Mindfulness Prevents Depression and Psychopathology in Elderly People with Mild to Moderate Alzheimer’s Disease: A Randomized Clinical Trial
Abstract
Background:
This longitudinal study addressed whether mindfulness practice prevents psychological and behavioral symptoms, especially mood disorders, in Alzheimer’s disease (AD).
Objective:
To assess the incidence of depression in the course of AD and to determine which non-pharmacological treatment (NPT) is most effective in preventing psychopathological symptoms.
Methods:
We conducted a longitudinal, non-inferiority and equivalence randomized clinical trial, repeated-measures design, with a control group and three experimental treatments: mindfulness, cognitive stimulation, and relaxation. Each experimental group performed three weekly sessions for two years. The pharmacological treatment of all participants was donepezil (10 mg). Participants were patients with probable AD without diagnosed depression from the public neurology services of the Canary Health Service, Spain. Psychological evaluation was performed using the Geriatric Depression Scale (GDS), Hamilton Depression Rating Scale (HDRS), and Neuropsychiatric Inventory (NPI-Q). The statistical analysis included only patients who attended at least 75% of the sessions. A nonparametric, repeated-measures analysis was performed with Kruskal-Wallis H test and between-group differences with Mann-Whitney U test with Bonferroni correction (p < 0.008). Effect size was calculated with partial eta-squared.
Results:
The results showed significant differences with large effect sizes (η2p>0.14) between mindfulness and the rest of the experimental groups as well as the control in the GDS, HDRS, and NPI-Q scales.
Conclusion:
Compared to the other experimental groups, only mindfulness prevented the onset of depression and other psychopathologies in early-stage AD. Based on its effectiveness in maintaining cognitive functions and preventing psychopathology, we recommend mindfulness as the first-choice NPT for mild to moderate AD.
INTRODUCTION
The impact of a diagnosis of Alzheimer’s disease (AD) is one of the most devastating stressful life events at the end of life. Depending on the premorbid psychopathological situation of patients, especially that related to mood disorders, comorbid disorders that accelerate cognitive, functional, and structural deterioration in frontotemporal brain areas are to be expected [1–3].
Different epidemiological studies have shown that the prevalence of AD is estimated to be between 5% and 8% of the world population over 65 years of age [4, 5]. These data are relevant, as approximately 5 million people suffer from this pathology worldwide, with an estimated 130 million people by 2050 [4]. In terms of incidence, all studies show that AD experiences an exponential growth from the age of 65 onwards. Recent reviews show an incidence rate of 17.1/1000 per year [5].
The prevalence rate of depression in AD ranges from 38% to 50% [6, 7]. These data are highly relevant, as approximately 2.5 million people may have comorbid depression at different stages of AD. Recent incidence studies show a comorbidity at 12-month follow-up of 13% [8]. On the other hand, prevalence studies in mild to moderate stages show an oscillation between 14% and 40% [9]. This variability in the data is mainly due to the underdiagnosis of major depressive disorder in AD [10], as there is an overlap of depressive symptomatology with that of AD. In this context, a mindfulness-based treatment was proposed as a preventive method to delay the onset of symptoms related to mood disorders in elderly people with AD.
Currently, the accepted definition of depression is that established by consensus by the WHO in ICD-11 [11] or the APA in DSM-5 [12]. However, in neither classification are there age subtypes, and the manifestation of depression during aging is not well defined. Depression in the elderly is an example of a non-specific and atypical manifestation that is underdiagnosed, since many physical symptoms can be the cause of it, such as pain, insomnia, constipation, diarrhea, paresthesia, etc. [13].
There is consensus in considering depression as a risk factor for AD [14]. Over the course of the latter, depressive symptoms manifest more frequently than in the general elderly population, doubling the prevalence of these symptoms [13]. It is important to note that the prevalence of depression increases in the mild-moderate stages of the disease, decreasing subsequently as depressive symptoms are diluted among the severity of the manifestations of AD itself [15].
The cognitive processes altered in the course of a mood disorder are attention, perception, processing speed, memory, and learning ability [14]. Thus, we can see how the neurodegenerative process itself shares the same cognitive impairment processes as AD. However, depression in AD can be characterized mainly by the following symptoms: “depressed mood, anhedonia, social withdrawal, decreased appetite, psychomotor disturbances, fatigue or lack of energy, feelings of worthlessness, suicidal ideation and a desire to die” [13].
In current neuroscientific research, mindfulness has been defined as the awareness that arises from paying attention in an intentional and nonjudgmental way to direct experience as it is in the present moment [16]. There are different proposals based on mindfulness to treat elderly people with depression that have shown a benefit on the reduction of symptomatology [17–23]. The mindfulness-based stress reduction (MBSR) program, the most widely used to reduce stress through mindfulness practice, has been adapted to elderly people with dementia, showing its usefulness in reducing behavioral disorders in residential settings [24, 25]. These programs are usually of short duration, usually 8 weeks, so longitudinal data are not available. However, the mindfulness-based Alzheimer’s stimulation (MBAS) program, used in this randomized clinical trial (RCT), is a program designed for three weekly sessions over 2 years [26].
The overall objective of this longitudinal study was to explore whether a mindfulness-based intervention can be effective as a treatment to prevent the onset of psychopathological symptomatology in mild or moderate stage AD, especially the comorbidity of depression. For this purpose, we conducted an equivalence or non-inferiority RCT with the usual non-pharmacological treatment (NPT) in AD, namely cognitive stimulation therapy (CST), as well as a progressive muscle relaxation (PMR) group.
The hypotheses from which we have started to test the efficacy of the treatment have been that the combined treatment based on mindfulness (MBAS) with donepezil will be more effective than:
1. Pharmacological treatment with donepezil without NPT (control group).
2. Cognitive stimulation (CST) with donepezil.
3. Progressive muscle relaxation (PMR) with donepezil.
MATERIALS AND METHODS
Design
A randomized, controlled, repeated-measures, double-blind, non-inferiority and equivalence, four groups parallel design clinical trial (4×5) was conducted in the Canary Islands, Spain. The RCT was approved by the ethics committee of La Laguna University (CEIBA2013-0079). To assess its non-inferiority or equivalence, the MBAS [26] program was compared to the most used NPTs, namely CST [27] and PMR [28], as well as to a usual caretaking-at-home group (control group). All patients took donepezil (10 mg) during the RCT. The main characteristics of the trial, inclusion and exclusion criteria, participants, randomization, non-pharmacological treatments, and blinding following the guidelines of Boutron et al. [29] can be found in our previous RCT publication [26].
Neuropsychological assessment scales
Behavioral and psychological symptoms of dementia (BPSD) were assessed by means of the Geriatric Depression Scale (GDS) [30], Hamilton Depression Rating Scale (HDRS) [31], and the Neuropsychiatric Inventory (NPI-Q) [32]. In all these scales, an increase in the score indicates a greater degree of psychopathology or behavioral disorders. The NPI-Q has a range of scores between 0 and 120, the GDS from 0 to 15, and the HDRS from 0 to 52 points.
