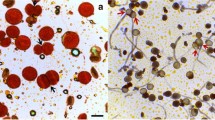
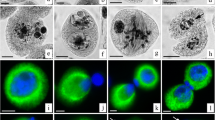
Abstract
Cytomixis and chromosomal polymorphism of microsporocytes in microsporogenesis of the species Lilium croceum Chaix, Allium fistulosum L., and A. cepa L. were studied. It was found that the main cytomictic events are associated with the early prophase of meiosis and are obligatory (constitutive) for the examined species. In metaphase microsporocytes, the so-called extra chromosomes, presumably of cytomictic origin, were identified. They are characterized by weakening of synapsis and/or disintegration of homologues and associations with bivalents of the basic karyotype with the formation of secondary associations of chromosomes. Extra chromosomes are present not only in hyperchromosomal but also in eu- and hypochromosomal microsporocytes. The majority of extra chromosomes seem to be a “genetic ballast” for the cell, which it gets rid of using a wide range of cellular tools, in particular, chromosomal rearrangements, chromatin diminution, asymmetry of division, cytomixis, and programmed cell death. Nevertheless, some extra chromosomes can participate in the rearrangement of the karyotype of microsporocytes and microspores.






Similar content being viewed by others
REFERENCES
Bala, S. and Gupta, R.C., Effect of secondary associations on meiosis, pollen fertility and pollen size in cape gooseberry (Physalis peruviana L.), Chromosome Bot., 2011, vol. 6, pp. 25–28. https://doi.org/10.3199/iscb.6.25
Bellucci, M., Roscini, C., and Mariani, A., Cytomixis in the pollen mother cells of Medicago sativa L., J. Hered., 2003, vol. 94, pp. 512–516. https://doi.org/10.1093/jhered/esg096
Bhattacharya, A. and Datta, A.K., Secondary chromosome associations in Uraria picta (Jacq.) DC (Family: Leguminosae), Cytologia, 2010, vol. 75, pp. 37–40. https://doi.org/10.1508/cyto-logia.75.37
Bretagnolle, F. and Thompson, J.D., Gametes with the somatic (sic) chromosome number: mechanisms of their formation and role in the evolution of autopolypoid plants, N. Phytol., 1995, vol. 129, no. 1, pp. 1–22. https://doi.org/10.1111/j.1469-8137.1995.tb03005.x
Cai, X. and Xu, S.S., Meiosis-driven genome variation in plants, Curr. Genomics, 2007, vol. 8, pp. 151–161. doi 10. 2174/138920207780833847
Cheng, K.C., Nie, X.W., Wang, Y.X., and Yang, Q.L., The relation between cytomixis and variation of chromosome numbers in pollen mother cells of rye (Secale cereale L.), Acta Bot. Sin., 1980, vol. 22, pp. 216–220.
Das, A., Datta, A.K., and Ghose, S., Cytogenetical studies on two varieties of Withania somnifera, J. Trop. Med. Plants, 2009, vol. 10, pp. 249–256.
De Storme, N. and Mason, A., Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance, Curr. Plant Biol., 2014, vol. 1, pp. 10–33. https://doi.org/10.1016/j.cpb.2014.09.002
Falistocco, E., Tosti, N., and Falcinelli, M., Cytomixis in pollen mother cells of diploid Dactylis, one of the origins of 2n gametes, J. Heredity, 1995, vol. 86, pp. 448–453. https://doi.org/10.1093/oxfordjournals. jhered.a111619
Fuentes, I., Stegemann, S., Golczyk, H., et al., Horizontal genome transfer as an asexual path to the formation of new species, Nature, 2014, vol. 511, pp. 232–235. https://doi.org/10.1038/nature13291
Gayen, P. and Sarkar, K.R., Cytomixis in maize haploids, Indian J. Genet. Plant Breed., 1996, vol. 56, no. 1, pp. 79–85.
