A Simple, Practical, and Novel Approach to Evaluate the Radiological Outcomes of Adult Chiari Malformation 1
Article Information
Asifur RahmanORCID ID1*, Nazmin AhmedORCID iD2, Abu Saleh Mohammad Abu ObaidaORCID iD1, Abu Naim Wakil UddinORCID iD1, Bipin ChaurasiaORCID iD3, Md Atikur RahmanORCID iD1, Md Shamsul AlamORCID iD1, Md Moududul HaqueORCID iD1
1Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Kazi Nazrul Islam Avenue
Shahbag, Dhaka 1000, Bangladesh
2Department of Neurosurgery, Ibrahim Cardiac Hospital & Research Institute, Kazi Nazrul Islam Avenue
Shahbag, Dhaka-1000, Bangladesh
3Consultant neurosurgeon, Neurosurgery clinic, Birgunj 44300, Nepal
*Corresponding Author: Asifur Rahman, Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Kazi Nazrul Islam Avenue Shahbag, Dhaka 1000, Bangladesh
Received: 28 December 2023; Accepted: 03 January 2024; Published: 02 February 2024
Citation: Asifur Rahman, Nazmin Ahmed, Abu Saleh Mohammad Abu Obaida, Abu Naim Wakil Uddin, Bipin Chaurasia, Md Atikur Rahman, Md Shamsul Alam, Md Moududul Haque. A Simple, Practical, and Novel Approach to Evaluate the Radiological Outcomes of Stealth Cranioplasty and Posterior Fossa Decompression with or without duraplasty for Adult Chiari 1 malformation. Journal of Surgery and Research. 7 (2024): 41-52.
View / Download Pdf Share at FacebookAbstract
Objective: Surgical techniques and outcomes of the enigmatic entity of Chiari malformation 1 (CM1) in the adult, which is often accompanied by syringomyelia (SM) vary widely and both the clinical and radiological outcomes are perplexing. The aims of this study were to assess the radiological outcomes of 2 surgical procedures namely the stealth cranioplasty (SC) and the posterior fossa decompression with or without duraplasty (PFD±DP) with the application of our novel approach to measure some radiological parameters.
Methods: This comparative cross-sectional study was conducted on adult CM1 patients with or without SM undergoing surgery by PFD±DP and SC from June 2019 to May 2021. Radiological outcomes of changes in pre and postoperative diameter of the foramen magnum (FM), the diameter of the SM, tonsillar position, and status of cisterna magna from computed tomography (CT) and/or magnetic resonance imaging (MRI) were evaluated and analyzed. The postoperative measurements of the FM diameter and tonsillar ectopia were measured by a new technique that we devised which has not been applied before.
Results: The study population comprised 37 male and 16 female (total of 53) symptomatic adult Chiari 1 malformation patients with or without SM ranging from 18 to 47 years of age (average 30.35 ± 7.49 years), where 30 (56.6%) underwent PFD±DP and 23 (43.4%) underwent SC. The postoperative change of the foramen magnum diameter was significantly better in the SC group (p 0.002) when an increase in the postoperative diameter was categorized as mild, moderate, and marked. And, while comparing the changes in millimeters, changes in the SC group were even better (p <0.001)
The postoperative tonsillar ascent was found to be significant (P <0.001) on both occasions when categorized into groups of mild, moderate, and marked, and when measured in millimeters. Postoperative SM size reduction was not significant when categorized as mild, moderate, and marked (p 0.085). However, when the changes were measured in millimeters, a significant reduction was observed in the SC groups (p 0.007). The postoperative appearance of Cisterna magna was found to be significant (p <0.001) in the SC group.
Conclusion: From this study, results of SC as a technique were found encouragingly to have better radiological outcomes in terms of postoperative changes in the diameter of the foramen magnum, tonsillar ascent, resolution of the SM, and postoperative appearance of cisterna magna. However, further multicenter studies on a larger population are recommended.
Keywords
Chiari malformation 1, Foramen magnum, Cerebellar tonsil, Stealth cranioplasty, Syringomyelia, Cisterna magna
Chiari malformation 1 articles; Foramen magnum articles; Cerebellar tonsil articles; Stealth cranioplasty articles; Syringomyelia articles; Cisterna magna articles
Chiari malformation 1 articles Chiari malformation 1 Research articles Chiari malformation 1 review articles Chiari malformation 1 PubMed articles Chiari malformation 1 PubMed Central articles Chiari malformation 1 2023 articles Chiari malformation 1 2024 articles Chiari malformation 1 Scopus articles Chiari malformation 1 impact factor journals Chiari malformation 1 Scopus journals Chiari malformation 1 PubMed journals Chiari malformation 1 medical journals Chiari malformation 1 free journals Chiari malformation 1 best journals Chiari malformation 1 top journals Chiari malformation 1 free medical journals Chiari malformation 1 famous journals Chiari malformation 1 Google Scholar indexed journals Foramen magnum articles Foramen magnum Research articles Foramen magnum review articles Foramen magnum PubMed articles Foramen magnum PubMed Central articles Foramen magnum 2023 articles Foramen magnum 2024 articles Foramen magnum Scopus articles Foramen magnum impact factor journals Foramen magnum Scopus journals Foramen magnum PubMed journals Foramen magnum medical journals Foramen magnum free journals Foramen magnum best journals Foramen magnum top journals Foramen magnum free medical journals Foramen magnum famous journals Foramen magnum Google Scholar indexed journals Cerebellar tonsil articles Cerebellar tonsil Research articles Cerebellar tonsil review articles Cerebellar tonsil PubMed articles Cerebellar tonsil PubMed Central articles Cerebellar tonsil 2023 articles Cerebellar tonsil 2024 articles Cerebellar tonsil Scopus articles Cerebellar tonsil impact factor journals Cerebellar tonsil Scopus journals Cerebellar tonsil PubMed journals Cerebellar tonsil medical journals Cerebellar tonsil free journals Cerebellar tonsil best journals Cerebellar tonsil top journals Cerebellar tonsil free medical journals Cerebellar tonsil famous journals Cerebellar tonsil Google Scholar indexed journals Stealth cranioplasty articles Stealth cranioplasty Research articles Stealth cranioplasty review articles Stealth cranioplasty PubMed articles Stealth cranioplasty PubMed Central articles Stealth cranioplasty 2023 articles Stealth cranioplasty 2024 articles Stealth cranioplasty Scopus articles Stealth cranioplasty impact factor journals Stealth cranioplasty Scopus journals Stealth cranioplasty PubMed journals Stealth cranioplasty medical journals Stealth cranioplasty free journals Stealth cranioplasty best journals Stealth cranioplasty top journals Stealth cranioplasty free medical journals Stealth cranioplasty famous journals Stealth cranioplasty Google Scholar indexed journals Syringomyelia articles Syringomyelia Research articles Syringomyelia review articles Syringomyelia PubMed articles Syringomyelia PubMed Central articles Syringomyelia 2023 articles Syringomyelia 2024 articles Syringomyelia Scopus articles Syringomyelia impact factor journals Syringomyelia Scopus journals Syringomyelia PubMed journals Syringomyelia medical journals Syringomyelia free journals Syringomyelia best journals Syringomyelia top journals Syringomyelia free medical journals Syringomyelia famous journals Syringomyelia Google Scholar indexed journals Cisterna magna articles Cisterna magna Research articles Cisterna magna review articles Cisterna magna PubMed articles Cisterna magna PubMed Central articles Cisterna magna 2023 articles Cisterna magna 2024 articles Cisterna magna Scopus articles Cisterna magna impact factor journals Cisterna magna Scopus journals Cisterna magna PubMed journals Cisterna magna medical journals Cisterna magna free journals Cisterna magna best journals Cisterna magna top journals Cisterna magna free medical journals Cisterna magna famous journals Cisterna magna Google Scholar indexed journals postoperative radiological changes articles postoperative radiological changes Research articles postoperative radiological changes review articles postoperative radiological