Statistical analysis
Nonparametric statistical analyses have been performed because there are two types of data with the three scales used. On the one hand, we have continuous data for the depression and BPSD measures and, on the other hand, we have ordinal data for the responses to each of the items of the HDRS and the NPI-Q. These data are relevant because they show qualitatively the efficacy of NPTs on specific psychopathological symptoms. All analyses were performed using SPSS 22.
To analyze the differences between the NPTs, an analysis was performed for several independent samples using the Kruskal-Wallis H test. This test allows us to resolve experimental situations similar to those studied with a completely randomized one-factor ANOVA. The fundamental advantage of this test over the F statistic of the one-factor ANOVA is twofold: 1) it does not require assumptions about the original population, and 2) it allows us to work with ordinal data, namely the analysis used for the items of the HDRS and NPI-Q scales.
The Kruskal-Wallis H statistic allows us to obtain a general probability of the existence of differences between the different treatment groups. However, it does not establish between which groups the difference occurs: to perform this analysis, we used the Mann-Whitney U test for two independent samples. In this case, we decided to apply the Bonferroni statistical correction to control the error rate. Since we have four groups, three experimental and one control, we need to make six two-by-two comparisons: 1) MBAS versus PMR, 2) MBAS versus CST, 3) MBAS versus control, 4) PMR versus CST, 5) PMR versus control, and 6) CST versus control. The application of the Bonferroni correction leads to decisions being based on a significance level of 0.05/6 = 0.008. In other words, two groups are considered to differ significantly from each other when the critical level obtained is less than 0.008; when the score is less than 0.05, it is simply a statistical trend.
Finally, eta-squared (η2p), a parametric effect size measure, was used to match a subgroup of patients with only mild-moderate AD.
RESULTS
The results showed that the mean of all the scores remained stable, below the cut-off points that would indicate a comorbid psychopathological state, as shown in Table 1. Considering the cut-off points established for each of the scales, Fig. 1 shows the number of subjects who presented depression scores throughout the treatment for each of the interventions, with MBAS presenting the lowest number of subjects with depression. Overall, the incidence of depression in the sample was 6.2% at 6 months, 7.5% at 12 months, 9.2% at 18 months, and 15.2% at 24 months.
Table 1
Descriptive statistics for the assessment of depressive symptoms
| Psychopathological Assessment | MBAS | PMR | CST | CONTROL | |
| MEAN (±SD) | MEAN (±SD) | MEAN (±SD) | MEAN (±SD) | ||
| GDS (Cut point 11) | Baseline | 2.68 (3.32) | 3.16 (3.76) | 2.70 (3.58) | 3.08 (3.48) |
| 6 months | 2.92 (3.62) | 4.47 (4.07) | 5.63 (3.77) | 5.50 (5.12) | |
| 12 months | 2.84 (2.91) | 6.22 (3.10) | 6.07 (3.55) | 6.46 (4.16) | |
| 18 months | 2.22 (2.47) | 6.44 (2.18) | 6.37 (1.67) | 6.75 ((3.21) | |
| 24 months | 1.83 (2.47) | 7.19 (2.99) | 6.11 (2.34) | 7.38 (1.95) | |
| HDRS (Cut point 14) | Baseline | 5.32 (7.66) | 6.25 (7.08) | 5.59 (8.31) | 5.88 (8.27) |
| 6 months | 5.68 (6.09) | 7.94 (5.79) | 9.85 (6.02) | 9.92 (8.45) | |
| 12 months | 5.54 (4.38) | 10.84 (5.44) | 10.70 (5.99) | 10.75 (6.63) | |
| 18 months | 5.24 (3.77) | 10.94 (4.85) | 11.26 (4.05) | 11.79 (5.66) | |
| 24 months | 5.83 (3.41) | 12.44 (5.84) | 11.00 (4.61) | 13.00 (4.33) | |
| NPI-Q (Cut point 60) | Baseline | 12.19 (18.96) | 11.81 (15.52) | 13.93 (21.13) | 15.17 (22.40) |
| 6 months | 10.81 (15.52) | 13.19 (13.71) | 21.15 (17.26) | 20.83 (22.85) | |
| 12 months | 8.97 (11.51) | 19.16 (14.43) | 23.44 (16.23) | 22.46 (17.74) | |
| 18 months | 8.62 (9.00) | 19.25 (12.81) | 24.74 (12.85) | 26.50 (16.84) | |
| 24 months | 9.29 (8.13) | 23.34 (16.16) | 22.48 (14.62) | 29.08 (14.59) | |
MBAS, Mindfulness-based Alzheimer’s Stimulation; CS, Cognitive Stimulation; PMR, Progressive Muscle Relaxation; GDS, Geriatric Depression Scale; HDRS, Hamilton Depression Rating Scale; NPI-Q, Neuropsychiatric Inventory Questionnaire.
Fig. 1
Subjects diagnosed with depression. MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
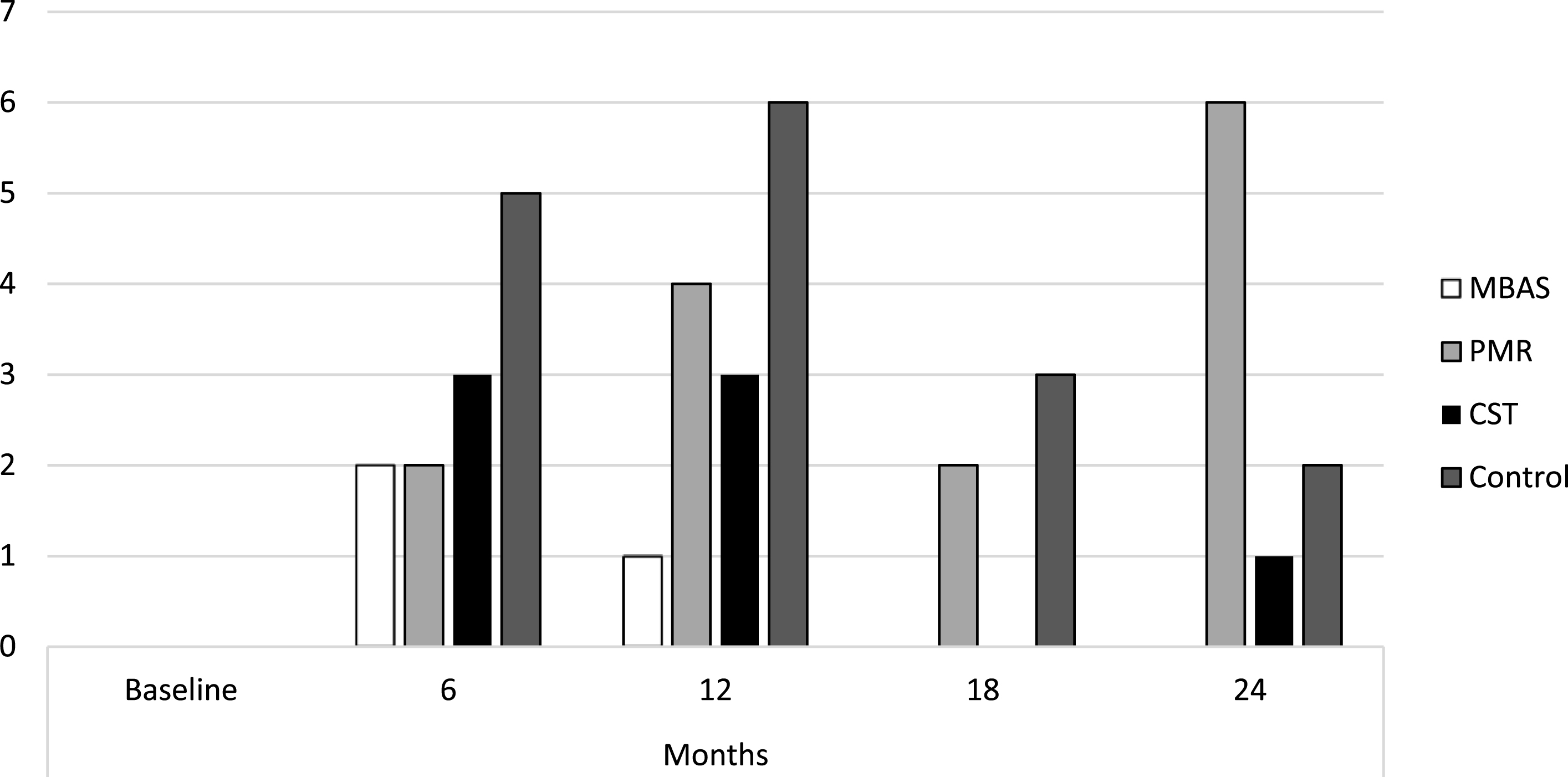
It should be noted that there were no significant differences in the pre-treatment phase (i.e., at baseline) as the absence of psychopathology was an inclusion or exclusion criterion in the RCT [26]. However, there were significant differences with all measures throughout the intervention phase from the first post-treatment measure at 6 months as shown in Table 2.
