The Development of A New Validated HPLC and Spectrophotometric Methods for the Simultaneous Determination of Daclatasvir and Sofosbuvir: Antiviral Drugs
Article Information
Amira S. Eldin1, Shereen M. Azab1*, Abdalla Shalaby2, Magda El-Maamly2
1Pharmaceutical Chemistry Dept., National Organization for Drug Control and Research [NODCAR], 6th Abu Hazem Street, Pyramids Ave, P.O. Box 29, Giza, Egypt
2Department of Analytical Chemistry, Faculty of Pharmacy, Zagazig University, P.C.44 519, Zagazig, Egypt
*Corresponding Author:Shereen M. Azab, Pharmaceutical Chemistry Dept., National Organization for Drug Control and Research [NODCAR], 6th Abu Hazem Street, Pyramids Ave, P.O. Box 29, Giza, Egypt, Tel: 202 01225591629;
Received:31 October 2017; Accepted:11December 2017; Published:18December 2017
View / Download Pdf Share at FacebookAbstract
Objective: Simple, rapid and reproducible reversed-phase high performance liquid chromatography (RP-HPLC) and ultraviolet derivative spectrophotometric (UVDS) methods for the simultaneous determination of daclatasvir (DAC) and sofosbuvir (SOF) in pure and in pharmaceutical dosage forms have been developed and validated.
Methods:The chromatographic separation of DAC and SOF was achieved using Agilent Zorbax SB C18 (4.6 x 250 mm, 5 μm) column at temperature of 40 °C. The mobile phase used was 9 mM dipotassium hydrogen orthophosphate buffer (pH 4±0.1): acetonitrile (60:40, v/v). The flow rate was maintained at 1 mL/min with UV detection at 265 nm.
Results: The calculated resolution was 4.56 (> 2), which ensures complete separation. The tailing factor was 1.13 and 1.40 (≤ 2) for DAC and SOF, respectively. Intermediate precision value was ≤ 2% indicate acceptable ruggedness.
Conclusion: The two methods were validated according to the International Conference on Harmonization (ICH) guidelines in terms of linearity, precision, accuracy, selectivity, specificity, detection limit, quantification limit, robustness and ruggedness.
Keywords
HPLC; Derivative spectrophotometry (2D); Validation, Daclatasvir; Sofosbuvir
Article Details
1. Introduction
Hepatitis C is a comprehensive liver disease produced by the hepatitis C virus (HCV) and can increase liver cirrhosis, liver failure, liver cancer and liver transplantation. The standard treatment for HCV is pegylated-interferon (Peg-IFN) and ribavirin (RBV) whoever these agents caused side effects such as bacterial infections, anemia, hematological toxicity, neutropenia and anorectal symptoms [1, 2]. Telaprevir and boceprevir were the first generation direct-acting protease inhibitors that developed and approved for the treatment of genotype I chronic hepatitis C However, they have to be co-administered with interferon and ribavirin therefore they were associated with their common side effects so their effectiveness were limited [3, 4].
Second-generation direct-acting antiviral drugs were developed and aimed to have a high pangenotypic activity with fewer undesirable side effects. These drugs include daclatasvir and sofosbuvir. Both medicines have effective antiviral activity and genotypic coverage [5, 6].
Daclatasvir, Methyl [(2S)-1-{(2S)-2-[4-(4’-{2-[(2S)-1-{(2S)-2-[(methoxycarbonyl) amino]-3-methylbutanoyl}-2-pyrrolidinyl]-1H-imidazol-4-yl}-4-biphenylyl)-1Himidazol-2-yl]-1-pyrrolidinyl}-3-methyl-1-oxo-2-butanyl] carbamate [7], is a nucleotide analogue NS5A polymerase inhibitor [8]. Only few techniques were reported for the quantitative investigation of DAC including different chromatographic methods [8-12], its chiral HPLC separation on a chiral stationary phase [13], stability indicating HPLC study in bulk and formulations and electrochemical detection with a Chitosan modified electrode [14].
Sofosbuvir,(S)-Isopropyl2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy) (phenoxy) phosphorylamino) propanoate, is a nucleotide analogue HCV NS5B polymerase inhibitor that is used in the treatment of chronic hepatitis C genotypes 1,2,3 or 4 [15]. Sofosbuvir (SOF) was determined by LC?MS/MS methods [8, 16], the degradation behavior of SOF under various stress conditions was also studied by Swain et al.[18]. Also SOF and its metabolite GS-331007 were determined in rat plasma by ultra-performance liquid chromatography tandem mass spectrometry (UPLC?MS/MS) method [19-22] and the dissolutionand forced degradation of SOF has also been studied by HPLC [15].
