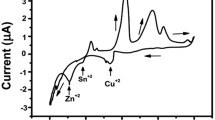
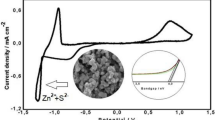
Abstract—
Aspects of electrochemical deposition of zinc sulfide from an aqueous electrolyte based on sodium sulfite and zinc sulfate are addressed, and conditions for electrochemical synthesis of ZnS films are identified. The range of potentials for ZnS deposition is demonstrated to be limited by the potential at which the current reaches the critical value associated with diffusion-limited electrochemical reduction of the sulfite ion. The synthesized films are found to contain excess sulfur, which can be removed by thermal treatment to obtain stoichiometrically correct films. A reaction mechanism leading to the formation of zinc sulfide is proposed.







Similar content being viewed by others
REFERENCES
Motevich, I.G., Strekal’, N.D., Shul’ga, A.V., and Maskevich, S.A., Fluorescent CdSe/ZnS nanoparticles as local pH nanoprobes in cancer diagnostics, Opt. Spektrosk., 2018, vol. 124, no. 5, p. 605. https://doi.org/10.21883/OS.2018.05.45939.277-17
Gurvich, A.M., Vvedenie v fizicheskuyu khimiyu kristallofosforov (Introduction to the Physical Chemistry of Crystal Phosphors), Moscow: Vysshaya shkola, 1971.
Temel, S., Gökmen, F.Ö., and Yaman, E., An energy efficient way to produce zinc-based semiconductor thin films via chemical bath deposition technique, J. Sustainable Dev. Energy Water Environ. Syst., 2019, vol. 7, no. 2, p. 253. https://doi.org/10.13044/j.sdewes.d6.0239
Goudarzi, A., Aval, G.M., Sahraei, R., and Ahmadpoor, H., Ammonia-free chemical bath deposition of nanocrystalline ZnS thin film buffer layer for solar cells, Thin Solid Films, 2008, vol. 516, p. 4953. https://doi.org/10.1016/j.tsf.2007.09.051
Vidal, J., De Melo, O., Vigil, O., Lopez, N., Contreras-Puente, G., and Zelaya-Angel, O., Influence of NH3 concentration and annealing in the properties of chemical bath deposited ZnS films, Thin Solid Films, 2002, vol. 419, no. 2, p. 118. https://doi.org/10.1016/S0254-0584(99)00130-3
Saib, S., Khan, M.A., and Bouarissa, N., Pressure-dependent dynamical properties of Zn-based II–VI semiconductors, Phys. B Condens. Matter, 2012, vol. 407, no. 17, p. 3570. https://doi.org/10.1016/j.physb.2012.05.027
Afifi, H.H., Mahmoud, S.A., and Ashour, A., Structural study of ZnS thin films prepared by spray pyrolysis, Thin Solid Films, 1995, vol. 263, p. 248. https://doi.org/10.1016/S0254-0584(00)00351-5
Elidrissi, B., Addou, M., Regragui, M., Bougrine, A., et al., Structure, composition and optical properties of ZnS thin films prepared by spray pyrolysis, Mater. Chem. Phys., 2001, vol. 68, p. 175.
Cheng, J., Fan, D.B., Wang, H., Liu, B.W., et al., Chemical bath deposition of crystalline ZnS thin films, Semicond. Sci. Tech., 2003, vol. 18, p. 676. https://doi.org/10.1088/0268-1242/18/7/313
Poulomi, R., Jyoti, R.O., and Suneel, K.S., Crystalline ZnS thin films by chemical bath deposition method and its characterization, Thin Solid Films, 2006, vol. 515, no. 4, p. 1912. https://doi.org/10.1016/j.tsf.2006.07.035
Ben Nasr, T., Kamoun, N., Kanzari, M., and Bennaceur, R., Effect of pH on the properties of ZnS thin films grown, Thin Solid Films, 2006, vol. 500, nos. 1–2, p. 4. https://doi.org/10.1016/j.tsf.2005.11.030
Shan, R., Yi, J., Zhong, J., and Yang, S., Effect of sulphur pressure on properties of ZnS thin film prepared by chemical bath deposition technique, J. Mater. Sci. Mater. Electron., 2019, vol. 30, no. 14, p. 13230. https://doi.org/10.1007/s10854-019-01686-2
Ichino, K., Ueyama, K., Yamamoto, M., Kariya, H., et al., High temperature growth of ZnS and ZnMgS by molecular beam epitaxy under high sulfur beam pressure, J. Appl. Phys., 2000, vol. 87, p. 4249. https://doi.org/10.1063/1.373061
Demidenko, I.V. and Ishimov, V.M., Electrodeposition of thin cadmium sulfide films from Na2SO3-based electrolyte, Russ. J. Appl. Chem., 2017, vol. 90, no. 8, p. 1225. https://doi.org/10.1134/S1070427217080055
Zarebska, K. and Skompska, M., Electrodeposition of CdS from acidic aqueous thiosulfate solution—investigation of the mechanism by electrochemical quartz microbalance technique, Electrochim. Acta, 2011, vol. 56, p. 5731. https://doi.org/10.1016/j.electacta.2011.04.046
Spravochnik po elektrokhimii (Reference Book on Electrochemistry), Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Kul’man, A.G., Obshchaya khimiya (General Chemistry), Moscow: Kolos, 1979, 3rd ed.
Tsitovich, I.K., Kurs analiticheskoi khimii (Analytical Chemistry Course), Moscow: Vysshaya shkola, 1968, 2nd ed.
Charlot, G., Les méthodes de la chimie analytique, Paris: Masson, 1961.
Skorchelletti, V.V., Teoreticheskaya elektrokhimiya (Theoretical Electrochemistry), Leningrad: Goskhimizdat, 1963, 2nd ed.
Gurevich, Yu.Ya. and Pleskov, Yu.V., Fotoelektrokhimiya poluprovodnikov (Photoelectrochemistry of Semiconductors), Moscow: Nauka, 1983.
ACKNOWLEDGMENTS
We are grateful to Prof. A.I. Dikusar for helpful discussion of the results of study and to L. Dermedzhi for elemental analysis of the prepared coatings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Kukharuk
About this article
Cite this article
Demidenko, I.V., Ishimov, V.M. Electrochemical Deposition of Zinc Sulfide from a Na2SO3-Based Electrolyte. Surf. Engin. Appl.Electrochem. 58, 109–115 (2022). https://doi.org/10.3103/S1068375522020028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375522020028