Diencephalic versus Hippocampal Amnesia in Alzheimer’s Disease: The Possible Confabulation-Misidentification Phenotype
Abstract
Background:
Alzheimer’s disease (AD) is clinically heterogeneous, including the classical-amnesic (CA-) phenotype and some variants.
Objective:
We aim to describe a further presentation we (re)named confabulation-misidentification (CM-) phenotype.
Methods:
We performed a retrospective longitudinal case-series study of 17 AD outpatients with the possible CM-phenotype (CM-ADs). Then, in a cross-sectional study, we compared the CM-ADs to a sample of 30 AD patients with the CA-phenotype (CA-ADs). The primary outcome was the frequency of cognitive and behavioral features. Data were analyzed as differences in percentage by non-parametric Chi Square and mean differences by parametric T-test.
Results:
Anterograde amnesia (100%) with early confabulation (88.2%), disorientation (88.2%) and non-infrequently retrograde amnesia (64.7%) associated with reduced insight (88.2%), moderate prefrontal executive impairment (94.1%) and attention deficits (82.3%) dominated the CM-phenotype. Neuropsychiatric features with striking misidentification (52.9%), other less-structured delusions (70.6%), and brief hallucinations (64.7%) were present. Marked behavioral disturbances were present early in some patients and very common at later stages. At the baseline, the CM-ADs showed more confabulation (p < 0.001), temporal disorientation (p < 0.02), misidentification (p = 0.013), other delusions (p = 0.002), and logorrhea (p = 0.004) than the CA-ADs. In addition, more social disinhibition (p = 0.018), reduction of insight (p = 0.029), and hallucination (p = 0.03) persisted at 12 months from baseline. Both the CA- and CM-ADs showed anterior and medial temporal atrophy. Compared to HCs, the CM-ADs showed more right fronto-insular atrophy, while the CA-ADs showed more dorsal parietal, precuneus, and right parietal atrophy.
Conclusion:
We described an AD phenotype resembling diencephalic rather than hippocampal amnesia and overlapping the past-century description of presbyophrenia.
INTRODUCTION
Six years ago, we reported the case of an amnesic patient, FM, diagnosed with Alzheimer’s disease (AD) supported by positive biomarkers in the cerebrospinal fluid (CSF), who presented with a rather unusual neuropsychiatric phenotype [1]. Specifically, early marked spontaneous confabulation associated with anterograde amnesia, executive disturbances, disorientation, mild hyperactivity-disinhibition syndrome, as well as marked misidentification and behavioral disturbances beginning in the intermediate stages, were the salient features of the phenotype [1]. This complex phenotype closely resembled the historical description of presbyophrenia, a well-known dementia syndrome at the beginning of the last century that then however passed into oblivion [2, 3]. This fact, together with the discovery in the literature of other sporadic cases of patients with AD who presented an unusual tendency to confabulation [4, 5], in some cases even early [6], suggested to us the idea that the FM phenotype might be an example of a defined syndrome rather than an idiosyncrasy of a single patient. Thus, we hypothesized the return of presbyophrenia [1]. From our initial observation to the present, we have observed other patients with AD at our Geriatric Unit who consistently presented with an amnestic neuropsychiatric phenotype similar to that of the FM patient. Considering the ancient and psychiatric sound of the term presbyophrenia, we decided to rename this phenotype as the confabulation-misidentification phenotype (CM-phenotype) based on its two most salient features. The discovery of a new AD presentation could have relevant clinical and research implications for early diagnosis, rehabilitation, and selective regional vulnerability in dementia. Bearing in mind this context, the objectives of this study were: 1) to report a detailed description of the possible CM-phenotype; 2) to preliminarily support the hypothesis that the CM-phenotype is an amnesic AD syndrome quite distinct from the classic amnesic presentation.
METHODS
Instruments
Informant reports about cognitive and behavioral status
We collected detailed informant reports about the cognitive and behavioral status at the baseline by interviewing a patient’s relative. We used a semi-structured interview designed by our group which assessed a definite spectrum of cognitive domains and behavioral features. The interview was not a validated tool, but we use it routinely in our Unit in the suspect of cognitive decline. More details about the standard version of the interview we used in this study are in the Supplementary Material.
