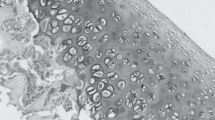
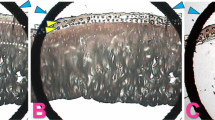
Abstract
The biochemical and molecular analysis of rat cartilage tissue with monosodium iodoacetateinduced osteoarthritis established the increase in the expression levels of Ptgs2 and Tgfb1 genes and the increase in ROS production compared to the corresponding control group of animals. This indicates the activation of inflammatory and destructive processes, impairment of the cellular redox balance, and the development of oxidative stress in the tissues. When using a chondroitin sulfate preparation under the same conditions, the expression levels of these genes, as well as the content of the superoxide anion radical and organic hydroperoxides, were closer to control values compared with experimental osteoarthritis, indicating the antiinflammatory and antioxidant properties of the drug used and its efficiency in osteoarthritis treatment.
Similar content being viewed by others
References
Dranitsina, A.S., Dvorshchenko, K.O., Grebinyk, D.M., and Ostapchenko, L.I., The impact of oxidative stress on Par2, Ptgs2 genes expression in rat duodenal epithelial cells under conditions of prolonged gastric hypochlorhydria and with administration of multiprobiotic, J. Appl. Pharm. Sci., 2016, vol. 6, no. 12, pp. 162–169.
Dranitsina, A.S., Taburets, O.V., Dvorshchenko, K.O., Grebinyk, D.M., Beregova, T.V., and Ostapchenko, L.I., Tgfb1, Ptgs2 genes expression during dynamics of wound healing and with the treatment of melanin, Res. J. Pharm. Biol. Chem. Sci., 2017, vol. 8, no. 1, pp. 2014–2023.
Sandell, L.J., Xing, X., Franz, C., Davies, S., Chang, L.W., and Patra, D., Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1β, Osteoarthritis Cartilage, 2008, vol. 16, no. 12, pp. 1560–1571.
Au, R.Y., Al-Talib, T.K., Au, A.Y., Phan, P.V., and Frondoza, C.G., Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages, Osteoarthritis Cartilage, 2007, vol. 15, no. 11, pp. 1249–1255.
Felson, D.T., Lawrence, R.C., Dieppe, P.A., Hirsch, R., Helmick, C.G., Jordan, J.M., Kington, R.S., Lane, N.E., Nevitt, M.C., Zhang, Y., Sowers, M., McAlindon, T., Spector, T.D., Poole, A.R., Yanovski, S.Z., Ateshian, G., Sharma, L., Buckwalter, J.A., Brandt, K.D., and Fries, J.F., Osteoarthritis: new insights. Part 1. The disease and its risk factors, Ann. Intern. Med., 2000, vol. 133, no. 8, pp. 635–646.
Goldring, S.R. and Goldring, M.B., The role of cytokines in cartilage matrix degeneration in osteoarthritis, Clin. Orthop. Relat. Res., 2004, no. 427, pp. S27–S36.
Wu, K.K., Cyclooxygenase 2 induction: molecular mechanism and pathophysiologic roles, J. Lab. Clin. Med., 1996, vol. 128, no. 3, pp. 242–245.
Benito, M.J., Veale, D.J., Fitzgerald, O., van den Berg, W.B., and Bresnihan, B., Synovial tissue inflammation in early and late osteoarthritis, Ann. Rheum. Dis., 2005, vol. 64, no. 9, pp. 1263–1267.
Pohlers, D., Beyer, A., Koczan, D., Wilhelm, T., Thiesen, H.J., and Kinne, R.W., Constitutive upregulation of the transforming growth factor-β pathway in rheumatoid arthritis synovial fibroblasts, Arthritis Res. Ther., 2007, vol. 9, p. R59.
Ota, K., Quint, P., Weivoda, M.M., Ruan, M., Pederson, L., Westendorf, J.J., Khosla, S., and Oursler, M.J., Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors, Bone, 2013, vol. 57, no. 1, pp. 68–75.
Shi-Wen, X., Leask, A., and Abraham, D., Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis, Cytokine Growth Factor Rev., 2008, vol. 19, no. 2, pp. 133–144.
Barnes, E.V. and Edwards, N.L., Treatment of osteoarthritis, South Med. J., 2005, vol. 98, no. 2, pp. 205–209.
Volpi, N., Quality of different chondroitin sulfate preparations in relation to their therapeutic activity, J. Pharm. Pharmacol., 2009, vol. 61, no. 10, pp. 1271–1280.
Lauder, R.M., Chondroitin sulfate: a complex molecule with potential impacts on a wide range of biological systems, Complemen. Ther. Med., 2009, vol. 17, no. 1, pp. 56–62.
Largo, R., Roman-Blas, J.A., Moreno-Rubio, J., Sánchez-Pernaute, O., Martínez-Calatrava, M.J., Castaneda, S., and Herrero-Beaumont, G., Chondroitin sulfate improves synovitis in rabbits with chronic antigeninduced arthritis, Osteoarthritis Cartilage, 2010, vol. 18, no. 1, pp. S17–S23.
