Tumoral Predisposition in type 1 Neurofibromatosis: A Case Report
Article Information
Maria Luísa Sequeiraa, Inês Salgadoa, Cláudia Pereira Rosab, Pedro Gomesa, Carlos Zagaloa,b,c
aHead and Neck Surgery Department, Instituto Português de Oncologia de Lisboa - Francisco Gentil, Lisbon, Portugal
bFaculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal
cCentro de Investigação Interdisciplinar Egas Moniz, City, Lisbon, Portugal
*Corresponding author: Maria Luisa de Oliveira Sequeira, Department of Head and Neck Surgery, Instituto Português de Oncologia de Lisboa Francisco Gentil Rua Prof. Lima Basto, Lisboa 1099-023, Portugal.
Received: 12 September 2021; Accepted: 18 September 2021; Published: 24 Novemberr 2021
Citation: Maria Luísa Sequeir, Inês Salgado, Cláudia Pereira Rosa, Pedro Gomes, Carlos Zagalo. Tumoral Predisposition in type 1 Neurofibromatosis: A Case Report. Journal of Surgery and Research 4 (2021): 694-701.
View / Download Pdf Share at FacebookAbstract
Neurofibromatosis type 1 (NF1) is an autosomal dominant condition that predisposes to a variety of primary neoplasias, having a wide presentation spectrum. Studies have suggested that the risk of developing benign and malignant tumors in NF1 patients could be approximately 2 to 5-fold higher than what is expected in the general population. We present the case of a 52-year-old caucasian man with NF1, recently diagnosed with a retroperitoneal Gastrointestinal Stromal Tumor (GIST). The patient had already been diagnosed with a Pheochromocytoma (PHEO) and a Papillary Thyroid Carcinoma (PTC) in the past. Here we discuss the rare concomitance of these three neoplasms in a NF1 patient, as well as the clinical presentation and proposed oncological pathways linking NF1 to each of the neoplasms presented by the patient. To the best of our knowledge a similar set of tumors in a NF1 patient has never been described in literature.
Keywords
Neurofibromatosis type 1, Pheochromocytoma, Gastrointestinal stromal tumor, Papillary thyroid carcinoma, Case report
Neurofibromatosis type 1 articles; Pheochromocytoma articles; Gastrointestinal stromal tumor articles; Papillary thyroid carcinoma articles; Case report articles
Neurofibromatosis type 1 articles Neurofibromatosis type 1 Research articles Neurofibromatosis type 1 review articles Neurofibromatosis type 1 PubMed articles Neurofibromatosis type 1 PubMed Central articles Neurofibromatosis type 1 2023 articles Neurofibromatosis type 1 2024 articles Neurofibromatosis type 1 Scopus articles Neurofibromatosis type 1 impact factor journals Neurofibromatosis type 1 Scopus journals Neurofibromatosis type 1 PubMed journals Neurofibromatosis type 1 medical journals Neurofibromatosis type 1 free journals Neurofibromatosis type 1 best journals Neurofibromatosis type 1 top journals Neurofibromatosis type 1 free medical journals Neurofibromatosis type 1 famous journals Neurofibromatosis type 1 Google Scholar indexed journals Pheochromocytoma articles Pheochromocytoma Research articles Pheochromocytoma review articles Pheochromocytoma PubMed articles Pheochromocytoma PubMed Central articles Pheochromocytoma 2023 articles Pheochromocytoma 2024 articles Pheochromocytoma Scopus articles Pheochromocytoma impact factor journals Pheochromocytoma Scopus journals Pheochromocytoma PubMed journals Pheochromocytoma medical journals Pheochromocytoma free journals Pheochromocytoma best journals Pheochromocytoma top journals Pheochromocytoma free medical journals Pheochromocytoma famous journals Pheochromocytoma Google Scholar indexed journals Gastrointestinal stromal tumor articles Gastrointestinal stromal tumor Research articles Gastrointestinal stromal tumor review articles Gastrointestinal stromal tumor PubMed articles Gastrointestinal stromal tumor PubMed Central articles Gastrointestinal stromal tumor 2023 articles Gastrointestinal stromal tumor 2024 articles Gastrointestinal stromal tumor Scopus articles Gastrointestinal stromal tumor impact factor journals Gastrointestinal stromal tumor Scopus journals Gastrointestinal stromal tumor PubMed journals Gastrointestinal stromal tumor medical journals Gastrointestinal stromal tumor free journals Gastrointestinal stromal tumor best journals Gastrointestinal stromal tumor top journals Gastrointestinal stromal tumor free medical journals Gastrointestinal stromal tumor famous journals Gastrointestinal stromal tumor Google Scholar indexed journals Papillary thyroid carcinoma articles Papillary thyroid carcinoma Research articles Papillary thyroid carcinoma review articles Papillary thyroid carcinoma PubMed articles Papillary thyroid carcinoma PubMed Central articles Papillary thyroid carcinoma 2023 articles Papillary thyroid carcinoma 2024 articles Papillary thyroid carcinoma Scopus articles Papillary