Although many studies proved separation methods [23-26] , daclatasvir with sofosbuvir that are co-administrated once per day oral dose achieved a high rate of virological response in patients with HCV genotype 1, 2 or 3. So the aim of this work was to develop and validate [17]two efficient methods using HPLC and UVDS for the simultaneous determination of DAC and SOF in pure form, binary mixtures and in pharmaceutical preparations.
2. Material and methods
2.1 Chemicals and Reagents
Daclatasvir hydrochloride and sofosbuvir were kindly supplied by the national organization for drug control and research (NODCAR), Cairo, Egypt. Daclenza® tablets labeled to contain 60 mg Daclatasvir per tablet produced by Memphis Pharmaceutical Industries, Egypt. Sofolanork® tablets labeled to contain 400 mg Sofosbuvir per tablet, produced by pharco pharmaceutical industries, Egypt. HPLC grade, acetonitrile supplied by El-Nasr Company, Egypt. The water for HPLC is doubly distilled and filtered through a 0.45 µm membrane filter. Distilled water was used for all the UVDS method
2.2 Instrumentation and Analytical Conditions
The HPLC system used was Agilent Technologies (Santa Clara, CA) 1100 composed of an isocratic pump, manual injector, variable wavelength detector and 2D-value solution ChemStation software. Chromatographic separation was attained on Agilent zorbax SB C18 (4.6 x 250 mm, 5 µm) column. Elution of samples was achieved using a mobile phase composed of 9 mM dipotassium hydrogen orthophosphate buffer (pH 4±0.1 adjusted with o-phosphoric acid): acetonitrile (60:40, v/v). The prepared mobile phase was filtered with 0.45 µm membrane filter and degassed for 30 min in an ultrasonic bath before being used. Flow rate used was 1 mL/min and the injection volume was 20 µL (using a 50 µL Agilent analytical syringe). Column temperature was maintained at 40 °C. The detection wavelength used was 265 nm. Equilibrium, conditioning and pre-washing of the stationary phase was done for 30 - 45 min [27].
A Shimadzu UV-visible recording spectrophotometer, model UV-2450, with 1 cm quartz cuvettes and connected to an IBM-PC computer loaded with UV Win PC software was used for all the absorbance measurements.
2.3 Preparation of Standard and Working Solutions
DAC and SOF stock solutions (1 mg/mL) and working solutions (0.2mg/mL) were prepared in the mobile phase for HPLC method. While DAC and SOF stock solutions (1 mg/mL) and working solution (0.1 mg/mL) were prepared in methanol for UVDS method.
2.4 Procedure for the HPLC method
Accurately measured aliquots of stock solutions (1 mg/mL) equivalent to 20-2000 µg of both DAC and SOF were separately transferred into two series of 10- ml volumetric flasks and completed to volume with the mobile phase. 20 µLof each dilution were injected in triplicate. The chromatograms were recorded using chromatographic conditions mentioned above. The recorded areas under peaks (AUP) were plotted versus the corresponding concentrations in µg/mL to obtain the calibration curves of DAC and SOF and the regression equations were calculated.
2.5 Procedure for the derivative method
The zero-order absorption spectra of 1 - 20 µg/mL for DAC and 2 ? 50 µg/mL for SOF were scanned within the wavelength range 200 ? 400 nm against a blank, and then the values of absorbance were measured at the selected wavelengths for both drugs [25].
As the above procedure, the obtained zero-order absorption spectra of DAC and SOF solutions, respectively, were derivatized to get the second derivative spectra.
Aliquots of DAC or SOF standard working solution equivalent to 8.0 µg/mL for both drugs, were transferred to two volumetric flasks, and the volume was completed with methanol. These two solutions were scanned against a blank methanol solution and their absorption spectra were computed. The D2 derivative spectra of these solutions were recorded under a certain selected instrumental parameters as: ??, scaling factor and wavelength range (200?400 nm). Then the working wavelengths of DAC or SOF, which are corresponding to the zero-crossing points, were recorded.
2.6 Application in Pharmaceutical Tablets
An accurate weight of the tablets equivalent to 25 mg of DAC and SOF respectively were dissolved in the mobile phase and filtered on 0.45 µm nylon syringes to produce stock solution of 200 µg/mL DAC and SOF, respectively. Different aliquots of the tablet working solution equivalent to 200 - 600 µg of both DAC and SOF were transferred into a series of volumetric flasks and completed to volume with the mobile phase.The method was performed by applying the standard addition technique.
For the UVDS method, 100 mg of DAC or SOF were transferred to 100-mL volumetric flask, dissolved in methanol to produce a stock tablet solution of 1.00 mg/mL of each drug. A 100 µg/mL working solution was prepared with further dilution of the stock solution with methanol.
