Synthesis of copper oxide nanoparticles on activated carbon for pollutant removal in tartrazine structure
DOI:
https://doi.org/10.29252/jcc.2.2.6Keywords:
Tartrazine, Activated carbon, Copper oxide, Removal efficiencyAbstract
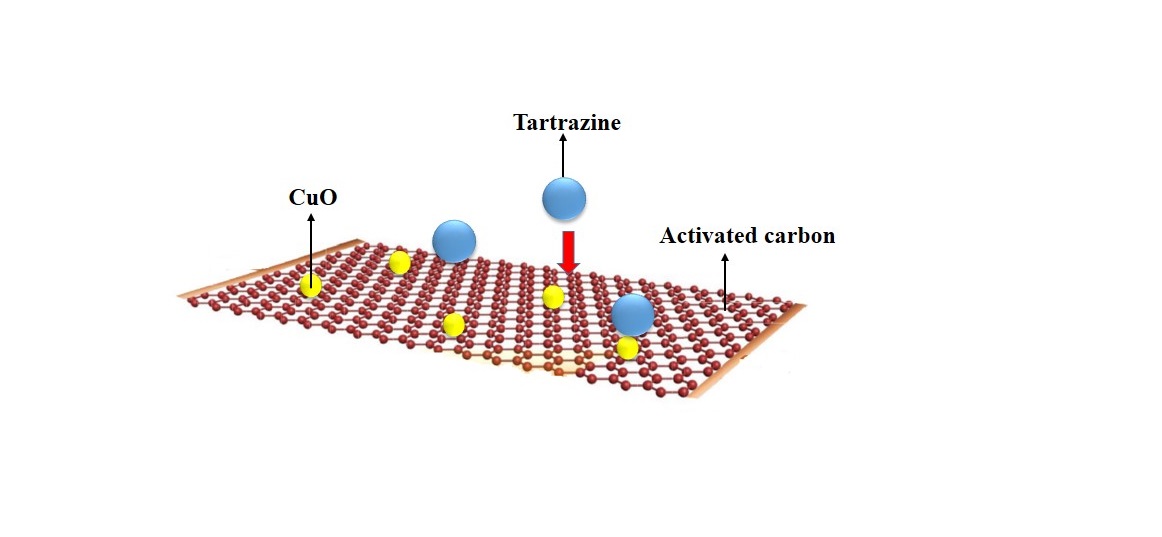
In this study, activated carbon particles were modified by copper oxide to remove the anionic Tartrazine dye from aqueous solutions. Adsorption studies were performed as batch studies and the influences of pH, initial dye concentrations, and contact times were evaluated. Maximum removal percentage was obtained for the initial concentration of 30 mg/L and the equilibrium of the adsorption was achieved within 60 minutes of contact time. The Langmuir and Freundlich kinetic models were used for analyzing the equilibrium data. It was shown that better fitting was observed by the Langmuir model. Pseudo-first-order and Pseudo-second-order kinetic models were also applied to understand the kinetics of the adsorption processes. It was found that the Tartrazine adsorption followed the pseudo-second-order kinetic model.
References
D. Wang, N. Zhao, T. Wang, C. Zhuang, Y. Wang, B. Yang, Crystal Struc-ture, Spectroscopy and Photocatalytic Properties of a Co(II) Complex Based on 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic Acid, Crystals 10(2) (2020).
M. Golmohammadi, M. Honarmand, S. Ghanbari, A green approach to synthe-sis of ZnO nanoparticles using jujube fruit extract and their application in photo-catalytic degradation of organic dyes, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 229 (2020) 117961.
A. Tkaczyk, K. Mitrowska, A. Posyniak, Synthetic organic dyes as contami-nants of the aquatic environment and their implications for ecosystems: A review, Science of The Total Environment 717 (2020) 137222.
J. Goscianska, R. Pietrzak, Removal of tartrazine from aqueous solution by carbon nanotubes decorated with silver nanoparticles, Catalysis Today 249 (2015) 259-264.
