Pregnancy Complicated by Hyperinsulinemic, Non-Insulinoma Hypoglycemia Syndrome: Case Report
Article Information
Enio Luis Damaso1, Conrado Sávio Ragazini2, Mariane Nunes de Nadai1, Patrícia Moreira Gomes3, Raphael Del Roio Liberatore Junior4, Elaine Christine Dantas Moisés2
1Department of Pediatric Dentistry, Orthodontics and Public Health, Bauru Dental School, University of São Paulo. Bauru, São Paulo, Brazil
2Department of Gynecology and Obstetrics, Ribeirao Preto Medical School, University of Sao Paulo. Ribeirão Preto, São Paulo, Brazil
3Department of Internal Medicine, Division of Endocrinology and Metabolism, Ribeirao Preto Medical School, University of Sao Paulo. Ribeirão Preto, Brazil
4Department of Pediatrics, Ribeirao Preto Medical School, University of São Paulo. Ribeirão Preto, São Paulo, Brazil
*Corresponding Author: Ênio Luis Damaso, Department of Pediatric Dentistry, Orthodontics and Public Health, Bauru Dental School, University of São Paulo, Brazil.
Received: 16 October 2023; Accepted: 27 October 2023; Published: 20 November 2023
Citation: Enio Luis Damaso, Conrado Sávio Ragazini, Mariane Nunes de Nadai, Patrícia Moreira Gomes, Raphael Del Roio Liberatore Junior, Elaine Christine Dantas Moisés. Pregnancy Complicated by Hyperinsulinemic, Non-Insulinoma Hypoglycemia Syndrome: Case Report. Obstetrics and Gynecology Research. 6 (2023): 270-274
View / Download Pdf Share at FacebookAbstract
Hyperinsulinemic hypoglycemia is an unusual metabolic disorder characterized by frequent episodes of hypoglycemia caused by uncontrolled insulin release. In pregnancy it can result in adverse perinatal outcomes. We report a rare case of a pregnant woman diagnosed with this disorder. During the first pregnancy, drug treatment was necessary, and the second pregnancy occurred after partial pancreatectomy, which allowed good control with nutritional support. Hyperinsulinemic hypoglycemia syndrome complicating pregnancy, although very rare, should be included in the differential diagnosis of hypoglycemia during pregnancy.
Keywords
Hypoglycemia; Hyperinsulinism; Pregnancy; High-Risk; Case Report
Hypoglycemia articles Hypoglycemia Research articles Hypoglycemia review articles Hypoglycemia PubMed articles Hypoglycemia PubMed Central articles Hypoglycemia 2023 articles Hypoglycemia 2024 articles Hypoglycemia Scopus articles Hypoglycemia impact factor journals Hypoglycemia Scopus journals Hypoglycemia PubMed journals Hypoglycemia medical journals Hypoglycemia free journals Hypoglycemia best journals Hypoglycemia top journals Hypoglycemia free medical journals Hypoglycemia famous journals Hypoglycemia Google Scholar indexed journals Hyperinsulinism articles Hyperinsulinism Research articles Hyperinsulinism review articles Hyperinsulinism PubMed articles Hyperinsulinism PubMed Central articles Hyperinsulinism 2023 articles Hyperinsulinism 2024 articles Hyperinsulinism Scopus articles Hyperinsulinism impact factor journals Hyperinsulinism Scopus journals Hyperinsulinism PubMed journals Hyperinsulinism medical journals Hyperinsulinism free journals Hyperinsulinism best journals Hyperinsulinism top journals Hyperinsulinism free medical journals Hyperinsulinism famous journals Hyperinsulinism Google Scholar indexed journals Pregnancy articles Pregnancy Research articles Pregnancy review articles Pregnancy PubMed articles Pregnancy PubMed Central articles Pregnancy 2023 articles Pregnancy 2024 articles Pregnancy Scopus articles Pregnancy impact factor journals Pregnancy Scopus journals Pregnancy PubMed journals Pregnancy medical journals Pregnancy free journals Pregnancy best journals Pregnancy top journals Pregnancy free medical journals Pregnancy famous journals Pregnancy Google Scholar indexed journals High-Risk articles High-Risk Research articles High-Risk review articles High-Risk PubMed articles High-Risk PubMed Central articles High-Risk 2023 articles High-Risk 2024 articles High-Risk Scopus articles High-Risk impact factor journals High-Risk Scopus journals High-Risk PubMed journals High-Risk medical journals High-Risk free journals High-Risk best journals High-Risk top journals High-Risk free medical journals High-Risk famous journals High-Risk Google Scholar indexed journals Case Report articles Case Report Research