3. Results and Discussion
3.1 RP-HPLC Method
3.1.1 Optimization of the Chromatographic Method
To optimize the HPLC assay parameters, the effect of acetonitrile composition and the apparent pH of the mobile phase on the capacity factor (K) were obtained. Different ratios of buffer and acetonitrile were tested [(50: 50), (60: 40), (70: 30), (30: 70) and (40: 60), v/v]. A satisfactory separation was obtained with a mobile phase containing acetonitrile and 0.05 M phosphate buffer (pH=4.0 ± 0.1) in the ratio 40: 60 (v/v) at 40 °C, where the injection volume was 20 µL. At lower acetonitrile concentration, the retention time of drug increased, whereas at higher or lower pH values, poor resolution was obtained. At apparent pH 4.0 improved resolution of the drug was observed. To investigate appropriate wavelength for the determination of DAC and SOF, we scanned the solutions of the two drugs individually in the UV-Visible region. Isosbestic point was calculated and found at 265 nm (Figure 1).
Then the quantitation of drug was achieved using UV detector at 265 nm. While the retention time for DAC and SOF were obtained at about 5.5 and 7.3 min, respectively and a flow rate of 1.0 mL min-1 with isocratic elution enable acceptable resolution of drug from possible impurities, in a short elution time at ambient temperature (Figure 2).This HPLC method does not need the addition of internal standard (IS) due to the purity of drug peaks with a clear baseline separation and no tailing.
Examining the separated peaks under the optimum conditions reveals the system suitability and validity of the method. The calculated resolution was 4.56 (> 2), which ensures complete separation. The tailing factor was 1.13 and 1.40 (? 2) for DAC and SOF, respectively, which shows the symmetry of the produced peaks within the stated Pharmacopeia’s range [22] used for quantitative analysis. The number of theoretical plate (N) and relative retention time (TR) were 3591 or 6242 and 5.5 or 7.3 for DAC or SOF, respectively, which were adequate for the separation of the two antiviral drugs. The RSD of area response was ? 2% for six replication injections. ‘’Peak purity test’’ results from the PDA detector confirmed that the DAC and SOF peaks obtained from their mixture samples analyzed were homogenous and pure, as shown in Figure 2.
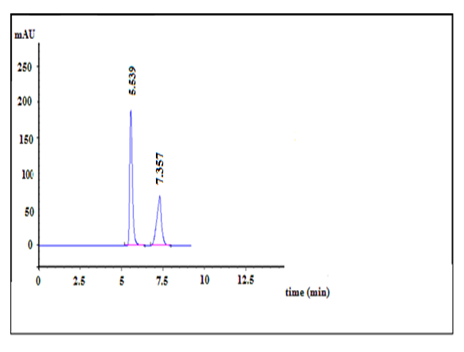
Figure 1:HPLC chromatogram of Dacla (100 µg/mL) (Rt =7.3 min), Sofo (100 µg/mL) (Rt =5.5 min) in mobile phase using 9 mM dipotassium hydrogen orthophosphate buffer (pH 4±0.1): acetonitrile (60:40, v/v) as the mobile phase at a flow rate of 1mL/min and UV detection at 265 nm.
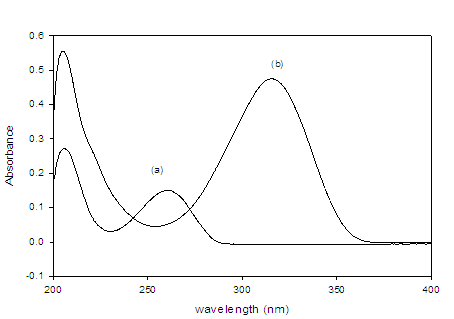
Figure 2:Absorption spectra of the both drugs of interest (a) DAC and (b) SOF.
3.2 Derivative Spectrophotometric Method
Absorption spectra: zero?order absorption spectra of DAC and SOF in methanol (Figure 3) show strong spectral overlap, which interfere with direct spectrophotometric analysis of the studied drugs. The suggested spectrophotometric method provide simple, convenient and accurate method for simultaneous analysis of DAC and SOF in their bulk and combined dosage forms without derivatization procedure and interference from overlapped peaks.
First to fourth derivative spectra of methanolic solutions of DAC and SOF at appropriate concentrations were taken and observed. The good resolution of the derivative spectra allowed selecting the optimal working wavelengths from several zero ?crossing wavelengths, i.e., those which exhibited the best linear response to analyte concentration and higher sensitivity.
The second derivative spectrophotometric method (2D) for determination of DAC as well as SOF in a mixture was the best than the other derivatives since the derivative absorbance peaks of these drugs were more sharper, smoother and well isolated (Figures 3 &4).The wavelengths at 350 nm for DAC and 288 nm for SOF were considered to be the zero-crossing wavelengths and would be examined to be the optimum working wavelengths for the second derivative (2D) method for the simultaneous determination of DAC and SOF in a binary mixture. The measurements were performed at ??= 8 and scaling factor (SF) = 100 for DAC and SOF.
