Association of Serum Protein C and Protein S Level in Unexplained Recurrent Pregnancy Loss
Article Information
Mousumi Saha1*, Jesika Rizvi Tamanna2, khadiza Begum3,Masuda Sultana4, Surayea Bul-Bul5,
Salauddin Shah6,Nahreen Akhtar7
1 Assistant Professor(OBGYN), OSD(DGHS), On Deputation, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
2 Registrar, Department of Gynecology & Obstetrics, Bangabandhu Sheikh Mujib Medical College and Hospital (BSMMCH), Faridpur, Bangladesh
3 Consultant (OBGYN). OSD (DGHS). On Deputation, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
4 Junior Consultant, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
5 Junior Consultant, Department of Gynecology & Obstetrics, Shaheed Suhrawardy Medical College and Hospital, Dhaka Bangladesh.
6 Chairman and Professor, Department of Haematology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
7 Chairman and Professor, Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
*Corresponding Author: Dr. Mousumi Saha, Assistant professor(OBGYN), OSD(DGHS), On deputation - Department of Fetomaternal Medicine, BSMMU, Dhaka, Bangladesh.
Received: 15 May 2023; Accepted: xx May 2023; Published: 05 October 2023
Citation: Dr. Mousumi Saha, Dr. Jesika Rizvi Tamanna, Dr. khadiza Begum, Dr. Masuda Sultana, Dr. Surayea Bul-Bul, Prof. Dr. Md. Salauddin Shah, Prof. Dr. Nahreen Akhtar. Association of Serum Protein C and Protein S Level in Unexplained Recurrent Pregnancy Loss. Obstetrics and Gynecology Research. 6 (2023): 260-269.
View / Download Pdf Share at FacebookAbstract
Background:
Recurrent pregnancy loss (RPL) is an intense personal calamity to the couples and an arduous clinical challenge to the obstetricians. One of the greatest challenges is not being able to identify the baseline etiology of RPL when all the routine and recommended investigations return normal or negative. RPL affects about 1-3% of women of reproductive age trying to conceive. Thrombophilia has been identified as one of main causes of RPL. Inherited thrombophilia includes Protein C deficiency, Protein S deficiency are linked to RPL and could be associated with severe obstetric complications such as preeclampsia, intrauterine growth restriction, or stillbirth which are further associated with microvascular thrombosis in placental blood vessels. Protein C and Protein S deficiencies are associated with a variably increased risk of thrombosis and are inherited independently in an autosomal dominant trait.
Objective:
The aim of the study was to determine the association of serum protein C and protein S level in patients with unexplained recurrent pregnancy loss.
Methods:
A case control study was carried out in the outpatient Department of Feto-maternal Medicine, Bangabandhu Sheikh Mujib Medical University, Dhaka. After obtaining the ethical clearance from the Institutional Review Board, a convenient sampling technique was applied for data collection. According to the availability of the patients as per inclusion and exclusion criteria, 30 patients with RPL and 30 ages and BMI matched control was taken and necessary information was recorded in a structured questionnaire. With all aseptic precaution 2 ml of venous blood was collected from antecubital vein of each participant by trained blood collector of Department of Hematology, BSMMU, in a supplied coagulation vial. After centrifuging, supernatant plasma was used for mixing with protein C and protein S reagent. By clotting method using a fully automated coagulation analyzer Sysmex CS-1600 machine results were obtained. The results were noted in the questionnaire and data was analyzed using SPSS software.
Result:
Mean age and BMI of the RPL patients was 26.85±4.17 years and 25.03±2.42 Kg/m2, which was matched with the control groups. The study found that RPL patients experienced an average of 4 pregnancy losses and about 70% pregnancy loss occurred during first trimester. A total of 3(10%) protein C deficient, 5(16.7%) protein S deficient and 2(6.7%) both protein C and S deficient patient were found in the case group. The mean of Protein C and S level among RPL patients was 78.46±13.18 and 81.69±14.06 respectively, which was significantly lower than the control group level. Protein C and S level did not vary for patients experienced 1st trimester loss or 2nd trimester loss. The odds ratio for RPL group due to protein C and protein S deficiency was considerably higher [3.22, 95% CI: 0.32-32.89 for Protein C and 5.80, 95% CI: 0.64-53.01 for PS] and a significant association of protein C and protein S level with RPL was found with adjusted binary logistic regression.
Conclusion:
The present study found higher incidence of protein C and S deficiency among RPL patients with most of the miscarriage occurring in the first trimester. Hence, pregnant women should be tested for protein C and S deficiencies after excluding common causes of RPL and treated accordingly.