changes PubMed articles postoperative radiological changes PubMed Central articles postoperative radiological changes 2023 articles postoperative radiological changes 2024 articles postoperative radiological changes Scopus articles postoperative radiological changes impact factor journals postoperative radiological changes Scopus journals postoperative radiological changes PubMed journals postoperative radiological changes medical journals postoperative radiological changes free journals postoperative radiological changes best journals postoperative radiological changes top journals postoperative radiological changes free medical journals postoperative radiological changes famous journals postoperative radiological changes Google Scholar indexed journals Craniocervical junction articles Craniocervical junction Research articles Craniocervical junction review articles Craniocervical junction PubMed articles Craniocervical junction PubMed Central articles Craniocervical junction 2023 articles Craniocervical junction 2024 articles Craniocervical junction Scopus articles Craniocervical junction impact factor journals Craniocervical junction Scopus journals Craniocervical junction PubMed journals Craniocervical junction medical journals Craniocervical junction free journals Craniocervical junction best journals Craniocervical junction top journals Craniocervical junction free medical journals Craniocervical junction famous journals Craniocervical junction Google Scholar indexed journals Wackenheim’s clivus canal line articles Wackenheim’s clivus canal line Research articles Wackenheim’s clivus canal line review articles Wackenheim’s clivus canal line PubMed articles Wackenheim’s clivus canal line PubMed Central articles Wackenheim’s clivus canal line 2023 articles Wackenheim’s clivus canal line 2024 articles Wackenheim’s clivus canal line Scopus articles Wackenheim’s clivus canal line impact factor journals Wackenheim’s clivus canal line Scopus journals Wackenheim’s clivus canal line PubMed journals Wackenheim’s clivus canal line medical journals Wackenheim’s clivus canal line free journals Wackenheim’s clivus canal line best journals Wackenheim’s clivus canal line top journals Wackenheim’s clivus canal line free medical journals Wackenheim’s clivus canal line famous journals Wackenheim’s clivus canal line Google Scholar indexed journals Tonsillar ectopia articles Tonsillar ectopia Research articles Tonsillar ectopia review articles Tonsillar ectopia PubMed articles Tonsillar ectopia PubMed Central articles Tonsillar ectopia 2023 articles Tonsillar ectopia 2024 articles Tonsillar ectopia Scopus articles Tonsillar ectopia impact factor journals Tonsillar ectopia Scopus journals Tonsillar ectopia PubMed journals Tonsillar ectopia medical journals Tonsillar ectopia free journals Tonsillar ectopia best journals Tonsillar ectopia top journals Tonsillar ectopia free medical journals Tonsillar ectopia famous journals Tonsillar ectopia Google Scholar indexed journals
Article Details
Highlights
- Chiari malformation 1 (CM1) is an enigmatic entity.
- The absence of ideal measurement parameters makes the assessment of postoperative radiological outcomes difficult in Chiari malformation 1.
- A novel approach of measuring postoperative radiological changes in CM1 is introduced which is simple, convenient, reliable, and useful.
- This unique technique of measuring the radiological outcome parameters would help clinicians and researchers discover newer aspects of pathophysiology to manage CM1 patients better in the future.
Abbreviations List
AP : Antero-posterior
BooA : Boogard’s angle
BSMMU : Bangabandhu Sheikh Mujib Medical University
C1 : First cervical vertebra
C2 : Second cervical vertebra
CM : Cisterna magna
CM1 : Chiari malformation 1
CSF : Cerebrospinal fluid
CT : Computer Tomography
CVJ : Craniocervical junction
FM : Foramen magnum
IRB : Institutional review board
McRL : McRae’s line
MRI : Magnetic Resonance Imaging
NIH : National Institutes of Health
PFD : Posterior fossa decompression
PFD±DP : Posterior fossa decompression with or without duraplasty
SC : Stealth cranioplasty
SM : Syringomyelia
TE : Tonsillar ectopia
WCCL : Wackenheim’s clivus canal line
Introduction
The diversity of presentations, management, and prognosis makes the Chiari malformation 1 (CM1) a puzzling entity. The presence of syringomyelia (SM) often complicates the commonest type of the Chiari spectrum, the CM1. The steady unfolding of the pathophysiology with advanced technologies and research is displaying newer dimensions of the complex nature of CM1. With all these odds, an absolute surgical protocol appears to be a far-off goal. Presently, many surgical procedures are in practice for CM1 with many variations and each has its own merits and demerits.
CM1 is defined as the downward displacement of the cerebellar tonsils below the level of the foramen magnum. Radiologically, CM1 in adults is defined as the descent of the cerebellar tonsils > 5 mm beyond the foramen magnum in magnetic resonance imaging (MRI) [1-4]. More CM1 patients are being detected with the advent of technologies, particularly MRI, and prevalence has been estimated to be 0.24%–3.6% of the population, including children. However, the annual incidence of symptomatic surgical candidates is significantly low, merely 0.06% [5-7].
Several theories are there to explain both the cerebellar tonsillar descent and its close association with frequently accompanying syringomyelia. The most recognized concept regarding the pathophysiology of CM1 is thought to be the discrepancy between shallow posterior fossa resulting from the underdevelopment of the mesochondrial component of the occipital bone and the overcrowded neural structures leading to cerebellar ectopia. Newer theories emphasize inadequate CSF flow around the craniovertebral junction (CVJ) hampering the CSF dynamics between the cranial and spinal compartments, which is also responsible for the development of SM in many cases [5,6,8-11].
The surgical options and outcomes are diverse, especially in the presence of SM and other associated disorders. The basic goal is to decompress the small posterior fossa and restore CSF flow and dynamics back to near normal. The most practiced surgical technique is the posterior fossa decompression with or without duraplasty (PFD±DP) with a variety of modifications. Although the outcomes are much improved lately, they are not very encouraging always [10,12-14]. A few years back, we developed a surgical technique, the “Stealth cranioplasty” (SC), where we tried to overcome some of the shortcomings of the common surgical practices which yielded good clinical outcomes [9,15,16]. However, the radiological parameters of outcome are ill-defined and less described in the literature owing to lack of any ideal assessment tool. Considering the need, we developed a novel technique of measurement for evaluation of the postoperative radiological outcomes of CM1 which is straightforward, consistent, and dependable.
In this study, using our technique, we compared the radiological outcomes between CM1 patients undergoing SC and PFD±DP in terms of the changes in the diameter of the foramen magnum (FM) and tonsillar Ectopia (TE) by our novel technique of measurement. The changes in the diameter of SM and the status of the Cisterna Magna (CM) were also studied. The results show that SC can achieve better radiological outcomes than the traditional PFD±DP in several aspects and our novel approach to measure the postoperative radiological parameters is a unique, reliable, and useful one.
Materials and Methods
Patient selection
After obtaining approval from the institutional review board (IRB) of Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, patients were recruited for this cross-sectional observational study between June 2019 to May 2021. All consecutive adult symptomatic CM1 patients with or without syringomyelia who underwent either SC or PFD±DP with a minimum of 3 months of postoperative follow-up with MRI were evaluated. In a few cases of SC, a CT scan was also performed.