articles Case Report review articles Case Report PubMed articles Case Report PubMed Central articles Case Report 2023 articles Case Report 2024 articles Case Report Scopus articles Case Report impact factor journals Case Report Scopus journals Case Report PubMed journals Case Report medical journals Case Report free journals Case Report best journals Case Report top journals Case Report free medical journals Case Report famous journals Case Report Google Scholar indexed journals metabolic disorder articles metabolic disorder Research articles metabolic disorder review articles metabolic disorder PubMed articles metabolic disorder PubMed Central articles metabolic disorder 2023 articles metabolic disorder 2024 articles metabolic disorder Scopus articles metabolic disorder impact factor journals metabolic disorder Scopus journals metabolic disorder PubMed journals metabolic disorder medical journals metabolic disorder free journals metabolic disorder best journals metabolic disorder top journals metabolic disorder free medical journals metabolic disorder famous journals metabolic disorder Google Scholar indexed journals pancreatic beta cells articles pancreatic beta cells Research articles pancreatic beta cells review articles pancreatic beta cells PubMed articles pancreatic beta cells PubMed Central articles pancreatic beta cells 2023 articles pancreatic beta cells 2024 articles pancreatic beta cells Scopus articles pancreatic beta cells impact factor journals pancreatic beta cells Scopus journals pancreatic beta cells PubMed journals pancreatic beta cells medical journals pancreatic beta cells free journals pancreatic beta cells best journals pancreatic beta cells top journals pancreatic beta cells free medical journals pancreatic beta cells famous journals pancreatic beta cells Google Scholar indexed journals gestation articles gestation Research articles gestation review articles gestation PubMed articles gestation PubMed Central articles gestation 2023 articles gestation 2024 articles gestation Scopus articles gestation impact factor journals gestation Scopus journals gestation PubMed journals gestation medical journals gestation free journals gestation best journals gestation top journals gestation free medical journals gestation famous journals gestation Google Scholar indexed journals glycemic levels articles glycemic levels Research articles glycemic levels review articles glycemic levels PubMed articles glycemic levels PubMed Central articles glycemic levels 2023 articles glycemic levels 2024 articles glycemic levels Scopus articles glycemic levels impact factor journals glycemic levels Scopus journals glycemic levels PubMed journals glycemic levels medical journals glycemic levels free journals glycemic levels best journals glycemic levels top journals glycemic levels free medical journals glycemic levels famous journals glycemic levels Google Scholar indexed journals Transabdominal ultrasound articles Transabdominal ultrasound Research articles Transabdominal ultrasound review articles Transabdominal ultrasound PubMed articles Transabdominal ultrasound PubMed Central articles Transabdominal ultrasound 2023 articles Transabdominal ultrasound 2024 articles Transabdominal ultrasound Scopus articles Transabdominal ultrasound impact factor journals Transabdominal ultrasound Scopus journals Transabdominal ultrasound PubMed journals Transabdominal ultrasound medical journals Transabdominal ultrasound free journals Transabdominal ultrasound best journals Transabdominal ultrasound top journals Transabdominal ultrasound free medical journals Transabdominal ultrasound famous journals Transabdominal ultrasound Google Scholar indexed journals
Article Details
Introduction
Hyperinsulinemic hypoglycemia is an unusual metabolic disorder caused by uncontrolled insulin release, secondary to either neoplastic or functionally defective pancreatic beta cells. The latter is known as Noninsulinoma Pancreatogenous Hypoglycemia Syndrome (NIPHS) or Congenital Hyperinsulinism, which is usually seen in newborns and rare in adults – only 0,5-5% of all organic hyperinsulinemic diseases in adults are caused by this condition [1]. Because this condition is rare in adults, histopathologic and clinical features are not well known [2].