The standard observational neuropsychological examination
All participants underwent a standard observational neuropsychological examination (NPE) routinely administered before the cognitive testing in our Unit. The NPE is a standardized instrument designed by our group that has received prior validation. It has been presented in more detail in previous articles [7, 8]. In brief, the NPE is a systematic collection of cognitive and behavioral features based on observation of patient behavior during interaction with the clinician, especially, but not exclusively, in the context of the preliminary clinical interview. Indeed, some features (signs in the original article) would emerge primarily during testing (e.g., provoked confabulation, attentive capture). The NPE is based on a semi-structured interview in which a defined list of signs of neuropsychological dysfunction is rated as absent/present based on how they appear (spontaneous or provoked by the questions) to the neuropsychologist observation. The semi-structured interview and the list of signs (features) used in the NPE, as well as further details, are in the Supplementary Material.
The clinical and instrumental workup
Each patient underwent a detailed past medical history and routine blood tests. A group of geriatricians performed the clinical and neurological examinations. Moreover, they evaluated the general cognitive status by the Mini-Mental State Examination (MMSE) [9], the functional autonomy by the Activities of Daily Living (ADL) [10] and Instrumental ADL (IADL) [11] scales, behavioral status by the Neuropsychiatric Inventory [12], dementia severity by the Clinical Dementia rating scale (CDR) [13], number of vascular risk factors (i.e., hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, atrial fibrillation, carotid atherosclerosis, smoking, stroke/TIA, obesity, hyperhomocysteinemia), and risk of vascular dementia by the Hachinski scale [14, 15]. Many different clinicians blinded to the aims of this study performed the follow-up geriatric visits. A group of neuropsychologists evaluated the cognitive and behavioral status of all participants through a semi-structured interview with informants, the standard observational NPE and the cognitive testing with patients. The administration order in our Unit was the following: NPE, cognitive testing, and informant reports. All AD patients underwent morphological brain imaging by CT and/or MRI scan. A subgroup underwent functional brain imaging by [18F]-FDG PET. A neurologist of the same Hospital reviewed all neurological examinations. Furthermore, he performed visual rating scales of atrophy and vascular burden on MRI-images for a subset of both CM-AD and CA-AD patients. In addition, he reviewed the [18F]-FDG PET charts and resumed the results by considering both cortical regions targeted and eventual asymmetries in cortical glucose hypometabolism. A biologist supervised genetic testing of APOE for all AD patients. In addition, all AD patients executed diagnostic lumbar puncture to dose the level of amyloid-β protein (Aβ), tau, and phosphorylated tau (p-tau) in the CSF. Finally, we revised the medical record of each patient at baseline and to the aim of this study we extracted the following data: months from symptoms onset to the first visit, the type of onset (i.e.: insidious, abrupt), the first cognitive symptom, the course (i.e.: gradual worsening, stable, stepwise), the eventual occurrence of fluctuations in cognition and/or attention, the presence of sundowning phenomenon, the familiarity for dementia, the occurrence of past delirium, and the current use of psychotropic medications (i.e., benzodiazepines, antidepressants, antipsychotics). Diagnosis of MCI and AD was made according to the current standards [16, 17]. For all patients diagnosed with AD in the registry, we had excluded medical conditions potentially causing cognitive decline including malnutrition, alcohol, and substance abuse, other different neurological diseases (e.g., stroke, traumatic brain injury, epilepsy, brain tumor, multiple sclerosis), infectious diseases, and psychiatric diseases.
Cognitive testing
Patients underwent a battery of neuropsychological tests that covered multiple cognitive domains (i.e., intellectual functions, prefrontal executive functions, psychomotor speed, visual selective attention, working memory, short-term memory, anterograde long-term memory, speech and language, praxis, visuoconstructional functions). The test battery was not a standardized tool as a whole; nonetheless, normative data from standardization studies on Italian samples were available for all tests in the battery. Impaired scores were established in the standardization studies. In detail, general intellectual functions were tested with Raven’s colored progressive matrices [18]. Executive functions were evaluated using a switching task (Trail Making Test, TMT, part B) [19], a phonological verbal fluency task [20], and a working memory task (digit span backward) [21]. Verbal short-term memory was rated with the digit span forward [22]. Anterograde memory (recall) was tested with delayed recall tasks, both visual and verbal (Rey-Osterrieth complex figure recall test and prose recall) [23, 24]. We tested visual attention with Bell’s test [25, 26] and a digit cancellation test [24], and measured psychomotor speed with TMT (part A) [19]. The language was examined with a picture naming task [27]. Limb and limb-kinetic apraxia were examined by using the De Renzi’s test [28]. Visuospatial and constructional abilities were explored using the copy of geometrical figures test [24] as well as the copy of the Rey-Osterrieth complex figure test [23].
