Group |
|||||
|
Parameters |
Group 1 (CRF) |
Group 11 (CSS) |
Group 111 (CISS) |
Group 1V (SISS) |
Group V (OISS) |
|
Total Protein (g/l) |
6.90±0.35b |
8.13±0.49a |
7.20±0.20b |
7.27±1.40b |
7.37±0.35b |
|
Albumin (g/l) |
2.83±0.25a |
3.27±0.15a |
2.97±0.35a |
2.97±0.21a |
3.07±0.15a |
|
Globulin (g/l) |
4.07±0.15a |
4.53±0.67a |
4.23±0.0.15a |
4.20±0.26a |
4.30±0.20a |
|
A/G Ratio |
0.67±0.06a |
0.67±0.06a |
0.67±0.15a |
0.67±0.06a |
0.70±0.00a |
|
AST (U/L) |
39.67±2.01a |
44.33±2.31a |
41.00±1.00a |
41.33±3.06a |
42.67±3.21a |
|
ALT (U/L) |
25.00±19.05a |
32.67±2.52a |
29.67±0.58a |
31.00±2.00a |
31.33±2.08a |
|
ALP (U/L) |
91.67±2.89c |
109.67±2.08b |
108.00±2.00b |
113.33±0.58a |
116.67±1.15a |
|
BUN (mg/dl) |
15.60±0.72a |
16.03±0.87a |
16.30±0.10a |
15.93±0.60a |
16.10±0.46a |
|
Creatinine (mg/dl) |
0.53±0.06a |
0.63±0.06a |
0.60±0.00a |
0.60±0.10a |
0.63±0.06a |
Values are the mean of the samples ± standard deviation of the triplicate determinations.
Values with different superscript letter along the same row are significantly different (p<0.05) using Duncan Multiple Range Test.
Group 1= Experimental Animal fed with Commercial Rat Feed (CRF)
Group 1I= Experimental Animal fed with Control Snacks Sample without Inulin (CSS)
Group 1II= Experimental Animal fed with Commercial Inulin Snacks Sample (CISS)
Group 1V= Experimental Animal fed with Sundried Inulin Snacks Sample (SISS)
Group V= Experimental Animal fed with Oven dried Inulin Snacks Sample (OISS)
A/G Ration = Packed Cell Volume (%)
AST (U/L) = Aspartate aminotransferase
ALT (U/L) = Alanine Aminotransferase
ALP (U/L) = Alkaline Phosphatase
BUN (mg/dl) = Blood Urea Nitrogen
Table 2: Biochemical Parameters of the Test Animals
|
Animals Group |
|||||
|
Parameters |
Group 1 (CRF) |
Group 11 (CSS) |
Group 111 (CISS) |
Group 1V (SISS) |
Group V (OISS) |
|
PCV (%) |
51.33±1.15a |
48.33±1.53a |
50.00±2.00a |
48.67±1.15a |
50.33±2.31a |
|
HB (mg/dl) |
16.77±0.06a |
15.57±0.86b |
16.50±0.20a |
16.93±0.31a |
16.60±0.60a |
|
RBC (X 103/µl) |
8.52±0.07a |
8.30±0.30a |
8.32±0.0.29a |
8.23±0.46a |
8.91±0.48a |
|
WBC (X 103/µl) |
7.65±0.05b |
5.37±0.21e |
6.88±0.10c |
5.60±0.10d |
7.88±0.06a |
|
Platelets (X 103) |
106.33±2.52d |
108.67±0.58d |
150.00±2.65a |
127.67±0.58c |
132.00±0.10b |
|
Lymphocytes (%) |
76.00±1.00a |
73.67±0.58a |
73.67±2.08a |
76.00±1.73a |
73.33±3.06a |
|
Heterophile (%) |
21.00±3.00b |
27.00±1.73a |
24.67±0.58a |
28.33±0.58a |
26.67±2.31a |
|
Monocyte (%) |
1.67±1.54a |
2.00±1.00a |
1.33±0.58a |
1.33±0.58a |
16.60±0.60a |
|
Eosinophil (%) |
1.33±1.15a |
1.00±0.00a |
1.33±0.58a |
1.33±0.53a |
1.67±1.15a |
Values are the mean of the samples ± standard deviation of the triplicates determinations.