Inclusion criteria
Inclusion criteria in recruiting the patients were: 1. Patients having a diagnosis of Chiari malformation 1 confirmed by tonsillar descent of >5 millimeters with or without syringomyelia in preoperative MRI. 2. Patients aged >18 years. 3. Patients having symptoms and signs related to CM1 and/or SM needing surgery. 4. Patients who had undergone either SC or PFD±DP. 5. Patients who had both preoperative and postoperative MRI.
Exclusion criteria
The followings were the exclusion criteria: 1. Patients having associated anomalies with CM1 like basilar invagination, atlanto-axial dislocation, or hydrocephalus. 2. Patients not having a minimum of 3 months of radiological follow-up with MRI. 3. Patients refusing to take part in the study.
Procedural details
Informed written consent was obtained from the patients or their legal guardians for surgery. Either of the two procedures was performed as per the surgeon’s choice. The posterior fossa decompression with or without duraplasty included suboccipital craniectomy (3 cm X 3 cm approx) and C1 laminectomy with removal of the dural band when present. When duraplasty was chosen to be performed, the best possible watertight duraplasty with pericranium or fascia lata was accomplished following an “Y” shaped dural opening after the suboccipital craniectomy and C1 laminectomy. During the dural opening, in a few cases, there were unintended minor arachnoid breaches which were sealed by low bipolar coagulation. The tonsils were not disturbed on any occasion. The stealth cranioplasty, which we described earlier [9,15,16], in brief, comprised 3 cm X 3 cm suboccipital craniectomy with C1 laminectomy (Figure 1B), linear arachnoid preserving durotomy in the midline (Figure 1C), duraplasty with the superficial layer of the deep cervical fascial graft (Figure 1D), cranioplasty with 5 cm X 5 cm titanium mesh pre-shaped in the configuration of a Stealth bomber cockpit with flat wings (Figure 1A) and by fixing with screws around the free margins of the craniectomy (Figure 1E) to cover the craniectomy gap as well as to enlarge the posterior fossa volume followed by tenting of the duraplasty with the titanium mesh (Figure 1E).
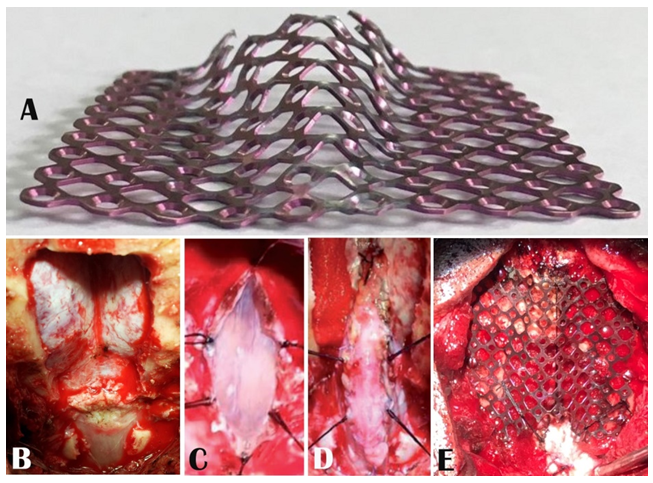
Figure 1: Peroperative picture showing A) the pre-shaped titanium mesh in the configuration of a Stealth bomber cockpit and flat wings, B) 3 cm X 3 cm suboccipital craniectomy with C1 laminectomy, C) linear midline arachnoid preserving durotomy and tacking sutures from the cut margins, D) duraplasty and E) cranioplasty fixed with screws and tenting of the duraplasty.
Although CT scans were performed to see the status of the Stealth cranioplasty in some cases, all the radiological parameters were measured, and evaluated from the preoperative and 3-month postoperative MRIs. The extent of changes in the diameter of the foramen magnum (FM), tonsillar ectopia (TE), SM diameter, and appearance of cisterna magna (CM) were studied and analyzed. The measurements were done with the freely available Java-based public-domain image processing and analysis program developed at the National Institutes of Health (NIH), ImageJ software version 1.53e, and the same procedure was followed in all the cases. The technique we devised to draw and measure the McRL and TE is particularly relevant and useful where the posterior margin of the foramen magnum is deficient following decompression. For drawing and measuring the McRL and TE the same procedure was followed in the case of both the SC and PFD±DP. In the preoperative MRI, the McRae line (McRL) is drawn by joining the basion and the opisthion (Figure 2A). The Wackenheim’s clivus canal line (WCCL) is drawn along the posterior margin of the clivus (Figure 2A). The angle, created at the meeting point of the McRL and the WCCL, is the Boogard’s angle (BooA) (Figure 2B). As the basion, the opisthion, and the clivus are fixed bony landmarks and the WCCL is a fixed line for any individual at any point in time and position, the BooA obviously turns out to be a static constant for that particular individual. Thus, keeping the WCCL, the basion, and the BooA as constant reference points, the trajectory of the McRL can be achieved perfectly. With this hypothesis, we developed our technique to draw the postoperative McRL. Once the BooA is measured from the preoperative MRI, the WCCL is drawn, and the BooA is set at the basion with the help of ImageJ software in the postoperative MRI (Figure 2D). The new trajectory from the basion is extended up to the posterior dural margin displayed by the posterior-most margin of the CSF to set the opisthion to complete the McRL (Figure 2E). Measuring the length of the new McRL with ImageJ software is straightforward.
The descent of the tonsil is measured by drawing a vertical line from the tip of the tonsil on the McRae line both in preoperative and postoperative MRIs (Figure 2C & F). Change in SM diameter is determined by measuring the maximum AP diameter of the SM in the preoperative midsagittal T2WI of MRI and by measuring the anteroposterior (AP) diameter at the corresponding level in the postoperative T2WI MRI (Figure 3A & B). The changes in the diameters of the FM and the SM and tonsillar ectopia were taken to be the difference between the pre and postoperative measurements (Figure 3A & B). The appearance of cisterna magna is noted in both pre and postoperative T2WI MRIs as well as in the MR myelograms whenever available (Figure 4).
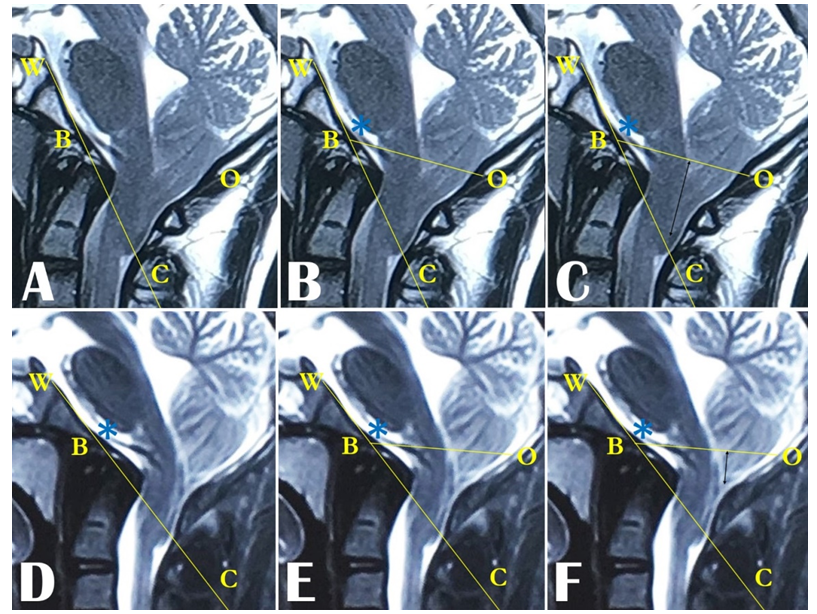
Figure 2: MRI images showing the points, lines, and angles to draw the McRL and BooA. In preoperative MRI, A) the basion (B) and the opisthion (O) are marked, the WCCL (WC) is drawn. B) The basion and the opisthion are joined to draw the McRL (BO) and the BooA is achieved (*). D) In the postoperative MRI, WCCL (WC) is drawn and the BooA (*) is placed on basion (B). E) Based on the BooA (*), achieved from the preoperative MRI, the trajectory is extended from the basion (B) on the WCCL (WC) up to the posteriormost margin of CSF, to mark the opisthion (O) to get the McRL (BO). In C) Preoperative and F) postoperative MRIs, vertical lines (black arrows) are drawn on the McRL (BO) from the tip of the tonsils to measure the tonsillar ectopia.