There are 3 cases published in scientific literature about this syndrome in pregnancy. The major concern of NIPHS and pregnancy is the well-known association between adverse perinatal outcomes and hypoglycemia [3,4], which occurs frequently in these patients.
This article describes the obstetric and clinical care involved and the evolution of two pregnancies of a woman who had the diagnosis of hyperinsulinemic hypoglycemia due noninsulinoma pancreatogenous hypoglycemia syndrome. This paper intends to increase clinical knowledge about this syndrome and report a successful case of treatment.
Case report
A 21-years old primigravida presented for the first time at 27 weeks and 5 days to our high-risk pregnancy antenatal clinic in 2013. She had a personal history of frequent episodes of hypoglycemia since she was three months of age. Her medical background included a family history of hypertension and dyslipidemia. At the presentation, her body mass index was 19.5 kg/m2 (body weight 58.4kg, height 160cm). The initial approach consisted of nutritional orientations, with a fractioned diet, poor in simple carbohydrates. After some weeks, as the hypoglycemic episodes continuously happened, at 33 weeks’ gestation she was admitted for diagnostic and treatment purposes. Episodes of glycemic levels of 18 mg/dL (1,0 mmol/l) were accompanied by hyperinsulinemia. Transabdominal ultrasound (US) and magnetic resonance imaging (MRI) showed a pancreas of normal shape and size, and no pathological findings. Critical sample obtained without starving showed blood glucose concentrations of 19 mg/dL (1,0 mmol/l), insulin level of 3 μl/ml, C peptide level 1,53 ng/ml and pro-insulin levels of 7,5 pmol/L (Table 1). These findings were strongly suggestive of NIPHS, leading to the prescription of diazoxide 100 mg 3 times a day and prednisone 15 mg once a day. This approach resulted in satisfactory glycemic control for a couple of weeks; at 35 weeks and 1 day of gestational age new hypoglycemic episodes with the need of intravenous glucose led to the indication of induction of labor. A cesarean delivery due to acute fetal distress of a male newborn weighing 2330g (INTERGROWTH-21 33th percentile(5)) occurred without further complications. Apgar Scores at 1 and 5 minutes of life were 9 and 10, respectively. They were discharged after 10 days.
We found a mutation in KCNJ11 gene, g.1350C>G (NM_000525) related to congenital hyperinsulinism. The patient refused pancreatic surgery after delivery as definitive treatment and dropped clinical follow-up for 3 years.
|
Parameter |
Patient |
Normal range |
NIPHS |
|
Glucose (mg/dL) |
19 |
>70 |
<55 |
|
Insulin (microU/mL) |
3 |
<3 |
≥3 |
|
C-peptide (nmol/L) |
1.53 |
<0.2 |
≥0.2 |
|
Proinsulin (pmol/L) |
7.5 |
<5 |
≥5 |
|
Beta-hydroxybutirate (mmol/L) |
- |
>2.7 |
≤2.7 |
Reference: Cryer et al. [13] and American Diabetes Association [33]
Table 1: Serum values of tests collected in 2013 of the reported case, as well as normal reference values and suggestive of NIPHS.
After 3 years she restarted follow-up at our center as the episodes of hypoglycemia had intensified. Verapamil was prescribed as treatment with no control of glycemic levels. Therefore, pancreatic surgery as definitive treatment was once again proposed. Subtotal pancreatectomy was performed, removing approximately 85% of the pancreas. Anatomopathological examination showed pancreatic islets with hypertrophic cells and giant nucleus in a diffuse distribution. Figure 1 shows this examination.