thyroid carcinoma impact factor journals Papillary thyroid carcinoma Scopus journals Papillary thyroid carcinoma PubMed journals Papillary thyroid carcinoma medical journals Papillary thyroid carcinoma free journals Papillary thyroid carcinoma best journals Papillary thyroid carcinoma top journals Papillary thyroid carcinoma free medical journals Papillary thyroid carcinoma famous journals Papillary thyroid carcinoma Google Scholar indexed journals autosomal dominant condition articles autosomal dominant condition Research articles autosomal dominant condition review articles autosomal dominant condition PubMed articles autosomal dominant condition PubMed Central articles autosomal dominant condition 2023 articles autosomal dominant condition 2024 articles autosomal dominant condition Scopus articles autosomal dominant condition impact factor journals autosomal dominant condition Scopus journals autosomal dominant condition PubMed journals autosomal dominant condition medical journals autosomal dominant condition free journals autosomal dominant condition best journals autosomal dominant condition top journals autosomal dominant condition free medical journals autosomal dominant condition famous journals autosomal dominant condition Google Scholar indexed journals neoplasms articles neoplasms Research articles neoplasms review articles neoplasms PubMed articles neoplasms PubMed Central articles neoplasms 2023 articles neoplasms 2024 articles neoplasms Scopus articles neoplasms impact factor journals neoplasms Scopus journals neoplasms PubMed journals neoplasms medical journals neoplasms free journals neoplasms best journals neoplasms top journals neoplasms free medical journals neoplasms famous journals neoplasms Google Scholar indexed journals oncological pathways articles oncological pathways Research articles oncological pathways review articles oncological pathways PubMed articles oncological pathways PubMed Central articles oncological pathways 2023 articles oncological pathways 2024 articles oncological pathways Scopus articles oncological pathways impact factor journals oncological pathways Scopus journals oncological pathways PubMed journals oncological pathways medical journals oncological pathways free journals oncological pathways best journals oncological pathways top journals oncological pathways free medical journals oncological pathways famous journals oncological pathways Google Scholar indexed journals Papillary Thyroid Carcinoma articles Papillary Thyroid Carcinoma Research articles Papillary Thyroid Carcinoma review articles Papillary Thyroid Carcinoma PubMed articles Papillary Thyroid Carcinoma PubMed Central articles Papillary Thyroid Carcinoma 2023 articles Papillary Thyroid Carcinoma 2024 articles Papillary Thyroid Carcinoma Scopus articles Papillary Thyroid Carcinoma impact factor journals Papillary Thyroid Carcinoma Scopus journals Papillary Thyroid Carcinoma PubMed journals Papillary Thyroid Carcinoma medical journals Papillary Thyroid Carcinoma free journals Papillary Thyroid Carcinoma best journals Papillary Thyroid Carcinoma top journals Papillary Thyroid Carcinoma free medical journals Papillary Thyroid Carcinoma famous journals Papillary Thyroid Carcinoma Google Scholar indexed journals
Article Details
1. Introduction
Type 1 Neurofibromatosis (NF1) is an autosomal dominant disease, with an estimated global prevalence of 1 per 3000 individuals [1]. Although rare, NF1 is the most common autosomal dominant disease affecting the nervous system and one the most frequent single gene disturbances in the general population [2]. NF1 occurs due to loss of function mutations in the NF1 gene, on chromosome 17q11.2, that codes neurofibromin-1 [3]. Neurofibromin-1’s function is related to tumor suppression. The protein plays an important role in the regulation of cellular proliferation, differentiation, transformation and apoptosis, namely by downregulating RAS proto-oncogene pathway. Loss of function of this protein leads to an overactivation of this pathway and consequent uncontrolled cellular proliferation, culminating in an increased tumor predisposition. Literature describes a two to five-fold increased risk of developing a benign or malignant tumor, when compared to the general population [1]. NF1-related tumors most frequently develop in the skin as well as central and peripheral nervous systems [2]. Nevertheless, NF1 can predispose to primary tumors of other organ systems [1]. We present a clinical case of a NF1 patient with metachronous occurrence of 3 different tumors: Pheochromocytoma, Gastrointestinal Stromal Tumor and Papillary Thyroid Carcinoma. The occurrence of these three primary tumors in a single NF1 patient is rare, with no other cases reported in English language to this date.