Table 2
Assessment of depression and psychological and behavioral symptoms
| Psychopathological Assessment | Baseline | 6 months | 12 months | 18 months | 24 months |
| GDS | |||||
| Chi-squared | 1.016 | 8.538 | 23.087 | 45.079 | 49.140 |
| Degree of freedom | 3 | 3 | 3 | 3 | 3 |
| p | 0.797 | 0.036 | 0.000 | 0.000 | 0.000 |
| HDRS | |||||
| Chi-squared | 2.536 | 9.003 | 22.741 | 37.994 | 44.761 |
| Degree of freedom | 3 | 3 | 3 | 3 | 3 |
| p | 0.469 | 0.029 | 0.000 | 0.000 | 0.000 |
| NPI-Q | |||||
| Chi-squared | 1.337 | 8.254 | 20.991 | 31.778 | 31.741 |
| Degree of freedom | 3 | 3 | 3 | 3 | 3 |
| p | 0.720 | 0.041 | 0.000 | 0.000 | 0.000 |
Kruskal-Wallis Statistic (p < 0,05). Grouping variable: treatment group. GDS, Geriatric Depression Scale, HDRS, Hamilton Depression Rating Scale, NPI-Q, Neuropsychiatric Inventory Questionnaire.
Evolution of GDS scores
Table 3 shows the significant differences and large effect sizes (i.e., η2p>0.14) between MBAS and the rest of the experimental groups and the control, with no significant differences between the rest of the experimental groups and the control, nor among themselves.
Table 3
Comparison for treatment group pairs in GDS
| Treatment group | Baseline | 6 months | 12 months | 18 months | 24 months | Effect Size |
| MBAS versus PMR | η2p | |||||
| U de Mann-Whitney | 521.000 | 445.000 | 264.500 | 137.500 | 95.500 | 0.304 |
| Z | –0.689 | –1.637 | –3.850 | –5.434 | –5.862 | |
| p | 0.491 | 0.102 | 0.000 | 0.000 | 0.000 | |
| MBAS versus CST | η2p | |||||
| U Mann-Whitney | 479.500 | 284.00 | 229.000 | 104.50 | 125.00 | 0.311 |
| Z | –0.023 | –2.816 | –3.558 | –5.336 | –4.978 | |
| p | 0.981 | 0.005 | 0.000 | 0.000 | 0.000 | |
| MBAS versus Control | η2p | |||||
| U Mann-Whitney | 432.50 | 346.500 | 209.500 | 125.000 | 85.000 | 0.326 |
| Z | –0.727 | –1.958 | –3.843 | –4.909 | –5.399 | |
| p | 0.467 | 0.050 | 0.000 | 0.000 | 0.000 | |
| PMR versus CST | η2p | |||||
| U Mann-Whitney | 361.500 | 356.000 | 383.000 | 370.500 | 336.000 | 0.000 |
| Z | –0.710 | –1.245 | –0.323 | –0.534 | –1.130 | |
| p | 0.478 | 0.213 | 0.747 | 0.593 | 0.258 | |
| PMR versus Control | η2p | |||||
| U Mann-Whitney | 402.000 | 362.000 | 357.500 | 323.500 | 371.500 | 0.004 |