She was discharged with normal glycemic levels and no exocrine dysfunction.
In her second pregnancy, 3 years after pancreatic surgery, she was 28 years old and started antenatal follow-up at 8 weeks of gestation. At the presentation, her body mass index was 21.8 kg/m2 (body weight 55.8 kg, height 160 cm). Nutritional support was based on a fractional diet, not fasting for long periods even during the night. Home glucose monitoring showed only a few episodes of glycemic levels between 60 and 70 mg/dl (3,3-3,8 mmol/l) during pregnancy with no need of additional care. At 39 weeks of gestation induction of labor was indicated and oxytocin after a cervical ripening balloon was used to induce labor. The patient had a vaginal delivery of a healthy female newborn, weighing 3155g (INTERGROWTH-21 40th percentile [5]), Apgar scores at 1 and 5 minutes of 8 and 9. They were discharged after 3 days.
Figure 1: Histological images from the anatomopathological examination after pancreatectomy
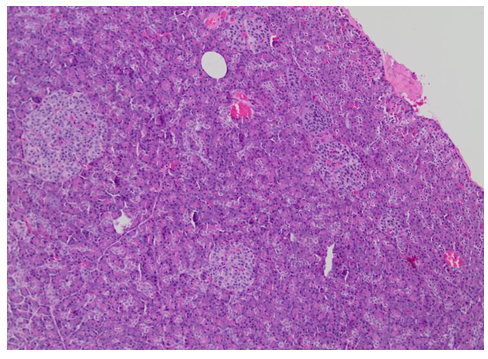
Figure 1a: Histologic section of the pancreas showed islets of variable sizes and shapes, irregularly distributed (H&E [Hematoxylin-eosin staining], 100X).
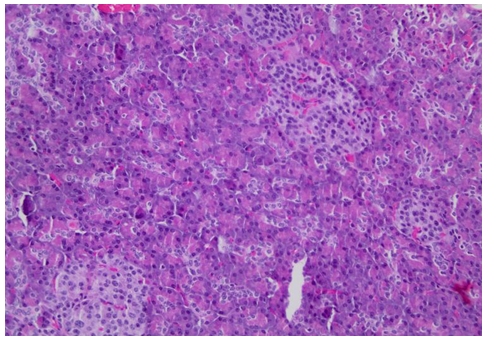
Figure 1b: Details of pancreatic islets (H&E, 200X)
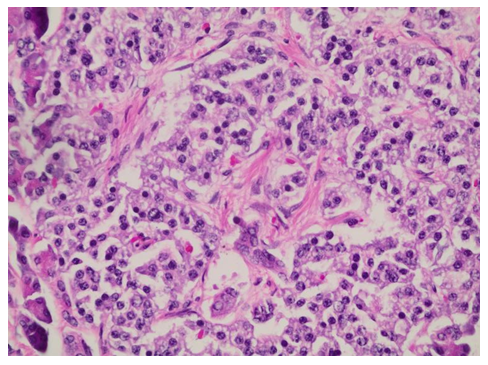
Figure 1c: Details of the islet cell showed hyperchromatic and enlarged nuclei (H&E, 400X)
Discussion
Hyperinsulinemic hypoglycemia is characterized by unregulated insulin release, leading to persistently low blood glucose concentrations with lack of alternative fuels [6]. Congenital Hyperinsulinism is characterized by endogenous hyperinsulinemic hypoglycemia that is not caused by an insulinoma. Pancreatic specimens from patients show beta cell hypertrophy, islets of Langerhans with enlarged and hyperchromatic nuclei, and increased islets budding from peri ductular epithelium [7-11]. Nesidioblastosis is a term that was introduced for the first time by Laidlaw in 1938 [10] to define the diffuse proliferation of pancreatic islet cells budding from ductal epithelium. After the knowledge of genes involved in the syntheses and secretion of insulin, the name nesidioblastosis was changed to congenital hyperinsulinism [12].