Different solvents were used to study their effects on the methods of analysis such as: methanol, ethanol, acetonitrile, 0.1 M HCl and 0.1 M NaOH. The criteria employed were the sensitivity of the method and availability of the solvent. Methanol was the solvent of choice for the suggested spectrophotometric methods owing to the high solubility of DAC and SOF in it, and also there is no shift in their absorbance maxima in methanol. Also the extraction of the drug with methanol from tablets eliminates the interference from most common excipients present, which is a good feature needed for any applicable method.
Method validation was carried out under ICH guidelines for validation of analytical procedure [23]. The assay was validated with respect to linearity, accuracy, precision, selectivity, specificity, LOD, LOQ, robustness and ruggedness.
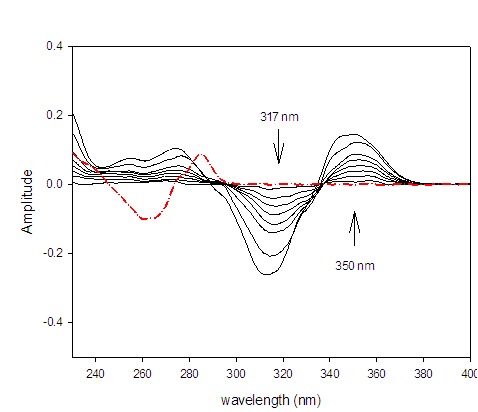
Figure 3:Second derivative (2D) spectra for DAC (1 ? 20 µg/mL) in methanol.
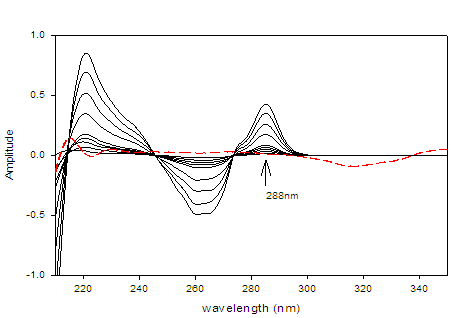
Figure 4:Second derivative (2D) spectra for SOF (1 ? 50 µg/mL) in methanol.
3.3 Linearity
Three series of different concentrations of DAC and SOF in methanol were prepared by dilution from 1.0 mg/mL standard solution. The first series consist of nine different concentrations of DAC or SOF (2 -200 µg mL-1). This series was used for the HPLC method. The second and third series consisted of eight different concentrations of DAC (1 - 20 µg mL-1) or SOF (2 ? 50 µg mL-1), these series were used for 2D method. Calibration curves of drug concentration versus peak area (HPLC method) or derivative amplitude (2D method) were plotted and subjected to regression analysis using the least squares method. The regression equations were recorded in Table 1.
Parameter |
HPLC method |
UVDS method |
||
DAC |
SOF |
DAC |
SOF |
|
Wavelength, nm |
265 |
265 |
350 |
288 |
Range of linearity |
2- 200 |
2- 200 |
|
|
Regression equation |
Y1=12.81X+30.40 |
Y1=18.24X+14.04 |
Y2=0.0078X+0.0029 |
Y2=0.0068X+0.0002 |
Regression coefficient(r2) |
0.9998 |
0.9999 |
0.9997 |
0.9999 |
LOD(µg mL-1) |
0.16 |
0.10 |
0.15 |
0.16 |
LOQ(µg mL-1) |
0.56 |
0.35 |
0.47 |
0.54 |
Standard deviation of slope (Sb) |
0.034 |
0.045 |
0.0003 |
0.0004 |
Standard deviation of intercept (Sa) |
0.100 |
0.135 |
0.0006 |
0.0008 |
Intra-day (RSD%)(a) |
0.16 |
0.55 |
0.44 |
0.85 |
Inter-day (RSD%)(b) |
0.17 |
0.54 |
0.64 |
0.80 |
Accuracy recovery%(c) |
100.50±0.034 |
99.95±0.045 |
100.60±0.0003 |
100.90±0.0004 |
Table 1:Results of assay validation of the proposed methods (HPLC and UVDS) for the simultaneous determination of DAC and SOF
(a) The intra-day is average of three concentrations of DAC and SOF (80, 100 and 120 µg mL-1) repeated three times during one day, and (b) at three different days. (c) mean of five determination±SD
3.4 Accuracy
The previously mentioned procedures under linearity were repeated three times for five different concentrations of pure samples. The concentrations were calculated from the regression equation; the recovery and RSD were calculated and tabulated in Table 1. The results indicate an adequate accuracy. Comparing our methods with other DAC previous works was performed and it was found thatthe best RSD result were achieved for both UVD2 (0.0006 %) and HPLC (0.1%) method, compared to Vikas et al. [28], jeevana et al. [29] , Hanaa et al. [30] and Ashoket al. [31] which were 0.121 %, 0.058%, 0.236 % and 0.15% , respectively.