Values with different superscript letter along the same row are significantly different (p<0.05) using Duncan Multiple Range Test.
Group 1 (CRF) = Experimental Animal fed with Commercial Rat Feed
Group 1I (CSS) = Experimental Animal fed with Control Snacks Sample without Inulin
Group 1II (CISS) = Experimental Animal fed with Commercial Inulin Snacks Sample
Group 1V (SISS) = Experimental Animal fed with Sundried Inulin Snacks Sample
Group V (OISS) = Experimental Animal fed with Oven dried Inulin Snacks Sample
PVC = Packed Cell Volume
HB = Haemoglobin concentration
RBC = Red Blood Cell counts
WBC = White Blood cell count
Table 3: Some haematological parameters of test animals
3.4 Effects of snack samples on some haematological parameters in wistar rats
The mean values obtained were not significantly different (p<0.05) from the control group (Table 3). packed cell volume (PCV): 51.33% (control group) and 48.33 – 50.33% (test group) and hemoglobin (Hb): 16.77mg/dl (control group) and 15.57 – 16.93mg/dl (test group). The red blood cell (RBC): 8.52x103/µl (control group) and 8.30x103 – 8.91x103/µl (test group); Haemoglobin concentration is the major indicator of assessment of tendency toward anemia and were not significantly lower than the control. This implies that the snack foods were neither toxic towards red blood cells nor an impediment towards erythropoiesis [25]. There was significant difference in white blood cells (WBC) values of the test group (5.37x103 – 7.88x103/µl) and the control (7.65x103/µl). This could be attributed to a suppression of leukocytosis in the bone marrow. Gnanamani et al. reported that blood urea nitrogen and creatinine are considered as suitable prognostic indicator of renal dysfunction and kidney failure for any toxic compounds [26]. The assessment of haematological parameters could be used to reveal the deleterious effect of foreign compounds on the blood constituents of animals [27]. Therefore, the study had shown that the snack samples had no adverse effect on the blood of the rats. There were no significant differences in blood urea nitrogen: [15.60 mg/dl (control group), 15.93-16.30 mg/dl (test group)]; creatinine [0.53 mg/dl (control group) and 0.60-0.63 mg/dl (test group)] of the rat fed the snack food over a duration of 6 weeks. This evidently meant that these foods had no adverse effect on the kidney.

Plate 1: Extruded Snack Samples
4. Conclusion
This work has shown that safe, acceptable extruded snack food samples with significant prebiotic potentials can be developed using okra pod inulin as a base. The result of the animal study indicated that the test samples had a profound salutary effect on the growth significant effect on the growth of the test animal, the growth of the probiotic organisms were enhanced beyond 30% more than the control.
Acknowledgements
We are grateful to the Staff and Students of the University of Ibadan that assisted us on the course of this research work.
Conflicts of Interest
The authors declare no conflicts of Interest.
References
- Sofyan M, Selma A, Radwan A, et al. Enhancing the nutritional value of gluten-free cookies with inulin. Advance Journal of Food Science and Technology 5 (2013): 866-870.
- Abdel-Salam AM. Functional food: hopefulness to good health. American Journal of Food Technology 5 (2010): 86-99.
- Ozen AE, Pons A, Tur JA. Worldwide consumption of functional foods: a systematic review. Nutrition Reviews 70 (2018): 472-481.
- Roberfroid M. Functional foods: concepts and application to inulin and oligofructose”, British Journal of Nutrition 87 (2002): S139-S143.
