Abstract
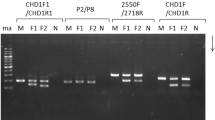
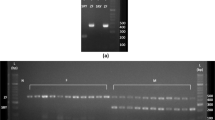
CHD gene polymorphism as a model object for molecular sex determination in Bubo bubo (Eurasian eagle-owl) was studied. Classical PCR methods for amplification of target regions of the CHD gene using P2/P8, 2550F/2718R, and 123L/1272H marker systems and restriction (PCR-RFLP) and heteroduplex methodological approaches were analyzed. It was shown that the use of the P2/P8 marker system within classical PCR is efficient for avian sexing only under electrophoretic separation of the fragments in polyacrylamide gel. The application of 2550F/2718R and 123L/127H markers demonstrated their lack of efficiency both in agarose and polyacrylamide gels because it was not possible to differentiate between genotypes CHDZZ and CHDZW. The presence of a polymorphic restriction site for HaeIII in CHDZ and CHDW fragments amplified with primers P2/P8 was proven. No polymorphic site was detected for DdeI restriction endonuclease. High efficiency of the heteroduplex analysis based on the P2/P8 markers for sexing individuals under electrophoretic separation of the amplified fragments both in agarose and polyacrylamide gels was proven. In case of experimental samples containing CHDZ and CHDW amplicons, additional fragments of heteroduplex DNA were found, the presence of which makes it possible to accurately determine the genotype of an avian.





Similar content being viewed by others
REFERENCES
Barros, T.B., Fraga, R.E., Ramos, C.N., and Tomazi, L., Improvement of the molecular sexing of parrots. in the State of Bahia, Acta Biol. Paran., 2017, vol. 46, nos. 3–4, pp. 89–107. https://doi.org/10.5380/abpr.v46i0.56731
Bermudez-Humaran, L.G., Chavez-Zamarripa, P., Guzman-Velasco, A., Leal-Garza, C.H., and Montes de Oca-Luna, R., Loss of restriction site DdeI, used for avian molecular sexing, in Oreophasis derbianus, Reprod. Domest. Anim., 2002a, vol. 37, pp. 321–323. https://doi.org/10.1046/j.1439-0531.2002.00362.x
Bermudez-Humaran, L.G., Garcia-Garcia, A., Leal-Garza, C.H., Riojas-Valdes, V.M., Jaramillo-Rangel, G., and Montes-de-Oca-Luna, R., Molecular sexing of monomorphic endangered Ara birds, J. Exp. Zool., 2002b, vol. 292, no. 7, pp. 77–80. https://doi.org/10.1002/jez.10070
Cakmak, E., Peksen, C.A., and Bilgin, C.C., Comparison of three different primer sets for sexing birds, J. Vet. Diagn. Invest., 2017, vol. 29, no. 1, pp. 59–63. https://doi.org/10.1177/1040638716675197
Casey, A.E., Jones, K.L., Sandercock, B.K., and Wisely, S.M., Heteroduplex molecules cause sexing errors in a standard molecular protocol for avian sexing, Mol. Ecol. Res., 2009, vol. 9, pp. 61–65. doi . 2008.02307.xhttps://doi.org/10.1111/j.1755-0998
Corters, O., Barroso, A., and Dunner, S., Avian sexing: an optimized protocol using polymerase chain reaction–single-strand conformation polymorphism, J. Vet. Diagn. Invest., 1999, vol. 11, pp. 297–299. 10.1177/10406387990110031
Delgado, M.D. and Penteriani, V., Gender determination of Eurasian Eagle-Owls (Bubo bubo) by morphology, J. Raptor Res., 2004, vol. 38, pp. 375–377.