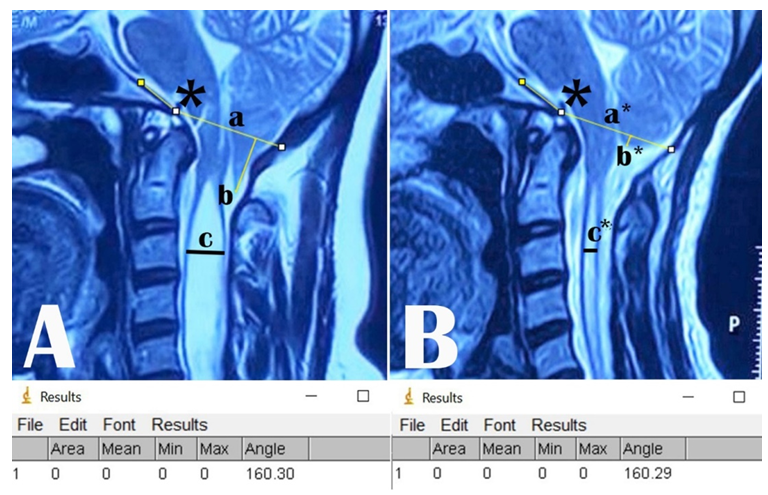
Figure 3: A) Preoperative, and B) postoperative T2WI MRI following PFD±DP showing the McRae’s line (a & a*), Boogard’s angle (*), tonsillar ectopia (b & b*), and syringomyelia diameter (c & c*). The measurements of the Boogard’s angle by ImageJ are in the lower panel.
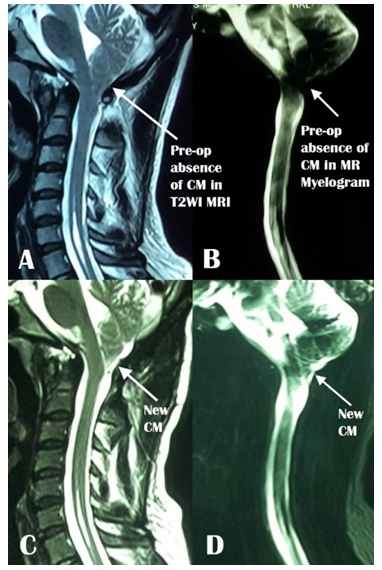
Figure 4: A) Preoperative and C) postoperative T2WI MRI and B) preoperative and D) postoperative MR myelogram showing the preoperative absence and postoperative appearance of cisterna magna (CM).
Statistical analysis
Parametric data were expressed as mean ± SD and compared via unpaired t-test and non-parametric data were expressed as medians and compared via Chi-square test and McNemar’s test. All statistical analysis was performed using SPSS 23 software. A p-value <0.05 was considered to be statistically significant.
Results
Overview
A total of 53 symptomatic adult Chiari 1 malformation patients with or without syringomyelia were included in the study where 23 and 30 patients underwent SC and PFD±DP respectively. The average age of the 37 males and 16 females recruited in the study ranged from 18 to 47 years with an average of 30.35 ± 7.49 years. All the patients had preoperative and 3-month postoperative CT and/or MRI scans to assess radiological outcomes.
Preoperative data
The preoperative AP diameter of the foramen magnum (length of McRae line) ranged from 22.92 to 35.57 mm with an average of 30.01 ± 2.70 millimeters (mm). Preoperatively the tonsillar ectopia was 9.50 ± 2.91 mm on average ranging from 5.70 to 14.92 mm in both the groups. 51 of 53 patients had syringomyelia at the time of presentation (SM was absent in 2 patients in the PFD±DP group) with an average diameter of 6.49 ± 2.56 mm which ranged from 2.15 to 13.66 mm in diameter at the widest. Only 2 of 23 patients showed the presence of cisterna magna in their preoperative MRIs in the SC group while none of the 30 patients in the PFD±DP group had CM.
Postoperative data
All the patients had postoperative MRIs and some had CT scans also at 3-month follow-ups. For the study, all the parameters were measured from the postoperative MRIs. The change in the anteroposterior diameter of the foramen magnum (FM) (length of McRae line) of the patients was categorized as mild (0 - 10%), moderate (11.1 - 20%), and marked (≥ 20.1%) depending on the changes in the diameter of the FM in the postoperative MRIs. Postoperatively, most of the patients in the SC group had moderate (47.8%) and marked (43.5%) increase in the diameter of the FM while patients in the PFD±DP group had mild (51.9%) and moderate (37.0%) increase (Table 1). However, 3 patients in the PFD±DP group had a decrease in AP diameter of the FM. The categorical change in diameter of the foramen magnum was significantly better (p 0.002) in the SC group than in the PFD±DP group. On the other hand, compared in millimeters, the increase in the FM diameter in the SC group was significantly better (p <0.001) than in the PFD±DP group (Table 2).
|
Surgery |
Change in Foramen Magnum Diameter |
p-value |
||
|
Mild |
Moderate |
Marked |
||
|
PFD±DP |
14 (14/27, 51.9%) |
10 (10/27, 37.0%) |
3 (3/27, 11.1%) |
0.002 |
|
STEALTH |
2 (2/23, 8.7%) |
11 (11/23, 47.8%) |
10 (10/23, 43.5%) |
|
Chi-square test was done
Mild -0 - 10%, Moderate -11.1 - 20%, Marked ≥ 20.1%
Table 1: Categorical Change in Diameter of Foramen Magnum
|
Surgery |
Foramen Magnum Diameter (in mm) |
p-value |
||
|
Pre-op |
Post-op |
Change |
||
|
PFD±DP |
30.03 ± 2.75 |
32.77 ± 2.78 |
2.73 ± 2.20 |
<0.001 |
|
STEALTH |
29.99 ± 2.71 |
35.90 ± 2.89 |
5.90 ± 2.36 |
|
Unpaired t test was done
Table 2: Postoperative Change in Diameter of Foramen Magnum in mm
Changes in tonsillar ectopia were categorized into mild, moderate, and marked by changes of 0-33%, 33.1-66%, and ≥66.1% of ascent respectively in the postoperative MRIs than the preoperative ones. Most of the patients in the SC group had moderate (52.2%) and marked (30.4%) ascent while in the PFD±DP group most of the patients had mild (66.7%) and moderate (33.3%) ascent which was significantly better in the SC group (p <0.001) (Table 3). Measuring in millimeters, the tonsillar ascent was 5.35 ± 1.91 mm in the SC group while that was only 2.58 ± 1.15 mm in the PFD±DP group, which was significantly better in the SC group (p <0.001) (Table 4).
