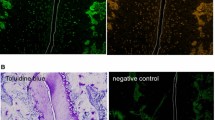
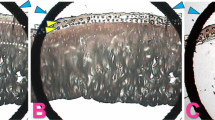
Abstract
Various genes level of activity in monolayer cultures of chondroprogenitor cells and chondrocytes isolated from articular cartilage of genetically modified mice was studied. Materials and methods: Monolayer cultures of chondrocytes and chondroprogenitor cells were subjected to qPCR study to determine the target genes activity: col 2a1, Sox 9, Il 1a, Il 1b, CCL 2, CCL 3, CCL 4, CCL 5, MMP 3, MMP 13 and aggrecan (Aggr). Results and its discussion: It was found that the activity of pro-inflammatory cytokines (Il1b, Il6, Il 8) is higher in chondroprogenitor cells, as well as the activity of metalloproteinases (MMP 3, MMP 13) responsible for the degradation of the matrix. Synthesis of type 2 collagen and agrecan in chondroprogenitor cells is higher than in chondrocytes, this pattern is observed in all strains of the studied animals. There is a high activity of type 2 collagen and aggrecan in both chondroprogenitor cells and chondrocytes of strain 6 isolated from the cartilage tissue, while the observed low activity of pro-inflammatory cytokines and metalloproteinases, which indicates a pronounced ability of this strain mice to repair damaged cartilage tissue.






Similar content being viewed by others
REFERENCES
Bedelbaeva, K., Snyder, A., Gourevitch, D., Clark, L., Zhang, X.M., Leferovich, J., et al., Lack of p21 expression links cell cycle control and appendage regeneration in mice, Proc. Natl. Acad. Sci. U. S. A., 2010, vol. 107, p. 5845e50. https://doi.org/10.1073/pnas.1000830107
Blankenhorn, E.P., Bryan, G., Kossenkov, A.V., Clark, L.D., Zhang, X.M., and Chang, C., Genetic loci that regulate healing and regeneration in LG/J and SM/J mice, Mamm. Genome, 2009, vol. 20, p. 720e33. https://doi.org/10.1007/s00335-009-9216-3
Blasioli, D.J., Kaplan, D.L., The roles of catabolic factors in the development of osteoarthritis, Tissue Eng. Part B Rev., 2014, vol. 20, no. 4, pp. 355–363. https://doi.org/10.1089/ten.teb.2013.0377
Brophy, R.H., Rai, M.F., Zhang, Z., Torgomyan, A., and Sandell, L.J., Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear, J. Bone Joint Surg. Am., 2012, vol. 94, no. 5, pp. 385–393. https://doi.org/10.2106/JBJS.K.00919
Deshpande, B.R., Katz, J.N., Solomon, D.H., Yelin, E.H., Hunter, D.J., Messier, S.P., Suter, L.G., and Losina, E., Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity, Arthritis Care Res. (Hoboken), 2016, vol. 68, p. 1743e50. https://doi.org/10.1002/acr.22897
Ding, C., Cicuttini, F., and Jones, G., How important is MRI for detecting early osteoarthritis?, Nat. Clin. Pract. Rheumatol., 2008, vol. 4, no. 1, pp. 4–5. https://doi.org/10.1038/ncprheum0676
Dowthwaite, G.P., Bishop, J.C., Redman, S.N., Khan, I.M., Rooney, P., et al., The surface of articular cartilage contains a progenitor cell population, J. Cell Sci., 2004, vol. 117, pp. 889–897. https://doi.org/10.1242/jcs.00912
Eltawil, N.M., De Bari, C., Achan, P., Pitzalis, C., and Dell’accio, F., A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury, Osteoarthritis Cartilage, 2009, vol. 17, no. 6, pp. 695–704. https://doi.org/10.1016/j.joca.2008.11.003
Fitzgerald, J., Rich, C., Burkhardt, D., Allen, J., Herzka, A.S., and Little, C.B., Evidence for articular cartilage regeneration in MRL/MpJ mice, Osteoarthritis Cartilage, 2008, vol. 16, p. 1319e26. https://doi.org/10.1016/j.joca.2008.03.014
Goldring, M., Tsuchimochi, K., Ijiri, K., The control of chondrogenesis, J. Cell Biochem., 2006, vol. 97, pp. 33–44. https://doi.org/10.1002/jcb.20652
Hunter, D.J., Snieder, H., March, L., and Sambrook, P.N., Genetic contribution to cartilage volume in women: a classical twin study, Rheumatology (Oxford), 2003, vol. 42, p. 1495e500. https://doi.org/10.1093/rheumatology/keg400
Lories, R.J. and Monteagudo, S., Review article: is Wnt signaling an attractive target for the treatment of osteoarthritis?, Rheumatol. Ther., 2020, vol. 7, no. 2, pp. 259–270. https://doi.org/10.1007/s40744-020-00205-8
Mihanfar, A., Shakouri, S.K., Khadem-Ansari, M.H., Fattahi, A., Latifi, Z., Nejabati, H.R., and Nouri, M., Exosomal miRNAs in osteoarthritis, Mol. Biol. Rep., 2020, vol. 47, no. 6, pp. 4737–4748. https://doi.org/10.1007/s11033-020-05443-1
Nelson, A.E., Osteoarthritis year in review 2017: clinical osteoarthritis and cartilage, 2018, vol. 26, p. 319e325.https://doi.org/10.1016/j.joca.2017.11.014
Rai, M.F., Hashimoto, S., Johnson, E.E., Janiszak, K.L., Fitzgerald, J., Heber-Katz, E., et al., Heritability of articular cartilage regeneration and its association with ear-wound healing, Arthritis Rheumatol., 2012, vol. 64, no. 7, pp. 2300–2310. https://doi.org/10.1002/art.34396
Rice, S.J., Beier, F., Young, D., and Loughlin, J., Interplay between genetics and epigenetics in osteoarthritis, Nat. Rev. Rheumatol., 2020, vol. 16, no. 5, pp. 268–281. https://doi.org/10.1038/s41584-020-0407-3
Torgomyan, A. and Saroyan, M., Molecular mechanisms of chondro- and osteogenesis disturbance in osteoarthritis and ways of their correction, Cytol. Genet., 2020, vol. 54, no. 4, pp. 347–352. ISSN 0095-4527. https://doi.org/10.3103/s0095452720040118
Vasilceac, F.A., Marqueti, R.C., Neto, I.V.S., Nascimento, D.C., Souza, M.C., Durian, J.L.Q., and Mattiello, S.M., Resistance training decreases matrix metalloproteinase-2 activity in quadriceps tendon in a rat model of osteoarthritis, Braz. J. Phys. Ther., 2020, S1413-3555(19)30309-0. https://doi.org/10.1016/j.bjpt.2020.03.002
ACKNOWLEDGMENTS
This study was funded by the Fulbright Program with the support of the Bureau of Educational and Cultural Affairs of the US Department of State and carried out at the University of Washington, USA, and was supported by YSMU; the authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
About this article
Cite this article
Torgomyan, A., Saroyan, M. Inflammatory and Anti-Inflammatory Cytokine Activity in the Cartilage Cells of Genetically Modified Mice. Cytol. Genet. 55, 396–403 (2021). https://doi.org/10.3103/S0095452721040125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721040125