Abstract
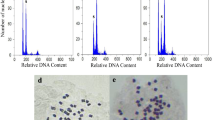
Primary screening of original diploid species and induced autotetraploids after induction of single node cutting of three populations of perennial diploid Medicago sativa ssp. caerulea: (Karaj1, Karaj2 and Tehran) and five annual diploid alfalfa species: (M. lupulina, M. radiata, M. rijidula, M. truncatula, M. turbinata) with three concentrations (0.10, 0.50, 1%) of colchicine counting the chloroplasts number in the stomata guard cells was done. In addition, the ploidy levels of species were determined using usual method of chromosomes counting in the metaphase cells of root tip meristems. Results of ANOVA showed significant effects of species, colchicine concentration and their interaction for survival and polyploidy induction rate (p < 0.01). The highest and lowest survival rate (80.2 and 8.9%) and tetraploidy induction (74.3 and 20.8%) were obtained in 0.10 and 1.0% colchicine concentration, respectively. In comparison, between species, the highest survival and tetraploidy induction rate were observed in M. truncatula (80.2 and 100%) followed by M. lupulina (73.6 and 73.6%), respectively. In contrast, the lowest survival and tetraploidy induction rate were observed in M. turbinata with 34 and 27.4%, respectively. Chloroplasts enumeration in stomata cell guards showed that in the survived species the number of chloroplasts of stomata guard cells were ranged between 8.07 to 9.15 in diploids and between 14.94 to 15.38 in tetraploids from induced diploids and five local tetraploid cultivated alfalfa cultivars, respectively. There was no significant difference between overall means of perennials and annual diploid alfalfa species for survival and tetraploidy induction. Ploidy levels have shown strong relationships with mean numbers of chloroplasts in stomata guard cells. It was concluded that enumeration of stomata guard cells, chloroplasts is an indirect and precise method for evaluating ploidy level in alfalfa.


Similar content being viewed by others
REFERENCES
Cardi, T., Carputo, D., and Frusciante, L., In vitro shoot regeneration and chromosome doubling in 2x and 3x potato clones, Am. Potato J., 1992, vol. 69, pp. 1–12. https://doi.org/10.1007/BF02853404
Callum, J.B., Dixon, R.A., Farmer, A.D., et al., The Medicago Genome Initiative: a model legume database, Nucleic Acids Res., 2001, vol. 29, no. 1, pp. 114–117. https://doi.org/10.1093/nar/29.1.114
Chen, L.L. and Gao, Sh.L., In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus memberanaceus, Sci. Hortic. (Amsterdam), 2007, vol. 112, no. 3, pp. 339–334. https://doi.org/10.1016/j.scienta.2006.12.045
Ghanavati, F. and Nematpajooh, N., Study of ploidy level of annual species of Onobrychis in Iran, Caryologia, 2012, vol. 65, no. 4, pp. 328–334. https://doi.org/10.1080/00087114.2012.760880
Ghanavati, F., Mozafari, J., and Masumi, A.A., Determination of ploidy level with counting the chloroplast number in stomatal guard cells in Medicago sp., Seed Plant J., 2004, vol. 20, pp. 117–127.
Gu, X.F., Yang, A.F., Meng, H., and Zhang, J.R., In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill. cv. Zhanhua, Plant Cell Rep., 2005, vol. 24, pp. 671–676. https://doi.org/10.1007/s00299-005-0017-1
Han, D.S., Niimi, Y., and Nakamo, M., Production of doubled haploid plants through colchicine treatment of anther-derived haploid calli in the Asiatic hybrid lily 'Connecticut King’, 1999, J. Jpn. Soc. Hortic. Sci., vol. 68, pp. 979–983. https://doi.org/10.2503/jjshs.68.979
Hawke, J.C. and Leech, R.M., Acetyl coenzyme A carboxylase in species of Triticum of different ploidy, Planta, 1990, vol. 181, no. 4, pp. 543–546. https://doi.org/10.1007/BF00193008
Koorneef, M., Van Diepen, J.A.M., Hanhart, C.J., et al., Chromosomal instability in cell- and tissue cultures of tomato haploids and diploids, Euphytica, 1989, vol. 43, pp. 179–186. https://doi.org/10.1007/BF00037911
Lapiņa, L., Grauda, D., and Rashal, I., Characterization of Latvian alfalfa Medicago sativa genetic resources, Acta Biol. Univ. Daugavp., 2011, vol. 11, no. 2, pp. 134–140.