|
Surgery |
Change in Tonsillar Ectopia |
p-value |
||
|
Mild |
Moderate |
Marked |
||
|
PFD±DP |
20/30 (66.7%) |
10/30 (33.3%) |
0/30 (0.0%) |
<0.001 |
|
STEALTH |
4/23 (17.4%) |
12/23 (52.2%) |
7/23 (30.4%) |
|
Chi-square test was done
Mild - 0-33%, Moderate - 33.1-66%, Marked - ≥66.1%
Table 3: Categorical Change in tonsillar ectopia.
|
Surgery |
Tonsillar Ectopia (mm) |
p-value |
||
|
Pre-op |
Post-op |
Change |
||
|
PFD±DP |
8.91 ± 2.51 |
6.33 ± 2.53 |
2.58 ± 1.15 |
<0.001 |
|
STEALTH |
10.28 ± 3.28 |
4.93 ± 3.60 |
5.35 ± 1.91 |
|
Unpaired t test was done
Table 4: Postoperative change in tonsillar ectopia in mm
While categorizing the postoperative change in SM diameter as mild, moderate, and marked, based on reduction of SM diameter by 0-33%, 33.1-66%, and ≥66.1% respectively, no significant change could be seen between the groups (p 0.085) as most of the patients in both the groups had mostly mild and moderate reductions (Table 5). However, when the postoperative SM diameter was measured in millimeters and compared, significantly better (p 0.007) reduction was seen in the SC group (Table 6).
In the PFD±DP group, none of the patients had the presence of cisterna magna preoperatively. Postoperatively, the development of CM was seen in only 5 (16.7%) out of the 30 patients in this group. In the SC group, only 2 (8.7%) out of 23 patients had CM preoperatively and 15 (71.4%) of the 21 patients not having any CM developed CM postoperatively. The appearance of post-operative CM was statistically significant only in the SC group (p <0.001) (Table 7).
|
Surgery |
Change in Syrinx Diameter |
p-value |
||
|
Mild |
Moderate |
Marked |
||
|
PFD±DP |
16/28 (57.1%) |
11/28 (39.3%) |
1/28 (3.6%) |
0.085 |
|
STEALTH |
7/23 (30.4%) |
12/23 (52.2%) |
4/23 (17.4%) |
|
Chi-square test was done
Mild - 0-33%, Moderate - 33.1-66%, Marked - ≥66.1%
Table 5: Categorical Change in Syringomyelia Diameter
|
Surgery |
Change in Syrinx Diameter (mm) |
p-value |
||
|
Pre-op |
Post-op |
Change |
||
|
PFD±DP |
5.95 ± 2.17 |
3.95 ± 1.69 |
1.99 ± 1.20 |
0.007 |
|
STEALTH |
7.20 ± 2.90 |
3.69 ± 2.20 |
3.43 ± 2.41 |
|
Unpaired t test was done
Table 6: Postoperative Change in Syringomyelia Diameter in mm
|
Surgery |
Pre-op |
Post-op |
p-value |
|
PFD±DP |
0/30 (0.0%) |
5/30 (16.7%) |
<0.001 |
|
STEALTH |
2/23 (8.7%) |
15/21 (71.4%) |
McNemar test was done
Table 7: Pre and postoperative status of Cisterna magna
Discussion
The decision-making in treating a CM1 patient is critical. Asymptomatic or minimally symptomatic patients can be observed with serial imaging. However, the symptomatic patients of CM1, particularly in the presence of SM need special consideration. As the outcomes are variable for different surgical techniques, decision-making for the choice of surgical technique should be based on thorough and meticulous clinical and radiological evaluations with the goal of having minimum complications. Generally, posterior fossa decompression and removal of the C1 posterior arch with or without duraplasty (PFD±DP), the commonest practice for CM1, have high rates of success in the alleviation of crowding of the posterior fossa neural element and reestablishing CSF flow and dynamics around the craniovertebral junction (CVJ). However, the outcomes are not very consistent, and are dependent on the type of surgery to a great extent. Combinations, modifications, and tailoring of the traditional PFD±DP are evolving continuously [2,3,14,17-20]. MRI, the imaging modality of choice for CM1, is a good imaging tool for assessing the radiological outcomes [2]. On average, the rate of postoperative radiological improvement is 81.1% [18].
A myriad of factors like the pathology, clinico-radiological status of the patient, judgment in decision-making, and choice of surgical technique influences the outcomes greatly. Different parameters and techniques have been devised and are used to measure the radiological outcomes of the surgery for CM1. Most of these often raise controversy and none has been proved to be absolute. Intending to ease and make perfect measurements, we devised a technique based on defining the McRL in postoperative MRI to measure some parameters to evaluate the radiological outcomes. Interestingly, during the measurements, we found the Boogards angles in most of the patients of both groups to be obtuse which can be a good topic for research in the future.
Measurement of the preoperative anteroposterior diameter of the foramen magnum (FM) in the images is straightforward. In the CT or MRI, the anteroposterior diameter as well as the plane of the foramen magnum can be drawn by joining the basion and the opisthion on the midsagittal plane of the images, which corresponds with McRae’s line [21-23]. Following posterior fossa decompression for CM1, because of the deficient posterior margin of the foramen magnum or the opisthion, it becomes difficult to delineate the McRL and measure the postoperative AP diameter of the FM. The method we devised is a simple and reliable one to measure the postoperative AP diameter of the FM despite the absence of the opisthion.
The AP diameter of the foramen magnum of CM1 patients and the normal population varies between different studies which is mostly due to the varying physical characteristics of the samples studied. Many studies found the FM AP diameter of CM1 patients larger than the normal population [24-27], some have found the AP diameter narrower in CM1 patients than in the normal population [28], while most of the studies found no significant difference in AP diameter of the FM in CM1 and normal populations [29-33]. The average AP diameter of the foramen magnum of CM1 patients ranged between 31.7 ± 6.1 mm to 40.1 mm in different studies [24-26,29,31,34,35]. We found the AP diameter of the foramen magnum to be slightly smaller than the lower range of other studies. This might be due to the ethnic difference of the population as people in our region are generally of shorter stature than the other study populations which consequently may have resulted in the smaller AP diameter of the foramen magnum from the anthropological point of view.
The increase in the postoperative AP diameter of the FM, which could be accurately measured from both the pre and postoperative MRIs, was remarkably more in the SC group than in the PFD±DP group (Table 2). We feel that the comparatively greater increase is due to persistent maintenance of the posterior surface of the posterior fossa and the posterior margin of the newly formed foramen magnum by the Stealth cranioplasty. In PFD±DP, initially, the posterior fossa volume along with the AP diameter of the FM increases but due to lack of any firm support in the back, gradually with time, the muscle bulk comes back or fibrosis ensues and the initial increase cannot be maintained anymore in all. Moreover, the increase in SC is relatively more from the very beginning owing to the cranioplasty which can make more room in the posterior fossa and around the FM.
Postoperative changes in foramen magnum diameter and plane have not been evaluated very frequently and literature related to this is hard to find. Most of the surgical techniques are centered around posterior fossa bony decompression and postoperatively, the posterior margin of the foramen magnum is deficient following surgery. Thus, any reference point following traditional CM1 surgery to identify the posterior end point of the McRae’s line (Opisthion), to measure the foramen magnum diameter is lacking. We feel that the technique that we introduced to identify the opisthion postoperatively, is a simple, easy, reliable, and useful one to mark and measure the McRae’s line to evaluate the postoperative foramen magnum diameter as well as the plane to see the status of tonsillar ectopia.