| Z | –0.017 | –0.669 | –0.737 | –1.069 | –0.272 | |
| p | 0.987 | 0.504 | 0.461 | 0.2850 | 0.272 | |
| CST versus Control | η2p | |||||
| U de Mann-Whitney | 301.500 | 324.000 | 320.500 | 286.500 | 251.500 | 0.007 |
| Z | –0.722 | –0.259 | –0.323 | –0.739 | –1.457 | |
| p | 0.471 | 0.796 | 0.747 | 0.460 | 0.145 |
Mann-Whitney Statistic with Bonferroni statistical correction (p < 0,008). MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
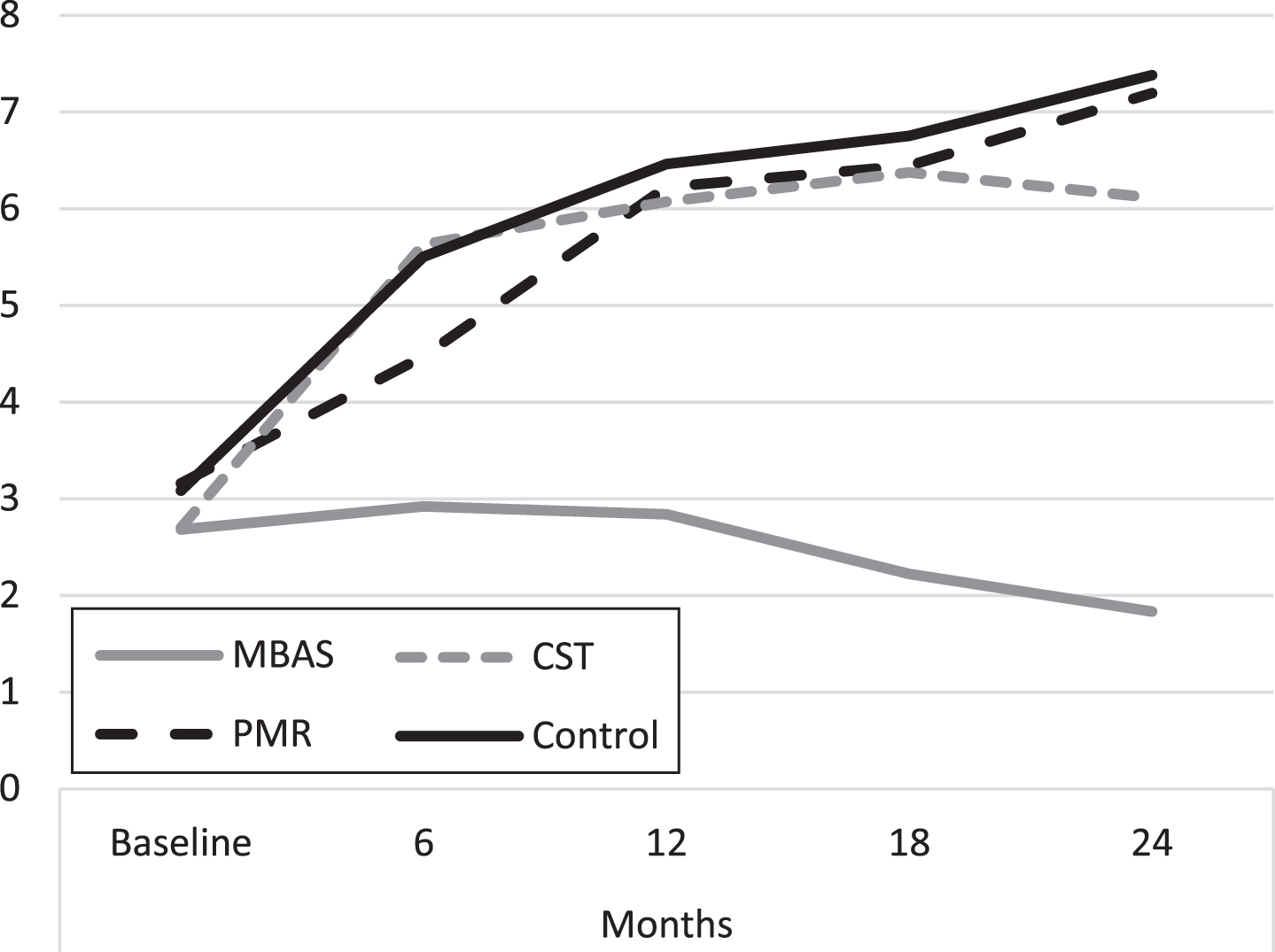
MBAS presented better scores than CST from 6 months to the end of the study and than PMR and the control from 12 months. Figure 2 shows the average evolution of the evaluations throughout the study. It is important to highlight that the PMR, CST, and control groups present a progressive increase in the scores throughout the study, whereas the tendency of the scores of the MBAS group is to remain constant.
Fig. 2
Evolution of depression scores (GDS). MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
Evolution of HDRS scores
As can be seen in Table 4, there are only significant differences and large effect sizes between MBAS and the rest of the groups, with no significant differences among the rest of the groups throughout the study, as in the case of the previous scale, the GDS. Figure 3 shows the evolution of the mean score throughout the study, along the same lines as the previous GDS scale.
Table 4
Comparison for treatment group pairs in HDRS
| Treatment group | Baseline | 6 months | 12 months | 18 months | 24 months | Effect Size |
| MBAS versus PMR | η2p | |||||
| U de Mann-Whitney | 469.500 | 417.500 | 256.000 | 182.000 | 117.000 | 0.208 |
| Z | –1.376 | –1.936 | –3.945 | –4.890 | –5.497 | |
| p | 0.169 | 0.053 | 0.000 | 0.000 | 0.000 | |
| MBAS versus CST | η2p | |||||
| U Mann-Whitney | 481.000 | 288.000 | 230.500 | 132.000 | 153.500 | 0.223 |
| Z | 0.001 | –2.722 | –3.523 | –4.907 | –4.418 | |
| p | 0.999 | 0.006 | 0.000 | 0.000 | 0.000 | |
| MBAS versus Control | η2p | |||||
| U Mann-Whitney | 462.500 | 339.500 | 217.000 | 147.500 | 68.500 | 0.230 |
| Z | –0.299 | –2.000 | –3.716 | –4.5560 | –5.557 | |
| p | 0.765 | 0.045 | 0.000 | 0.000 | 0.000 | |
| PMR versus CST | η2p | |||||