Zhang, R., Brennan, M.L., Shen, Z., MacPherson, J.C., Schmitt, D., Molenda, C.E., and Hazen, S.L., Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation, J. Biol. Chem., 2002, vol. 277, no. 48, pp. 46116–46122.
Caraglia, M., Beninati, S., Giuberti, G., D’Alessandro, A.M., Lentini, A., Abbruzzese, A., Bove, G., Landolfi, F., Rossi, F., Lampa, E., and Costantino, M., Alternative therapy of earth elements increases the chondroprotective effects of chondroitin sulfate in mice, Exp. Mol. Med., 2005, vol. 37, no. 5, pp. 476–481.
Janusz, M.J., Little, C.B., King, L.E., Hookfin, E.B., Brown, K.K., Heitmeyer, S.A., Caterson, B., Poole, A.R., and Taiwo, Y.O., Detection of aggrecanase-and MMP-generated catabolic neoepitopes in the rat iodoacetate model of cartilage degeneration, Osteoarthritis Cartilage, 2004, vol. 12, no. 9, pp. 720–728.
Sutherland, M.W. and Learmonth, B.A., The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase, Free Radic. Res., 1997, vol. 27, no. 3, pp. 283–289.
Able, A.J., Guest, D.I., and Sutherland, M.W., Use of new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophtora parasitica varnicotianae, Plant Physiol., 1998, vol. 177, no. 2, pp. 491–499.
Jiang, Z.Y., Woollard, A.C., and Wolff, S.P., Hydrogen peroxide production during experimental protein glycation, FEBS Lett., 1990, vol. 268, no. 1, pp. 69–71.
Nourooz-Zadeh, J., Tajaddini-Sarmadi, J., and Wolff, S.P., Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay, Anal. Biochem., 1994, vol. 220, no. 2, pp. 403–409.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, vol. 193, no. 1, pp. 265–275.
Chomczynski, P. and Sacchi, N., Single-step method of RNA isolation by acid guanidinium thiocyanatephenolchloroform extraction, Anal. Biochem., 1987, vol. 162, no. 1, pp. 156–159.
Livak, E.J. and Schmittgen, T.D., Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method, Methods, 2001, vol. 25, no. 4, pp. 402–408.
Kamata, H. and Hirata, H., Redox regulation of cellular signalling, Cell Signal., 1999, vol. 11, no. 1, pp. 1–14.
Starkman, B.G., Cravero, J.D., Delcarlo, M., and Loeser, R.F., Igf-i stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK, Biochem. J., 2005, vol. 389, pp. 723–729.
Haringman, J.J., Smeets, T.J.M., Reinders-Blankert, P., and Tak, P.P., Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis, Ann. Rheum. Dis., 2006, vol. 65, no. 3, pp. 294–300.
Conte, A., Volpi, N., Palmieri, L., Bahous, I., and Ronca, G., Biochemical and pharmacokinetic aspects of oral treatment with chondroitin sulfate, Arzneimittelforschung, 1995, vol. 45, no. 8, pp. 918–925.
Pelletier, J.P., Martel-Pelletier, J., and Abramson, S.B., Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets, Arthritis Rheum., 2001, vol. 44, no. 6, pp. 1237–1247.
Chan, P.S., Caron, J.P., Rosa, G.J., and Orth, M.W., Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E2 in articular cartilage explants, Osteoarthritis Cartilage, 2005, vol. 13, no. 5, pp. 387–394.
Jomphe, C., Gabriac, M., Hale, T.M., Héroux, L., Trudeau, L.E., Deblois, D., Montell, E., Vergés, J., and Souich, P., Chondroitin sulfate inhibits the nuclear translocation of nuclear factor-kappa B in interleukin-1beta-stimulated chondrocytes, Basic Clin. Pharmacol. Toxicol., 2008, vol. 102, no. 1, pp. 59–65.
Kwan Tat, S., Pelletier, J.-P., Lajeunesse, D., Fahmi, H., Lavigne, M., and Martel-Pelletier, J., The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor κB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells, Clin. Exp. Rheumatol., 2008, vol. 26, no. 2, pp. 295–304.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Ukrainian Text © A.S. Dranitsina, K.O. Dvorshchenko, A.G. Korotkiy, D.M. Grebinyk, L.I. Ostapchenko, 2018, published in Tsitologiya i Genetika, 2018, Vol. 52, No. 3, pp. 33–39.
About this article
Cite this article
Dranitsina, A.S., Dvorshchenko, K.O., Korotkiy, A.G. et al. Expression of Ptgs2 and Tgfb1 Genes in Rat Cartilage Cells of the Knee under Conditions of Osteoarthritis. Cytol. Genet. 52, 192–197 (2018). https://doi.org/10.3103/S0095452718030039
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452718030039