3.5 Precision
The intraday precision (repeatability) was evaluated by assaying freshly prepared drug solutions at concentrations of 50, 100 and 200 µg mL-1 (HPLC method) and 5, 10 and 20 or 10, 20 and 40 µg mL-1 (2D method for DAC or SOF, respectively), within the same day and in three successive days (interday precision). The results listed in Table 1, show that no significant difference for the assay, which tested within day (repeatability) and between day (reproducibility). The relative standard deviation (RSD) was less than 1% indicates high degree of precision of the proposed methods.
3.6 Selectivity
Method selectivity for each drug in presence of the other was attained by preparing different mixtures of DAC and SOF within linearity range. The results obtained in Table 2 are good indication of the high selectivity of the two methods and their potential for the simultaneous determination of drugs from their mixtures.
HPLC method |
UVDS method (2D) |
||||||||||
Taken, µg mL-1 |
Founda, µg mL-1 |
Recovery(%) |
Taken, µg mL-1 |
Founda, µg mL-1 |
Recovery(%) |
||||||
DAC |
SOF |
DAC |
SOF |
DAC |
SOF |
DAC |
SOF |
DAC |
SOF |
DAC |
SOF |
20.0 |
100.0 |
20.03 |
100.65 |
100.15 |
100.60 |
2.0 |
18.0 |
1.98 |
18.04 |
99.00 |
100.22 |
40.0 |
80.0 |
40.50 |
80.15 |
101.40 |
100.20 |
4.0 |
16.0 |
4.03 |
15.97 |
100.75 |
99.81 |
60.0 |
60.0 |
60.00 |
59.53 |
100.00 |
99.23 |
8.0 |
12.0 |
7.98 |
12.03 |
99.75 |
100.25 |
80.0 |
40.0 |
79.89 |
40.10 |
99.80 |
100.32 |
12.0 |
8.0 |
12.03 |
7.96 |
100.25 |
99.50 |
100.0 |
20.0 |
100.32 |
20.06 |
100.30 |
100.31 |
16.0 |
4.0 |
15.95 |
4.03 |
99.69 |
100.75 |
Mean |
|
100.33 |
100.13 |
|
99.69 |
100.11 |
|||||
RSD% |
0.63 |
0.52 |
0.59 |
0.47 |
|||||||
Table 2:Determination of DAC and SOF in laboratory prepared mixtures applying the proposed methods (HPLC and UVDS)
a average of three determinations.
3.7 Specificity
The specificity of the methods was investigated by observing any interference encountered from the common tablet excipients such as talc, glucose, sucrose, starch and magnesium stearate. These excipients did not interfere with the proposed methods. This fact indicates good selectivity of the methods to determine of these drugs in drug formulations.
3.8 Detection and Quantification Limits
3.8.1 For HPLC method
The limit of detection (LOD) represents the concentration of analyte that would yield a signal-to-noise ratio of 3:1 and the limit of quantification (LOQ) represents the concentration of analyte that would yield a signal-to-noise ratio of 10:1. The results are tabulated in Table 1.
3.8.2 For 2D method
According to ICH recommendation [22], the approach based on the SD of the response and the slope (b) of Beer’s law was used for determining the detection and quantification limits. The values of low limits of detection (LOD) and quantification (LOQ) were assessed practically and listed in Table 1.
3.9 Stability of Standard and Sample Solutions
Stability of standard and sample solutions was evaluated at room temperature for 3 days. The relative standard deviation was found < 2%. It indicates both standard and sample solutions were stable up to 3 days at room temperature.
3.10 Recovery studies
The accuracy of the HPLC and UVDS methods was also checked by performing recovery experiments using the standard addition method. Known amount of drug were added to reanalyzed tablet solution, then the spiked samples were analyzed by two methods. The results of the recovery analyses are tabulated in table 3. It was concluded that the proposed methods are sufficiently accurate and precise and can be applied to pharmaceutical dosage forms and high recovery data show that the two methods are free from the interference of the excipients used in the formulations.