Keywords
Recurrent Pregnancy Loss; Protein C; Protein S; Thrombophilia
Recurrent Pregnancy Loss articles Recurrent Pregnancy Loss Research articles Recurrent Pregnancy Loss review articles Recurrent Pregnancy Loss PubMed articles Recurrent Pregnancy Loss PubMed Central articles Recurrent Pregnancy Loss 2023 articles Recurrent Pregnancy Loss 2024 articles Recurrent Pregnancy Loss Scopus articles Recurrent Pregnancy Loss impact factor journals Recurrent Pregnancy Loss Scopus journals Recurrent Pregnancy Loss PubMed journals Recurrent Pregnancy Loss medical journals Recurrent Pregnancy Loss free journals Recurrent Pregnancy Loss best journals Recurrent Pregnancy Loss top journals Recurrent Pregnancy Loss free medical journals Recurrent Pregnancy Loss famous journals Recurrent Pregnancy Loss Google Scholar indexed journals Protein C articles Protein C Research articles Protein C review articles Protein C PubMed articles Protein C PubMed Central articles Protein C 2023 articles Protein C 2024 articles Protein C Scopus articles Protein C impact factor journals Protein C Scopus journals Protein C PubMed journals Protein C medical journals Protein C free journals Protein C best journals Protein C top journals Protein C free medical journals Protein C famous journals Protein C Google Scholar indexed journals Protein S articles Protein S Research articles Protein S review articles Protein S PubMed articles Protein S PubMed Central articles Protein S 2023 articles Protein S 2024 articles Protein S Scopus articles Protein S impact factor journals Protein S Scopus journals Protein S PubMed journals Protein S medical journals Protein S free journals Protein S best journals Protein S top journals Protein S free medical journals Protein S famous journals Protein S Google Scholar indexed journals Thrombophilia articles Thrombophilia Research articles Thrombophilia review articles Thrombophilia PubMed articles Thrombophilia PubMed Central articles Thrombophilia 2023 articles Thrombophilia 2024 articles Thrombophilia Scopus articles Thrombophilia impact factor journals Thrombophilia Scopus journals Thrombophilia PubMed journals Thrombophilia medical journals Thrombophilia free journals Thrombophilia best journals Thrombophilia top journals Thrombophilia free medical journals Thrombophilia famous journals Thrombophilia Google Scholar indexed journals consecutive pregnancy loss articles consecutive pregnancy loss Research articles consecutive pregnancy loss review articles consecutive pregnancy loss PubMed articles consecutive pregnancy loss PubMed Central articles consecutive pregnancy loss 2023 articles consecutive pregnancy loss 2024 articles consecutive pregnancy loss Scopus articles consecutive pregnancy loss impact factor journals consecutive pregnancy loss Scopus journals consecutive pregnancy loss PubMed journals consecutive pregnancy loss medical journals consecutive pregnancy loss free journals consecutive pregnancy loss best journals consecutive pregnancy loss top journals consecutive pregnancy loss free medical journals consecutive pregnancy loss famous journals consecutive pregnancy loss Google Scholar indexed journals ectopic pregnancies articles ectopic pregnancies Research articles ectopic pregnancies review articles ectopic pregnancies PubMed articles ectopic pregnancies PubMed Central articles ectopic pregnancies 2023 articles ectopic pregnancies 2024 articles ectopic pregnancies Scopus articles ectopic pregnancies impact factor journals ectopic pregnancies Scopus journals ectopic pregnancies PubMed journals ectopic pregnancies medical journals ectopic pregnancies free journals ectopic pregnancies best journals ectopic pregnancies top journals ectopic pregnancies free medical journals ectopic pregnancies famous journals ectopic pregnancies Google Scholar indexed journals Fetomaternal articles Fetomaternal Research articles Fetomaternal review articles Fetomaternal PubMed articles Fetomaternal PubMed Central articles Fetomaternal 2023 articles Fetomaternal 2024 articles Fetomaternal Scopus articles Fetomaternal impact factor journals Fetomaternal Scopus journals Fetomaternal PubMed journals Fetomaternal medical journals Fetomaternal free journals Fetomaternal best journals Fetomaternal top journals Fetomaternal free medical journals Fetomaternal famous journals Fetomaternal Google Scholar indexed journals cervical anatomical abnormalities articles cervical anatomical abnormalities Research articles cervical anatomical abnormalities review articles cervical anatomical abnormalities PubMed articles cervical anatomical abnormalities PubMed Central articles cervical anatomical abnormalities 2023 articles cervical anatomical abnormalities 2024 articles cervical anatomical abnormalities Scopus articles cervical anatomical abnormalities impact factor journals cervical anatomical abnormalities Scopus journals cervical anatomical abnormalities PubMed journals cervical anatomical abnormalities medical journals cervical anatomical abnormalities free journals cervical anatomical abnormalities best journals cervical anatomical abnormalities top journals cervical anatomical abnormalities free medical journals cervical anatomical abnormalities famous journals cervical anatomical abnormalities Google Scholar indexed journals ovarian dysfunction articles ovarian dysfunction Research articles ovarian dysfunction review articles ovarian dysfunction PubMed articles ovarian dysfunction PubMed Central articles ovarian dysfunction 2023 articles ovarian dysfunction 2024 articles ovarian dysfunction Scopus articles ovarian dysfunction impact factor journals ovarian dysfunction Scopus journals ovarian dysfunction PubMed journals ovarian dysfunction medical journals ovarian dysfunction free journals ovarian dysfunction best journals ovarian dysfunction top journals ovarian dysfunction free medical journals ovarian dysfunction famous journals ovarian dysfunction Google Scholar indexed journals endocrinological articles endocrinological Research articles endocrinological review articles endocrinological PubMed articles endocrinological PubMed Central articles endocrinological 2023 articles endocrinological 2024 articles endocrinological Scopus articles endocrinological impact factor journals endocrinological Scopus journals endocrinological PubMed journals endocrinological medical journals endocrinological free journals endocrinological best journals endocrinological top journals endocrinological free medical journals endocrinological famous journals endocrinological Google Scholar indexed journals
Article Details
INTRODUCTION
