Abstract
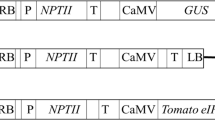
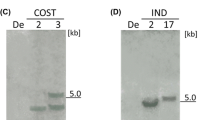
The human lactoferrin gene was transferred into genomes of several potato (Solanum tuberosum) cultivars of Ukrainian breeding using the Agrobacterium-mediated transformation. The plasmid vector pBIN35LF carrying the human lactoferrin gene hLf controlled by the 35S cauliflower mosaic virus promoter (CaMV35S) and the octopine synthase terminator, as well as the selective marker neomycin phosphotransferase II gene (nptII) conferring the resistance to kanamycin, was used. As a result of selection, 44 lines of Vernisage, 26 lines of cv. Levada, 25 lines of cv. Svitanok Kyivskyi, and 16 lines of cv. Zarevo cultivars resistant to 100 mg/L of kanamycin were obtained. PCR and Western blot analyses were carried out for transformed lines with primers specific to the hLf gene and a monoclonal antibody against lactoferrin to confirm the transgenic nature of selected tomato plants and hLf gene expression. The selected transgenic potato lines were tested for resistance to bacterial and fungal phytopathogens. The disk diffusion assay revealed that the juice of transgenic potato lines possesses an antibacterial effect against phytopathogenic bacteria Ralstonia solanacearum (causing potato brown rot) and Clavibacter michiganensis subsp. sepedonicus (causing potato ring rot). The resistance of transgenic potato plants to late blight was investigated by in vitro infection of plants with the Phytophthora infestans isolate. As a result, it was found that the obtained transgenic potato lines have enhanced resistance to P. infestans as compared to the control. Thus, the obtained data show that the transfer of the hLf gene into the potato genome enhances potato’s resistance to bacterial and fungal pathogens.







Similar content being viewed by others
REFERENCES
Melnyk, S.I., Kovchi, A.L., Stefkivska, Y.L., Kravchuk, O.O., and Gorytska, T.V., Potato market in Ukraine, Plant Var. Stud. Protect., 2017, vol. 13, no. 2, pp. 206–210. https://doi.org/10.21498/2518-1017.13.2.2017.105419
Cook, E., Agriculture, Forestry and Fishery Statistics, Luxembourg: Publications Office of the European Union, 2018.
Kolobayev, V.A. and Rogozina, Ye.V., Cultivars immunity to phytopathogens as a factor of environmental safety: the cases of potato and sugarcane, Biosphere, 2014, vol. 6, no. 3, pp. 222–230.
Erenkova, L.A., Molyavko, A.A., Marukhlenko, A.V., and Borisova, N.P., New generation potato varieties with resistance to pathogens, Breeding, Seed Product.Genet., 2018, vol. 4, pp. 47–50. https://doi.org/10.24411/2413-4112-2018-10007
Fiers, M., Edel-Hermann, V., Chatot, C., Alabouvette, C., and Steinberg, C., Potato soil-borne diseases. A review, Agron. Sustainable Dev., 2012, vol. 32, pp. 93–132. https://doi.org/10.1007/s13593-011-0035-z
Gordienko, V.V. and Zaharchuk, N.A., Creation of initial breeding material of potato with complex resistance to Fusarium dry rot and tuber late blight, Plant Varieties Stud. Protect., 2017, vol. 13, no. 3, pp. 239–244.https://doi.org/10.21498/2518-1017.13.3.2017
Kolomiets, Y.V., Grygoryuk, I.P., and Butsenko, L.M., Bacterial diseases of tomato plants in terms of open and covered growing of Ukraine, Ann. Agrar. Sci., 2017, vol. 15, no. 2, pp. 213–216. https://doi.org/10.1016/j.aasci.2017.05.010
Kolomiets, Y.V., Grygoryuk, I.P., Butsenko, L.M., and Kalinichenko, A.V., Biotechnological control methods against phytopathogenic bacteria in tomatoes, Appl. Ecol. Environ. Res., 2019, vol. 17, no. 2, pp. 3215– 3230. https://doi.org/10.15666/aeer/1702_32153230