Brain imaging
All AD patients in the study underwent morphological brain imaging by CT scan and/or MRI scan. Moreover, a subgroup underwent functional brain imaging by [18F]-fluorodeoxyglucose-positron emission tomography (FDG-PET). We executed MRI- and FDG PET scans in the context of the first baseline visit. Since some MRI scans were performed in the radiology Unit of our Hospital, we selected those patients with MRI available for volumetric analysis. A protocol of 6 different visual rating scales, as described in previously published papers [29, 30] was applied by an expert rater blinded for clinical and demographical information to assess atrophy in T1 images. In particular, the scales used were: Orbitofrontal (OF), Anterior Cingulate (AC), Anterior Temporal (AT), Fronto-Insular (FI), Medial Temporal (MTA), and Posterior scale (PA). Right and left sides were assessed separately for each scale. To evaluate vascular burden in Flair images Fazekas scales for white matter hyperintensities (WMH) and periventricular (PV) were used. The software used to display images was MRIcron [31]; images have been rated in the native space, in keeping with standard clinical reads. We used as reference data the atrophy scores obtained by the same visual scales from a small sample of 15 healthy controls recruited in the Neurologic Unit of the same Hospital. This sample has been described in a previous article [32]. Considering the functional imaging, we resumed the [18F]-FDG PET scan outcomes and made a comparison between CM-AD and CA-AD patients on them, by considering medial temporal hypo-metabolism, cortical regions targeted, and asymmetries in glucose metabolism reduction.
Biomarkers in the cerebrospinal fluid (CSF)
All patients underwent lumbar puncture to assess biomarkers in the CSF supporting a diagnosis of AD (or of prodromal-AD). CSF Aβ, tau, and phosphorylated tau (p-tau) were evaluated by ELISA (Innogenetics, Ghent, Belgium). Concentration threshold for Aβ1 - 42 was set at 640 pg/ml, for tau protein at 580 pg/mL, and for p-tau at 61 pg/mL [33–36].
Apolipoprotein E genetic testing
Genetic testing of the apolipoprotein E (APOE) was carried out in all AD patients. The APOE genotype was determined as previously described [37].
Retrospective observational case-series study
To describe in detail the possible CM-phenotype, we performed a retrospective observational case-series study of some outpatients seen in our Geriatric Unit of IRCCS Ca’ Granda Foundation Hospital, in the central district of Milan, Italy, over a 10-year period, between 2008 and 2018. All patients came to our observation with suspected cognitive decline. We retrieved all data from the registry of AD outpatients with positive biomarkers in the CSF. The main eligibility criterion for patients to be selected with CM-phenotype was the occurrence of confabulation and/or misidentification in the mild cognitive impairment (MCI) stage (Clinical dementia rating, CDR: 0.5) or mild dementia (CDR: 1). We considered patients’ cognitive and behavioral features as primary outcomes, and we collected them from the first baseline multidimensional assessment to follow-up geriatric visits performed in the same Unit over 10-years. In particular, baseline data were collected from both a detailed informant report and a standard pre-test NPE performed at baseline. Instead, longitudinal cognitive and behavioral features were retrieved by reviewing medical records from follow-up geriatric visits that included brief informant reports and clinical observations of geriatricians. We measured the total number of patients with a defined cognitive or behavioral characteristic (absolute frequency) that emerged in at least one of the two assessments (detailed informant report and NPE) at baseline as the primary outcome. We used cumulative frequencies instead of absolute frequencies of features at geriatric follow-ups, since we expected longitudinal data to be likely incomplete and missing, especially later than 24 months from baseline. On the other hand, cumulative frequencies effectively capture how common a feature was in the phenotype. For this study, we considered the three types of assessments (detailed informant report, NPE, geriatric follow-up visit) to be equivalent. To ensure comparability of the different methods, we classified the features simply binary as present/absent in all assessments. We took note of the details of the reported main features, but we did not consider the severity of manifestation for data analysis. Neuropsychiatric Inventory (NPI) score collected exclusively at baseline was our secondary outcome. We collected additional data at baseline to better characterize the case-series (see the Supplementary Material for detail).
