Abstract
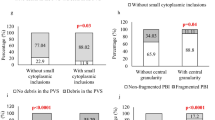
The expedience of using oocytes with morphological dysmorphism in assisted reproduction technology programs is debatable. This study aimed to estimate morphological and molecular cytogenetic characteristics of enlarged oocytes and to predict results of their further fertilization. Among 774 oocytes retrieved from 80 patients, 83 (11%) cells were enlarged. Morphometric analysis showed the diameter of the ooplasm to be (176.67 ± 1.76) μm and the diameter with ZP (200.8 ± 4.24) μm. Among these giant oocytes, 47 (56.6%) were at the metaphase II stage, 26 (31.3%) at the metaphase I stage, and 10 (12.1%) at the prophase I stage. The retrieved giant oocytes were characterized by dysmorphism of endo- and exocytoplasmic structures. Among 47 cells at the metaphase II stage, 40 (85.1%) oocytes had two polar bodies, three (6.4%) oocytes had one polar body, and four (8.5%) oocytes had a fragmented polar body. The assessment of the presence of meiotic spindle by polarization microscopy showed that 39 (83%) oocytes had two meiotic spindles, 3 (6.4%) oocytes had one meiotic spindle, and 5 oocytes (10.6%) had no visualized meiotic spindles despite the presence of polar bodies. After fertilization of 47 oocytes at the metaphase II stage, pronuclei were detected in 42 (89.4%) cells, including 9 (21.4%) oocytes with two pronuclei, 27 (63.3%) oocytes with 3 pronuclei, and 6 (15.3%) oocytes with four pronuclei. The molecular cytogenetic analysis demonstrated that the embryos obtained after fertilization of giant oocytes had a polyploid chromosome set number. Thus, the results of our study showed the inexpediency of using giant oocytes for further fertilization.



Similar content being viewed by others
REFERENCES
Almeida, P.A. and Bolton, V.N., Immaturity and chromosomal abnormalities in oocytes that fail to develop pronuclei following insemination in vitro, Hum. Reprod., 1993, vol. 8, no. 2, pp. 229–232. doihttps://doi.org/10.1093/oxfordjournals.humrep.a138028
Balaban, B. and Urman, B., Effect of oocyte morphology on embryo development and implantation, Reprod. Biomed. Online, 2006, vol. 12, no. 5, pp. 608–615. https://doi.org/10.1016/s1472-6483(10)61187-x
Balakier, H., Bouman, D., Sojecki, A., et al., Morphological and cytogenetic analysis of human giant oocytes and giant embryos, Hum. Reprod., 2002, vol. 17, no. 9, pp. 2394–2401. https://doi.org/10.1093/humrep/17.9.2394
Bilinski, S.M., Kloc, M., and Tworzydlo, W., Selection of mitochondria in female germline cells: is Balbiani body implicated in this process?, J. Assist. Reprod. Genet., 2017, vol. 34, no. 11, pp. 1405–1412. https://doi.org/10.1007/s10815-017-1006-3
Bosch, E., Labarta, E., Kolibianakis, E., et al., Super-ovulation induced changes of lipid metabolism in ovaries and embryos and its probable mechanism, PLoS One, 2015, vol. 10, no. 7. e0132638. https://doi.org/10.1371/journal.pone.0132638
Brunet, S. and Verlhac, M.H., Positioning to get out of meiosis: the asymmetry of division, Hum. Reprod. Update, 2011, vol. 17, no. 1, pp. 68–75. https://doi.org/10.1093/humupd/dmq044
Buderatska, N.O. and Petrushko, M.P., Variability of morphological parameters as a prognostic criterion of human oocyte cryopreservation, Morphologia, 2017, vol. 10, no. 4, pp. 18– 22. https://doi.org/10.26641/1997-9665.2016.4.18-22
Buderatska, N., Gontar, J., Ilyin, I., et al., Does human oocyte cryopreservation affect equally on embryo chromosome aneuploidy?, Cryobiology, 2020, vol. 93, pp. 33–36. https://doi.org/10.1016/j.cryobiol.2020.03.002
Burkhardt, S., Borsos, M., Szydlowska, A., et al., Chromosome cohesion established by rec8-cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice, Curr. Biol., 2016, vol. 26, no. 5, pp. 678–685. https://doi.org/10.1016/j.cub.2015.12.073