Recurrent pregnancy loss (RPL) is a devastating & challenging condition for couples as well as clinicians which needs sensitive and reassuring care as well as optimum management. One of the greatest challenges is not being able to identify the baseline etiology of RPL when all the routine and recommended investigations return normal or negative. RPL may be defined as the loss of three or more consecutive pregnancies (RCOG, 2011)[1]. According to ASRM,2012[2] and ESHRE, 2017 RPL[3] may be defined as two or more clinical and consecutive pregnancy losses with either ultrasound or histopathological documentation, with exclusion of molar and ectopic pregnancies. Thus, the couples should be evaluated after two losses. RPL is a common disorder that affects 3-5% of pregnant women. Identification of causes of RPL is the most challenging issue for the Fetomaternal specialists. Several causes may lead to RPL, such as chromosomal anomalies, congenital or acquired uterine and cervical anatomical abnormalities, ovarian dysfunction, endocrinological problems, maternal thrombophilia, infection, autoimmune disorders[4]. However, in about 50% cases, the reason of miscarriage is unknown[5]. Unfortunately, rest half never have a cause identified even after extensive investigations. These cases are referred to as unexplained recurrent pregnancy loss and serve as the submerged portion of iceberg for the researchers[6]. Thrombophilia has been identified as one of the main causes of RPL [7]. After chromosomal abnormality, thrombophilic disorders have generated considerable interest in the field of RPL of genetic origin, especially in the unexplained cases[8]. Thrombophilia is a blood coagulation disorder that increases the risk of venous thromboembolism. It also causes recurrent miscarriages in early weeks of pregnancy[9], and development of preeclampsia, intrauterine growth restriction, abruptio placentae, stillbirth in later part of gestation which are associated with microvascular thrombosis in placental blood vessels[10]. Inherited thrombophilia includes Antithrombin-III, protein C deficiency, protein S deficiency, Factor V Leiden mutation, Prothrombin gene mutation (20210A)[11]. Normal pregnancy is associated with increased procoagulants, decreased fibrinolysis & decreased anticoagulants to maintain placental hemostasis during pregnancy. The hemostatic system plays an important role in the success of pregnancy and the process of implantation and placentation. Implantation of the fertilized egg into the uterine decidua establishes a contact between the fetus, the placenta and the maternal circulation. This contact is crucial for the success of pregnancy. However, thrombophilia might be a risk factor with these changes especially in women with deficiency of any of natural anticoagulant factors. Protein C and protein S are natural anticoagulants and their deficiency was found to be associated with placental thrombosis, hypoperfusion, fetal loss and fetal death[12]. Relationship between RPL and thrombophilia is a much- debated topic with well entrenched expert opinion on both sides. Potential association between two is based on the theory of thrombosis in decidual vessels and inhibition of trophoblast differentiation causing fetal loss[13]. Protein C is a vitamin K-dependent plasma glycoprotein activated by thrombin-thrombomodulin complex on the surface of endothelial cells[14]. Activation of protein C into the serine protease - like enzyme, activated protein C[15] is catalyzed by thrombin when it is bound to the endothelial proteoglycan thrombomodulin. Activated protein C exerts its anticoagulant activity primarily through inactivation of coagulation factors Va and VIIIa, which are required for factor ? activation & thrombin generation. Protein S is a cofactor for APC. In the presence of protein S, phospholipids and calcium, APC inactivates membrane bound F Va and F VIIIa so results in attenuation of thrombin generation which leads to inhibit clot formation[16]. Protein C deficiency is generally subdivided into two types: type I decreased levels of protein C and type II decreased functional activity of protein C. Protein C deficiency is inherited as autosomal disorders and, in most cases, derived from heterozygous mutations[17]. There are two main types of assays: activity assay which is either clotting time - based assay or chromogenic by spectrophotometer. Quantitative assay for protein C antigen which are immunoassays generally done by using ELISA; it is considered to measure the quantity of protein C irrespective of its function. Genetic testing by DNA sequencing is indicated if the results of functional and antigenic assays do not approve the diagnosis clearly[18]. Acquired protein C deficiency can develop with vitamin K deficiency, liver disease, treatment with vitamin K antagonists, autoimmune syndromes, nephritic syndrome, disseminated intravascular coagulation [19]. A recent study stated an observation of a higher rate of late fetal loss in patients with protein C deficiency compared to non- deficient patients[20]. Protein S is a vitamin K-dependent glycoprotein which acts as natural anticoagulant. Protein S exists in plasma both free (40%) and bound to the compliment C4b binding protein (60%)[21]. Protein S has both APC-dependent and independent anticoagulant properties and consequently is an important protector in controlling thrombin generation and fibrinolysis[22]. The protein S deficiency is identified more than protein C deficiency and its prevalence has been assessed with about 0.5% in the healthy population and 2% to 12% of thrombophilia patients[23]. Regarding pregnancy complications, in a meta-analysis showed that protein S deficiency consulted an overall 15-fold increased risk of recurrent pregnancy loss and a 7-fold higher risk of late fetal loss[24]. Hereditary protein S deficiency is an autosomal dominant disorder. Acquired deficiency of protein S is detected in several pathological conditions including nephrotic syndrome, DIC, liver disease, oral anticoagulant drugs and could be associated with an increased risk of thrombosis[25]. There are two types of PS assays: Immunoassays for the determination of total and free PS levels and clotting assays to measure APC cofactor activity. Immunoassays for free and total PS are preferred for screening pregnancy loss. This study was designed to determine the association of serum protein C and protein S level in unexplained recurrent pregnancy loss.
OBJECTIVES
General objective
The general objective of the study to evaluate the association of serum protein C and protein S level in patients with unexplained recurrent pregnancy loss.
Specific objectives
- To estimate the serum protein C and protein S level in RPL patients
- To estimate the serum protein C and protein S level in age and BMI matched control
- To compare serum protein C and protein S level in RPL group with the women with normal reproductive history
- To figure out the association of serum protein C and protein S deficiency with recurrent pregnancy loss.
METHODOLOGY
This was a case-control study; a convenient sampling technique was applied for this study. This case-control study was conducted in the outpatient Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), and Dhaka, Bangladesh from December 2020 to November 2021. A total of 60 participants were included in this study, 30 for RPL and 30 for control participants group. The participant’s age and BMI matched women (Patient’s attendants) from both outpatient and in patients of BSMMU.
Inclusion criteria
The case groups
Women aged 18-40 years attending Feto-maternal Medicine OPD for preconceptional counseling for RPL who
- Had H/O consecutive two or more failed clinical pregnancies
- Had given consent to participate in the study.
Control groups
Age and BMI matched women who had at least one successful pregnancy with no history of spontaneous pregnancy loss from Outpatient and inpatient Department of Fetomaternal Medicine, Bangabandhu Sheikh Mujib Medical University, Dhaka.