Ellegren, H., First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds, Proc. Biol. Sci., 1996, vol. 263, no. 1377, pp. 1635–1641. https://doi.org/10.1098/rspb.1996.0239
Fridolfsson, A. and Ellegren, H., A simple and universal method for molecular sexing of non-ratite birds, J. Avian Biol., 1999, vol. 30, pp. 116–121. https://doi.org/10.2307/3677252
Garcia, C.B., Insausti, J.A., Gil, J.A., Frutos, A., Alcantara, M., Gonzalez, J., Cortes, M.R., Bonafonte, J.I., and Arruga, M.V., Comparison of different procedures of DNA analysis for sex identification in the endangered bearded vulture (Gypaetus barbatus), Eur. J. Wild Res., 2009, vol. 55, pp. 309–312. https://doi.org/10.1007/s10344-008-0239-y
Griffiths, R., Daan, S., and Dijkstra, C., Sex identification in birds using two CHD genes, Proc. R. Soc. London B, 1996, vol. 263, pp. 1251–1256. https://doi.org/10.1098/rspb.1996.0184
Griffiths, R., Double, M., Orr, K., and Dawson, R., A DNA test to sex most birds, Mol. Ecol., 1998, vol. 7, pp. 1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Griffiths, R. and Tiwari, B., Sex of the last wild Spix’s macaw, Nature, 1995, vol. 375, p. 454. https://doi.org/10.1038/375454a0
Insee, J., Kamolnorranath, S., Baicharoen, S., Chumpadang, S., Sawasu, W., and Wajjwalku, W., PCR-based method for sex identification of eastern sarus crane (Grus antigone sharpii): implications for reintroduction programs in Thailand, Zool. Sci., 2014, vol. 31, pp. 95–100. https://doi.org/10.2108/zsj.31.9
Kahn, N.W., John, J.S.T., and Quinn, T.W., Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds, Auk, 1998, vol. 115, no. 4, pp. 1074–1078. https://doi.org/10.2307/4089527
Krüger, O., The evolution of reversed sexual size dimorphism in hawks, falcons and owls: a comparative study, Evol. Ecol., 2005, vol. 19, no. 5, pp. 467–486. https://doi.org/10.1007/s10682-005-0293-9
Lee, M.Y., Hong, Y.J., Park, S.K., Kim, Y.J., Choi, T.Y., Lee, H., and Min, M.S., Application of two complementary molecular sexing methods for East Asian bird species, Genes Genom., 2008, vol. 30, no. 4, pp. 365–372.
Mario Leyn-Ortega, M., del Mar Delgado, M., Martinez, J.E., Penteriani, V., and Calvo, J.F., Factors affecting survival in Mediterranean populations of the Eurasian eagle owl, Eur. J. Wild Res., 2016, vol. 62, pp. 643–651. https://doi.org/10.1007/s10344-016-1036-7
Mataragka, A., Balaskas, C., Sotirakoglou, K., and Ikonomopoulos, J., Comparative evaluation of the performance of the PCR assays commonly used for the determination of sex in avian species, J. King Saud. Univ.–Sci., 2020, vol. 32, pp. 228–234. https://doi.org/10.1016/j.jksus.2018.04.020
Medeiros, R.T., Chaves, F.G., Vecchi, M.B., Nogueira, D.M., and Alves, M.A.S., Molecular sexing and inter-sexual differences in the morphometry of the Hang-nest tody-tyrant Hemitriccus nidipendulus (Passeriformes: Rhynchocyclidae), Zoologia, 2019. https://doi.org/10.3897/zoologia.36.e32771
Miyaki, C.Y., Sex identification of parrots, toucans, and curassows by PCR: perspectives for wild and captive population studies, Zoo Biol., 1998, vol. 17, pp. 415–423. https://doi.org/10.1002/(SICI)1098-2361(1998)17:5<415::AID-ZOO6>3.0.CO;2-2
Patino, L., Cruz, M., Martinez, P., and Cedeno-Escobar, V., Using PCR-RFLP for sexing of the endangered Galapagos petrel (Pterodroma phaeopygia), Genet. Mol. Res., 2013, vol. 12, no. 2, pp. 4760–4767. https://doi.org/10.4238/2013.October.18.13