Conti, M. and Franciosi, F., Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events, Hum. Reprod. Update, 2018, vol. 24, no. 3, pp. 245–266. https://doi.org/10.1093/humupd/dmx040
Delhanty, J.D., SenGupta, S.B., and Ghevaria, H., How common is germinal mosaicism that leads to pre-meiotic aneuploidy in the female?, J. Assist. Reprod. Genet., 2019, vol. 36, no. 12, pp. 2403–2418. https://doi.org/10.1007/s10815-019-01596-6
Egozcue, S., Blanco, J., Vidal, F., et al., Diploid sperm and the origin of triploidy, Hum. Reprod., 2002, vol. 17, no. 1, pp. 5–7. https://doi.org/10.1093/humrep/17.1.5
Elkouby, Y.M., Jamieson-Lucy, A., and Mullins, M.C., Oocyte polarization is coupled to the chromosomal bouquet, a conserved polarized nuclear configuration in meiosis, PLoS Biol., 2016, vol. 14, no. 1. e1002335. https://doi.org/10.1371/journal.pbio.1002335
Escobar-Aguirre, M., Zhang, H., Jamieson-Lucy, A., et al., Microtubule–actin crosslinking factor 1 (Macf1) domain function in Balbiani body dissociation and nuclear positioning, PLoS Genet., 2017, vol. 13, no. 9. e1006983. https://doi.org/10.1371/journal.pgen.1006983
Garcia-Cruz, R., Brieco, M.A., Roig, I., et al., Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes, Hum. Reprod., 2010, vol. 25, no. 9, pp. 2316–2327. https://doi.org/10.1093/humrep/deq180
Heim, A.E., Hartung, O., Rothhamel, S., et al., Oocyte polarity requires a Bucky ball-dependent feedback amplification loop, Development, 2014, vol. 141, no. 4, pp. 842–854. https://doi.org/10.1242/dev.090449
Lehner, A., Kaszas, Z., Murber, A., et al., Giant oocytes in human in vitro fertilization treatments, Arch. Gynecol. Obstet., 2015, vol. 292, no. 3, pp. 697–703. https://doi.org/10.1007/s00404-015-3679-0
Machtinger, R., Politch, J.A., Hornstein, M.D., et al., A giant oocyte in a cohort of retrieved oocytes: does it have any effect on the in vitro fertilization cycle outcome?, Fertil. Steril., 2011, vol. 95, no. 2, pp. 573–576. https://doi.org/10.1016/j.fertnstert.2010.06.037
Mahadevan, M.M., Fleetham, J., Long-Simpson, L., et al., Recovery of a preovulatory binucleate oocyte in a patient following induction of ovulation for in vitro fertilization, J. In Vitro Fert. Embryo Transf., 1988, vol. 5, no. 5, pp. 299–300. https://doi.org/10.1007/BF01132182
Martin, R.H., Meiotic errors in human oogenesis and spermatogenesis, Reprod. Biomed. Online, 2008, vol. 16, no. 4, pp. 523–531. https://doi.org/10.1016/s1472-6483(10)60459-2
McFadden, D.E., Jiang, R., and Langlois, S., Dispermy—origin of diandric triploidy: brief communication, Hum. Reprod., 2002, vol. 17, no. 12, pp. 3037–3038. https://doi.org/10.1093/hum-rep/17.12.3037
Munné, S., Alikani, M., and Cohen, J., Monospermic polyploidy and atypical embryo morphology, Hum. Reprod., 1994, vol. 9, no. 3, pp. 506–510. https://doi.org/10.1093/oxfordjournals.humrep.a138536
Pampalona, J., Frías, C., Genesca, A., et al., Progressive telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells, PLoS Genet., 2012, vol. 8, no. 4. e1002679. https://doi.org/10.1371/journal.pgen.1002679
Pellestor, F., Andréo, B., Arnal, F., et al., Mechanisms of non-disjunction in human female meiosis: the coexistence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes, Hum. Reprod., 2002, vol. 17, no. 8, pp. 2134–2145. https://doi.org/10.1093/humrep/17.8.2134
Petrushko, M.P., Yurchuk, T.O., and Buderatska, N.O., Oolemma invagination of fresh and cryopreserved human oocytes during in vitro fertilization by ICSI, Probl. Cryobiol. Cryomed., 2018, vol. 28, no. 3, pp. 258–265. https://doi.org/10.15407/cryo28.03.258
Reader, K.L., Stanton, J.L., and Juengel, J.L., The role of oocyte organelles in determining developmental competence, Biology (Basel), 2017, vol. 6, no. 3, p. 35. https://doi.org/10.3390/biology6030035