We report a rare case of Congenital Hyperinsulinism on a patient who has been pregnant twice. During her first pregnancy, laboratory tests (blood glucose concentrations, insulin, C peptide and pro-insulin) (Table 1) and radiological exams (US and MRI) were compatible with NIPHS. Drug treatment was prescribed due to insufficient glycemic control with diet alone. The second pregnancy occurred after a partial pancreatectomy, which resulted in satisfactory glucose levels with nutritional support alone.
In adults, hypoglycemia due to endogenous hyperinsulinism can be caused by the following [13]: beta cell secretagogues, such as a sulfonylurea; beta cell tumors (insulinoma); functional beta cell disorders that can occur as a feature of the noninsulinoma pancreatogenous hypoglycemia syndrome; after gastric bypass and insulin autoimmune hypoglycemia.
If nutritional support fails to prevent hypoglycemia, some drugs have been proposed to improve hypoglycemic symptoms in patients with NIPHS [15-18]. Octreotide is a somatostatin analog that inhibits insulin secretion [19]. Verapamil is a calcium-channel blocker that also acts in beta cells inhibiting insulin secretion [16]. Diazoxide is a non diuretic benzothiadiazine and an agonist of the sulfonylurea receptor, used for inhibiting insulin secretion in such cases of hypoglycemia [20]. Finally, acarbose, an alpha-glucosidase that inhibits postprandial glucose and its insulin response, can also be used [21].
For patients with NIPHS and severe postprandial hypoglycemia (e.g., neuroglycopenia with loss of consciousness) or with symptoms despite medical management, surgery is the pillar of therapy. In case series and reports, partial or subtotal pancreatectomy successfully relieved hypoglycemic symptoms in the majority of patients [8,9,14].
Early pregnancy is associated with a reduction in fasting glucose levels by 5 to 10 mg/dL [22,23], presumably secondary to an increase in glucose uptake by the fetoplacental unit, as well as a decrease in glycogenolysis and hepatic glucose production. As pregnancy progresses, a state of "accelerated starvation" develops with increased glucose consumption and reduction in hepatic glucose production, resulting in a lowered blood glucose and a compensatory increase in fatty acid utilization [24]. Cousins et al. [25] demonstrated the wide excursions in glucose and insulin necessary to maintain relative euglycemia throughout pregnancy. Therefore, early pregnancy, in particular, is associated with a reduction in peripheral glucose levels and can potentially increase the risk of hypoglycemia [26].
The occurrence of hypoglycemia during pregnancy raises concerns for the well-being of the pregnant woman and her fetus. It has been hypothesized that hypoglycemia during pregnancy can induce potential adverse effects that lead to fetal malformations, small for gestational age and poor neuropsychiatric development [4,27]. The association between the level of hypoglycemia and diabetic embryopathy remains unclear [28].
No fetal malformation or growth retardation has yet been reported in pregnant patients with insulinomas, even in a patient in whom the diagnosis of insulinoma was not made until delivery [29-31]. This case confirms this, as both newborns had similar birth weight percentiles (33 and 40, respectively). On the other hand, hypoglycemia in pregnant women with insulinomas can have serious maternal consequences, especially repeated seizures, and misdiagnosis can be even fatal [32]. Insulinomas can be surgically treated during the second trimester of pregnancy.
There are 3 more cases published of pregnancy in congenital hyperinsulinism patients. In all cases, the treatment used was octreotide.
In the first case, published in 2014, a 36 years woman with nesidioblastosis was treated until 33 weeks of gestation with octreotide with no effects on the newborn [34].
In the second case, published in 2017, a 24 years old woman with congenital hyperinsulinism caused by a GCK mutation, was treated with octreotide from 23 to 35 weeks of gestation. Intrauterine growth retardation was observed although she was also a smoker [35].
In the third one, published in 2022, a 29 year old woman was treated with octreotide until 38 weeks of gestational age with no complications [36].