2. Case Presentation
We report the case of a 52-year-old male patient, caucasian, independent in daily life activities. With a personal history of NF1, diagnosed in 2009, bilateral pheochromocytoma treated with bilateral adrenalectomy in 2009, PTC treated with right hemithyroidectomy in 2018, intracranial neurofibroma diagnosed in 2018 and dyslipidemia since 2019. He’s usually medicated with hydrocortisone, fludrocortisone and simvastatin, and presents a smoking history with a load of 45 pack-years, that ended in 2019. The patient was sent to an Endocrinology appointment in August/2018 because of a de novo increase in total urinary metanephrine (1413mcg/24h) and normetanephrine (1007mcg/24h) with normal plasma metanephrine and 3-methoxytyramine. He reported light to moderate headaches, for which he took acetaminophen with a good response, denying any other symptoms. At the physical examination, multiple subcutaneous neurofibromas, regions of hyperpigmented skin and multiple cafe-au-lait macules previously known in the context of NF1, were documented. No other alterations were found, namely in the blood pressure and heart rate. In the context of this history, and suspecting a relapse of PHEO, a 123I-MIBG Scintigraphy was carried out. This exam revealed an abdominal hypercaptation focus, adjacent to the right diaphragmatic crus (figure 1). To further investigate, a 68-Gallium-DOTANOC PET was requested, documenting a retroperitoneal nodule between the diaphragmatic crus and the inferior vena cava (IVC) (figure 2), compatible with malignant neoplastic tissue with high expression of somatostatin receptors. To clarify these findings, an MRI was requested. This exam confirmed the presence of two retroperitoneal nodules: one located posteriorly to the IVC, with an estimated dimension of 50 x 20 mm (figure 3); and the other anterior to the IVC and inferior to the right kidney, with 20 mm. Numerous augmented lymphatic nodes were identified between the left renal hilum and the pancreas tail, and adjacent to the mesenteric blood vessels. At this point a relapsed PHEO was considered as a probable diagnosis. The patient was seen in a Surgery appointment, and surgical resection was performed in September 2019. The histopathological exam, however, showed a low grade (G1) GIST, with a fusocelular pattern, with 17mm of diameter, 0 mitosis/50 fields of 400x. No metastasis were found in the resected lymph nodes. The patient began to attend Gastroenterology consultations for follow-up. Given the grade of the tumor and the clinical stability, without any gastroenterological signs or symptoms, a watchful waiting protocol was planned with periodical clinical appointments and twice-a-year laboratorial and radiological vigilance. Adjuvant therapy was not performed.
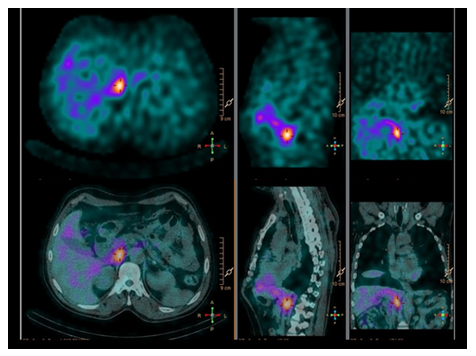
Figure 1: MIGB-scintigraphy showing a hypercaptating mass adjacent to the right diaphragmatic crux.
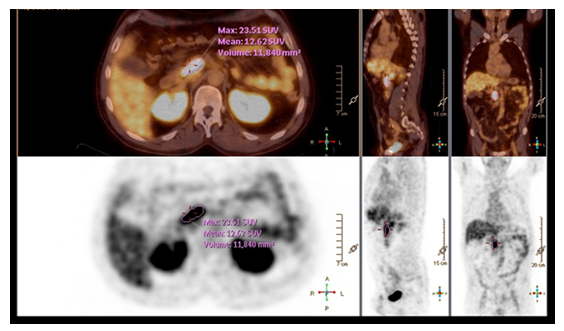
Figure 2: Gallium-68 DOTANOC PET showing a retroperitoneal mass between the right diaphragmatic crux and the inferior vena cava.