Kolomiets, Y.V., Grygoryuk, I.P., Likhanov A., But-senko L.M., and Blume Ya., Induction of bacterial canker resistance in tomato plants using plant growth promoting Rhizobacteria, Open Agric. J., 2019, vol. 13, pp. 215–22.https://doi.org/10.2174/18743315019
Boroday, V.V. and Parfeniuk, A.I., Spreading and development of the main potato (Solanum tuberosum L.) diseases in Ukraine, Agroecol. J., 2018, vol. 4, pp. 82–87. https://doi.org/10.33730/2077-4893.4.2018.161774
Lopez-Garcia, B., San Segundo, B., and Coca, M., Antimicrobial peptides as a promising alternative for plant disease protection, in Small Wonders:Peptides for Disease Control, 2012, pp. 263–294. https://doi.org/10.1021/bk-2012-1095.ch013
Moosa, A., Farzand, A., Sahi, S.T., and Khan, S.A., Transgenic expression of antifungal pathogenesis-related proteins against phytopathogenic fungi—15 years of success, Israel. J. Plant Sci., 2017. doi 80/07929978.2017.1288407
Jung, Y., Kang, K., Application of antimicrobial peptides for disease control in plants, Plant Breed. Biotechnol., 2014, vol. 2, no. 1, pp. 1–13. https://doi.org/10.9787/PBB.2014.2.1.001
Patil, V.U., Gopal, J., and Singh, B.P., Improvement for bacterial wilt resistance in potato by conventional and biotechnological approaches, Agric. Res., 2012, vol. 1, pp. 299–316. https://doi.org/10.1007/S40003-012-0034-6
Yemets, A.I., Tanasienko, I.V., Krasylenko, Y.A., and Blume, Y.B., Plant-based biopharming of recombinant human lactoferrin, Cell Biol. Int., 2014, vol. 38, pp. 989–1002. https://doi.org/10.1002/cbin.10304
Murashige, T. and Skoog, F., A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiol. Plant.,1962, vol. 15, pp. 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Buziashvili, A. and Yemets, A., Obtaining of tomato and potato plants with human lactoferrin gene hLf, Rep. Natl. Acad. Sci. Ukr., 2018, vol. 10, pp. 88–94. https://doi.org/10.15407/dopovidi2018.10.088
Sambrook, J.F. and Russell, D.W., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, 2001.
Jeroen, S., Damm, B., Melchers, L., and Hoekema, A., Factors influencing transformation frequency of tomato (Lycopersicon esculentum), Plant Cell Rep., 1993, vol. 12, pp. 644–647. https://doi.org/10.1007/BF00232816
Rogers, S.O., Bendich, A.J., Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues, Plant Mol. Biol., 1985, vol. 5, pp. 69–76. https://doi.org/10.1007/BF00020088
Tanasienko, I.V., Yemets, A.I., Pirko, Y.V., Korhkovyy, V.I., Abumhadi, N., and Blume, Y.B., Generation of transgenic barley lines producing human lactoferrin using mutant alpha-tubulin gene as the selective marker, Cytol. Genet., 2011, vol. 45, no. 1, pp. 3–10. https://doi.org/10.3103/S0095452711010026
Baskaran, P., Soys, V., Balazs, E., and Van Stadena, J., Shoot apical meristem injection: A novel and efficient method to obtain transformed cucumber plants, S. Afr. J. Bot., 2016, vol. 103, pp. 210–215. https://doi.org/10.1016/j.sajb.2015.09.006
Mitra, A., Zhang, Z., Expression of a human lactoferrin cDNA in tobacco cells produces antibacterial protein(s), Plant Physiol., 1994, vol. 106, no. 3, pp. 977–981.
Rachmawati, D., Mori, T., Hosaka, T., Takaiwa, F., Inoue, E., and Anzai, H., Production and characterization of recombinant human lactoferrin in transgenic Javanica rice, Breed. Sci., 2005, vol. 55, no. 2, pp. 213–222. https://doi.org/10.1270/jsbbs.55.213
Bradford, M., A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, vol. 72, no. 1–2, pp. 248–254.
Laemmli, U., Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, vol. 227, pp. 680–5.