In our case, during the first pregnancy, we used diazoxide, an anti hypertensive drug used for treatment of congenital hyperinsulinism and a category C drug for use during pregnancy. During 2 weeks of using diazoxide and glucocorticoid, the nutritional approach and medication were able to maintain normal glucose levels. During the second pregnancy and after pancreatectomy, she had no hypoglycemic episodes. Both newborns were normal.
Octreotide and diazoxide can cross the placental and potentially affect the fetus [37,38]. There was no robust experience on treatment of congenital hyperinsulinism during pregnancy and this is probably the first case of diazoxide use.
It is also uncommon that a woman who had a pancreatectomy becomes pregnant and gives birth to a normal newborn.
Conclusion
This report reminds us that episodes of hypoglycemia can be unrelated to the treatment of diabetes mellitus. Physicians need to be aware of differential diagnoses and their main therapeutic approaches. The already lower blood glucose levels during pregnancy associated with NIPHS pose even higher risks for these women and their offspring.
Noninsulinoma pancreatogenous hypoglycemia syndrome complicating pregnancy, although very rare, should be included in the differential diagnosis of hypoglycemia during pregnancy.
We report the use of diazoxide for two weeks during pregnancy with partial control of hypoglycemic episodes and a successful pregnancy after pancreatectomy.
Ethical Approval
The Ethics Committee of the University Hospital, Ribeirão Preto Medical School approved this study under protocol number 5.043.448.
Contributions:
ELD e CSR foram os responsáveis por reunir as informações clínicas e obstétricas do caso relatado. PMG e RDRL foram responsáveis por reunir as informações endocrinológicas e moleculares do caso relatado. MNN e ECDM foram respondidos pela revisão final do manuscrito. Todos os autores contribuíram para a confecção e revisão do manuscrito
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Then C, Nam-Apostolopoulos Y-C, Seissler J, et al. Refractory idiopathic noninsulinoma pancreatogenous hypoglycemia in an adult: case report and review of the literature. JOP J Pancreas 14 (2013): 264-268.
- Raffel A, Krausch M M, Anlauf M, et al. Diffuse nesidioblastosis as a cause of hyperinsulinemic hypoglycemia in adults: a diagnostic and therapeutic challenge. Surgery 141 (2007):179-184; discussion 185-186.
- Jensen VFH, Mølck A-M, Lykkesfeldt J, et al. Importance of gestational hypoglycaemia for fetal malformations and skeletal development in rats. Reprod Toxicol 91 (2020): 14-26.
- ter Braak EWMT, Evers IM, Willem Erkelens D, et al. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev 18 (2002): 96-105.
- Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet Lond Engl 384 (2014): 857-868.
- Rizza RA, Haymond MW, Verdonk CA, et al. Pathogenesis of hypoglycemia in insulinoma patients: suppression of hepatic glucose production by insulin. Diabetes 30 (1981): 377-381.
- Anlauf M, Wieben D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol 29 (2005): 524-533.
- Service FJ, Natt N, Thompson GB, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab 84 (1999): 1582–1589.
- Thompson GB, Service FJ, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia syndrome: an update in 10 surgically treated patients. Surgery 128 (2000): 937-944; discussion 944-945.
- Laidlaw GF. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol 14 (1938): 125-135.
- Karnauchow PN. Nesidioblastosis in adults without insular hyperfunction. Am J Clin Pathol 78 (1982): 511-513.
- Sandler R, Horwitz DL, Rubenstein AH, et al. Hypoglycemia and endogenous hyperinsulinism complicating diabetes mellitus. Am J Med 59 (1975): 730-736.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 94 (2009): 709–728.
- Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 4 (2008): 492–499.
- Arao T, Okada Y, Hirose A, et al. A rare case of adult-onset nesidioblastosis treated successfully with diazoxide. Endocr J 53 (2006): 95-100.
- Nadelson J, Epstein A. A rare case of noninsulinoma pancreatogenous hypoglycemia syndrome. Case Rep Gastrointest Med 2012: 164305.