Exclusion criteria:
Women whom cause for RPL was identified such as:
- Parental chromosomal abnormalities
- Anatomical defect of cervix and uterus
- Type I and type II diabetes mellitus
- Thyroid disorders
- Chronic hypertension
- Diagnosed case of chronic renal disease
- Autoimmune disorders
- Polycystic ovarian disease
Study procedure
All patients underwent a complete diagnostic work up for RPL including relevant history, clinical examination and investigation to exclude chronic hypertension, diabetes mellitus, thyroid disorders, chronic renal disease, polycystic ovarian disease, chromosomal analysis of both partners, and uterine anomalies. Regarding investigation CBC, blood sugar, serum TSH, TPO antibody, ANA, antids DNA, Lupas anticoagulant, anticardiolipin antibody, TVS, chromosomal analysis of each partner was done to exclude exclusion criteria. Then 30 participants were ultimately regarded as cases after scrutiny. Age and BMI matched 30 healthy participants who had no history of recurrent pregnancy loss and having at least one healthy child were selected as controls. A convenient sampling technique was used. Then physical examination findings and relevant laboratory investigation findings were recorded. After taking weight and height for each patient, BMI was calculated by the following formula: weight in kg/ height in meter2(kg/m2), and recorded in individual data sheet. Determination of the functional activity of protein C and protein S was performed in the Department of Hematology, BSMMU. After collection of specimens from antecubital vein in a supplied coagulation vial to obtain plasma, carefully 1 part of sodium citrate solution was mixed with 9 parts of venous blood, avoiding the formation of foam then was centrifuged at no less than 1500 rpm for at least 15 minutes at room temperature. During removal of plasma, carefulness was maintained regarding withdrawn of no platelets. Plasma sample was stored at -180c then thawed within 10 minutes at +370c after which the assay was to be performed by clotting method within 4 hours by using protein S Ac and protein C reagent in Sysmex CS-1600 Auto analyzer Machine. Internal quality control test was ensured. The results were obtained using a reference curve prepared beforehand by serially diluting standard Human plasma with protein S and protein C deficient plasma. Results were usually presented in term of percentage. Expected value of protein C range from 70-140% of normal activity. Expected value of protein S range from 70-125% of normal activity. In this study protein C and protein S level <70% was considered as deficiency. All information and investigation result were recorded in a predesigned data collection sheet.
Data Analysis of the Study
Statistical analysis was performed using the SPSS version 23.0 software for windows. The results of the study were presented in tables, figures and diagrams. The descriptive statistics of the study was presented in tables, figures, mean as per the requirement of qualitative and quantitative variables. Mean comparison between two groups was done by independent sample t-test or Mann-Whitney U test. Mean comparison between more than two groups was done by ANOVA test or Kruskal-Wallis test. Chi-square test was conducted to find out any association between categorial variables. Binary logistic regression analysis was conducted to figure out the association of protein C and protein S with RPL P<0.05 was considered as statistically significant.
Ethical consideration
Ethical clearance for the study was taken from the Institutional Review Board of BSMMU. Permission for the study was taken from the concerned departments. The entire study population was thoroughly appraised about the nature, purpose and implications of the study, as well as entire spectrum of benefits and risks of the study. Written consent of all the study participants.
RESULTS
|
Age groups |
RPL patient |
Control |
P value |
||
|
(n=30) |
% |
(n=30) |
% |
||
|
18-24 yrs. |
8 |
26.7 |
4 |
13.3 |
|
|
25-34 yrs. |
16 |
53.3 |
20 |
66.7 |
|
|
35-40 yrs. |
6 |
20 |
6 |
20 |
|
|
Mean ± SD |
26.85 ± 4.17 |
29.30 ± 4.63 |
0.462 |
||
Table 1: Age of the patients (N=60)
Table 1 showed it appears that maximum (53.3 %) of the patients in RPL group and 67.7% in control group were from 25 to 34 years of age. Only 6(20%) patients in RPL group were from 35-40 years of age. The mean of age of the RPL patients was (26.85±4.17), whereas the mean of age of the Controls was (29.30 ±4.63). There was no significant difference between these two groups in terms of age.
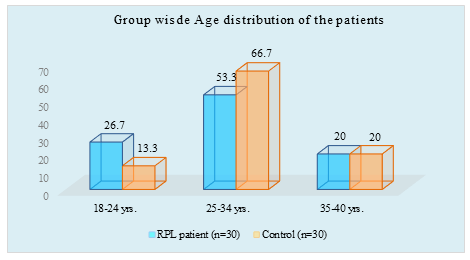
Figure I: Column chart showed age wise patients (N=60)
|
BMI (kg/m2) |
RPL (n=30) |
Control (n=30) |
P value |
||
|
n |
% |
n |
% |
||
|
18.5-24.9 |
16 |
52.9 |
19 |
62.9 |
0.367 |
|
25-29.9 |
9 |
35.3 |
7 |
24.3 |
|
|
30 ± |
5 |
11.8 |
4 |
12.8 |
|
|
Mean ± SD |
25.03±2.42 |
24.43±1.96 |
|||
|
Range |
19-34.4 |
18.1-31.4 |
|||
Table 2: Body mass index (BMI) (N=60)
Table 2 showed A Mann Whitney test was used to compare the mean BMI between RPL group and Control patient group. The test was not statistically significant. So, it can be said that BMI of RPL patient (25.03±2.42) was not significantly different from Control patient (24.43±1.96).
|
Parameters |
Frequency (n) |
Percentage (%) |
|
Pregnancy loss |
|
|
|
3 losses |
18 |
60 |
|
4 losses |
5 |
17 |
|
5 loss or more |
7 |
23 |
|
RPL type |
|
|
|
Primary |
24 |
80 |
|
Secondary |
6 |
20 |
|
Pregnancy loss as per trimester* |
|
|
|
1st trimester |
|
28(93.3) |
|
2nd trimester |
|
15(50) |
|
Combined (1st + 2nd) |
|
13(43.3) |
*Multiple responses considered
Table 3: Obstetric history among RPL Patients (N=30)
Table 3 showed among the RPL patients, 18(60%) had pregnancy loss for 3 times. Moreover, 7(23%) of the RPL patients had pregnancy loss for 5 times or more. In addition, 80% of the cases were primary RPL. It also appeared that 28(93.3%) RPL patient experienced pregnancy loss in 1st trimester.
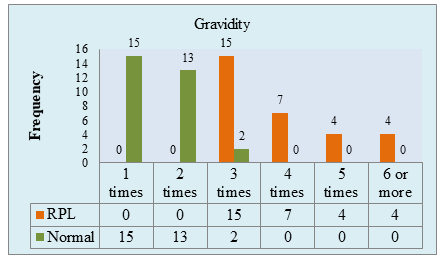
Figure II: Gravidity of the study population. (N=60)
Figure II showed, gravidity of the study sample was seen for both RPL and control group. In RPL group, all patients had been pregnant at least thrice. In addition, pregnancy-time ranges from 3 to 7 for RPL patients and majority were pregnant thrice. In control group, pregnancy-time ranges from 1 to 3 where most of the sample in this group had been pregnant ones or twice.