Following posterior fossa decompression, owing to the lack of posterior margin of the FM, most studies measured the preoperative tonsillar herniation only. Preoperatively, McRae’s line is used and described as the reference line to measure the tonsillar ectopia of CM1 patients [11,36,37]. However, the failure to delineate the opisthion and the McRL in the postoperative MRI made the postoperative measurements of tonsillar ectopia scarce in the literature. Different techniques have been applied, which were always neither uniform nor ideal as different reference points were used pre and postoperatively. With the introduction of our technique, we defined some fixed reference points, angles, and lines for postoperative measurement of the AP diameter of the FM and the tonsillar ectopia which are substantially constant and reliable. The McRae’s line is a fixed reference line that can be easily drawn on postoperative MRIs by our technique and the tonsillar ectopia can also be measured easily by drawing a vertical line from the tip of the tonsil on the McRae’s line.
The preoperative extent of tonsils from the foramen magnum (McRae’s line) ranged from 5-38 mm with an average of 7.4mm to 13.03 ± 5.31 mm in different literature [3,11,24-26,29,34,35,38-40]. We found the preoperative tonsillar ectopia to be matching with the range of other studies. Postoperatively, different studies observed changes in tonsillar ectopia ranging differently which varies greatly depending on the measuring techniques. One study, without describing the technique of measurement observed 9.4±2.4 mm of change in tonsillar ectopia postoperatively [40]. Interestingly, in another study, the change in tonsillar ectopia was 3.8 mm when measured from the line through the C2 vertebral end plate and 5.4 mm when measured from the line through C2 synchondrosis in the same study population (6). This finding implies that the measurements can be inconsistent even in the same population with different techniques of measurements owing to inconsistent measuring landmarks. The directions and dimensions of the measurement parameter lines have a propensity to change with the position of the neck during flexion and extension. During scanning, it is not possible always to keep the patient in the scanner exactly in the same position during both the pre and postoperative scans. That is why one needs to have constant landmarks to draw lines, that would make the lines constant also. This is how, our technique effectively gives better reading than the others in measuring the change in tonsillar ectopia postoperatively as the clival line, the basion, and the Boogard’s angle, all three are constant, whatever the position of the patient may be. However, there is the possibility of a change in the position of the tonsil, both pre and postoperatively, due to two factors. Firstly, the tonsils would have different positions, if the neck is not exactly in the same position in the scanner both pre and postoperatively due to a change in flexion or extension. Secondly, even if the positions are the same, there is a chance of discrepancy in the position of the tonsils as they move quite a bit during inspiration and expiration as well as the cardiac cycle. Even after considering all these, we feel that our technique is comparatively a better way of drawing and measuring the McRae’s line and thus measuring the diameter of the foramen magnum as well as the changes in tonsillar ectopia postoperatively. Nevertheless, absolute measurements seem to be practically daunting.
The postoperative changes in tonsillar ectopia were better in the SC group than in the PFD±DP group which was highly significant (p <0.001). This can be explained by the improvement of 2 postoperative factors. In the SC group, both the increase in AP diameter of foramen magnum and development of cisterna magna was more than in the PFD±DP group and more importantly, both the FM diameter and the CM were maintained in a more sustained way by the support of the Stealth cranioplasty. The postoperative additional increase in FM diameter helped the tonsils to pull back to the extra voluminous posterior fossa aided by the push of CSF from the spinal compartment below. Furthermore, the new position of the tonsil was maintained better by the buoyancy of more CSF in the posterior fossa, particularly with the aid of the newly formed CM.
SM associated with Chiari malformation is a mysterious entity. This has bothered the scientists ever since this has caught their eyes and that continues till today. The management protocol for SM associated with CM1 has evolved all the way to today, yet no conclusive solution could be achieved. As a result, many modalities of treatment protocols are followed in practice and the results vary a great deal as the occurrences of SM with CM1 vary greatly also.
Different studies reported occurrences of the SM in a wide range of percentages in CMI operative series which varies from 20% to 100% in adult preoperative MRIs [3,6,20,38,40-43]. 96 % (51 / 53) of patients presented with SM in their preoperative MRIs in our series. The presence of such a high percentage of syringes in our series might be due to the late presentation of the patients to us. The patients might have neglected their symptoms initially and presented late, which is very common in this part of the world, and harbored the CM1 for a long time to establish the SM sturdily until they had the obvious SM-related symptoms. Another possible reason might be the ethnic differences in the study populations. The smaller FM diameter in our population may also have some role in the discrepancy of CSF dynamics more than usual around the foramen magnum leading to the formation of the SM in this group of population earlier and more in numbers than others.
Dismayingly, improvement in SM is highly variable in different series and an unfavorable outcome is not very uncommon. Various series describe different accounts of improvement of the SM, ranging between 39 -100% [2,6,14, 18-20,38,40,41,44]. No improvement of the SM is also described in 10% to 40% of cases following surgery [11,19,20]. Postoperatively, 5.72% to 9.1% of patients may even experience an increase in the size of the SM [6,19,20]. Most of the studies found that no single surgical technique is better than the others in SM reduction [11,19].
All the patients that had SM (n= 51) in our series had some resolution of the SM, ranging from a minimum of 0.16% to a maximum of 89.7% of the change in postoperative diameter. However, the follow-up period was only 3 months and longer follow-ups might have a different picture.
We found more SM resolution in the SC group than in the PFD±DP group. The possibility is that we create more space in the posterior fossa by doing the cranioplasty and maintain that better than in PFD±DP and there is little chance of restenosis. The newly formed bigger cisterna magna in SC offers more possibility of restoration and maintenance of near normal CSF dynamics and equilibrium between the cranial and the spinal compartments which may have an additional role in resolution of the SM.
The absence of cisterna magna is one of the important key features other than the tonsillar herniation and the presence of SM. The absence of CM plays a significant role in CM1 patients as this heralds the disequilibrium of CSF dynamics between the cranial and spinal CSF compartments. Hence, postoperative reestablishment of CM can be an ideal sign of adequate decompression and therapeutic success.
The impact of stenosed or absent CM in preoperative MRI has been given supreme importance in producing clinical manifestations as well as in prognosis by various authors and it has been stressed that satisfactory restoration of the cisterna magna is a substantial feature of good prognosis [30,34,35,45-47]. Stenosis or absence of CM in preoperative MRI is one of the most common radiological findings and has been described in several studies ranging from 55% to 100% [24,30,34,35,48,49].
One of the goals of surgery for CM1 remains to be reestablishing CSF flow around the CVJ by creating or enlarging the CM and a number of techniques to do so have been adapted. Various studies showed success in creating or enlarging the CM in 36.4% to 100% of patients as evidenced in postoperative MRIs [45,50-56].
Postoperative CM development in the SC group was significantly better than in the PFD±DP group in our study population (p <0.001). The absence of the CM in the preoperative MRI might have resulted from late presentations when both the clinical and radiological features of CM1 were established overtly.
The CM has a dense lattice of arachnoid all around the cerebellum through which CSF makes passage. Once the tonsils herniate and get impacted at the foramen magnum, the CSF pathways through the arachnoid webbings start to get squeezed and the interlacing networks adhere to one another. With time, the more the cerebellum is pushed down the more the remaining CSF passages are choked cyclically and obstruct the remaining pathways of CSF leading to more adhesion of the arachnoid meshwork. Enlarging the posterior fossa and foramen magnum with or without duraplasty not only makes room for the overcrowded neural elements but also serves greatly in re-establishing the CSF flow and dynamics around the foramen magnum. The newly created space helps the CSF to flow freely in larger volumes to disentangle the adhesions to open up the previously closed channels. With decompression, the neural elements get ample space to breathe and the CSF gets a chance to flow freely aided by the brain pulsation.