- Spanakis E, Gragnoli C. Successful medical management of status post-Roux-en-Y-gastric-bypass hyperinsulinemic hypoglycemia. Obes Surg 19 (2009): 1333-1334.
- Moreira RO, Moreira RBM, Machado NAM, et al. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg 18 (2008): 1618-1621.
- Barrons RW. Octreotide in hyperinsulinism. Ann Pharmacother 31 (1997): 239-241.
- Drash A, Wolff F. Drug therapy in leucine-sensitive hypoglycemia. Metabolism 13 (1964): 487-492.
- Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord EWD 3 (1998): 136-140.
- Catalano PM, Tyzbir ED, Wolfe RR, et al. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am J Obstet Gynecol 167 (1992): 913-919.
- Catalano PM, Hollenbeck C. Energy Requirements in Pregnancy: A Review. Obstet Gynecol Surv 47 (1992): 368-372.
- Felig P, Lynch V. Starvation in Human Pregnancy: Hypoglycemia, Hypoinsulinemia, and Hyperketonemia. Science 170 (1970): 990-992.
- Cousins L, Rigg L, Hollingsworth D, et al. The 24-hour excursion and diurnal rhythm of glucose, insulin, and C-peptide in normal pregnancy. Am J Obstet Gynecol 136 (1980): 483-488.
- Reece EA, Homko C, Wiznitzer A. Metabolic changes in diabetic and nondiabetic subjects during pregnancy. Obstet Gynecol Surv 49 (1994): 64-71.
- Rosenn BM, Miodovnik M. Glycemic control in the diabetic pregnancy: is tighter always better? J Matern Fetal Med 9 (2000): 29-34.
- Rizzo T, Metzger BE, Burns WJ, et al. Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med 325 (1991): 911-916.
- Garner PR, Tsang R. Insulinoma complicating pregnancy presenting with hypoglycemic coma after delivery: a case report and review of the literature. Obstet Gynecol 73 (1989): 847-849.
- Smythe AR, McFarland KF, Yousufuddin M, et al. Multiple endocrine adenomatosis type I in pregnancy. Am J Obstet Gynecol 163 (1990): 1037-1039.
- Galun E, Ben-Yehuda A, Berlatzki J, et al. Insulinoma complicating pregnancy: Case report and review of the literature. Am J Obstet Gynecol 155 (1986): 64-65.
- Dobrindt EM, Mogl M, Goretzki PE, et al. Insulinoma in pregnancy (a case presentation and systematic review of the literature). Rare Tumors 13 (2021): 2036361320986647.
- ADA’s Standards of Medical Care in Diabetes. Clin Diabetes Publ Am Diabetes Assoc 39 (2021): 128.
- Boulanger C, Vezzosi D, Bennet A, et al. Normal pregnancy in a woman with nesidioblastosis treated with somatostatin analog octreotide. J Endocrinol Invest 27 (2014): 465-470.
- Geilswijk M, Andersen LL, Frost M, et al. Octreotide therapy and restricted fetal growth: pregnancy in familial hyperinsulinemic hypoglycemia. Endocrinol Diabetes Metab Case Rep 1 (2017): 1-5.
- Barsi Á, Beke A, Sármán B. Case report: A particularly rare case of endogenous hyperinsulinemic hypoglycemia complicated with pregnancy treated with short-acting somatostatin analog injections. Front Endocrinol (Lausanne) 13 (2022): 964481.
- Caron P, Gerbeau C, Pradayrol L. Maternal-fetal transfer of octreotide. N Engl J Med 333 (1995): 601-602.
- Nuwayhid B, Brinkman CR, Katchen B, Symchowicz S, MARTINEK H, Assali NS. Maternal and fetal hemodynamic effects of diazoxide. Obstet Gynecol 46 (1975): 197-203.


 Impact Factor: * 3.2
Impact Factor: * 3.2 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 