Rienzi, L., Balaban, B., Ebner, T., et al., The oocyte, Hum. Reprod., 2012, vol. 27, suppl. 1, pp. i2–i21. https://doi.org/10.1093/hum-rep/des200
Roeles, J. and Tsiavaliaris, G., Actin-microtubule interplay coordinates spindle assembly in human oocytes, Nat. Commun., 2019, vol. 10, no. 1, p. 4651. https://doi.org/10.1038/s41467-019-12674-9
Rosenbusch, B., Mechanisms giving rise to triploid zygotes during assisted reproduction, Fertil. Steril., 2008, vol. 90, no. 1, pp. 49–55. https://doi.org/10.1016/j.fertnstert.2007.06.031x
Rosenbusch, B., The potential significance of binovular follicles and binucleate giant oocytes for the development of genetic abnormalities, J. Genet., 2012, vol. 91, no. 3, pp. 397–404. https://doi.org/10.1007/s12041-012-0195-x
Soewarto, D., Schmiady, H., and Eichenlaub-Ritter, U., Consequences of non-extrusion of the first polar body and control of the sequential segregation of homologues and chromatids in mammalian oocytes, Hum. Reprod., 1995, vol. 10, no. 9, pp. 2350–2360. https://doi.org/10.1093/oxford-journals.humrep.a136298
Storchova, Z. and Kuffer, C., The consequences of tetraploidy and aneuploidy, J. Cell Sci., 2008, vol. 121, pt. 23, pp. 3859–3866. https://doi.org/10.1242/jcs.039537
Tsutsumi, M., Fujiwara, R., Nishizawa, H., et al., Age-related decrease of meiotic cohesins in human oocytes, PLoS One, 2014, vol. 9, no. 5. e96710. https://doi.org/10.1371/journal.pone.0096710
Ullah, Z., Lee, C.Y., and Lilly, M.A., Developmentally programmed endoreduplication in animals, Cell Cycle, 2009, vol. 8, no. 10, pp. 1501–1509. https://doi.org/10.4161/cc.8.10.8325
Verlhac, M.H. and Terret, M.E., Oocyte maturation and development, F1000Res, 2016, vol. 5. https://doi.org/10.12688/f1000re-search.7892.1
Wang, L.Y., Wang, N., Le, F., et al., Superovulation induced changes of lipid metabolism in ovaries and embryos and its probable mechanism, PLoS One, 2015, vol. 10, no. 7. e0132638. https://doi.org/10.1371/journal.pone.0132638
Yi, K. and Li, R., Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation, Cytoskeleton (Hoboken), 2012, vol. 69, no. 10, pp. 727–737. https://doi.org/10.1002/cm.21048
Yurchuk, T.O., Buderatska, N.O., Ilyin, I.E., et al., Morphological characteristics of the first polar body of oocytes and results of embryos PGT-A, Morphologia, 2019, vol. 13, no. 4, pp. 50–54. https://doi.org/10.26641/1997-9665.2019.4.50-54
Funding
This work was carried out by the authors without funding from state or nonstate funds or financial institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement of compliance with standards of research involving humans as subjects. All studies were carried out in accordance with the rules of biomedical ethics. To work with oocytes and embryos, written, voluntary, and informed consent of patients was obtained. All manipulations with oocytes and preimplantation embryos were carried out in accordance with the order of the Ministry of Health of Ukraine from September 9, 2013, no. 787 “On Approval of the Procedure for the Use of Assisted Reproductive Technologies in Ukraine” and the European Protocol on Embryo Protection. The research was carried out in accordance with the principles of the Helsinki Declaration of Human Rights, the European Convention of Human Rights and Biomedicine, and the recommendations of ESHRE and ARSM. The studies were approved by the Bioethics Committee of the Institute of Problems of Cryobiology and Cryomedicine of the National Academy of Sciences of Ukraine (minutes no. 7, 2015, and no. 1, 2019).
Additional information
Translated by K. Lazarev
About this article
Cite this article
Petrushko, M.P., Buderatska, N.O., Gontar, J.V. et al. Morphological and Molecular Cytogenetic Characteristics of Giant Human Oocytes. Cytol. Genet. 55, 132–137 (2021). https://doi.org/10.3103/S0095452721020110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721020110