The smaller number of developments of CM in the PFD±DP group, particularly in those who had an arachnoid breach, might have been due to seepage of blood from the cut dural margin or from some other source into the subarachnoid space instigating chemical meningitis and causing inflammation and more adhesion. Peroperative manipulation of the arachnoid itself may also lead to arachnoiditis blocking the existing narrow CSF passages further with time. These factors are particularly relevant when the arachnoid is opened. Moreover, whatever improvement is achieved by decompression of the neural elements by PFD±DP, as time passes, there is every possibility of blockage of the opened-up CSF passages again by meningitis, arachnoiditis, or compression from behind by muscles, or fibrosis. With SC we tried to cover these problems and it seems to work well. On the other hand, the higher percentage of the re-establishment of CM in SC can be attributed to the fact that in SC, not only the CM is reconstructed and enlarged by duraplasty, but it is also well maintained through the cranioplasty and tenting. All these together allow for opening up more CSF channels that are blocked by the adhesion of the arachnoid trabeculae. This is further enhanced by forceful voluminous CSF flow aided by the constant rhythmic brain pulsation.
Conclusion
Stealth cranioplasty seems to be a better option than the traditional posterior fossa decompression with or without duraplasty for symptomatic adult Chiari malformation 1 considering the postoperative radiological outcomes of changes in the diameter of the foramen magnum (FM), tonsillar Ectopia (TE), the diameter of syringomyelia (SM) and the formation of the Cisterna Magna (CM). The approach we innovated to evaluate postoperative radiological changes in CM1 patients is a unique but simple, convenient, reliable, and useful one. We believe it will be helpful to other researchers to measure the radiological outcomes consistently. This technique will also show ways for researchers to find newer dimensions of pathophysiology to treat CM1 patients better in the future.
Strength
Our technique of postoperative radiological measurement of Chiari malformation 1 is a novel one. This simple, easy, and dependable technique can be of much use on a regular basis and can be beneficial in future research.
Limitations
The calculations that were made for different measurements were done manually with the help of ImageJ software. Although the measurements were made at least 3 times for each parameter to reduce the margin of human error, it is very likely and predictable that these measurements cannot be the most accurate ones. Regrettably, due to the COVID-19 pandemic, the number of patients was less than anticipated and the follow-up period had to be cut short owing to restrictions on movement during the study period.
Recommendations
The technique that we followed can be used conveniently to measure the changes in McRL and tonsillar ectopia. Nevertheless, the development of new software to draw these lines and measure the parameters would give more accuracy in results. Further multicenter studies on a larger population are also recommended.
Disclosure of conflict of interest
None
Acknowledgement
We are grateful to our nursing and other staff and the patients.
Funding
This study was partially funded by the research grant of Bangabandhu Sheikh Mujib Medical University (BSMMU).
Credit author statement
Asifur Rahman: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing - original draft, Writing - review & editing
Nazmin Ahmed: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing
Abu Saleh Mohammad Abu Obaida: Conceptualization, Data curation, Investigation, Writing - review & editing
Abu Naim Wakil Uddin: Conceptualization, Data curation, Investigation, Writing - review & editing
Bipin Chaurasia: Conceptualization, Data curation, Investigation, Writing - review & editing
Md Atikur Rahman: Conceptualization, Data curation, Investigation, Writing - review & editing
Md Shamsul Alam: Conceptualization, Data curation, Investigation, Writing - review & editing
Md Moududul Haque: Conceptualization, Data curation, Investigation, Writing - review & editing
References
- Barkovich A, Wippold F, Sherman J, Citrin C. Significance of cerebellar tonsillar position on MR. American journal of neuroradiology 7 (1986): 795-799.
- McClugage SG, Oakes WJ. The Chiari I malformation: JNSPG 75th anniversary invited review article. Journal of Neurosurgery: Pediatrics 24 (2019): 217-226.
- Rangari K, Das KK, Singh S, et al. Type I Chiari malformation without concomitant bony instability: assessment of different surgical procedures and outcomes in 73 patients. Neurospine 18 (2021): 126.
- Tubbs RS, Lyerly MJ, Loukas M, et al. The pediatric Chiari I malformation: a review. Child's Nervous System 23 (2007):1239-1250.
- Holly LT, Batzdorf U. Chiari malformation and syringomyelia: JNSPG 75th Anniversary Invited Review Article. Journal of Neurosurgery: Spine 31 (2019): 619-628.
- Jussila MP, Nissilä J, Vakkuri M, et al. Preoperative measurements on MRI in Chiari 1 patients fail to predict outcome after decompressive surgery. Acta Neurochirurgica 163 (2021): 2005-2014.
- Luciano MG, Batzdorf U, Kula RW, Rocque BG, Maher CO, Heiss J, et al. Development of common data elements for use in Chiari malformation type I clinical research: an NIH/NINDS project. Neurosurgery 85 (2019): 854-860.
- Ciaramitaro P, Ferraris M, Massaro F, et al. Clinical diagnosis-part I: what is really caused by Chiari I. Child's Nervous System 35 (2019): 1673-1679.
- Rahman A, Rana M, Bhandari P, et al. "Stealth cranioplasty:" A novel endeavor for symptomatic adult Chiari I patients with syringomyelia: Technical note, appraisal, and philosophical considerations. Journal of Craniovertebral Junction & Spine 8 (2017): 243-252.
- Grangeon L, Puy L, Gilard V, et al. Predictive factors of headache resolution after Chiari type 1 malformation surgery. World Neurosurgery 110 (2018): e60-e6.
- Tosi U, Lara-Reyna J, Chae J, et al. Persistent syringomyelia after posterior fossa decompression for chiari malformation. World neurosurgery 136 (2020): 454-461.
- Hale AT, Adelson PD, Albert GW, et al. Factors associated with SM size in pediatric patients treated for Chiari malformation type I and syringomyelia: a study from the Park-Reeves Syringomyelia Research Consortium. Journal of Neurosurgery: Pediatrics 25 (2020): 629-639.
- Sadler B, Kuensting T, Strahle J, Park TS, Smyth M, Limbrick DD, et al. Prevalence and impact of underlying diagnosis and comorbidities on Chiari 1 malformation. Pediatric neurology 106 (2020): 32-37.
- Takeshima Y, Matsuda R, Nishimura F, Nakagawa I, Motoyama Y, Park Y-S, et al. Sequential enlargement of posterior fossa after duraplasty for Chiari malformation type 1. World Neurosurgery: X 2 (2019):100004.
- Rahman A. “Stealth Cranioplasty” for Adult Chiari Malformation Type 1: A Philosophical Journey of Innovation, Adaptation, and Evolution. Neurosurgical Procedures-Innovative Approaches: IntechOpen (2019).
- Rahman A. Role of Cranioplasty in Management of Chiari Malformation. Neurosurgical Procedures-Innovative Approaches: IntechOpen (2020).
- Lara-Reyna J, Chae J, Tosi U, Souweidane MM, Uribe-Cardenas R, Greenfield JP. Syringomyelia Resolution Following Chiari Surgery: A Novel Scale for Communication and Research. Neurosurgery 88 (2021): E60-E66.
- Perrini P, Anania Y, Cagnazzo F, Benedetto N, Morganti R, Di Carlo DT. Radiological outcome after surgical treatment of syringomyelia-Chiari I complex in adults: a systematic review and meta-analysis. Neurosurgical review 44 (2021): 177-187.
- Walker-Palmer TK, Cochrane DD, Singhal A, et al. Outcomes and complications for individual neurosurgeons for the treatment of Chiari I malformation at a children’s hospital. Child's Nervous System 35 (2019): 1895-904.