Ghaffari, S.M., Occurrence of diploid and polyploid microspores in Sorghum bicolor (Poaceae) is the result of cytomixis, Afr. J. Biotech., 2006, vol. 5, pp. 1450–1453. https://doi.org/10.5897/AJB06.338
Grishanin, A.K., Shekhovtsov, S.V., Boykova, T.V., Akifyev, A.P., and Zhimulev, I.F., The problem of chromatin diminution at the border of the XX and XXI centuries, Cytology, 2006, vol. 48, no. 5, pp. 379–397. pmid: 16892848
Guan, J.Z., Wang, J.J., Cheng, Z.H., Liu, Y., and Li, Z.Y., Cytomixis and meiotic abnormalities during micro-sporogenesis are responsible for male sterility and chromosome variations in Houttuynia cordata, Genet. Mol. Res., 2012, vol. 11, pp. 121–130. https://doi.org/10.4238/2012.January.17.2
Hizume, M., Sato, S., and Tanaka, A., A highly reproducible method of nucleolus organizer regions staining in plants, Stain Technol., 1980, vol. 55, pp. 87–90. pmid: 6157230
Jelesko, J.G., Harper, R., Furuya, M., and Gruissem, W., Rare germinal unequal crossing-over leading to recombinant gene formation and gene duplication in Arabidopsis thaliana, Proc. Natl. Acad. Sci. U. S. A., 1999, vol. 18, pp. 10302–10307. https://doi.org/10.1073/pnas.96.18.10302
Kravets, E.A., Cellular and tissue mechanisms of recovery processes in Hordeum distichum L. under irradiation, Cytol. Genet., 2009, vol. 43, no. 1, pp. 9–17. https://doi.org/10.3103/S0095452709010022
Kravets, E.A., Nature, significance, and cytological consequences of cytomixis, Cytol. Genet., 2012, vol. 46, no. 3, pp. 188–195. https://doi.org/10.3103/S0095452712030061
Kravets, E.A., Cytomixis and its role in the regulation of plant fertility, Russ. J. Dev. Biol., 2013, vol. 44, no. 3, pp. 113–128. https://doi.org/10.1134/s1062360413030028
Kravets, E.A., Cytomixis as a primary form of sexual process, Adv. Cytol. Pathol., 2018, vol. 3, no. 5, pp. 88–91. https://doi.org/10.15406/acp.2018.03.00059
Kravets, E.A., Mykheyev, A.N., Ovsyannikova, L.G., and Grodzynsky, D.M., Critical level of radiation damage of root apical meristem and mechanisms for its recovery in Pisum sativum L., Cytol. Genet., 2011, vol. 45, no. 1, pp. 18–26. https://doi.org/10.3103/S0095452711010051
Kravets, E.A., Berezhnaya, V.V., Sakada, V.I., Rashydov, N.M., and Grodzinsky, D.M., Structural architectonics of the root apical meristem in connection with quantitative evaluation of its radiation damage, Cytol. Genet., 2012, vol. 46, no. 2, pp. 63–73. https://doi.org/10.3103/S0095452712020016
Kravets, E.A., Sidorchuk, Yu.V., Horyunova, I.I., Plohovskaya, S.H., Mursalimov, S.R., Deineko, E.V., Yemets, A.I., and Blume, Ya.B., Intra- and intertissular cytomictic interactions in the microsporogenesis of mono- and dicotyledonous plants, Cytol. Genet., 2016, vol. 50, no. 5, pp. 267–277. https://doi.org/10.3103/s0095452716050054
Kravets, E.A., Yemets, A.I., and Blume, Ya.B., Cellular mechanisms of nuclear migration, Cytol. Genet., 2017, vol. 51, no. 3, pp. 192–201. https://doi.org/10.3103/S0095452717030069
Kravets, E.A., Yemets, A.I., and Blume, Ya.B., Cytoskeleton and nucleoskeleton involvement in processes of cytomixis in plants, Cell Biol. Int., 2019, vol. 43, no. 9, pp. 999–1009. https://doi.org/10.1002/cbin.10842
Kumar, P. and Singhal, V.K., Male meiosis, morphometric analysis and distribution pattern of 2x and 4x cytotypes of Ranunculus hirtellus Royle (Ranunculaceae) from the cold regions of Northwest Himalayas (India), Comp. Cytogenet., 2011, vol. 5, pp. 143–161. https://doi.org/10.3897/CompCytogen.v5i3.1359
Kumar, G. and Chaudhary, N., Secondary chromosomal association in kidney bean (Phaseolus vulgaris L.), Jordan J. Biol. Sci., 2014, vol. 7, no. 1, pp. 71–74. https://doi.org/10.12816/0008217
Kumar, G. and Singh, S., Enigmatic phenomenon of secondary association among bivalents in Guar (Cyamopsis tetragonoloba (L.) Taub.), Cytol. Genet., 2018, vol. 52, no. 6, pp. 478–483. https://doi.org/10.3103/S0095452718060075
Kumar, G. and Singh, S., Induced cytomictic crosstalk behaviour among micro-meiocytes of Cyamopsis tetragonoloba (L.) Taub. (cluster bean): reasons and repercussions, Caryologia, 2020, vol. 73, no. 2, pp. 111–119.https://doi.org/10.13128/caryologia-544
Lattoo, S.K., Khan, S., Bamotra, S., and Dhar, A.K., Cytomixis impairs meiosis and influences reproductive success in Chlorophytum comosum (Thunb) Jacq.—an additional strategy and possible implications, J. Biosci., 2006, vol. 31, pp. 629–637. https://doi.org/10.1007/BF02708415
Malallah, G.A. and Attia, T.A., Cytomixis and its possible evolutionary role in a Kuwaiti population of Diplotaxis harra (Brassicaceae), Bot. J. Linn. Soc., 2003, vol. 143, pp. 169–175. https://doi.org/10.1046/j.1095-8339.2003.00218.x
Malgwi, M.M., Oyewole, S.O., and Khan, A.U., Chromosomes and secondary associations in tetraploid Cleome polyanthera L., Nucleus, 1997, vol. 40, pp. 20–25.