- Wichienchot S, Thammarutwasik, P, Jongjareonrak A, et al. Extraction and analysis of prebiotics from selected plants from Southern Thailand. Journal of Science and Technology 33 (2011): 517-523.
- Wichienchot S, Thammarutwasik P, Jongjareonrak A, et al. Extraction and analysis of prebiotics from selected plants from Southern Thailand. Journal of Science and Technology 33 (2011): 517-523.
- Roberfroid MB, Prebiotics: The Concept revisited” The Journal of Nutrition 137 (2007): 830S–837S.
- Lu S, Walker CE. Laboratory preparation of ready to eat breakfast flakes from grain sorghum flour. Journal of American Association of Cereal Chemistry 65 (1988): 377-379.
- Muhammad WB, Aamir N, Muhammad WG, et al. A Review: Role of inulin in animal nutrition. Journal of Food Technology Research 6 (2019):18-27.
- Huebner J, Wehling RL, Hutkins RW. Functional activity of commercial prebiotics. International Dairy Journal 17 (2017): 770-775.
- Muhammad Y, Nur FUA. Effect of inulin on physiochemical and sensory products cocoa liquor and milk chocolate free cocoa beans local south Sulawesi: Indonesia. International Journal of Botany Studies 5 (2020): 122-129.
- Deshpande HW, Poshadri A. Physical and sensory characteristics of extruded snacks prepared from foxtail millet based composite flours. International Food Research Journal 18 (2011): 751-756.
- Satter M, Jabin SA, Abedin N, et al. Development of nutritionally enriched instant weaning food and its safety aspects. African Journal of Food Science 7 (2013): 238-245.
- Fawole MO, Oso BA. Characterization of Bacteria Laboratory Manual of Microbiology, 4th edition Spectrum Books Limited, Ibadan, (2004): 24-33.
- Huebner J, Wehling RL, Hutkins RW. Functional activity of commercial prebiotics. International Dairy Journal 17: (2007): 770–775.
- Botham PA. Acute systematic toxicity- prospects for tired testing strategies. Toxicology in Vitro. 18 (2004): 227-230.
- Burtis CA, Ashwood ER, Bruns DE. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th Saunders (2011): 122-138.
- Adesiji OF. Production, storage stability sensory properties and nutritional quality of tempe-fortified maize based weaning food. A University of Ibadan Ph.D. Thesis Department of Food Technology (1999).
- Wahua TAT. Applied Statistics for Scientific Studies. African Links Publishing Press, Aba, Nigeria (1999): 129-155.
- Zhu L, Qin S, Zhai S, et al. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiology Letters 364 (2017): 1-7.
- Samanta A, Senani S, Kolt AP, et al. Effect of prebiotic on digestibility of total mixed ration. Indian Veterinary Journal 89 (2012): 41 - 42.
- Muhammad WB, Aamir N, Muhammad WG, et al. A Review: Role of inulin in animal nutrition. Journal of Food Technology Research 6 (2019 ): 18-27.
- Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Applied Physiology, Nutrition, and Metabolism 35 (2010): 9-16.
- Plahar WA, Onuma OB, Annam NT. Nutritional quality and storage stability of extruded weaning foods based on peanut, maize and soybean. Plant Food Human Nutrition 58 (2003): 1-16.
- Dacie JV, Lewis SM, Practical Haematology, 12th Edition Churchill Livingstone, Edunburg, London (2017): 22-37.
- Gnanamani A, Sudha M, Deepa G, et al. (2008) Haematological and biochemical effects of polyphenolics in animal models. Chemosphere 72 (2008): 1321-1326.
- Ajayi IA, Ifedi EN. Chemical and preliminary toxicological evaluation of Chrysophyllum albidum seed floor in dietary formulation of Albino rats. Journal of Environmental Science, Toxicology and Food Technology 9 (2015): 59-67.


 Impact Factor: * 3.8
Impact Factor: * 3.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 