- Zhao JL, Li MH, Wang CL, et al. A systematic review of Chiari I malformation: techniques and outcomes. World neurosurgery 88 (2016): 7-14.
- Nishikawa M, Sakamoto H, Hakuba A, et al. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. Neurosurgical Focus 1 (1996): E1.
- Nishikawa M, Sakamoto H, Hakuba A, et al. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. Journal of neurosurgery 86 (1997): 40-47.
- Poretti A, Ashmawy R, Garzon-Muvdi T, et al. Chiari type 1 deformity in children: pathogenetic, clinical, neuroimaging, and management aspects. Neuropediatrics 47 (2016): 293-307.
- Aydin S, Hanimoglu H, Tanriverdi T, et al. Chiari type I malformations in adults: a morphometric analysis of the posterior cranial fossa. Surgical neurology 64 (2005): 237-241.
- Dagtekin A, Avci E, Kara E, et al. Posterior cranial fossa morphometry in symptomatic adult Chiari I malformation patients: comparative clinical and anatomical study. Clinical neurology and neurosurgery 113 (2011): 399-403.
- Houston JR, Eppelheimer MS, Pahlavian SH, et al. A morphometric assessment of type I Chiari malformation above the McRae line: a retrospective case-control study in 302 adult female subjects. Journal of Neuroradiology 45 (2018): 23-31.
- Tastemur Y, Sabanciogullari V, Salk I, et al. The relationship of the posterior cranial fossa, the cerebrum, and cerebellum morphometry with tonsiller herniation. Iranian Journal of Radiology 14 (2017): e24436.
- Hwang HS, Moon JG, Kim CH, et al. The comparative morphometric study of the posterior cranial fossa: what is effective approaches to the treatment of Chiari malformation type 1? Journal of Korean Neurosurgical Society 54 (2013): 405-410.
- Alperin N, Loftus JR, Oliu CJ, et al. Magnetic resonance imaging measures of posterior cranial fossa morphology and cerebrospinal fluid physiology in Chiari malformation type I. Neurosurgery 75 (2014): 515-522.
- Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 44 (1994): 1005-1017.
- Milhorat TH, Nishikawa M, Kula RW, et al. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta neurochirurgica 152 (2010): 1117-1127.
- Sekula RF, Jannetta PJ, Casey KF, et al. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal fluid research 2 (2005): 1-7.
- Urbizu A, Poca MA, Vidal X, et al. MRI-based Morphometric Analysis of Posterior Cranial Fossa in the Diagnosis of Chiari Malformation Type I. Journal of neuroimaging 24 (2014): 250-256.
- Alkoç OA, Songur A, Eser O, et al. Stereological and morphometric analysis of MRI Chiari malformation type-1. Journal of Korean Neurosurgical Society 58 (2015): 454-461.
- Karagöz F, Izgi N, Sencer SK. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta neurochirurgica 144 (2002): 165-171.
- Erdogan E, Cansever T, Secer HI, et al. The evaluation of surgical treatment options in the Chiari Malformation Type I. Turkish neurosurgery 20 (2010): 303-313.
- Ladner TR, Dewan MC, Day MA, et al. Evaluating the relationship of the pB–C2 line to clinical outcomes in a 15-year single-center cohort of pediatric Chiari I malformation. Journal of Neurosurgery: Pediatrics 15 (2015): 178-188.
- Feghali J, Marinaro E, Xie Y, et al. Family History in Chiari Malformation Type I: Presentation and Outcome. World neurosurgery 142 (2020): e350-e356.
- Halvorson KG, Kellogg RT, Keachie KN, et al. Morphometric analysis of predictors of cervical SM formation in the setting of Chiari I malformation. Pediatric Neurosurgery 51 (2016): 137-141.
- Lei Z, Wu S, Zhang Z, et al. Clinical characteristics, imaging findings and surgical outcomes of Chiari malformation type I in pediatric and adult patients. Current Medical Science 38 (2018): 289-295.
- Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult Chiari malformation Type I surgical series 1965-2013: a review of demographics, operative treatment, and outcomes. Journal of Neurosurgery Pediatrics 15 (2015): 161-77.
- Koueik J, Sandoval-Garcia C, Kestle JR, et al. Outcomes in children undergoing posterior fossa decompression and duraplasty with and without tonsillar reduction for Chiari malformation type I and syringomyelia: a pilot prospective multicenter cohort study. Journal of Neurosurgery: Pediatrics 25 (2019): 21-29.
- Soleman J, Roth J, Constantini S. Direct SM drainage in patients with Chiari I malformation. Child's Nervous System 35 (2019): 1863-1868.
- Lloyd RA, Fletcher DF, Clarke EC, Bilston LE. Chiari malformation may increase perivascular cerebrospinal fluid flow into the spinal cord: a subject-specific computational modelling study. Journal of biomechanics 65 (2017): 185-193.
- Lee HS, Lee SH, Kim ES, et al. Surgical results of arachnoid-preserving posterior fossa decompression for Chiari I malformation with associated syringomyelia. Journal of Clinical Neuroscience 19 (2012): 557-560.
- Ventureyra EC, Aziz HA, Vassilyadi M. The role of cine flow MRI in children with Chiari I malformation. Child's Nervous System 19 (2003): 109-113.
- Yuh WT, Kim CH, Chung CK, et al. Surgical outcome of adult idiopathic Chiari malformation type 1. Journal of Korean Neurosurgical Society 59 (2016): 512-517.
- Lou Y, Yang J, Wang L, et al. The clinical efficacy study of treatment to Chiari malformation type I with syringomyelia under the minimally invasive surgery of resection of Submeningeal cerebellar Tonsillar Herniation and reconstruction of Cisterna magna. Saudi Journal of Biological Sciences 26 (2019): 1927-1931.
- Quon JL, Grant RA, DiLuna ML. Multimodal evaluation of CSF dynamics following extradural decompression for Chiari malformation Type I. Journal of Neurosurgery Spine 22 (2015): 622-630.
- Assina R, Meleis AM, Cohen MA, et al. Titanium mesh-assisted dural tenting for an expansile suboccipital cranioplasty in the treatment of Chiari 1 malformation. Journal of clinical neuroscience 21 (2014):1641-1646.
- Bao C, Yang F, Liu L, et al. Surgical treatment of Chiari I malformation complicated with syringomyelia. Exp Ther Med 5 (2012): 333-337.
- Deng X, Wu L, Yang C, et al. Surgical treatment of Chiari I malformation with ventricular dilation. Neurologia medico-chirurgica 53 (2013): 847-852.
- Deng X, Yang C, Gan J, et al. Long-term outcomes after small-bone-window posterior fossa decompression and duraplasty in adults with Chiari malformation type I. World neurosurgery 84 (2015): 998-1004.
- Liu B, Wang Y, Liu S, et al. Tonsillectomy with modified reconstruction of the cisterna magna with and without craniectomy for the treatment of adult Chiari malformation type I with syringomyelia. Acta Neurochirurgica 162 (2020): 1585-1595.
- Penfield W, Coburn DF. Arnold-Chiari malformation and its operative treatment. Archives of Neurology & Psychiatry 40 (1938): 328-336.
- Pinna G, Alessandrini F, Alfieri A, Rossi M, Bricolo A. Cerebrospinal fluid flow dynamics study in Chiari I malformation: implications for SM formation. Neurosurgical focus 8 (2000): 1-8.


 Impact Factor: * 4.2
Impact Factor: * 4.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 