Mandal, A. and Datta, A.K., Secondary chromosome associations and cytomixis in Corchorus spp., Cytologia, 2011, vol. 76, no. 3, pp. 337–343. https://doi.org/10.1508/cytologia.76.337
Mandal, A., Datta, A.K., Gupta, S., et al., Cytomixis—a unique phenomenon in animal and plant, Protoplasma, 2013, vol. 250, no. 5, pp. 985–996. https://doi.org/10.1007/s00709-013-0493-z
Mursalimov, S. and Deineko, E., Cytomixis in plants: facts and doubts, Protoplasma, 2017, vol. 255, no. 3, pp. 719–731. https://doi.org/10.1007/s00709-017-1188-7
Mursalimov, S., Sidorchuk, Yu., and Deineko, E., New insights into cytomixis: specific cellular features and prevalence in higher plants, Planta, 2013, vol. 238, no. 3, pp. 415–423. https://doi.org/10.1007/s00425-013-1914-0
Mursalimov, S.R., Sidorchuk, Y.V., and Deineko, E.V., Analysis of cytoskeleton in the cells involved in cytomixis: the migrated chromatin displays an MT-organizing activity and can interact with the spindle, Biologia, 2019, vol. 74, no. 5, pp. 555–562. https://doi.org/10.2478/s11756-019-00203-4
Pierre, M.O. and de Sousa, S.M., Citomixia em plantas: causas, mecanismos e consequencias, R. Bras. Biosci., 2011, vol. 9, pp. 231–240.
Reis, A.C., Sousa, S.M., and Viccini, L.F., High frequency of cytomixis observed at zygotene in tetraploid Lippia alba, Plant Syst. Evol., 2015, vol. 302, no. 1, pp. 121–127. https://doi.org/10.1007/s00606-015-1249-3
Sapre, A.B. and Deshpande, D.S., A change in chromosome number due to cytomixis in an interspecific hybrid of Coix L., Cytologia, 1987, vol. 52, pp. 167–174. https://doi.org/10.1508/cytologia.52.167
Sidorchuk, Yu.V., Kravets, E.A., Mursalimov, S.R., Plokhovskaya, S.G., Goryunova, I.I., Emets, A.I., Blume, Y.B., and Deineko, E.V., Efficiency of the induction of cytomixis in the microsporogenesis of dicotyledonous (N. tabacum L.) and monocotyledonous (H. distichum L.) plants by thermal stress, Russ. J. Dev. Biol., 2016, vol. 47, no. 6, pp. 335–347. https://doi.org/10.1134/s1062360413030028
Singhal, V.K. and Kumar, P., Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeate Royle), J. Biosci., 2008, vol. 33, pp. 371–380. https://doi.org/10.1007/s12038-008-0057-0
Singhal, V.K., Rana, P.K., Kumar, P., and Kaur, D., Persistent occurrence of meiotic abnormalities in a new hexaploid cytotype of Thalictrum foetidum from Indian cold deserts, Biologia, 2011, vol. 66, pp. 58–464. https://doi.org/10.2478/s11756-011-0033-2
Streit, A., Silencing by throwing away: a role for chromatin diminution, Dev. Cell, 2012, vol. 23, no. 5, pp. 918–919. https://doi.org/10.1016/j.devcel.2012.10.022
Wu, W., Zheng, Y.L., Yang, R.W., Chen, L., et al., Variation of the chromosome number and cytomixis of Houttuynia cordata from China, J. Syst. Evol., 2003, vol. 41, pp. 245–257.
Zheng, G.C., Yang, Q.R., and Zheng, Y.R., The relationship between cytomixis and chromosome mutation and karyotype evolution in lily, Caryologia, 1987, vol. 40, pp. 243–259. https://doi.org/10.1080/00087114.1987.10797827
Zickler, D. and Kleckner, N., Recombination, pairing, and synapsis of homologs during meiosis, Cold Spring Harb. Perspect. Biol., 2015. https://doi.org/10.1101/cshperspect.a016626
Funding
This work was not funded from any sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies using humans and animals as objects of study.
Additional information
Translated by K. Lazarev
About this article
Cite this article
Kravets, E.A., Plohovskaya, S.H., Horyunova, I.I. et al. Sources of Chromosomal Polymorphism of Microsporocytes in Species of Lilium L. and Allium L.: Cytomixis, Extra Chromosomes, and Chromatin Diminution. Cytol. Genet. 55, 107–116 (2021). https://doi.org/10.3103/S0095452721020080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721020080