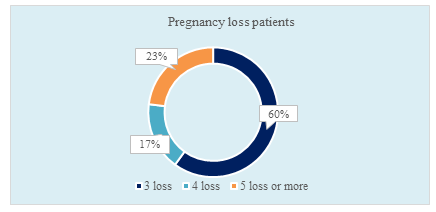
Figure III: Ring chart showed pregnancy loss in RPL patients. (N=60)
Figure III showed, among the RPL patients, 60% had pregnancy loss for 3 times. Moreover, 23% of the RPL patients had pregnancy loss for 5 times or more.
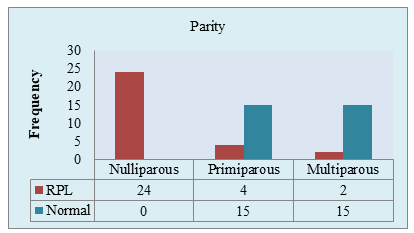
Figure IV: Parity of two groups of the study population (N=60)
Figure IV showed, among the RPL patient, 80% (24/30) were not able to give birth to any child. Only 6.7% RPL patient was multiparous. In contrast, 50% of the control patient was primiparous and 50% multiparous.
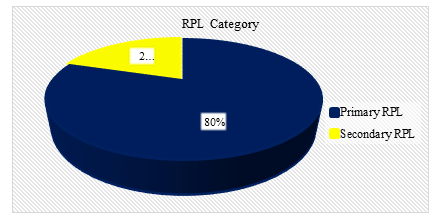
Figure V: Pie chart showed RPL category of the s (N=60)
Figure V showed among RPL patients, 80% of the cases were primary RPL.
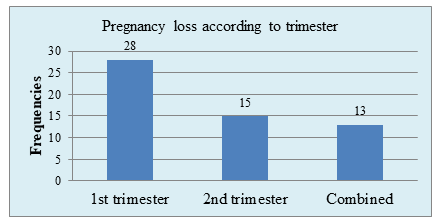
Figure VI: Bar chart showed Pregnancy loss according to trimester (N=60)
Figure VI showed, that 28(93.3%) RPL patient experienced pregnancy loss in 1st trimester.
|
Gestational age |
Frequency (n=111) |
Percentage (%) |
|
1st trimester (1-12 weeks) |
77 |
69.4 |
|
2nd trimester (13-24 weeks) |
34 |
30.6 |
Table 4: Total Pregnancy loss as per trimester
Table 4 showed most (69.4%) of the pregnancy loss occurred during first trimester. About 30.6% (34/111) pregnancy loss occurred during 2nd trimester.
|
Total pregnancy loss |
111 |
|
Number of RPL patients |
30 |
|
Average |
3.7~4 |
Table 5: Average pregnancy loss in RPL patient
Table 5 showed among the RPL patients, a total of 111 pregnancy loss was found. That is on average 3.7~4 losses occurred among RPL subjects.
|
Tests of Normality |
Kolmogorov-Smirnov |
||
|
Statistics |
df |
P value |
|
|
Protein C |
0.134 |
60 |
0.009 |
|
Protein S |
0.097 |
60 |
0.2 |
|
BMI |
0.304 |
60 |
<0.001 |
Table 6: Normality test of continuous variable (N=60)
Table 6 showed, normal distribution of the variables was checked first before comparing the means between RPL and control patients. Then according to the result parametric and non-parametric test was selected for comparing means of the two groups.
Parametric test
Parametric (independent sample t test) test was run to determine a significance difference of protein S between RPL and Control group since these data were normally distributed.
Non parametric test
Non parametric (Mann Whitney test) test was run to determine a significance difference of Protein C and BMI between RPL and Control patient group since these data were not normally distributed.
|
Deficiency |
Frequency (n) |
Percentage (%) |
|
Protein C deficiency |
3 |
10 |
|
Protein S deficiency |
5 |
16.7 |
|
Both Protein C & S deficiency |
2 |
6.7 |
Table 7: Protein C and S deficiency among RPL patients (n=10)
Table 7 showed among the RPL patients a total of 3(10%) protein C deficient, 5(16.7%) protein S deficient and 2(6.7%) both protein C and S deficient patient were found in the study.
|
Parameter |
RPL |
Control Mean ± SD |
P value |
|
Protein C level |
78.46±13.18 |
112.84 ± 17.57 |
<0.001 |
Table 8: Protein C level difference between the two groups
Table 8 showed (Mann Whitney test) to compare the mean Protein C level between RPL group and Control group. The test result was statistically significant (U= 831.500; P <0.001). So, it can be said that Protein C level of RPL patient (78.46 ± 13.18) was significantly lower than Control patient (112.84 ± 17.57).
|
Parameter |
RPL = 30 |
P value |
||
|
1st Trimester loss |
2nd Trimester loss |
(1st + 2nd) Trimester loss (Combined) |
||
|
Mean ±SD |
Mean ±SD |
Mean ±SD |
||
|
Protein C |
78.20±13.49 |
79.60 ±10.37 |
79.20 ±10.77 |
0.929 |
Table 9: Comparison of protein C level in different trimester (n=30)
Table 9 showed, that there were no significant differences in protein C level between patients who had pregnancy loss in 1st Trimester, 2nd Trimester or together.
