Abstract
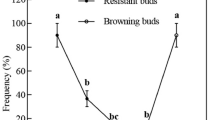
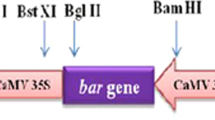
Sugar beet is highly sensitive to imidazolinone herbicides thus rotational restrictions exist. In order to develop imidazolinone tolerant sugar beets als gene from Arabidopsis thaliana encoding acetolactate synthase with S653N mutation was used for genetic transformation. Transgenic sugar beet plants were obtained by Agrobacterium-mediated transformation of aseptic seedlings using vacuum-infiltration. The efficiency of genetic transformation was 5.8%. RT-PCR analysis of obtained plants revealed accumulation of specific als transcript. The resistance to imidazolinone was proved for developed transgenic sugar beet plants in vitro and in greenhouse conditions after spraying with imazethapyr (Pursuit®, BASF).
Similar content being viewed by others
References
Renner, K.A. and Powell, G.E., Response of Sugar Beet (Beta vulgaris) to Herbicides Residues in Soil, Weed Technol., 1991, vol. 5, pp. 622–627.
Alister, C. and Kogan, M., Efficacy of Imidazolinone Herbicides Applied to Imidazolinone Resistant Maize and Their Carryover Effect on Rotational Crops, Crop Prot., 2005, vol. 24, pp. 375–379.
Shaner, D.L., Anderson, P.C., and Stidham, M.A., Imidazolinones: Potent Inhibitors of Acetohydroxyacid Synthase, Plant Physiol., 1984, vol. 76, pp. 545–546.
Singh, B.K., Stidham, M.A., and Shaner, D.L., Assay of Acetohydroxyacid Synthase, Anal. Biochem., 1988, vol. 171, pp. 173–179.
Chatteff, R.S. and Mauvais, C.J., Acetolactate Synthase Is the Site of Action of Two Sulfonylurea Herbicides in Higher Plants, Science, 1984, vol. 224, pp. 1443–1445.
Ray, T.B., Site of Action of Chlorsulfuron: Inhibition of Valine and Isoleucine Biosynthesis in Plants, Plant Physiol., 1984, vol. 75, pp. 827–831.
Gerwick, B.C., Subramanian, M.V., Loney-Gallant, V., and Chander, D.P., Mechanism of Action of the 1,2,4-Triazolo[L,5-A]Pyrimidine, Pestic. Sci., 1990, vol. 29, pp. 357–364.
Sprague, C.L., Frasier, A.L., and Penner, D., Identifying Acetolactate Synthase Inhibitors for Potential Control of Quackgrass (Elytrigia repens) and Canada Thistle (Cirsium arvense) in Corn (Zea mays), Weed Technol., 1999, vol. 13, pp. 54–58.
Geier, P.W., Stahlman, P.W., Hargett, J.G., Dose Responses of Weeds and Winter Wheat to MKH 6561, Weed Sci., 2001, vol. 49, pp. 788–791.
Winder, T. and Spalding, M., Imazaquin and Chlorsulfuron Resistance and Cross Resistance in Mutants of Chlamydomonas reinhardtii, Mol. Gen. Genet., 1988, vol. 213, pp. 394–399.
Saxena, P. and King, J., Herbicide Resistance in Datura innoxia: Cross-Resistance of Sulfonyl Urea Resistance Lines to Imidazolinones, Plant Physiol., 1988, vol. 86, pp. 863–867.
Haughn, G.W. and Somerville, C.R., A Mutation Causing Imidazolinone Resistance Maps to Csrl Locus in Arabidopsis thaliana var. columbia, Plant Physiol., 1990, vol. 92, pp. 1081–1085.
Trucco, F., Eager, A.G., and Tranel, P.J., Acetolactate Synthase Mutation Conferring Imidazolinone-Specific Herbicide Resistance in Amaranthus hybridus, J. Plant Physiol., 2006, vol. 163, pp. 475–479.
Ott, K.M., Kwagh, J.-G., Stockton, G.W., Sidorov, V., and Kake-Juda, G., Rational Molecular Design and Genetic Engineering of Herbicide Resistant Crops by Structure Modeling and Site-Directed Mutagenesis of Acetohydroxyacid Synthase, J. Mol. Biol., 1996, vol. 263, pp. 359–368.
Chang, A.K. and Duggleby, R.G., Herbicide-Resistant Forms of Arabidopsis thaliana Acetohydroxyacid Synthase: Characterization of the Catalytic Properties and Sensitivity to Inhibitors of Four Defined Mutants, Biochem. J., 1998, vol. 333, pp. 765–777.