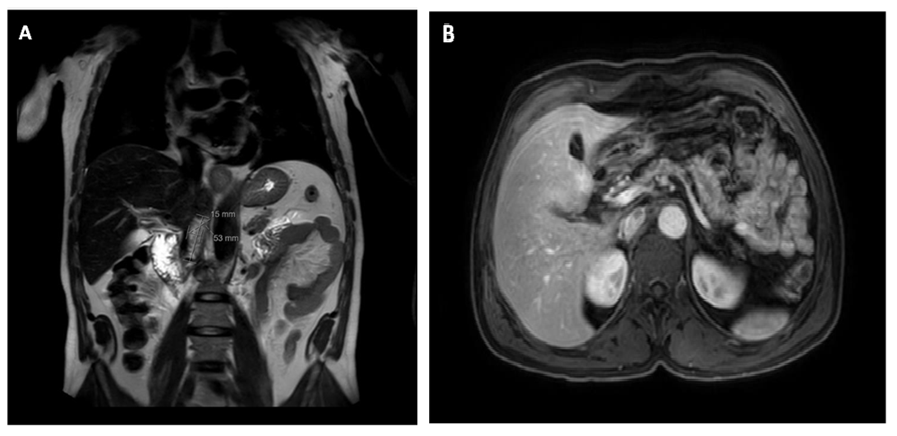
Figure 3: (A) MRI (T2 sequence, coronal cut) showing a retroperitoneal nodule measuring approximately 50mm of greater diameter. (B) MRI (mDixon sequence, axial cut) showing the same nodule posterior to the inferior vena cava.
3. Disucssion
NF1 predisposes to multiple neoplasms, thus augmenting the probability of occurrence of successive primary tumours in the same individual. The association of NF1 with GIST and PHEO is well described, and an increased predisposition to some tumors of the thyroid gland is known as well.
3.1 Gastrointestinal Stromal Tumor (GIST)
GISTs occur in the general population at a rate of approximately 1.2 per 100.000 individuals per year [4] however in NF1 patients GISTs are reported in 5% to 25% of the patients [5]. These tumours originate from Cajal Interstitial Cells and, in sporadic forms, are most commonly associated with a gain of function mutation in c-kit proto-oncogene. In the case of NF1, the physiopathology is related with the loss of function of neurofibromin [6]. NF-1 related GISTs tend to develop
in the small intestine and its occurrence outside the gastrointestinal tract, as in the case we presented, is rare [5]. The clinical manifestations are variable and often unspecific or even nonexistent, especially in earlier stages of disease [7]. In NF1 patients, GISTs tend to be less aggressive than sporadic forms and have lower mitotic activity and smaller sizes at diagnosis, and consequently are associated with less symptoms and better prognosis [5]. In multiple cases the diagnosis is accidental in imaging studies [7]. When there is a clinical suspicion, endoscopy and/or imaging studies should be carried out, as well as a biopsy of the mass when feasible [5,8]. Treatment of NF1-related GISTs relies thoroughly on surgical resection. In sporadic forms with unresectable tumors, tyrosine kinase inhibitors, such as imatinib, are proven to increase overall survival, however, given the different pathophysiology, NF1-associated GISTs show a primary resistance to these drugs. [4,5,8].
3.2 Pheochromocytoma (PHEO)
Although PHEOs occur in approximately 2-9.1 per 1.000.000 individuals in general population [9], it is estimated that 1-7% of NF1 patients develop these tumors [10]. PHEOs have their origin in chromaffin cells of the adrenal medulla therefore, the vast majority are metabolically active, secreting catecholamines (epinephrine, norepinephrine, and dopamine) as well as their inactive metabolites (metanephrine, normetanephrine and 3-methoxytyramine) [9,11]. Around 70% of PHEOs are hereditary, having been identified mutations in more than 30 genes associated its physiopathology, some of these related to clinical syndromes. In NF1, the constitutional activation of RAS/MAPK and PI3/AKT pathways, caused by the loss of function of neurofibromin, is responsible for the hyperproliferation of chromaffin cells found in PHEO [9]. The symptoms of PHEO are variable, being the most commonly reported symptom hypertension [9]. However, according to literature, 10-15% of patients are asymptomatic [12]. In clinical suspicion, initial biochemical screening with plasma free metanephrines or urinary fractionated metanephrines measurement is recommended. If metanephrine levels are increased, imaging studies should be performed. CT scan is the preferred study for the initial evaluation. 123I-MIBG scintigraphy can be indicated when there is an increased risk for metastatic disease, namely in the presence of extra-adrenal or recurrent disease, such as in the case we present. 18F-FDG PET/CT is recommended in patients with known metastatic disease, and in this group of patients is even superior to 123I-MIBG scintigraphy in detection of metastasis. The treatment is mainly surgical, being laparoscopic adrenalectomy recommended in most cases. In spite of this, open resection can be of use in some patients to ensure complete tumor resection, prevent tumor rupture and avoid local recurrence [13].