Retrospective observational cross-sectional study
To preliminarily support the idea that the CM-phenotype is an amnesic syndrome of AD quite distinct from the classic amnesic phenotype (CA-phenotype), we performed a retrospective cross-sectional study. We compared patients with the possible CM-phenotype (CM-ADs) with a group of AD patients presenting with the CA-phenotype (CA-ADs) at both baseline multidimensional assessment and follow-up geriatric visits at successive 12-month intervals after baseline. A group of healthy controls (HCs) served as a reference in the baseline assessment. We selected CA-ADs from the same outpatient registry of AD patients with positive biomarkers in the CSF of our Geriatric Unit used for the retrospective observational case-series study over the same period, from 2008 to 2018. In particular, after the removal of CM-ADs among all AD patients remaining in the registry over the 10-year interval, we extracted those classified as having a CA-phenotype. A detailed delineation of the dementia phenotype based on a multidimensional assessment usually performed at our Unit is reported in the Supplementary Material. HCs were consecutively selected among persons referred to our Unit in the same time interval who were found to be unimpaired after neuropsychological assessment. We retrieved data on HCs from the general registry of outpatients in the same Unit. Primary outcomes, data collection methods, and measures were the same as those adopted in the case-series study. In detail, the primary outcomes were any differences between CM-ADs and CA-ADs on the frequency of cognitive and behavioral features revealed in the detailed informant report and/or NPE performed at baseline. Considering longitudinal data, the primary outcomes were any differences between the same two groups on the frequency of cognitive and behavioral features reported in the chart of follow-up geriatric visits at successive 12-month intervals after baseline. Secondary outcomes at baseline were any differences in the following clinical variables and assessments: type of onset, first cognitive symptom, course, fluctuations, sundowning, score on the NPI, scores on cognitive tests, atrophy scores on visual rating scales on T1 MRI images, and frequency of the different [18F]-FDG-PET imaging diagnoses reported on the chart. Finally, a neurologist reviewed all neurological examinations at baseline and follow-up visits and made a preliminary qualitative comparison between the two clinical groups of AD patients. We considered as potential confounders at baseline any differences between the two AD groups on the following variables and assessments: demographic data (sex, mean age, mean years of education, handedness), months since onset, cognitive screening (MMSE score), dementia severity (CDR score, sum of CDR boxes, number of MCIs, and mild dementia stage), vascularity (mean number of cardiovascular risk factors, Hachinski score), use of psychotropic medications (benzodiazepines, antidepressants, antipsychotics), level of AD biomarkers in the CSF (Aβ, tau, phospho-tau), carriers of APOE ɛ4 allele, and vascular scores at Fazekas scales on T1 MRI images.
Both patients and their caregivers provided written informed consent for participation in both studies. All data used in the study were collected for clinical purposes. The local Ethical Committee of the IRCCS Ca’ Granda Foundation Hospital statutorily approved research based on retrospective analysis of already available routinely collected health data. All examinations and tests patients underwent in the study were performed following the ethical standards laid out in the 1964 Declaration of Helsinki and its later amendments.
Statistical analyses plan
Statistical analyses were run using SPSS software, version 27.0 (SPSS, Chicago, IL). The Kolmogorov-Smirnov test was used to verify the normal distribution of continuous variables. Comparisons among the characteristics of the three groups of participants were performed by one-way ANOVA or Kruskal–Wallis H test according to variables’ distribution. Pairwise comparisons were conducted by t-test or Mann–Whitney U test. The chi-square test was applied to determine whether a significant association was present between groups (HC, CM, or CA) and the total frequency of the cognitive and behavioral features. Fisher exact test was considered when one or more expected values were less than 5. For all analyses, a p-value lower than 0.05 was considered statistically significant. A Bonferroni adjustment for multiple comparisons was performed to minimize type I error. Overall, the longitudinal data collected were rather incomplete especially after 24 months from baseline (see Supplementary Tables 2 and 3). Thus, we decided to perform exclusively simple nonparametric comparisons (Chi square) between the two groups at 12 and 24 months.