Method |
Concentration of drug (µg mL-1) |
||||||||
Taken |
Added |
Found |
Recovery(%)a |
Added |
Found |
Recovery(%)a |
|||
HPLC |
|
DAC |
SOF |
||||||
60 |
20 |
20.20 |
101.00 |
20 |
20.17 |
100.65 |
|||
60 |
40 |
39.98 |
99.95 |
40 |
40.30 |
100.75 |
|||
60 |
60 |
59.80 |
99.67 |
60 |
60.41 |
100.68 |
|||
60 |
80 |
80.34 |
100.42 |
80 |
79.94 |
99.93 |
|||
Mean |
|
100.26 |
|
100.55 |
|||||
RSD(%) |
0.58 |
0.42 |
|||||||
UVDS(2D)
|
6 |
3 |
3.04 |
100.28 |
3 |
2.98 |
99.33 |
||
6 |
6 |
5.97 |
99.50 |
6 |
6.01 |
100.17 |
|||
6 |
9 |
9.08 |
100.94 |
9 |
9.06 |
100.67 |
|||
6 |
12 |
11.95 |
99.58 |
12 |
12.10 |
100.83 |
|||
Mean |
|
100.32 |
|
100.25 |
|||||
RSD(%) |
0.91 |
0.67 |
|||||||
Table 3:Recovery of DAC and SOF in tablets by standard addition analyses.
(a) Average of three determinations.
3.11 Robustness and ruggedness
Small deliberate variations of the experimental conditions for HPLC method were applied in order to determine the effect on RT and resolution, three different C18 columns were tested and showed that any stationary phase with strongly deactivated silica could be used. Changes in mobile phase composition (±2%) or the flow rate ±0.2 mL/min, pH of the mobile phase (±0.1) and column temperature was varied ±50C, did not significantly affect the HPLC method, illustrating the robustness of the method.
The ruggedness of the proposed methods was evaluated by applying the developed procedure to assay of drug using the same instrument by two different analysts under the same optimized conditions at different days. Intermediate precision value (RSD) in both instances were ? 2% indicate acceptable ruggedness.
3.12 Analytical Applications
The developed and validated HPLC and UVDS (2D) methods were successfully applied to bulk powder and commercially available tablets of studied drugs. The DAC and SOF content of tablets were determined using regression equation method. The obtained amount of DAC or SOF and statistical analysis are given in table 4 (HPLC method) and Table 5 (UVDS method, 2D). Satisfactory results were obtained for DAC and SOF and were in good agreement with label claims. The results of the two drugs were compared with those obtained by the HPLC manufacture method, using t- and F- tests. The values at 95 % confidence limit did not exceed the theoretical values of t- and F- tests (listed in Tables 4&5), indicate no significant difference between the performance of these methods regarding accuracy and precision.
Statistical value |
DAC |
Manufacture procedure (a) |
SOF |
Manufacture procedure (a) |
Pure sample |
||||
Mean ±SD(%) |
100.38±0.72 |
100.40±0.80 |
100.12±0.65 |
100.60±0.64 |
Variance |
0.52 |
0.64 |
0.42 |
0.41 |
SAE(%)(b) |
0.32 |
0.36 |
0.29 |
0.28 |
N |
5 |
5 |
5 |
5 |
T-test |
0.04 |
|
1.18 |
(2.306) © |
F-test |
1.23 |
1.02 |
(6.39) © |
|
Pharmaceutical formulations |
||||
|
Daclenza tablets |
|
Sofolanork tablets |
|
Mean ±SD(%) |
100.07±0.95 |
100.29±0.46 |
100.46±0.58 |
100.34±0.63 |
Variance |
0.90 |
0.21 |
0.34 |
0.40 |
SAE(%)(b) |
0.39 |
0.19 |
0.24 |
0.26 |
NO(c) |
6 |
6 |
6 |
6 |
T-test |
0.51 |
|
0.34 |
(2.23)(d) |
F-test |
4.29 |
1.18 |
(5.05)(d) |
|
Table 4: Accuracy data for the analysis of pure samples and tablets of DAC and SOF by the proposed HPLC method and compared with HPLC manufacture procedure.
(a) HPLC manufacture procedure (Memphis pharmaceutical industries (cairo, Egypt), personal communication.
(b) Standard analytical error,% =( SD(%))/(?N).
(c) N= number of experiments
(d) Theoretical values of t- and f- tests at p = 0.05.
Statistical value |
DAC |
Manufacture procedure (a) |
SOF |
Manufacture procedure (a) |
Pure sample |
||||
Mean ±SD(%) |
100.19±0.74 |
100.40±0.80 |
100.13±0.87 |
100.60±0.64 |
Variance |
0.55 |
0.64 |
0.76 |
0.41 |
SAE(%)(b) |
0.33 |
0.36 |
0.39 |
0.29 |
N © |
5 |
5 |
5 |
5 |
T-test |
0.43 |
|
0.97 |
(2.306) (d) |
F-test |
1.16 |
1.85 |
(6.93) (d) |
|
Pharmaceutical formulations |
||||
|
Daclenza tablets |
|
Sofolanork tablets |
|
Mean ±SD(%) |
100.12±0.39 |
100.29±0.46 |
100.50±0.65 |
100.34±0.63 |
Variance |
0.15 |
0.21 |
0.42 |
0.40 |
SAE(%) |
0.16 |
0.19 |
0.27 |
0.26 |
N |
6 |
6 |
6 |
6 |
T-test |
0.69 |
|
0.43 |
(2.23) |
F-test |
1.40 |
1.05 |
(5.05) |
|
Table 5:Accuracy data for the analysis of pure and tablets of DAC and SOF by the UVDS method (2D) and compared with HPLC manufacture procedures
(a) HPLC manufacture procedure (Memphis pharmaceutical industries (cairo, Egypt), personal communication.