Lee, Y.-T., Chang, A.K., and Duggleby, R.G., Effect of Mutagenesis at Serine 653 of Arabidopsis thaliana Acetohydroxyacid Synthase on the Sensitivity to Imidazolinone and Sulfonylurea Herbicides, FEBS Lett., 1999, vol. 452, pp. 341–345.
Oh, K.J., Park, E.J., Yoon, M.Y., Han, T.R., and Choi, J.D., Roles of Histidine Residues in Tobacco Acetolactate Synthase, Biochem. Biophys. Res. Commun., 2001, vol. 282, pp. 1237–1243.
Duggleby, R.G., Pang, S.S., Yu, K., and Guddat, L.W., Systematic Characterization of Mutations in Yeast Acetohydroxyacid Synthase: Interpretation of Herbicide-Resistance Data, Eur. J. Biochem., 2003, vol. 270, pp. 2895–2904.
Le, D.T., Yoon, M.Y., Kim, Y.T., and Choi, J.D., Roles of Conserved Methionine Residues in Tobacco Acetolactate Synthase, Biochem. Biophys. Res. Communs, 2003, vol. 306, pp. 1075–1082.
Le, D.T., Yoon, M.Y., Kim, Y.T., and Choi, J.D., Two Consecutive Aspartic Acid Residues Conferring Herbicide Resistance in Tobacco Acetohydroxy Acid Synthase, Biochim. Biophys. acta. Proteins Proteomics, 2005, vol. 1749, pp. 103–112.
Kolkman, J.M., Slabaugh, M.B., Bruniard, J.M., Berry, S., Bushman, B.S., Olungu, C., Maes, N., Abratti, G., Zambelli, A., Miller, J.F., Leon, A., and Knapp, S.J., Acetohydroxyacid Synthase Mutations Conferring Resistance to Imidazolinone or Sulfonylurea Herbicides in Sunflower, Theor. Appl. Genet., 2004, vol. 109, pp. 1147–1159.
Preston, C., Stone, L.M., Rieger, M.A., and Baker, J., Multiple Effects of a Naturally Occurring Proline to Threonine Substitution within Acetolactate Synthase in Two Herbicide-Resistant Populations of Lactuca serriola, Pestic Biochem. Physiol., 2006, vol. 84, pp. 227–235.
Newhouse, K.E., Singh, B.K., Shaner, D.L., and Stidham, M.A., Mutations in Corn (Zea mays L.) Conferring Resistance to Imidazolinone Herbicides, Theor. Appl. Genet., 1991, vol. 83, pp. 65–70.
Newhouse, K.E., Smith, W.A., Starrett, M.A., Schaefer, T.J., and Singh, B.K., Tolerance to Imidazolinone Herbicides in Wheat, Plant Physiol., 1992, vol. 100, pp. 882–886.
Wright, T.R. and Penner, D., Cell Selection and Inheritance of Imidazolinone in Sugarbeet (Beta vulgaris), Theor. Appl. Genet., 1998, vol. 96, pp. 612–620.
Andersson, M., Trifonova, A., Andersson, A.-B., Johansson, M., Bulow, L., and Hofvander, P., A Novel Selection System for Potato Transformation Using a Mutated AHAS Gene, Plant Cell. Rep, 2003, vol. 22, pp. 261–267.
Murashige, T. and Skoog, F., A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Culture, Physiol. Plant., 1962, vol. 15, pp. 473–497.
Kishchenko, E.M., Komarnitskii, I.K., and Kuchuk, N.V., Production of Transgenic Sugarbeet (Beta vulgaris L.) Plants Resistant to Phosphinothricin, Cell. Biol. Int., 2005, vol. 29, pp. 15–19.
Morel, G. and Wetmore, R.H., Fern Callus Tissue Culture, Am. J. Bot., 1951, vol. 38, pp. 141–143.
Doyle, J.J. and Doyle, J.L., Isolation of Plant DNA from Fresh Tissue, Focus, 1990, vol. 12, pp. 13–15.
Logemann, J., Schell, J., and Willmitzer, L., Improved Method for the Isolation of RNA from Plant Tissues, Ann. Biochem., 1987, vol. 163, pp. 16–20.
Tertivanidis, K., Goudoula, C., Vasilikiotis, C., Hassiotou, E., Perl-Treves, R., and Tsaftaris, A., Superoxide Dismutase Trans-Genes in Sugarbeets Confer Resistance to Oxidative Agents and the Fungus C. beticola, Transgenic Res., 2004, vol. 13, pp. 225–233.
Norouzi, P., Malboobi, M.A., Zamani, K., and Yazdi-Samadi, B., Using a Competent Tissue for Efficient Transformation of Sugarbeet (Beta vulgaris L.), In Vitro Cell. Dev. Biol. Plant, 2005, vol. 41, pp. 11–16.