3.3 Papillary Thyroid Carcinoma (PTC)
PTCs are the most frequent malignant neoplasm of the thyroid gland, corresponding to 80-95% of thyroid malignancies [14]. These carcinomas are well differentiated and have their origin in thyroid follicular cells. Most PTCs occur in a sporadic fashion, having a multifactorial etiology and are typically associated with excellent prognosis and overall survival [15]. In spite of this, a minority of thyroid cancers are associated with familial syndromes and, in these cases, patients are typically younger and have more aggressive carcinomas, frequently presenting lymph node metastasis and extraglandular invasion at diagnosis [15].
4. Conclusion
NF1 associated PTCs are rare, with few reported cases and seldom information available in literature. Therefore, genetic and molecular mechanisms for this association are not yet fully understood [16,17]. RAS proto-oncogene mutations and consequent molecular pathways hyperactivation have been associated with the pathogenesis of sporadic PTC [14]. For this reason, some authors propose that the hyperactivation of RAS protein observed in NF1 may contribute to the pathogenesis of PTCs in these patients [16,17]. However, robust evidence that supports this hypothesis is still lacking. We present a patient with metachronous occurrence of three different primary tumors outside the nervous system. Although NF1 is associated with an increased incidence of GIST, PHEO and thyroid cancer, in our review of the current literature we found no other publications reporting this set of tumors in the same patient. In addition to this a rare combination of tumors, this case as some other particular features such as the atypical clinical presentation of both GIST and PHEO. Furthermore, GIST, PHEO and PCT can have a very subtle clinical presentation in earlier stages, demanding a high clinical suspicion, to make an accurate definitive diagnosis and timely directed treatment. The case we present exemplifies the importance of a thorough follow-up of NF1 patients, to detect de novo primary tumors, as well as recurrence, in time for a potentially curable treatment.
Acknowledgement
We The authors would like to thank Instituto Português de Oncologia de Lisboa Francisco Gentil.
Funding
No source of funding.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Gutmann D, Ferner R, Listernick R, et al. Neurofibromatosis type 1. Nat Rev Dis Primers 3 (2017): 17004.
- Blakeley J, Plotkin, S. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol 18 (2016): 624-638.
- Gutmann D, Wood D, Collins F. Identification of the neurofibromatosis type 1 gene product. Proc. Natl Acad Sci 88 (1991): 9658-9662.
- Blay JY, Kang YK, Nishida T et al. Gastrointestinal stromal tumours. Nat Rev Dis Primers 7 (2021): 22.
- Garrouche N, Abdallah A, Arifa N, et al. Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights into imaging 9 (2018): 661-671.
- Maertens O, Prenen H, Debiec-Rychter M, et al. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Human molecular genetics 15 (2006): 1015-1023.
- Martins S, Lamelas J, Pinheiro A et al. Tumores do estroma gastrointestinal (GIST): casuística do Departamento de Cirurgia. Bol Hosp S Marcos 22 (2006): 193-195.
- Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29 (2018): 267.
- Farrugia FA, Charalampopoulos A. Pheochromocytoma. Endocr Regul 53 (2019): 191-212.
- Dare A, Gupta A, Thipphavong S, et al. Abdominal neoplastic manifestations of neurofibromatosis type 1. Neuro-oncology advances 2 (2020):124-133.
- Aygun N, Uludag M. Pheochromocytoma and Paraganglioma: From Clinical Findings to Diagnosis. Sisli Etfal Hastanesi tip bulteni 54 (2020): 271-280.
- Sbardella E, Grossman AB. Pheochromocytoma: An approach to diagnosis. Clinical endocrinology & metabolism 34 (2019): 101-346.
- Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. pp. The Journal of Clinical Endocrinology & Metabolism 99 (2014): 1915-1942.
- Tang, KT, Lee CH. BRAF Mutation in Papillary Thyroid Carcinoma: pathogenic role and clinical implications. J Chin Med Assoc 73 (2010): 113-128.
- Guilmette J, Nosé V. Hereditary and familial thyroid tumours. Histopathology 72 (2018): 70-81.
- Koksal Y, Sahin M, Koksal H, et al. Neurofibroma adjacent to the thyroid gland and a thyroid papillary carcinoma in a patient with Neurofibromatosis Type 1: Report of a Case. Surg Today 39 (2009): 884-887
- Kim B, Choi Y, Gwoo S, et al. Neurofibromatosis type 1 associated with papillary thyroid carcinoma incidentally detected by thyroid ultrasonography: a case report. Journal of medical case reports 6 (2012): 179.


 Impact Factor: * 4.2
Impact Factor: * 4.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 