(b) Standard analytical error,% =( SD(%))/(?N).
(c) N= number of experiments
(d) Theoretical values of T and F- tests at p = 0.05.
4. Conclusion
This study investigates the first developed and validated RP-HPLC and UVDS methods for the determination of DAC and SOF in a mixture. The method was validated in terms of linearity, precision, accuracy, selectivity, detection limit, quantification limit and robustness and can be used for the determination of DAC and SOF either alone or in combined form. The availability of the instrumentation, simplicity of procedures, speed, precision and accuracy of the suggested techniques make these methods more valuable and attractive for uses.
The present HPLC method can be considered simple, rapid, and easy to apply, making it very suitable for routine analysis of DAC and SOF in pharmaceutical formulations. It involves a single step procedure for the preparation of the samples and direct injection. Sample preparation and analytical procedure run times are short so this method can be successfully applied for routine analysis in quality control. The UVDS method of analysis is simple, rapid, sensitive and accurate analytical method for routine quantitative determination of samples where they reduce unnecessary tedious sample preparations.
5. Acknowledgment
The authors would like to acknowledge the financial support by the National Organization for Drug Control and Research (NODCAR, Egypt). We also like to express our very great appreciation to Professor Dr Fatma Abdel gawad, Professor of analytical Chemistry, National Organization for Drug Control and Research,(NODCAR),for her patient guidance, enthusiastic encouragement and useful critiques of this research work and also Dr Manal El masry, Assistant Professor of analytical Chemistry, Faculty of Pharmacy, Zagazig University,for her valuable suggestions during the development of this research work.
References
- Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol49 (2008): 274-287.
- Wang CS, Ko HH, Yoshida EM, Marra CA. Richardson K.Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: A review and quantitative analysis. Am J Transplant 6 (2006): 1586-1599.
- Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S. et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: A multicenter experience. J Hepatol60 (2014):78-86.
- Butt AA, Kanwal F. Boceprevir and Telaprevir in the Management of Hepatitis C Virus-Infected Patients. ClinInfect Dis 54 (2012):96-104.
- Sundaram V, Kowdley KV. Dual daclatasvir and sofosbuvir for treatment of genotype 3 chronic hepatitis C virus infection. Expert Rev GastroenterolHepatol10 (2016):13-20.
- Liao H, Tan P, Zhu Z, Yan X, Huang J. Sofosbuvir in combination with daclatasvir in liver transplant recipients with HCV infection: A systematic review and meta-analysis.Clin Res HepatolGastroenterol41 (2017): 262-271.
- Sumathi K, Thamizhvanan K, Vijayraj S. Development and validation of stability indicating RP-HPLC method for the estimation of Daclatasvir in bulk and formulation. Der Pharm Lett8 (2016):107-113.
- Ariaudo A, Favata F, De Nicolň A, Simiele M, Paglietti L, Boglione L. et al. A UHPLC?MS/MS method for the quantification of direct antiviral agents simeprevir, daclatasvir, ledipasvir, sofosbuvir/GS-331007, dasabuvir, ombitasvir and paritaprevir, together with ritonavir, in human plasma. J Pharm Biomed Anal 125 (2016):369-375.
- Chakravarthy VA, Sailaja BBV. Method development and validation of assay and dissolution methods for the estimation of daclatasvir in tablet dosage forms by reverse phase HPLC. Eur J Pharm Med Res 3 (2016):356-364.
- Jiang H, Kandoussi H, Zeng J, Wang J, Demers R, Eley T. et al. Multiplexed LC-MS/MS method for the simultaneous quantitation of three novel hepatitis C antivirals, daclatasvir, asunaprevir, and beclabuvir in human plasma. J Pharm Biomed Anal 107 (2015):409-418.
- Nannetti G, Messa L, Celegato M, Pagni S, Basso M, Parisi SG. et al. Development and validation of a simple and robust HPLC method with UV detection for quantification of the hepatitis C virus inhibitor daclatasvir in human plasma. J Pharm Biomed Anal 134 (2017):275-281.
- Rezk MR, Bendas ER, Basalious EB, Karim IA. Development and validation of sensitive and rapid UPLC?MS/MS method for quantitative determination of daclatasvir in human plasma: Application to a bioequivalence study. J Pharm Biomed Anal 128 (2016):61-66.