Penteriani, V., Alonso-Alvarez, C., del Mar Delgado, M., Sergio, F., and Ferrer, M., Brightness variability in the white badge of the eagle owl Bubo bubo, J. Avian Biol., 2006, vol. 37, no. 1, pp. 110–116. https://doi.org/10.1111/j.0908-8857.2006.03569.x
Ravindran, S., Saufi, S., Amni, W.N., Ishak, I., Hamid, N.H., Abidin, C.M.R.Z., Ahmad, A.H., Azzam, G., and Salim, H., Sex identification comparison of barn owls (Tyto alba javanica) using morphological features and molecular-based methods, Slovak Raptor J., 2018, vol. 12, pp. 47–54.https://doi.org/10.2478/srj-2018-0005
Ravindran, S., Woo, W.K., Saufi, S., Amni, W.N., Hamid, N.H., Abidin, C.M.R.Z., Ishak, I., Azzam, G., and Salim, H., Molecular sexing of Southeast Asian barn owl, Tyto alba javanica, using blood and feather, Trop. Life Sci. Res., 2019, vol. 30, no. 2, pp. 13–23. https://doi.org/10.21315/tlsr2019.30.2.2
Sacchi, P., Soglia, D., Maione, S., Meneguz, G., Campora, M., and Rasero, R., A non-invasive test for sex identification in short-toed Eagle (Circa etus gallicus), Mol. Cell Prob., 2004, vol. 18, no. 3, pp. 193–196. https://doi.org/10.1016/j.mcp.2004.01.002
Seidensticker, M.T., Holt, D.W., Detienne, J., Talbot, S., and Gray, K., Sexing young snowy owls, J. Raptor Res., 2011, vol. 45, no. 4, pp. 281–289. https://doi.org/10.3356/JRR-11-02.1
Tornberg, R., Mikkola, H., and Rytkönen, S., Morphometric sex determination of Great Grey Owls Strix nebulosa, Ornis Norvegica, 2016, vol. 39, pp. 6–10. https://doi.org/10.15845/on.v39i0.991
Valadan, R., Nejatollahi, F., Ehsaninori, H., Habibi, H., Amini, H., and Aliabadian, M., Avian gametologs as molecular tags for sex identification in birds of prey of Iran, Zoo Biol., 2017, vol. 36, no. 8, pp. 289–293. https://doi.org/10.1002/zoo.21363
Vucicevic, M., Stevanov-Pavlovic, M., Stevanovic, J., Bosnjak, J., Gajic, B., Aleksic, N., and Stanimirovic, Z., Sex determination in 58 bird species and evaluation of CHD gene as a universal molecular marker in bird sexing, Zoo Biol., 2013, vol. 32, pp. 269–276. https://doi.org/10.1002/zoo.21010
Wang, L.C., Severinghaus, L.L., Chen, C.T., Liu, L.Y., Pan, C.H., Huang, D., Lee, H.Y., Lir, J.T., Chin, S.C., and Pu, C.E., Sex identification of owls (family Strigidae) using oligonucleotide microarrays, J. Hered., 2008, vol. 99, no. 2, pp. 187–192. doi.org/https://doi.org/10.1093/jhered/esm107
Wang, P.H., Hsu, H.A., Chao, M.C., Chan, F.T., Wang, L.M., Lin, P.I., Tsao, H.S., Yuan, H.W., Chen, C.C., and Ding, S.T., Sex identification in the Collared Scops Owl (Otus bakkamoena) with novel markers generated by random amplified polymorphic DNA, Conserv. Genet Res., 2013, vol. 5, pp. 239–242. https://doi.org/10.1007/s12686-012-9778-3
Funding
The research was not funded by third-party commercial organizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. Studies were carried out using Bubo bubo feathers as a source of biological material without direct manipulations concerning birds.
Additional information
Translated by K. Lazarev
About this article
Cite this article
Kulibaba, R.O., Liashenko, Y.V. Analysis of CHD Gene Polymorphism as a Model Object for Molecular Sexing of Eurasian Eagle-Owl (Bubo bubo). Cytol. Genet. 55, 324–330 (2021). https://doi.org/10.3103/S0095452721040071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721040071