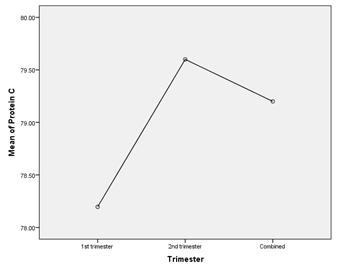
Figure IX: Comparison of protein C among groups.
|
Parameter |
RPL (Mean ± SD) |
Control Mean ± SD |
P value |
|
Protein S level |
81.69±14.06 |
104.23±15.13 |
<0.001 |
Table 10: Protein S level difference between the two groups (N=60)
Table 10 showed, that the mean Protein S level between RPL group and Control group. The test result was statistically significant (P<0.001). So, it can be said that Protein S level of RPL patient (81.69±14.06) was significantly lower than Control patient (104.23±15.13).
|
Parameter |
RPL = 30 |
P value |
||
|
1st Trimester loss |
2nd Trimester loss |
(1st + 2nd) Trimester loss (Combined) |
||
|
Mean ±SD |
Mean ±SD |
Mean ±SD |
||
|
Protein S |
82.76 ± 13.61 |
79.88 ± 12.99 |
81.89 ± 11.96 |
0.79 |
Table 11: Comparison of protein S level in different trimester (n=30)
Table 11 showed, that there were no significant differences in protein S level between patients who had pregnancy loss in 1st Trimester, 2nd Trimester or together.
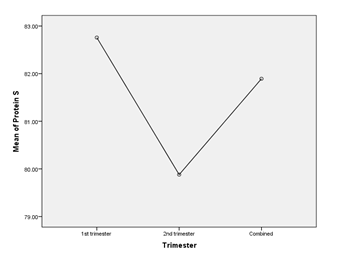
Figure X: Comparison of protein S among groups
|
Gestational age |
Number of losses (n) |
Percentage (%) |
|
1st trimester (1-12 weeks) |
10 |
71.4 |
|
2nd trimester (13-24 weeks) |
4 |
28.6 |
|
Protein S deficient |
||
|
1st trimester (1-12 weeks) |
15 |
75 |
|
2nd trimester (13-24 weeks) |
5 |
25 |
|
Both deficient |
||
|
1st trimester (1-12 weeks) |
6 |
66.7 |
|
2nd trimester (13-24 weeks) |
3 |
33.3 |
Table 12: Total pregnancy loss of Protein C and Protein S deficient patient as per trimester
According to table 12, it appeared that both in protein C and S deficient group, most of the pregnancy loss occurred during 1st trimester. Moreover, even for both deficient patient, 66.7% had loss in first trimester.
|
|
RPL |
Control |
OR |
95% CI |
P value |
|
Protein C Deficiency (<70%) |
3 |
1 |
3.22 |
0.32 - 32.89 |
0.612 |
|
Protein C Normal (70-140%) |
27 |
29 |
|||
|
Total |
30 |
30 |
Table 13: Association of protein C deficiency with RPL
According to table 13, it appeared that no significant association was found between Protein C deficiency (<70) and RPL (P>0.05). However, the odds for RPL due to protein C deficiency (<70) was 3.22 [95% CI: 0.32-32.89].
|
RPL |
Control |
OR |
95% CI |
P value |
|
|
Protein S Deficiency (<70%) |
5 |
1 |
5.8 |
0.64 - 53.01 |
0.195 |
|
Protein S Normal (70-125%) |
25 |
29 |
|||
|
Total |
30 |
30 |
Table 14: Association of protein S deficiency with RPL
Table 14 stated, no significant association was found between Protein S deficiency (<70) and RPL (P>0.05). However, the odds for RPL due to protein S deficiency (<70) was 5.80 [95% CI: 0.64 - 53.01].
|
OR |
95% C.I. for OR |
P value |
||
|
Lower |
Upper |
|||
|
Protein C |
1.196 |
1.064 |
1.344 |
0.003 |
|
Protein S |
1.201 |
1.037 |
1.39 |
0.014 |
Table 15: Association of Protein C and Protein S with RPL
Table 15 stated, binary logistic regression analysis was conducted to figure out the association of protein C and protein S with RPL [χ2 (2, N=60) = 63.270; P<0.001]. The odds for RPL with 1-unit decrease of Protein C level was 1.196 [95% CI: 1.064-1.344] and the odds for RPL with 1-unit decrease of Protein S level was 1.201 [95% CI: 1.037-1.390]. Both variables had significant association with RPL (P<0.05).
DISCUSSION
For both patients and obstetricians, recurrent pregnancy loss is heartbreaking and disappointing because a causative etiology cannot be found in around half of the cases. Maternal thrombophilia has recently been identified as a major cause of adverse pregnancy outcome, including recurrent pregnancy loss, still-birth, severe pre-eclampsia, placental abruption, and intrauterine growth restriction[26,27]. However, deficiencies of the natural anticoagulant like protein C, S and antithrombin occur much less than 1% to 2% in RPL patients[27]. The present study found 3patients had protein C deficiency, 5patients had protein S deficiency and 2patients had both protein C and protein S deficiency. On the other hand, only three patients with protein C deficiency and no patients with Antithrombin III deficiency were found among 93 cases[28]. The prevalence of protein C, and protein S deficiencies are higher in patients with thromboembolic events compared with those in the healthy population[29]. On the other hand, the present study found limited number of protein C (10%) and protein S deficient (16.7%) patient which contradict with the above-mentioned literature. Similarly, Yamada et al. (2011)[30] could not find an increased incidence of protein C deficiency in patient with RPL. In the present study, mean age and BMI of the RPL group was 26.85±4.17 years and 2.42kg/m2, which was matched with the control group. Moreover, primary RPL was more frequent in the study. Other than that, in this study, most of the pregnancy loss for Protein C and protein S deficient women occurred during 1st trimester. However, Alshammary et al. (2015)[31] found that Protein C and protein S deficiency had a significant association with second trimester pregnancy loss. Jyotsna et al. (2011) [32] found a significant association in the mean value of protein C in patient group comparing with control group. Regarding the comparison of mean value of protein C level in RPL group and control group, the present study found a significant difference of protein C and protein S between RPL and controls. To exemplify, Protein C level of RPL patient (78.46±13.18) was significantly lower than Control patient (112.84± 17.57). However, protein C had no significant difference between patients and controls, found in Vora et al. (2018), Raziel et al. (2011)[33,34]. Moreover, according to a meta-analysis by Rey et al. (2013)[35], PC deficiencies are not linked with fetal loss. The prevalence of protein S deficiency in healthy individuals is 0.03-1.3%, [24] but the risks associated with PS deficiency are similar to those of PC deficiency[36]. A significant association of RPL with protein S deficiency was found in the present study, which supports the studies of Raziel et al. (2011) and Parand et al. (2013)[27,34]. Moreover, PS deficiency was also found associated with late-term fetal loss (Rey et al., 2013)[35]. However, Laghoff-Ross