A. Kazemzadeh, H. Kazemzadeh, L. Bazli, Determination of Hg2+ by Diphenyl-carbazone Compound in Polymer Film, Composites and Compounds 1(1) (2019).
H. Won Jang, A. Zareidoost, M. Moradi, A. Abuchenari, A. Bakhtiari, R. Pouri-amanesh, B. Malekpouri, A. Jafari Rad, Photosensitive nanocomposites: environ-mental and biological applications, Journal of Composites and Compounds 1(1) (2020).
S. Saadi, B. Nazari, Submission Title: Recent Developments and Applications of Nanocomposites in Solar Cells: a Review, Composites and Compounds 1(1) (2019).
A. Kazemzadeh, M.A. Meshkat, H. Kazemzadeh, M. Moradi, R. Bahrami, R. Pouriamanesh, Submission Title: Preparation of Graphene Nanolayers through Surfactant-assisted Pure Shear Milling Method, Composites and Compounds 1(1) (2019).
L. Bazli, M. Siavashi, A. Shiravi, A Review of Carbon Nanotube/TiO2 Com-posite Prepared via Sol-Gel Method, Composites and Compounds 1(1) (2019).
E. Asadi, A. Fassadi Chimeh, S. Hosseini, S. Rahimi, B. Sarkhosh, L. Bazli, R. Bashiri, A.H. Vakili Tahmorsati, A Review of Clinical Applications of Graphene Quantum Dot-based Composites, Composites and Compounds 1(1) (2019).
V.S. Rizi, F. Sharifianjazi, H. Jafarikhorami, N. Parvin, L.S. Fard, M. Irani, A. Esmaeilkhanian, Sol–gel derived SnO2/Ag2O ceramic nanocomposite for H2 gas sensing applications, Materials Research Express 6(11) (2019) 1150g2.
F. Sharifianjazi, M. Moradi, N. Parvin, A. Nemati, A.J. Rad, N. Sheysi, A. Abouchenari, A. Mohammadi, S. Karbasi, Z. Ahmadi, Magnetic CoFe2O4 nanopar-ticles doped with metal ions: a review, Ceramics International (2020).
S. Muthukrishnan, A. Eswaran, Phytochemical and antimicrobial profile of nanobased liv-pro-09 polyherbal formulation.
F.S. Jazi, N. Parvin, M. Rabiei, M. Tahriri, Z.M. Shabestari, A.R. Azadmehr, Effect of the synthesis route on the grain size and morphology of ZnO/Ag nano-composite, Journal of Ceramic Processing Research 13(5) (2012) 523-526.
F.S. Jazi, N. Parvin, M. Tahriri, M. Alizadeh, S. Abedini, M. Alizadeh, The relationship between the synthesis and morphology of SnO2-Ag2O nanocomposite, Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 44(5) (2014) 759-764.
J. Peternela, M.F. Silva, M.F. Vieira, R. Bergamasco, A.M.S. Vieira, Synthesis and Impregnation of Copper Oxide Nanoparticles on Activated Carbon through Green Synthesis for Water Pollutant Removal, Materials Research 21 (2018).
A. Moghanian, F. Sharifianjazi, P. Abachi, E. Sadeghi, H. Jafarikhorami, A. Sedghi, Production and properties of Cu/TiO2 nano-composites, Journal of Alloys and Compounds 698 (2017) 518-524.
S. Periyasamy, I.A. Kumar, N. Viswanathan, Activated Carbon from Different Waste Materials for the Removal of Toxic Metals, in: M. Naushad, E. Lichtfouse (Eds.), Green Materials for Wastewater Treatment, Springer International Publish-ing, Cham, 2020, pp. 47-68.
P.P. Bhave, D. Yeleswarapu, Removal of Indoor Air Pollutants Using Acti-vated Carbon—A Review, in: V. Sivasubramanian, S. Subramanian (Eds.) Global Challenges in Energy and Environment, Springer Singapore, Singapore, 2020, pp. 65-75.