Onuoha, S.C., Alisa, C.O., Antimicrobial potential of leaf juice and extracts of Moringa oleifera lam against some human pathogenic bacteria, J. Pharm. Biol. Sci., 2013, vol. 5, no. 4, pp. 37–42. https://doi.org/10.9790/3008-0543742
Chahardooli, M., Farzaneh, A., and Sohrabi, A., Expression of recombinant Arabian camel lactofer-ricin-related peptide in Pichia pastoris and its antimicrobial identification, J. Sci. Food Agricult., 2016, vol. 96, no. 2, pp. 569–575. https://doi.org/10.1002/jsfa.7125
Yabuuchi, E., Kosako, Y., Oyaizu, H., Yano, I., Hotta, H., Hashimoto, Y., Ezaki, T., and Arakawa, M., Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov., Microbiol. Immunol., 1992, vol. 36, no. 12, pp. 1251–75. https://doi.org/10.1111/j.1348-0421.1992.tb02129.x
Podgorskiy, V.S., Ukrainian National Collection of Microorganisms, 2nd ed., Kyiv: Naukova Dumka, 2007.
Tournas, V., Stack, M.E., Mislivec, P.B., Koch, H.A., and Bandler, R., FDA Bacteriological Analytical Manual, chapter 18: Yeasts, Molds, and Mycotoxins, 8th ed., Revision A, USA: AOAC International, 1998.
Tkachyk, S.O., Methods of Phytopathological Researches with Artificial Infection of Plants, Kyiv: Nylan-LTD, 2014.
Cherednichenko, L.M., Evaluation of the potato selection material for the resistance of the aboveground plant parts to late blight with the use of detached leaf particles, Potato Grow. Ukr., 2013, vols. 1–2, pp. 19–24.
Podhaietskyi, A.A., Kravchenko, N.V., Kovalenko, V.M., Bondus, R.O., Hordienko, V.V., Cherednichenko, L.M., and Sobra, V.M., Ecological testing of potatoes, Ukr. J. Ecol., 2018, vol. 8, no. 4, pp. 17–25.
Michalska, A.M., Sobkowiak, S., Flis, B. Zimnoch-Guzowska, E., Virulence and aggressiveness of Phytophthora infestans isolates collected in Poland from potato and tomato plants identified no strong specificity, Eur. J. Plant Pathol., 2016, vol. 144, pp. 325–336. https://doi.org/10.1007/s10658-015-0769-6
Buziashvili A.Yu. and Yemets, A.I., Agrobacterium-mediated transformation of potato with human lactoferrin gene, Fact. Exp. Evol. Org., 2019, vol. 25, pp. 184–189.
Han, E.H., Goo, Y.M., Lee, M.K., and Lee, S.W., An efficient transformation method for a potato (Solanum tuberosum L. var. Atlantic), J. Plant Biotechnol., 2015, vol. 42, pp. 77–82. https://doi.org/10.5010/JPB.2015.42.2.77
Shin, D.Y., Seong, E.S., Na, J.K., Yoo, J.H., Kang, W.H., Ghimire, B.K., Lim, J.D., Yu, C.Y., Conditions for regeneration and transformation of Solanum tuberosum cultivars using the cotton glutathione S-transferase (Gh-5) gene, Afr. J. Biotechnol., 2011, vol. 10, no. 67, pp. 15135–15141. https://doi.org/10.5897/AJB11.1895
Soto, N., Enriquez, G.A., Ferreira, A., Corrada, M., Fuentes, A., Tiel, K., and Pujol, M., Efficient transformation of potato stems segments from cultivar Desiree, using phosphinothricin as selection marker, Biotechnol. Apl., 2007, vol. 24, no. 2, pp. 139–144.
Craze, M., Bates, R., Bowden, S., and Wallington, E.J., Highly efficient Agrobacterium-mediated transformation of potato (Solanum tuberosum) and production of transgenic microtubers, Curr. Protoc. Plant Biol., 2018, vol. 3, no. 1, pp. 33–41. doi 10.1002/cppb.20065
Alimohammadi, M., Bagherieh-Najjar, M., Agrobacterium-mediated transformation of plants: basic principles and influencing factors, Afr. J. Biotechnol., 2009, vol. 8, no. 20, pp. 5142–5148.