| U Mann-Whitney | 329.500 | 324.500 | 388.500 | 356.000 | 352.000 | 0.000 |
| Z | –1.261 | –1.263 | –0.234 | –0.762 | –0.822 | |
| p | 0.207 | 0.207 | 0.815 | 0.446 | 0.411 | |
| PMR versus Control | η2p | |||||
| U Mann-Whitney | 346.000 | 364.500 | 372.500 | 327.000 | 351.000 | 0.003 |
| Z | –0.971 | –0.619 | –0.492 | –1.011 | –0.604 | |
| p | 0.332 | 0.536 | 0.623 | 0.312 | 0.546 | |
| CST versus Control | η2p | |||||
| U de Mann-Whitney | 325.000 | 320.500 | 326.500 | 298.000 | 2456.000 | 0.003 |
| Z | –0.280 | –0.322 | –0.212 | –0.512 | –1.496 | |
| p | 0.779 | 0.748 | 0.832 | 0.609 | 0.135 |
Mann-Whitney Statistic with Bonferroni statistical correction (p < 0,008). MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
Fig. 3
Evolution of depression scores (HDRS). MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
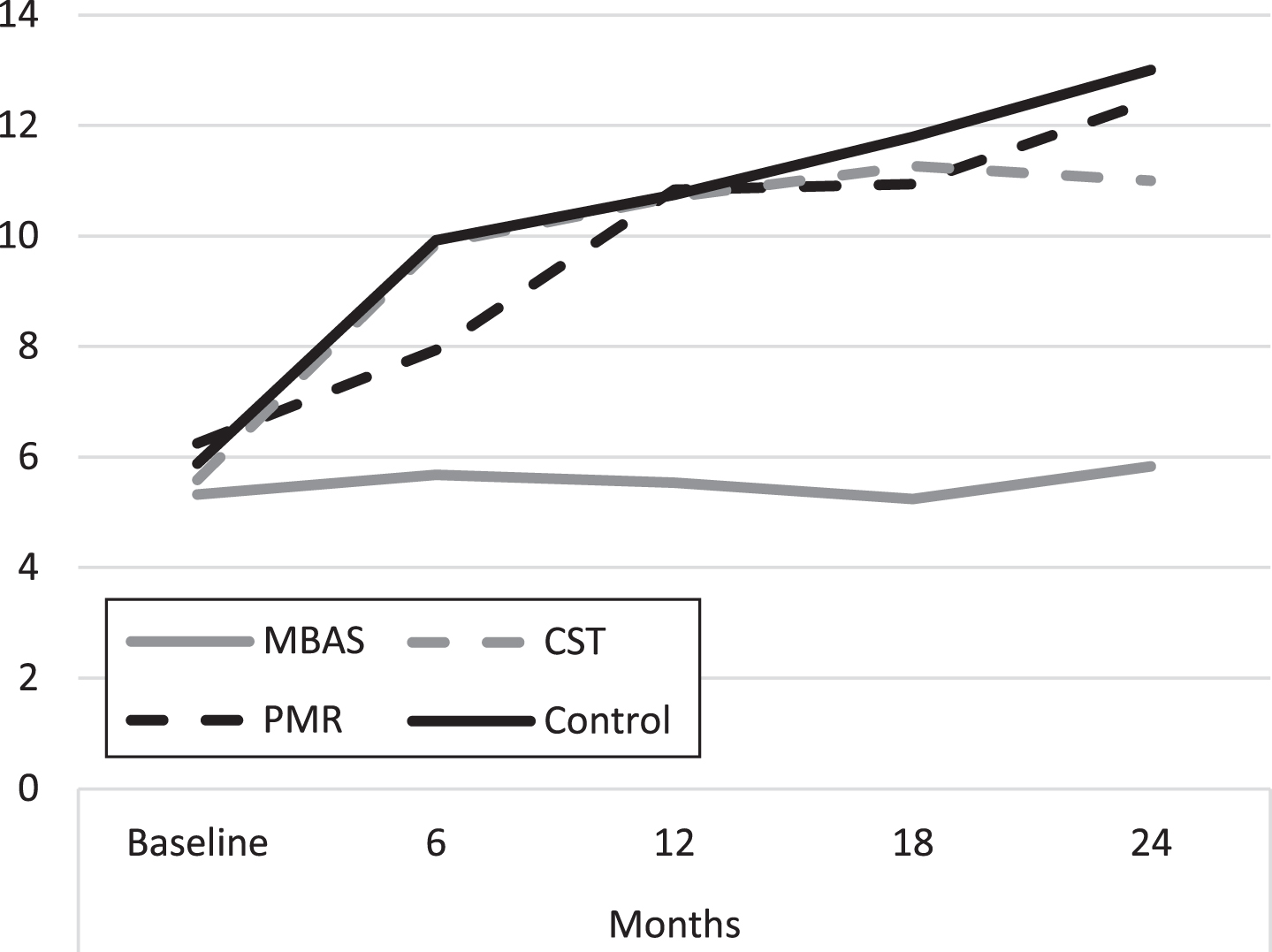
Fig. 4
Evolution of behavioral and psychological symptoms of dementia (NPI-Q). MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
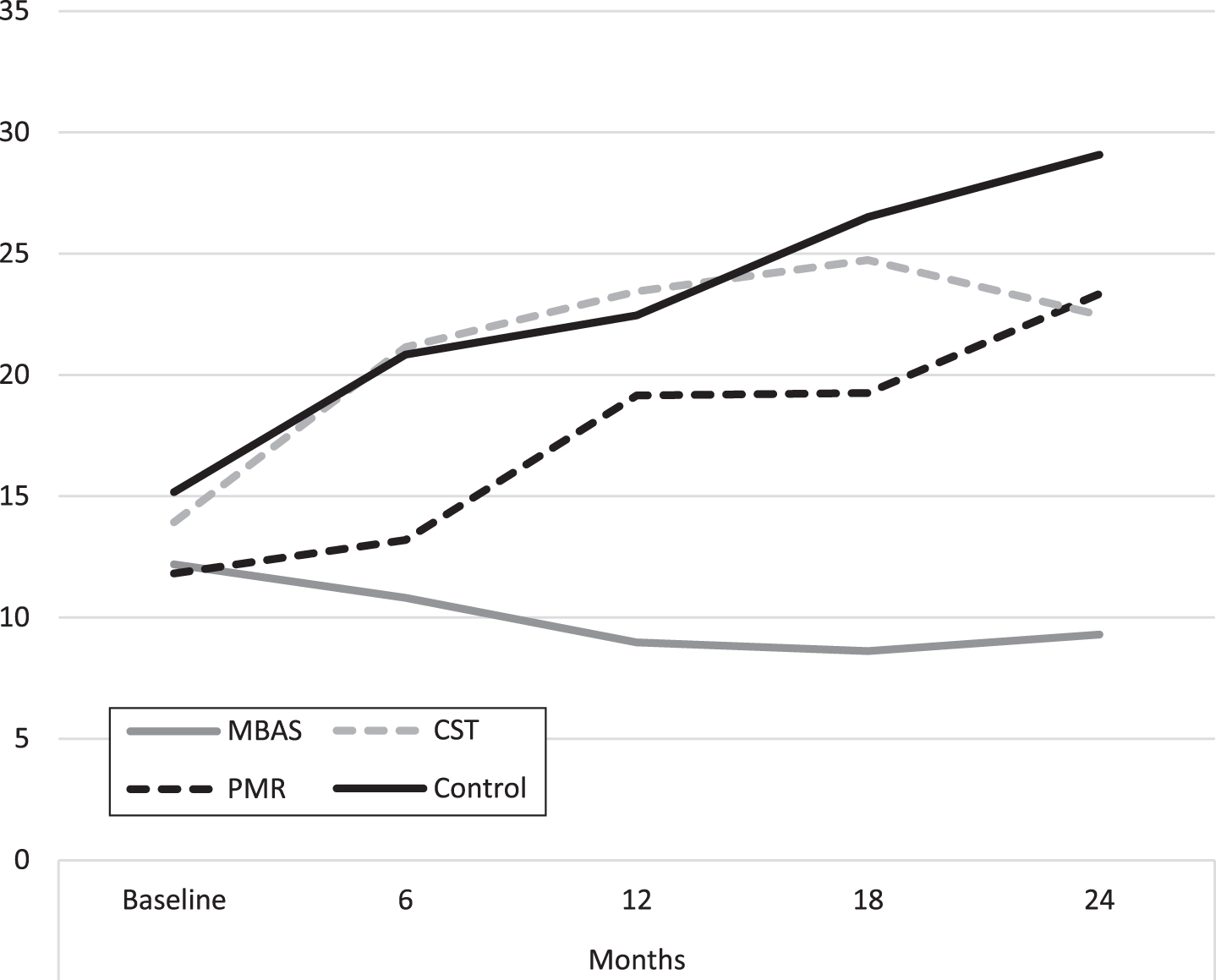
HDRS scores qualitatively show that MBAS presented lower scores throughout the study, keeping these scores in a range of values considered as normal. The rest of the groups increased their scores, passing through a mild depressive stage until they developed a depressive disorder in 15.2% of the cases.
Nonparametric analysis of each of the HDRS items shows that MBAS outperforms the other NPTs on the following items:
1. Depressed Mood: differences with PMR (Z = –2.472, p = 0.013) from 18 months onwards, with CST (Z = –3.041, p = 0.002) and control (Z = –3.404, p = 0.001) from 6 months.
2. Insomnia, early in the night: differences with PMR (Z = –2.824, p = 0.009) and with CST (Z = –3.711 p = 0.000) from 6 months, and from 12 months with control (Z = –3.420, p = 0.001).
3. Insomnia, middle of the night: differences with PMR (Z = –2.596, p = 0.009), and CST (Z = –2.209 p = 0.027) from 6 months, and with control (Z = –2.592, p = 0.010) from 12 months.
4. Insomnia, early hours of the morning: differences with PMR (Z = –2.710, p = 0.007) and with CST (Z = –2.172, p = 0.030) from 6 months, and with control (Z = –2.092, p = 0.036) from 12 months.