Z. Heidarinejad, M.H. Dehghani, M. Heidari, G. Javedan, I. Ali, M. Sillanpää, Methods for preparation and activation of activated carbon: a review, Environmen-tal Chemistry Letters 18(2) (2020) 393-415.
K. Gong, X. Li, H. Liu, X. Cheng, D. Sun, Q. Shao, M. Dong, C. Liu, S. Wu, T. Ding, B. Qiu, Z. Guo, Residue metals and intrinsic moisture in excess sludge improve pore formation during its carbonization process, Carbon 156 (2020) 320-328.
C.H. Nguyen, H.N. Tran, C.-C. Fu, Y.-T. Lu, R.-S. Juang, Roles of adsorption and photocatalysis in removing organic pollutants from water by activated car-bon–supported titania composites: Kinetic aspects, Journal of the Taiwan Institute of Chemical Engineers 109 (2020) 51-61.
F.S. Arakawa, Q.L. Shimabuku-Biadola, M. Fernandes Silva, R. Bergamas-co, Development of a new vacuum impregnation method at room atmosphere to produce silver–copper oxide nanoparticles on activated carbon for antibacterial applications, Environmental Technology (2019) 1-12.
M. Ghaedi, A.M. Ghaedi, M. Hossainpour, A. Ansari, M.H. Habibi, A.R. Asghari, Least square-support vector (LS-SVM) method for modeling of methy-lene blue dye adsorption using copper oxide loaded on activated carbon: Kinetic and isotherm study, Journal of Industrial and Engineering Chemistry 20(4) (2014) 1641-1649.
M.K.T. Al-Zain, Removal of Toxic Organic Compounds and Dyes from Water by Magnesium Oxide Nanostructure, Al-Azhar University-Gaza, 2019.
K. Rovina, S. Siddiquee, S. Md Shaarani, An electrochemical sensor for the determination of tartrazine based on CHIT/GO/MWCNTs/AuNPs composite film modified glassy carbon electrode, Drug and Chemical Toxicology (2019) 1-11.
K.G. Pavithra, S.K. P, J. V, S.R. P, Removal of colorants from wastewater: A review on sources and treatment strategies, Journal of Industrial and Engineering Chemistry 75 (2019) 1-19.
N.A. Al-Shabib, J.M. Khan, M.S. Khan, M.S. Ali, A.M. Al-Senaidy, M.A. Alsenaidy, F.M. Husain, H.A. Al-Lohedan, Synthetic food additive dye “Tartra-zine” triggers amorphous aggregation in cationic myoglobin, International Journal of Biological Macromolecules 98 (2017) 277-286.
G.A.P. Mateus, T.R.T. dos Santos, I.S. Sanches, M.F. Silva, M.B. de Andrade, M.P. Paludo, R.G. Gomes, R. Bergamasco, Evaluation of a magnetic coagulant based on Fe3O4 nanoparticles and Moringa oleifera extract on tartrazine removal: coagulation-adsorption and kinetics studies, Environmental Technology (2018) 1-16.
S. Sahnoun, M. Boutahala, C. Tiar, A. Kahoul, Adsorption of tartrazine from an aqueous solution by octadecyltrimethylammonium bromide-modified benton-ite: Kinetics and isotherm modeling, Comptes Rendus Chimie 21(3-4) (2018) 391-398.
Article DOR: 20.1001.1.26765837.2020.2.3.6.6
Published
How to Cite
License
Copyright (c) 2020 JCC Research Group

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors will be asked, upon acceptance of an article, to transfer copyright of the article to the Publisher. This will ensure the widest possible dissemination of information under copyright laws. The submitted materials may be considered for inclusion but can not be returned.
Licensing: The JCC articles are licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source (appropriate citation), provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
*Author rights
As an author you (or your employer or institution) have certain rights to reuse your work.