Opabode, J., Agrobacterium-mediated transformation of plants: emerging factors that influence efficiency, Biotechnol. Mol. Biol. Rev., 2006, vol. 1, no. 1, pp. 12–20.
Bakhsh, A., Anayol, E., and Ozcan, S., Comparison of transformation efficiency of five Agrobacterium tumefaciens strains in Nicotiana tabacum L., Emirates J. Food Agricult., 2014, vol. 26, no. 3, pp. 259–264. https://doi.org/10.9755/ejfa.v26i3.16437
Gelvin, S., Liu, C., Genetic manipulation of Agrobac-terium tumefaciens strains to improve transformation of recalcitrant plant species, Plant Mol. Biol. Manual., 1994, pp. 85–97.
Stefanova, G., Slavov, S., Gecheff, K., Vlahova, M., and Atanassov, A., Expression of recombinant human lactoferrin in transgenic alfalfa plants, Biol. Plant., 2013, vol. 57, no. 3, pp. 457–464.
Vlahova, M., Stefanova, G., Petkov, P., Barbulova, A., Petkova, D., Kalushkov, P., and Atanassov, A., Genetic modification of alfalfa (Medicago sativa L.) for quality improvement and production of novel compounds, Biotechnol. Biotechn. Equipm., 2005, vol. 19, no. 3, pp. 56–62. doi 0/13102818.2005.10817286
Legrand, D., Salmon, V., Spik, G., Gruber, V., Bournat, P., and Merot, B., Recombinant lactoferrin, methods of production from plants and uses, US Patent No. 6569831 B1, May 27, 2003.
Chong, D.K., Langridge, W.H., Expression of full-length bioactive antimicrobial human lactoferrin in potato plants, Transgen. Res., 2000, vol. 9, no. 1, pp. 71–78. https://doi.org/10.1023/A:1008977630179
On the Amendments to the List of Regulated Pests, The edition of the order of the Ministry of Agrarian Policy and Food of Ukraine, July 16, 2019.
Fukuta, S., Kawamoto, K., Mizukami, Y., Yoshimura, Y., Ueda, J., and Kanbe, M., Transgenic tobacco plants expressing antimicrobial peptide bovine lactoferricin show enhanced resistance to phyto-pathogens. Plant Biotechnol., 2012, vol. 29, no. 4, pp. 383–389. https://doi.org/10.5511/plantbiotechnology.12.0619a
Sohrabi, S.M., Niazi, A., Chahardooli, M., and Aram, F., Isolation and expression of antimicrobial camel lactoferrin (cLf) gene in tobacco, Plant OMICS, 2014, vol. 7, pp. 298–307.
Chahardooli, M., Fazeli, A., Niazi, A., and Ghabooli, M., Recombinant expression of LFchimera antimicrobial peptide in a plant-based expression system and its antimicrobial activity against clinical and phytopathogenic bacteria, Biotechnol. Biotechn. Equipm., 2018, vol. 32, no 3, pp. 714–723. https://doi.org/10.1080/13102818.2018.1451780
Humphrey, B.D., Huang, N., and Klasing, K.C., Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks, J. Pediatr. Gastroenterol. Nutr., 2003, vol. 36, no. 2, pp. 190–199. https://doi.org/10.1093/jn/132.6.1214
Lee, T.T., Chang, C.C., Juang, R.S., Chen, R.B., Yang, H.Y., Chu, L.W., Wang, S.R., Tseng, T.H., Wang, C.S., Chen, L.J., and Bi, Yu., Porcine lactoferrin expression in transgenic rice and its effects as a feed additive on early weaned piglets, J. Agric. Food Chem., 2010, vol. 58, no. 8, pp. 5166–5173. https://doi.org/10.1021/jf903904s
Takase, K., Hagiwara, K., Onodera, H., Nishizawa, Y., Ugaki, M., Omura, T., Numata, S., Akutsu, K., Kumura, H., and Shimazaki, K., Constitutive expression of human lactoferrin and its N-lobe in rice plants to confer disease resistance, Biochem. Cell Biol., 2005, vol. 83, no. 2, pp. 239–49. https://doi.org/10.1139/o05-022
Franco, I., Castillo, E., Perez, M.D., Calvo, M., and Sanchez, L., Effects of hydrostatic high pressure on the structure and antibacterial activity of recombinant human lactoferrin from transgenic rice, Biosci. Biotechnol. Biochem., 2012, vol. 76, no. 1, pp. 53–59. https://doi.org/10.1271/bbb.110433