et al. (2016) reported that PS is not associated with recurrent early fetal loss. In the present study 2(6.7%) both protein C and S deficient patient in the RPL group was found. Other studies didn’t find any statistically significant for both protein C and S deficiency in RPL patients comparing with control group[31,32] described a significant risk of RPL in pregnant Indian women with thrombophilia. Moreover, In the European Prospective Cohort on Thrombophilia study, which involved 1384 women, the authors reported that the risk of fetal loss was increased in women with thrombophilia compared with controls (168/571 vs. 93/395; OR: 1.35 [1.01-1.82]) [37]. In the same study, the authors reported increased fetal losses in women with familial thrombophilia and stillbirths [37]. A meta-analysis of 31 retrospective study which had shown that the relationship of thrombophilia with late pregnancy loss is stronger than early miscarriages[35]. Alonso et al. (2012)[38] also stated that the prevalence of thrombophilia was more prominent in second trimester, also in agreement with studies done by Rey and Regan (2011) [39] which established that protein C deficiency has been associated with an increased risk of second trimester miscarriage and stillbirth. Robertson et al. (2016)[40] conducted a systematic review of thrombophilia in pregnancy that included a total of 79 studies (three randomized controlled trials, eight prospective cohorts, and 68 retrospective studies) to evaluate the risk of early pregnancy loss in thrombophilia patients. They found that women with thrombophilia were more likely to experience miscarriage than those without it. It was determined that there was an ORs for Antithrombin III, PC, and PS deficiency were 0.88 (0.17-4.48), 2.29 (0.20-26.43), and 3.35 (0.35-35.72) respectively. In the present study, the odds for RPL due to protein C and protein S deficiency (<70) were 3.22 [95% CI: 0.32-32.89] and 5.80 [95% CI: 0.64-53.01] respectively. Moreover, the odds for RPL with 1(one) unit decrease of Protein C and protein S level were 1.196 [95% CI: 1.064-1.344] and 1.201 [95% CI: 1.037-1.390] respectively. The protein C and protein S deficits that occur during pregnancy are key contributors to thrombophilia, which is related with an increased risk of venous thromboembolisms in the pregnant women[41]. When combined with thrombophilia (a broad spectrum of, the hypercoagulable state of pregnancy may increase the risk of thromboembolism during pregnancy or postpartum[42]. Among the top causes of maternal death in the United States, pulmonary embolism ranks first[41]. Since, the present study found a considerable number of protein C and protein S deficiency among RPL pregnant women, therefore, screening and subsequent therapy for thrombophilia is required among pregnant women coming for check-up in hospitals. There are contradictory hypotheses concerning the role of thrombophilia as a cause of abortions. According to many authors, abortions do not occur in all cases of thrombophilia[43]. While the study of Cosmi B. et al. (2013) and Yildizet al. (2012)[44,45] stated that the combination of more than one inherited thrombophilic gene defect has been recognized as a cause of early and late RPL. In spite of that, the incidence of thrombophilia is not clear[46]. Moreover, Sibai et al. (2017)[41] found that approximately 0.2-1% of patients with a combined deficiency of PC and PS have normal pregnancy outcomes. Nevertheless, to detect an association between thrombophilia and recurrent pregnancy loss, it would be necessary to increase considerably the sample size but with unlikely clinical significance.
RECOMMENDATION
Large population-based studies are required to assess the relationship of Protein C and protein S with the risk of RPL. Laboratory facilities should be made available and reagents should be cost-effective so that the affected patients can be evaluated. Routine thrombophilia screening test can be done for evaluation of RPL patients. Low Molecular Weight Heparin should be given when patient has been diagnosed with thrombophilia.
CONCLUSION
The present study found that patients with RPL had a higher incidence of protein C and S deficit when compared to healthy controls. It has been found that protein C and protein S deficiency are associated with recurrent miscarriage, with the rate of miscarriage occurring in the first trimester being the most prevalent. Based on the findings of the current study, it is possible to conclude that inherited thrombophilia due to protein C and protein S deficiency may be a contributing factor of unexplained recurrent pregnancy loss. Limitations of the study Time and resources were limited. This study was carried out in a specialized tertiary care hospital which perhaps not the true representation of all Bangladeshi RPL patients. The study was conducted in a relatively small sample size.
REFERENCES
- Green Top Guide Line: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines
- American Society for Reproductive Medicine (ASRM-12):https://www.medscape.com/viewcollection/32652
- ESHRE,2017RPLfile:///C:/Users/Dell/Downloads/ESHRE%20RPL%20Guideline%20_%20Update%
202022_%20Final%20Version%20January%202023_v2.pdf - Mc Namee K, Dawood F & Farquharson R. 2012. Recurrent miscarriage and thrombophilia: an update. Current Opinion in Obstetrics and Gynecology. 24, pp. 229-234.
- Farahmand, et al. 2016. Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. The Journal of Maternal-Fetal & Neonatal Medicine. 29, pp. 1269-1273.
- Grimstad F & Krieg S. 2016. Immunogenetic contributions to recurrent pregnancy loss. Journal of Assisted Reproduction and Genetics. 33(7), pp. 833-847.
- Micco P D & D’uva, M. 2019. Recurrent pregnancy loss and thrombophilia. The Open Atherosclerosis & Thrombosis Journal, 2.
- Teremmahi et al., 2013. Case control study of the factor V Leiden and factor II G20210A mutation frequency in women with recurrent pregnancy loss. Iran J Reprod Med. 11(1), pp. 61-64
- Younis J S, Ohel G, Brenner B & Ben-Ami M. 2017. Familial thrombophilia-the scientific rationale for thrombophylaxis in recurrent pregnancy loss? Human Reproduction. 12, pp.1389-1390.