Leech, R.M., Observation of the mechanism of chloroplast division in higher plants, New Phytol., 1981, vol. 87, no. 1, pp. 1–9. https://doi.org/10.1111/j.1469-8137.1981.tb01686.x
Malekzadeh Shafaroodi, S.A., Ghani, A.S., Habibi, M.P., and Amiri, A., Evaluation of polyploidy induction in basil (Ocimumbasilicum. L), J. Hortic. Sci., 2011, vol. 25, no. 4, pp. 461–469.
Melnychuk, O.V., Ozheredov, S.P., Rakhmetov, D.B., et al., The technology used for synthetic polyploid production of Miscanthus as cellulosic biofuel feedstock, Open Agric. J., 2020, vol. 14, pp. 164–173. https://doi.org/10.2174/1874331502014010164
Melnychuk, O.V., Ozheredov, S.P., Rakhmetov, D.B., et al., Induction of polyploidy in giant miscanthus (Miscanthus × giganteus Greef et Deu.), Proc. Latv. Acad. Sci., Sect. B, 2020, vol. 74, no. 3, pp. 206–214. https://doi.org/10.2478/prolas-2020-0032
Mirzai NeduShen, H., Annual Medics (Genetics and Breeding), Tehran: Res. Inst. For. Rangel., 2001.
Murray, H. and Standing, L., Genomic constancy during the development of Lathyrus odoratus cultivars, Heredity, 1992, vol. 68, pp. 321–327. https://doi.org/10.1038/hdy.1992.46
Niazian, M. and Nalousi, A.M., Artificial polyploidy induction for improvement of ornamental and medicinal plants, Plant Cell, Tissue Organ Cult., 2020, vol. 142, no. 3, pp. 447–469. https://doi.org/10.1007/s11240-020-01888-1
Omidbaigi, R., Mirzaee, M., Hassani, M.E., and Sedghi Moghadam, M., Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment, 2020, Int. J. Plant Prod., vol. 4, no. 2, pp. 87–98. https://doi.org/10.22069/IJPP.2012.686
Quan, K., Guolu, L., Qigao, G., and Xiaolin, L., Polyploid induction of Arctium lappa by colchicine, Plant Physiol. Commun., 2004, vol. 40, no. 2, pp. 157–158.
Sakiroglu, M., Sherman-Broyles, S., Story, A., Moore, K.J., Doyle, J.J., and Brummer, E.C., Patterns of linkage disequilibrium and association mapping in diploid alfalfa (M. sativa L.), Theor. Appl. Genet., 2012, vol. 125, no. 3, pp. 577–590. https://doi.org/10.1007/s00122-012-1854-2
Sarathum, S., Hegele, M., Tantiviwat, S., and Nanakorn, M., Effect of concentration and duration of colchicine treatment on polyploidy induction in Dendrobium scabrilingue, L., Eur. J. Hortic. Sci., 2010, vol. 75, no. 3, pp. 123–127.
Singsit, C. and Ozias-Akins, P., Rapid estimation of ploidy levels of in vitro regenerated interspecific Arachis hybrids, Euphytica, 1992, vol. 64, pp. 183–188. https://doi.org/10.1007/BF00046047
Sleper, D.A. and Poehlman, J.M., Breeding Field Crops, Oxford: Blackwell, 2006.
Su-Jin, B., Md Mazharul, I., Hong-Yul, K., and Ki-Byung, L., Induction of tetraploidy in watermelon with oryzalin treatments, Hortic. Sci. Technol., 2020, vol. 38, no. 3, pp. 385–393. https://doi.org/10.7235/HORT.20200037
Touchell, D.H., Palmer, I.E., and Ranney, T.G., In vitro ploidy manipulation for crop improvement, Front. Plant Sci., 2020, vol. 11, art. ID 722. https://doi.org/10.3389/fpls.2020.00722
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
About this article
Cite this article
Ansari, E., Khosrowshahli, M., Etminan, A. et al. Polyploidy Induction and Ploidy Level Determination in Annual and Perennial Diploid Medicago Species Using the Enumeration of Chloroplasts of Stomata Guard Cells. Cytol. Genet. 56, 164–171 (2022). https://doi.org/10.3103/S0095452722020025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722020025