Funakoshi, T., Hosaka, T., Inoue, E., and Anzai, H., Gene expression of lactoferrin-derived antimicrobial peptides in rice, Plant Physiol. Biochem., 2017, vol. 123. https://doi.org/10.1016/j.plaphy.2017.12.037
Lee, T. J., Coyne, D. P., Clemente, T. E., and Mitra, A., Partial resistance to bacterial wilt in transgenic tomato plants expressing antibacterial lactoferrin gene, J. Am. Soc. Horticult. Sci., 2002, vol. 127, no. 2, pp. 158–164. https://doi.org/10.21273/JASHS.127.2.158
Jo, S.H., Kwon, S.Y., Park, D.S., Yang, K.S., Kim, J.W., Lee, K.T., Kwak, S.S., and Lee, H.S., High-yield production of functional human lactoferrin in transgenic cell cultures of Siberian ginseng (Acanthopanax senticosus), Biotechnol. Bioprocess Eng., 2006, vol. 11, no. 5, pp. 442–448. https://doi.org/10.1007/BF02932312
Malnoy, M., Venisse, J.S., Brisset, M.N., and Chevreau, E., Expression of bovine lactoferrin cDNA confers resistance to Erwinia amylovora in transgenic pear, Mol. Breed., 2003, vol. 12, no. 3, pp. 231–244. https://doi.org/10.1023/A:1026365311067
Nguyen, T.C., Lakshman, D.K., Han, J., Galvez, L.C., and Mitra, A., Transgenic plants expressing antimicrobial lactoferrin protein are resistant to a fungal pathogen, J. Plant Mol. Biol. Biotechnol., 2011, vol. 2, no.1, pp. 1–8.
Han, J., Lakshman, D.K., Galvez, L.C., Mitra, S., Baenziger, P.S., and Mitra, A., Transgenic expression of lactoferrin impacts enhanced resistance to head blight of wheat caused by Fusarium graminearum, BMC Plant Biol., 2012, vol. 12, no. 33. https://doi.org/10.1186/1471-2229-12-33
Bruni, N., Capucchio, M.T., Biasibetti, E., Pessione, E., Cirrincione, S., Giraudo, L., Corona, A., and Dosio, F., Antimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicine, Molecules, 2016, vol. 21, no. 6. https://doi.org/10.3390/molecules21060752
Fernandes, K.E., Carter, D.A., The antifungal activity of lactoferrin and its derived peptides: mechanisms of action and synergy with drugs against fungal pathogens, Front. Microbiol., 2017, vol. 8, no. 2. https://doi.org/10.3389/fmicb.2017.00002
Fry, W., Phytophthora infestans: the plant (and R gene) destroyer, Mol. Plant Pathol., 2008, vol. 9, no. 3, pp. 385–402.https://doi.org/10.1111/j.1364-3703.2007
Dominguez, P., Gomez, I., Barbero, M., Fellmann, T., Chatzopoulos, T., Jensen, H., and Philippidis, G., Eu Commodity Market Development: Medium-Term Agricultural Outlook, Luxembourg: Publ. Office. Eur. Union, 2018, ISBN 978-92-79-97850-0, https://doi.org/10.2760/4519, JRC113987
Funding
This research was financially supported for AB, YB and AY by the grant “Application of lactoferrin gene for production of phytopathogen resistant plant lines from Solanaceaå family” in the frames of complex interdisciplinary research program “Molecular and Cell Biotechnologies for the Needs of Medicine, Industry, and Agriculture” of National Academy of Sciences of Ukraine (no. 0115U005021, 2015–2019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any research using organisms or organs of humans and animals as objects.
Additional information
Translated by K. Lazarev
About this article
Cite this article
Buziashvili, A., Cherednichenko, L., Kropyvko, S. et al. Obtaining Transgenic Potato Plants Expressing the Human Lactoferrin Gene and Analysis of Their Resistance to Phytopathogens. Cytol. Genet. 54, 179–188 (2020). https://doi.org/10.3103/S0095452720030020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452720030020