Jafari, M., Norouzi, P., Malboobi, M.A., Ghareyazie, B., Valizadeh, M., Mohammadi, S.A., and Mousavi, M., Enhanced Resistance to a Lepidopteran Pest in Transgenic Sugar Beet Plants Expressing Synthetic CrylAb Gene, Euphytica, 2009, vol. 165, pp. 333–344.
Krens, F.A., Trifonova, A., Keizer, L.C.P., and Hall, R.D., The Effect of Exogenously-Applied Phytohormones on Gene Transfer Efficiency in Sugarbeet (Beta vulgaris L.), Plant Sci., 1996, vol. 116, pp. 97–106.
Mishutkina, Ya.V., Kamionskaya, A.M., and Skryabin, K.G., Sozdanie transgennykh rastenii sakharnoi svekly, ekspressiruyushchikh gen bar, Prikl. Biokhim. Mikrobiol., 2010, vol. 46, no. 1, pp. 89–95.
Hisano, H., Kimoto, Y., Hayakawa, H., et al., High Frequency Agrobacterium-Mediated Transformation and Plant Regeneration via Direct Shoot Formation from Leaf Explants in Beta vulgaris and Beta maritima, Plant Cell. Rep., 2004, vol. 22, pp. 910–918.
Lindsey, K. and Gallois, P., Transformation of Sugarbeet (Beta vulgaris) by Agrobacterim tumefaciens, J. Exp. Bot., 1990, vol. 41, pp. 529–536.
Snyder, G.W., Ingersoll, J.C., Smigocki, A.C., and Owens, L.D., Introduction of Pathogen Defense Genes and a Cytokinin Biosynthesis Gene into Sugarbeet (Beta vulgaris L.) by Agrobacterium or Particle Bombardment, Plant Cell. Rep., 1999, vol. 18, pp. 829–834.
Zhang, C.-L., Chen, D.-F., McCormac, A.C., Scott, N.W., Elliot, M.C., and Slater, A., Use of the GFP Reporter Gene as a Vital Marker for Agrobacterium-Mediated Transformation of Sugar Beet (Beta vulgaris L.), Mol. Biotechnol., 2001, vol. 17, pp. 109–117.
Joersbo, M., Donaldson, I., Kreiberg, J., Petersen, S.G., Brunstedt, J., and Okkels, F.T., Analysis of Mannose Selection Used for Transformation of Sugar Beet, Mol. Breed., 1998, vol. 4, pp. 111–117.
Kuykendall, L.D., Stocked, T.M., and Saunders, J.W., Rhizobium radiobacter Conjugation and Callus-Independent Shoot Regeneration Used to Introduce the Cercosporin Export Gene Cfp from Cercospora into Sugar Beet (Beta vulgaris L.), Biotechnol. Lett., 2003, vol. 25, pp. 739–744.
Yang, A.F., Duan, X.G., Gu, X.F., Gao, F., and Zhang, J.R., Efficient Transformation of Beet (Beta vulgaris) and Production of Plants with Improved Salt-Tolerance, Plant Cell Tissue Organ Cult., 2005, vol. 83, pp. 259–270.
D’alluin, K., Bossut, M., Bonne, E., Mazur, B., Leemans, J., and Botterman, J., Transformation of Sugarbeet (Beta vulgaris L.) and Evaluation of Herbicide Resistance in Transgenic Plants, Bio. Technology, 1992, vol. 10, pp. 309–314.
Ivic-Haymes, S.D. and Smigocki, A.C., Biolistic Transformation of Highly Regenerative Sugar Beet (Beta vulgaris L.) Leaves, Plant Cell Rep., 2005, vol. 23, pp. 699–704.
Hall, R.D., Riksen-Bruinsma, T., Weyens, G.J., et al., A High Efficiency Technique for the Generation of Transgenic Sugar Beets from Stomatal Guard Cells, Nat. Biotechnol., 1996, vol. 14, pp. 1133–1138.
Mannerlöf, M., Tuvesson, S., Steen, P., and Tenning, P., Transgenic Sugar Beet Tolerant to Glyphosate, Euphytica, 1997, vol. 94, pp. 83–91.
Winder, T. and Spalding, M., Imazaquin and Chlorsulfuron Resistance and Cross Resistance in Mutants of Chlamydomonas reinhardtii, Mol. Gen. Genet., 1988, vol. 231, pp. 394–399.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Kishchenko, E.M., Komarnitskii, I.K. & Kuchuk, N.V. Transgenic sugar beet tolerant to imidazolinone obtained by Agrobacterium-mediated transformation. Cytol. Genet. 45, 148–152 (2011). https://doi.org/10.3103/S0095452711030030
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452711030030