RESULTS
General results
Participants
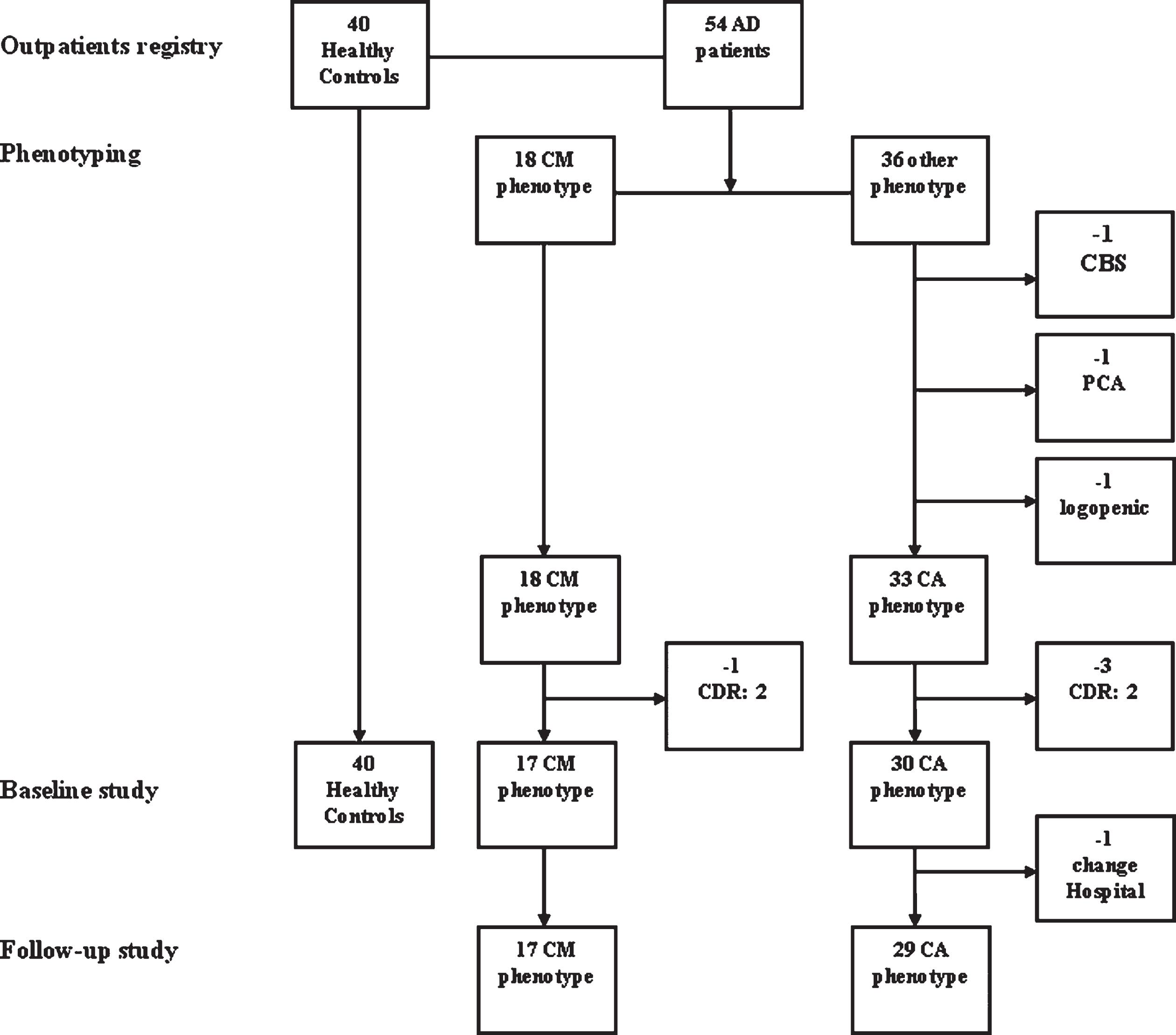
Seventeen patients with CM-AD participated in the case series study. The same group of 17 CM-AD patients, plus 30 CA-AD patients, and 40 cognitively unimpaired HCs participated in the cross-sectional study at baseline (T0). Seventeen CM-AD patients and 29 CA-AD patients participated in the longitudinal study, as one CA-AD patient was excluded because the follow-up visits were performed at another hospital. We stopped the recruitment of HCs at 40 because it was an adequate number for comparison. We have reported the flowchart of the study sample in Fig. 1.
Fig. 1
Flow-chart of the study sample. We first identified 18 outpatients presenting with a possible confabulation-misidentification phenotype (CM-phenotype) among the patients with AD diagnosis supported by positive biomarkers in the CSF visited in a ten-year period at the Geriatric Unit of the Ca’ Granda Foundation hospital. Then, we excluded one CM-AD patient from this group because she was at moderate stage of dementia (CDR: 2) when she came to our first observation. At the same time, we first selected 33 outpatients presenting with a classical amnesic phenotype (CA-phenotype) among the 36 outpatients with AD diagnosis supported by positive biomarkers in CSF remaining in the outpatient registry in the same period after the extraction of the 18 CM-AD patients. Instead, 3 patients were excluded because presenting an atypical phenotype of AD (i.e., corticobasal syndrome, PCA syndrome, logopenic aphasia). Then, we excluded 3 CA-ADs because they were at advanced stage of dementia (all CDR: 2) at the time of the first visit. In total, 17 CM-ADs and 30 CA-ADs, all at early dementia stage (CDR 0.5 or 1), participated in the cross-sectional study at the time of the baseline assessment. Instead, 17 CM-ADs and 29 CA-ADs participated in the longitudinal study, due to the fact that one CA-AD patient underwent follow-up visits into a different hospital. Finally, we selected 40 people taken from the general outpatient registry in the same Unit among those patients resulted cognitively unimpaired after the neuropsychological assessment, who served as healthy controls (HC) group in the first baseline assessment.