5. Agitation: differences with PMR (Z = –3.326, p = 0.001) at 24 months, with CST (Z = –3.793, p = 0.000) and control (Z = –2.968, p = 0.003) from 12 months.
6. Anxiety Psychic: differences with PMR (Z = –2.817, p = 0.005) and control (Z = –2.354, p = 0.019) from 6 months, and with CST (Z = –3.913, p = 0.000) from 12 months.
7. Anxiety Somatic: differences with CST (Z = –2.788, p = 0.005) from 6 months, and with PMR (Z = –3.331, p = 0.001) and control (Z = –3.516, p = 0.000) from 12 months.
8. Somatic symptoms gastrointestinal: differences with CST (Z = –2.366, p = 0.018) and control (Z = –2.366, p = 0.018) from 12 months, and with PMR (Z = –2.104, p = 0.035) from 18 months.
9. General somatic symptoms: differences with PMR (Z = –2.515, p = 0.012), CST (Z = –2.820, p = 0.005) and control (Z = –2.891, p = 0.004) from 12 months.
10. Loss of weight according to measurements: differences with CST (Z = –2.564, p = 0.010) and control (Z = –3.166, p = 0.002) from 12 months.
11. Insight: differences with CST (Z = –2.562, p = 0.010) and control (Z = –2.401, p = 0.016) from 18 months.
Evolution of NPI-Q scores
Lastly, the results of the NPI are shown, where not only results associated with mood, but also psychological and behavioral symptoms associated with AD and the degree of discomfort for the caregiver are assessed. Table 5 also shows that there are only significant differences with large effect sizes between MBAS and the rest of the NPTs and the control group. There is also a significant difference at 18 months between PMR and control, but this is not maintained in the evaluation at 24 months. There are also no significant differences between the rest of the groups throughout the study.
Table 5
Comparison for treatment group pairs in NPI-Q
| Treatment group | Baseline | 6 months | 12 months | 18 months | 24 months | Effect Size |
| MBAS versus PMR | η2p | |||||
| U de Mann-Whitney | 496.000 | 467.500 | 314.500 | 300.000 | 205.500 | 0.120 |
| Z | –1.020 | –1.373 | –3.222 | –3.391 | –4.340 | |
| p | 0.308 | 0.170 | 0.001 | 0.001 | 0.00 | |
| MBAS versus CST | η2p | |||||
| U Mann-Whitney | 474.000 | 298.000 | 210.000 | 156.000 | 188.000 | 0.218 |
| Z | –0.113 | –2.686 | –3.839 | –4.574 | –3.912 | |
| p | 0.910 | 0.007 | 0.000 | 0.000 | 0.000 | |
| MBAS versus Control | η2p | |||||
| U Mann-Whitney | 451.500 | 359.000 | 235.000 | 156.000 | 124.000 | 0.226 |
| Z | –0.472 | –1.845 | –3.493 | –4.450 | –4.719 | |
| p | 0.637 | 0.065 | 0.000 | 0.000 | 0.000 | |
| PMR versus CST | η2p | |||||
| U Mann-Whitney | 352.500 | 289.500 | 319.000 | 281.500 | 392.500 | 0.020 |
| Z | –0.862 | –1.837 | –1.347 | –1.949 | –0.168 | |
| p | 0.389 | 0.066 | 0.178 | 0.051 | 0.866 | |
| PMR versus Control | η2p | |||||
| U Mann-Whitney | 371.000 | 358.500 | 345.000 | 256.000 | 308.000 | 0.034 |
| Z | –0.542 | –0.729 | –0.930 | –2.170 | –1.311 | |
| p | 0.588 | 0.466 | 0.352 | 0.030 | 0.190 | |
| CST versus Control | η2p | |||||
| U de Mann-Whitney | 319.500 | 318.500 | 331.000 | 287.500 | 249.000 | 0.003 |
| Z | –0.393 | –0.364 | –0.128 | –0.708 | –1.434 | |
| p | 0.694 | 0.716 | 0.898 | 0.479 | 0.152 |
Mann-Whitney Statistic with Bonferroni statistical correction (p < 0,008). MBAS, Mindfulness based Alzheimer Stimulation; PMR, Progressive Muscle Relaxation; CST, Cognitive Stimulation Therapy.
The scores shown in each of the items qualitatively show that the mindfulness group presented lower scores throughout the study, keeping these scores at normal values. The rest of the groups increased their scores, producing the behavioral and psychological disorders characteristic of the disease. Therefore, it can be affirmed that mindfulness-based practice seems to favor the prevention of the onset of psychological and behavioral symptoms in mild or moderate AD in our study.
Nonparametric analysis of each of the NPI-Q items shows that MBAS outperforms the other NPTs on the following items:
1. Delusions: differences with PMR (Z = –2.517, p = 0.012) from 18 months, with CST (Z = –2.221, p = 0.026) from 12 months and control (Z = –2.180, p = 0.029) from 6 months.
2. Agitation/aggression: differences with PMR (Z = –2.927, p = 0.003) only at 24 months, while with CST (Z = –3.456 p = 0.001) and control (Z = –3.409, p = 0.001) from 12 months.
3. Depression/Dysphoria: differences with PMR (Z = –2.495, p = 0.013) from 18 months, with CST (Z = –2.519 p = 0.012) from 12 months and with control (Z = –2.651, p = 0.008) from 6 months.
4. Anxiety: differences with PMR (Z = –2.712, p = 0.007), CST (Z = –3.422 p = 0.001) and control (Z = –3.468 p = 0.001) from 12 months.
5. Apathy/Indifference: difference with control (Z = –3.250, p = 0.001) at 18 months.
6. Irritability/Lability: differences with PMR (Z = –2.712, p = 0.007) from 6 months, with CST (Z = –3.755 p = 0.000) from 12 months and with control (Z = –3.056 p = 0.002) from 18 months.
7. Night-time Behaviors: differences with PMR (Z = –2.480, p = 0.013), CST (Z = –2.582 p = 0.010) and control (Z = –2.707 p = 0.007) from 12 months.
8. Appetite/Eating: differences with PMR (Z = –2.880, p = 0.004), CST (Z = –2.593 p = 0.010) and control (Z = –3.527 p = 0.000) from 12 months.
DISCUSSION
The treatment of depression and BPSD in AD remains one of the greatest challenges in clinical practice to delay the functional deterioration of patients and avoid institutionalization. In this RCT, comorbidity was 15.2%, similar to the rate found in other incidence studies [8].
Traditionally, the main approach to depression in this disease is pharmacological treatment with antidepressants, although there are insufficient data to justify this and research needs to improve, especially in depression comorbid with AD [7]. However, there is some consensus suggesting that the main approach in AD should start with a NPT [15].
In this study, a non-pharmacological treatment based on CST was included because there is a widespread emphasis on training these abilities to improve the overall condition of patients, including psychopathology. However, this study shows that despite maintaining cognitive abilities in at least 2 years of follow-up [26], CST is not sufficient to prevent comorbidity with depression and BPSD, as shown by the data presented. These data are in line with other studies, because despite improving cognition, CST does not improve depressive symptomatology [33, 34]. Therefore, a non-pharmacological treatment that specifically addresses the prevention of depression and psychopathology is necessary. In this sense, functional stimulation in daily activities based on mindfulness may be a solution, as it favors the prevention of psychopathology as also shown in this study.