- Srinivasu G, Kumar KN, Thirupathi C, Narayana CL, Murthy CP. Development and Validation of the Chiral HPLC Method for Daclatasvir in Gradient Elution Mode on Amylose-Based Immobilized Chiral Stationary Phase. Chromatographia79 (2016):1457-1467.
- Azab SM, Fekry AM. Electrochemical design of a new nanosensor based on cobalt nanoparticles, chitosan and MWCNT for the determination of daclatasvir: a hepatitis C antiviral drug. RSC Adv7 (2017):1118-1126.
- Hassouna MEM, Abdelrahman MM, Mohamed MA. Assay and Dissolution Methods Development and Validation for Simultaneous Determination of Sofosbuvir and Ledipasvir by RP-HPLC Method in Tablet Dosage Forms. J Forensic SciCrimInvestig1 (2017):555-562.
- Elkady EF, Aboelwafa MA. A Rapid and Optimized LC-MS/MS Method for the Simultaneous Extraction and Determination of Sofosbuvir and Ledipasvir in Human Plasma. J AOAC Int99 (2016):1252-1259.
- Ravichandran V, Shalini S, Sundram K, Harish R. Validation of analytical methods?strategies & importance. J Pharm PharmSci2 (2010):18-22.
- Swain D, Samanthula G, Bhagat S, Bharatam PV, Akula V, Sinha BN. Characterization of forced degradation products and in silico toxicity prediction of Sofosbuvir: A novel HCV NS5B polymerase inhibitor, J Pharm Biomed Anal 120 (2016):352-363.
- Pan C, Chen Y, Chen W, Zhou G, Jin L, Zheng Y. et al. Simultaneous determination of ledipasvir, sofosbuvir and its metabolite in rat plasma by UPLC?MS/MS and its application to a pharmacokinetic study, J Chromatogr B 1008 (2016):255-259.
- Rezk MR, Basalious EB, Karim IA. Development of a sensitive UPLC-ESI-MS/MS method for quantification of sofosbuvir and its metabolite, GS-331007, in human plasma: Application to a bioequivalence study, J Pharm Biomed Anal 114 (2015):97-104.
- Rezk MR, Bendas ER, Basalious EB, Karim IA. Quantification of sofosbuvir and ledipasvir in human plasma by UPLC?MS/MS method: Application to fasting and fed bioequivalence studies, J Chromatogr B 1028 (2016):63-70.
- Shi X, Zhu D, Lou J, Zhu B, Hu A, Gan D. Evaluation of a rapid method for the simultaneous quantification of ribavirin, sofosbuvir and its metabolite in rat plasma by UPLC?MS/MS, J Chromatogr B 1002 (2015): 353-357.
- Topagi K. S., Jeswani R. M., Sinha P. K., Damle M. C. A validated normal phase HPLC method for simultaneous determination of drotaverine hydrochloride and omeprazole in pharmaceutical formulation, Asian JPharmClinic Res 3 (2010):20-24.
- Rajeev K G, Anand C,Brijeshkunvar M. Simultaneous estimation of propafenone and its two metabolites in human plasma by liquid chromatography/tandem mass spectrometry LC-MS/MS. Int J Pharm PharmSci9 (2017):192-199.
- Himaja M, Kalpana J, Anbarasu C. Validated zero order and first order derivative spectrophotometric methods for invitro analysis of Tenofovir disoproxil fumarate tablets using azeotropic mixture, Int J Pharm PharmSci6 (2014):302-304.
- Lakshmi K., Rajesh T., Sharma S. Simultaneous determination of metformin and pioglitazone by reversed phase HPLC in pharmaceutical dosage forms, Int J Pharm PharmSci1 (2009):162-166.
- Armstrong DW, Zhang B. Product review: chiral stationary phases for HPLC. ACS Publications 2001: 557.
- Vikas K, Sachin G, Omprakash B. Development, Validation and Stability Study of UV Spectrophotometric Method for Determination of Daclatasvirin Bulk and Pharmaceutical Dosage Forms.Int J ChemTech Res 10 (2017): 281 -287.
- Jeevana JB, Padmaja G. UV spectrophotometric method for estimation ofnew drug, Daclatasvir dihydrochloride, Int Res J Pharm 7 (2016):1-3.
- Hanaa S, Gamal HR, Mohamed AO. Stability indicating hplc method development and validation for determination of daclatasvir in pure and tablets dosage forms. Indo Am J Pharm Sci3 (2016):1565-1572.
- Ashok CV, Sailaja BBV, Praveen KA. Method development and validation of ultraviolet-visible spectroscopic method for the estimation of hepatitis-c drugs - daclatasvir and sofosbuvir in active pharmaceutical ingredient form, Asian JPharmClin Res 9 (2016):61-66.


 Impact Factor: * 2.5
Impact Factor: * 2.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 