- Kovalevsky G, Gracia C R, Berlin J A, Sammel, M. D. & Barnhart, K. T. 2014. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss: a meta-analysis. Archives
- Prothrombin G20210A; https://www.stoptheclot.org/learn_more/prothrombin-g20210a-factor-ii-mutation/
- Sarig G, Younis J, Hoffman R, Lanir N, Blumenfeld Z & Brenner B. (2012). Thrombophilia is common in women with idiopathic pregnancy loss and is associated with late pregnancy wastage.
- Alfirevic Z, Roberts D & Martlew V. 2012. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? European Journal of Obstetrics & Gynecology and Reproductive Biology. 101(1), pp.6-14.
- Heit J A. 2012. Predicting the risk of venous thromboembolism recurrence. Am J Hematol. 87(1): pp 63-67.
- Esmon CT. 2013. The protein C pathway. Chest. 124. pp 26-32.
- Dahlbäck B. 2018. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 112. pp 19-27.
- Whitlatch NL & Ortel TL. 2018 Thrombophilias: when should we test and how does it help? Semin Respir Crit Care Med. 29. pp 25-39.
- Bereczky Z, Kovács KB & Muszbek L. 2010. Protein C and protein S deficiencies: similarities and differences between two brothers playing in the same game. Clin Chem Lab Med. 48. Pp 53-66.
- Bernard K and Van-Cott EM. 2010. Laboratory tests for protein C deficiency. Am. J. Hematol. 85. pp 440-442.
- Korteweg et al., 2012. Fetal loss in women with hereditary thrombophilic defects and concomitance of other thrombophilic defects: a retrospective family study. BJOG. 119. pp 422-430.
- Dahlback B. 2017. The tale of protein S and C4b-binding protein, a story of affection. Thromb Haemost. 98. pp 90- 96.
- Rezende S M, Simmonds R E & Lane D A. 2014. Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S-C4b binding protein complex. Blood. 103 pp 1192-201.
- Garcia-Tsao G, Sanyal A J, Grace N D & Carey W, 2017. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 46(3). pp 922-938.
- Dawood F. 2013. Pregnancy and Thrombophilia. J Blood Disorders Transf. 4. pp 5.
- Armando D and Silvana V D. 2018. Protein S deficiency. Haematologica. 93(4). pp 112-166
- Girling J & de Swiet M. 2018. Inherited thrombophilia and pregnancy. Curr Opin Obtet Gynecol 10. pp 135-144.
- Parand A, Zolghadri J, Nezam M, Afrasiabi A, Haghpanah S & Karimi M. 2013. Inherited Thrombophilia and Recurrent Pregnancy Loss. Iran Red Cres Med J. 15(12): e13708.
- Cardona H, et al., 2012. Lack of Association between Recurrent Pregnancy Loss and Inherited Thrombophilia in a Group of Colombian Patients. Thrombosis (2012).
- Mekaj et al., 2015. Prevalence and role of antithrombin III, protein C and protein S deficiencies and activated protein C resistance in Kosovo women with recurrent pregnancy loss during the first trimester of pregnancy. J Hum Reprod Sci. 8. pp 224-9
- Yamada et al., 2011. Recurrent pregnancy loss: Etiology of thrombophilia. Semin Thromb Haemost. 27. pp 121-9.
- Alshammary H, Almosawi H & Hadi, F. 2015. Deficiency of Protein C and Protein S in Recurrent Pregnancy Loss. Medical Journal of Babylon. 12 (2). Pp 348-356.
- Jyotsna P L, Sharma S & Trivedi S S. 2011. Coagulation inhibitors and activated protein C resistance in recurrent pregnancy losses in Indian women. 54(4). pp 752-755.
- Vora S, Shetty S, Salvi V, Satoskar P & Ghosh K. 2018. Thrombophilia and unexplained pregnancy loss in Indian patients. Natl Med J India. 21. pp 116-119.
- Raziel A, Kornberg Y, Friedler S, Schachter M, Sela B A & Ron-El R. 2011. Hypercoagulable thrombophilic defects and hyperhomocysteinemia in patients with recurrent pregnancy loss. Am J Reprod Immunol. 45(2). pp 65-71.
- Rey E, Kahn S R, David M & Shrier I. 2013. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 361(9361). Pp 901-908.
- Beauchamp et al., 2014. The prevalence of, and molecular defects underlying, inherited protein S deficiency in the general population. Br J Haematol. 125. pp 647-654.
- Preston et al., 2016. Increased fetal loss in women with heritable thrombophilia. Lancet 1996. 348. pp 913- 916.
- Alonso A, Soto I, Urgelles M F, Corte J R, Rodriguez M J & Pinto C R. 2012. Acquired and inherited thrombophilia in women with unexplained fetal losses. Am J Obstet Gynecol. 187(5). pp 1337-1342.
- Rey R. and Regan L. 2011. Thrombophilic and adverse pregnancy outcome. Semin.Reprod. Med. 18. pp 369-377.
- Robertson et al., 2016. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 132. pp 171-196.
- Sibai B M, How H Y & Stella C L, 2017. Thrombophilia in pregnancy: Whom to screen, when to treat. Obg Management, 19(1), pp.50-64.
- Paidas M J, De-Hui W K & Arkel Y S. 2014. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 31. 783-805
- Dykes A C, Walker I D, McMahon A D, Islam S I & Tait R C. 2011. A study of Protein S antigen levels in 3788 healthy volunteers: Influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol. 113. pp 636 41.
- Cosmi et al., 2013. The influence of factor V Leiden and G20210A prothrombin mutation on the presence of residual vein obstruction after idiopathic deep-vein thrombosis of the lower limbs. Thromb Haemost. 109. pp 510-516.
- Yildiz G, Yavuzcan A, Yildiz P, Suer N & Tandogan N. 2012. Inherited thrombophilia with recurrent pregnancy loss in Turkish women--A real phenomenon. Gine kol Pol. 83. pp 598-603.
- Abu-Heija A. 2014. Thrombophilia and Recurrent Pregnancy Loss: Is heparin still the drug of choice? Sultan Qaboos Univ Med J. 14(1) pp26-36.


 Impact Factor: * 3.2
Impact Factor: * 3.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 