Intervention on mood disorders, anxiety, or other BPSD in mild or moderate AD should be approached with a treatment that improves coping strategies to stabilize psychopathology and prevent cognitive symptoms due to depression from increasing cognitive impairment. In this regard, mindfulness-based interventions in older people with AD are proving to be an effective treatment for depression [17, 21–24]. Therefore, the longitudinal findings of this study reinforce the need to include continued mindfulness-based treatment as a way to prevent or manage the presence of psychopathology.
MBAS can be considered as a preventive tool in the onset of depressive symptomatology in the first place and, in the second place, as a treatment for the control of anxiety, insomnia, agitation, and somatic symptoms. MBAS would be in the same line of action as the mindfulness-based cognitive therapy (MBCT) for depression developed especially for relapse and suicide prevention [3].
Finally, we note that the data from this study corroborate the competence-environmental pressure interaction model, which holds that the ability of an elderly person to interact competently with the environment decreases as functionality is lost [35]. Functionality decreases in AD patients in the following order: first, advanced daily living activities such as working, followed by instrumental ones such as shopping, and finally basic ones such as eating or showering. It is important to highlight this aspect for at least two reasons: the first is that it is usually assumed that training cognition improves functionality and thus improves the ability to cope with environmental demands; the second is that the family tends to reduce the demands on advanced and instrumental activities of daily living, limiting the patient’s development.
This study showed that the stimulation of cognitive abilities did not prevent psychopathology, as the CST group evolved negatively, worsening its psychopathological situation throughout the two-year treatment phase of the RCT. This aspect is especially relevant in the case of the CST group, because although cognitive abilities are better maintained than with the control group [26], scores in the psychopathological area worsen in general. Therefore, it is necessary to address all mental processes involved in the psychopathology of elderly people with AD and not only to attribute the changes to the loss of cognitive abilities.
Likewise, this study has also shown that the psychopathological situation of the PMR and control groups progressively worsens over the two-year treatment phase of the RCT, both displaying significant differences with MBAS.
The main objective of this work was to show that a psychological treatment program based on mindfulness, from the joint attention approach [36], is useful to prevent the onset of comorbid psychopathology throughout the course of mild-moderate AD. Whereas some studies have shown that mindfulness-based programs improve depressive symptoms [17–23] and others based on MBSR decrease BPSD in advanced stages [24, 25], this is the first study to show that mindfulness practice in a population of AD patients without psychopathology at baseline prevents the onset of BPSD, especially anxiety and depression.
It is important to highlight that participants in the mindfulness group have maintained stable scores on the depression and BPSD scales during the two years of the intervention. Furthermore, compared to the rest of the participants, the mindfulness training program in daily activities MBAS shows better overall scores from 12 months and in some cases from 6 months, especially in the areas related to depression, anxiety, and sleep disorders. This is very relevant, since the rest of the experimental groups or the control group treated only with donepezil do not show a maintenance of scores in depression measured with GDS or HDRS or in BPSD measured with the NPI-Q.
Therefore, the first conclusion that can be derived from this research work is that mindfulness-based training can be proposed as an adjuvant to prevent psychopathology in AD.
The second conclusion is that mindfulness training is particularly effective in preventing the onset of depressive symptomatology during two years of intervention. Importantly, this shows that continued mindfulness practice can slow the mood-associated cognitive decline that occurs in the early stages of AD and, therefore, mindfulness can potentially slow cognitive decline that is not directly related to neurodegeneration but to depression. For this reason, the course of cognitive decline in the MBAS group is insidious, maintaining a degeneration curve with little cognitive loss [26].
The third conclusion is the main novelty of this study, the application of mindfulness jointly with caregivers in the development of activities of daily living, based on the paradigm of joint attention [36]. Joint attention is the essential condition on which communication and cognitive development are built. The perceptual experience changes when attending to an object alone versus when attending jointly with another person. In this case, in addition to the object, the existence of the other person attending jointly becomes a constituent element of the experience. This aspect is important because the patient does not feel judged for not responding to a task with an externally determined outcome. Shared mindfulness tasks implicitly involve the development of different cognitive capacities that allow the patient to develop a state of serenity while the practice enables the consolidation of an affective bond with the caregiver.
In summary, this is the first comparative longitudinal study showing that the combination of a mindfulness-based treatment with donepezil is helpful in preventing the onset of psychopathology in the course of AD. It had previously been shown that mindfulness also allows cognitive and functional stability [26], being in this sense equivalent to CST. The equivalence of MBAS to CST in maintaining cognitive function and its superiority in preventing psychopathology lead us to suggest that mindfulness should be considered the NPT of first choice for the treatment of mild to moderate AD. New multicenter RCT studies will be necessary to reproduce these results and validate our conclusion.
Limitations
This RCT was the first study that sought to demonstrate longitudinally and comparatively that mindfulness practice has a benefit as a NPT. However, one of its major limitations lies in the exclusive evaluation with psychometric scales, since it was not possible to use brain imaging markers due to their cost. It is equally important to highlight that the decision to compare mindfulness with other NPTs reduced the number of experimental subjects in each group. Nevertheless, we considered that we had to compare the mindfulness program with other established treatments for this population, since further research on a mindfulness-based treatment for AD is not justified if it does not provide convincing comparative data.
The MBAS program has shown that mindfulness is effective compared to other treatments, and a larger clinical trial with brain imaging measures, biomarkers and new psychometric tests should now be conducted. New data with these clinical follow-up measures could ratify in AD the same results found in populations with depression after applying the MBSR program [37], thus verifying the change or maintenance of brain structure in AD.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0889r1).
REFERENCES
[1] | Loreto F , Fitzgerald A , Golemme M , Gunning S , Win Z , Patel N , Carswell C , Perry R , Kennedy A , Edison P , Malhotra P ((2022) ) Prevalence of depressive symptoms in a memory clinic cohort: A retrospective study. J Alzheimers Dis 88: , 1179–1187. |
[2] | Son JH , Han DH , Min KJ , Kee BS ((2013) ) Correlation between gray matter volume in the temporal lobe and depressive symptoms in patients with Alzheimer’s disease. Neurosci Lett 548: , 15–20. |
[3] | Teasdale JD , Segal ZV , Williams JM , Ridgeway VA , Soulsby JM , Lau MA ((2000) ) Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 68: , 615–623. |
[4] | Garre-Olmo J ((2018) ) [Epidemiology of Alzheimer’s disease and other dementias]. Rev Neurol 66: , 377–386. |
[5] | Lopez OL , Kuller LH ((2019) ) Chapter 9 - Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer’s disease and other dementias. In Handbook of Clinical Neurology, Dekosky ST, Asthana S, eds. Elsevier, pp. 139–148. |
[6] | Leung DKY , Chan WC , Spector A , Wong GHY ((2021) ) Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. Int J Geriatr Psychiatry 36: , 1330–1344. |
[7] | Orgeta V , Tabet N , Nilforooshan R , Howard R ((2017) ) Efficacy of antidepressants for depression in Alzheimer’s disease: Systematic review and meta-analysis. J Alzheimers Dis 58: , 725–733. |
[8] | Ryu S-H , Jung H-Y , Lee KJ , Moon SW , Lee DW , Hong N , Kee BS , Kim DH , Han C , Lee CU ((2017) ) Incidence and course of depression in patients with Alzheimer’s disease. Psychiatry Investig 14: , 271–280. |
[9] | Botto R , Callai N , Cermelli A , Causarano L , Rainero I ((2022) ) Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol Sci 43: , 4107–4124. |
[10] | Starkstein SE , Mizrahi R ((2006) ) Depression in Alzheimer’s disease. Expert Rev Neurother 6: , 887–895. |
[11] | International Classification of Diseases (ICD). WHO, International Statistical Classification of Diseases and Related Health Problems (ICD), World Health Organization, http://www.who.int/classifications/icd/en |
[12] | American Psychiatric Association (2014) DSM-5: Manual diagnóstico y estadístico de los trastornos mentales. |
[13] | Antón-Jiménez M , Gálvez-Sánchez N , Esteban-Sáiz R (2006) Depresión y ansiedad. Sociedad Española de Geriatría y Gerontología (SEGG), Madrid (España), pp 243-249. |
[14] | Wallin K , Boström G , Kivipelto M , Gustafson Y ((2013) ) Risk factors for incident dementia in the very old. Int Psychogeriatr 25: , 1135–1143. |
[15] | Weiner MF , Garrett R , Bret ME (2010) Enfermedad y diagnósico neuropsiquiátrico. Enfermedad de Alzheimer y otras demencias. Panamericana, pp. 39-70. |
[16] | Kabat-Zinn J ((1982) ) An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry 4: , 33–47. |
[17] | Paller KA , Creery JD , Florczak SM , Weintraub S , Mesulam M-M , Reber PJ , Kiragu J , Rooks J , Safron A , Morhardt D , O’Hara M , Gigler KL , Molony JM , Maslar M ((2015) ) Benefits of mindfulness training for patients with progressive cognitive decline and their caregivers. Am J Alzheimers Dis Other Demen 30: , 257–267. |
[18] | Cladder-Micus MB , Speckens AEM , Vrijsen JN , T Donders AR , Becker ES , Spijker J ((2018) ) Mindfulness-based cognitive therapy for patients with chronic, treatment-resistant depression: A pragmatic randomized controlled trial. Depress Anxiety 35: , 914–924. |
[19] | Li SYH , Bressington D ((2019) ) The effects of mindfulness-based stress reduction on depression, anxiety, and stress in older adults: A systematic review and meta-analysis. Int J Ment Health Nurs 28: , 635–656. |
[20] | Lee KC , Tang WK , Bressington D ((2019) ) The experience of mindful yoga for older adults with depression. J Psychiatr Ment Health Nurs 26: , 87–100. |
[21] | Innis AD , Tolea MI , Galvin JE ((2021) ) The effect of baseline patient and caregiver mindfulness on dementia outcomes. J Alzheimers Dis 79: , 1345–1367. |
[22] | Larouche E , Hudon C , Goulet S ((2019) ) Mindfulness mechanisms and psychological effects for aMCI patients: A comparison with psychoeducation. Complement Ther Clin Pract 34: , 93–104. |
[23] | Vespa A , Fabbietti P , Giulietti MV ((2022) ) Study of the effects of mindfulness training on quality of life of patients with Alzheimer’s disease and their caregivers (Dyad Mindfulness Project). Aging Clin Exp Res 34: , 65–71. |
[24] | Lantz MS , Buchalter EN , McBee L ((1997) ) The Wellness Group: A novel intervention for coping with disruptive behavior among [corrected] elderly nursing home residents. Gerontologist 37: , 551–556. |
[25] | McBee L , Westreich L , Likourezos A ((2004) ) A psychoeducational relaxation group for pain and stress management in the nursing home. J Soc Work Long-Term Care 3: , 15–28. |
[26] | Quintana-Hernández DJ , Miró-Barrachina MT , Ibáñez-Fernández IJ , Pino AS-D , Quintana-Montesdeoca MP , Rodríguez-de Vera B , Morales-Casanova D , Pérez-Vieitez MDC , Rodríguez-García J , Bravo-Caraduje N ((2016) ) Mindfulness in the maintenance of cognitive capacities in Alzheimer’s disease: A randomized clinical trial. J Alzheimers Dis 50: , 217–232. |
[27] | Tárraga Mestre L ((1998) ) Terapias blandas: Programa de Psicoestimulación Integral. Alternativa terapéutica para las personas con enfermedad de Alzheimer. Rev Neurol 27: , 51. |
[28] | Amutio A (2002) Estrategias de manejo del estrés: El papel de la relajación. Cuadernos de Medicina Psicosomática y Psiquiatría de Enlace. |
[29] | Boutron I , Altman DG , Moher D , Schulz KF , Ravaud P , CONSORT NPT Group ((2017) ) CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann Intern Med 167: , 40–47. |
[30] | Martí D , Miralles R , Llorach I , García-Palleiro P , Esperanza A , Guillem J , Cervera AM ((2000) ) Trastornos depresivos en una unidad de convalecencia: Experiencia y validación de una versión española de 15 preguntas de la escala de depresión geriátrica de Yesavage. Rev Esp Geriatría Gerontol 35: , 7–14. |
[31] | Bobes J , Bulbena A , Luque A , Dal-Ré R , Ballesteros J , Ibarra N , Grupo de Validacion en Espanol de Escalas Psicometricas ((2003) ) [A comparative psychometric study of the Spanish versions with 6, 17, and 21 items of the Hamilton Depression Rating Scale]. Med Clin (Barc) 120: , 693–700. |
[32] | Boada M , Cejudo JC , Tàrraga L , López OL , Kaufer D ((2002) ) [Neuropsychiatric inventory questionnaire (NPI-Q): Spanish validation of an abridged form of the Neuropsychiatric Inventory (NPI)]. Neurologia 17: , 317–323. |
[33] | Aguirre E , Woods RT , Spector A , Orrell M ((2013) ) Cognitive stimulation for dementia: A systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Res Rev 12: , 253–262. |
[34] | Doshi K , Henderson SL , Fan Q , Wong KF , Lim J ((2021) ) Mindfulness-based training does not improve neuropsychological outcomes in mild cognitive impairment more than spontaneous reversion rates: A randomized controlled trial. J Alzheimers Dis 84: , 449–458. |
[35] | Lawton MP , Nahemow L , Tsong-Min-Yeh null ((1980) ) Neighborhood environment and the wellbeing of older tenants in planned housing. Int J Aging Hum Dev 11: , 211–227. |
[36] | Gabouer A , Bortfeld H ((2021) ) Revisiting how we operationalize joint attention. Infant Behav Dev 63: , 101566. |
[37] | Hölzel BK , Carmody J , Vangel M , Congleton C , Yerramsetti SM , Gard T , Lazar SW ((2011) ) Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res 191: , 36–43. |