Descriptive data
We reported the demographic and clinical data of the 17 CM-ADs at baseline in Table 1. In addition, we included confabulation and misidentification, which were the two most salient features of the CM-phenotype. Patients were all late-onset AD, as expected since recruitment was from a Geriatric Unit. The mean age was 75.8±4.6 years. Women were 58.8%. Mean education was 9.9±4.4 years. All patients were in the early stage of dementia at the initial visit (8 MCI/9 mild dementia). The mean MMSE score at baseline was 24.7±2.6. The mean NPI score was 12.2±8.6. All 17 patients (100%) showed at least one confabulation or misidentification. In total, 15 patients (88.2%) presented with confabulation, 9 (52.9%) misidentification, and 7 (41.2%) both confabulation and misidentification. Demographic and clinical data of the CA-AD and HC groups were in Table 5. Considering geriatric follow-up visits, there was great heterogeneity in the number of visits (mean: 7.7±4.4, range: 2–17) and duration of follow-up (mean: 40.8±24.8 months, range: 13–100) in the CM-AD group (see Supplementary Tables 6 and 7). No differences emerged between the CM- and CA-AD groups on the number and duration of follow-ups, MMSE scores at baseline and last follow-up, and age at baseline and last follow-up (see Supplementary Table 7).
Table 1
Demographic characteristics and some clinical features of the 17 AD patients with the CM phenotype collected at baseline
| n. | Sex | Age | Edu | Han | MMSE | NPI | VRF | Hac | IADL | ADL | CDR | CDR SOB | Syndr | Spontaneous confabulation | Provoked confabulation | Misidentification |
| Case#1 | m | 79 | 13 | R | 25 | 6 | 4 | 3 | 4(5)° | 6 | 1 | 3 | Mild dem | yes | yes (verbal) | (wife, home, TV celebrities)+ |
| Case#2§ | f | 69 | 5 | R | 25 | 10 | 0 | 1 | 5(8)° | 6 | 0,5 | 3.5 | MCI(sd+) | yes | yes (ver/vis) | (husband, daughter, home, holiday home, TV celebrities, people displayed in an advertisement poster) |
| Case#3 | m | 76 | 5 | L | 25 | 2 | 4 | 4 | 4(5) | 5 | 1 | 4 | Mild dem | – | yes (visual) | – |
| Case#4 | m | 81 | 8 | R | 22 | 16 | 5 | 2 | 3(5) | 6 | 0.5 | 3.5 | MCI(md+) | – | yes (ver/vis) | – |
| Case#5 | f | 80 | 8 | R | 24 | 14 | 0 | 2 | 6(8) | 5 | 0.5 | 4 | MCI(md+) | yes | – | a relative (unspecified), home of the relative, her dog |
| Case#6 | m | 77 | 13 | R | 23 | 18 | 1 | 3 | 1(5) | 2 | 1 | 8 | Mild dem | yes | yes (verbal) | sun, wife, home, TV celebrities, himself in the mirror |
| Case#7 | f | 79 | 4 | R | 22 | 4 | 1 | 2 | 7(8) | 6 | 1 | 4 | Mild dem | – | yes (visual) | (relatives unspecified) |
| Case#8 | m | 68 | 18 | R | 29 | 6 | 6 | 2 | 5(5) | 6 | 0.5 | 2.5 | MCI(md+) | – | yes (ver/vis) | – |
| Case#9 | f | 78 | 13 | R | 24 | 22 | 2 | 2 | 4(8) | 5 | 1 | 5 | Mild dem | – | – | husband, home |
| Case#10 | f | 78 | 8 | R | 24 | 22 | 4 | 1 | 3(8) | 5 | 1 | 6.5 | Mild dem | yes | – | (home) |
| Case#11 | f | 73 | 8 | R | 27 | 0 | 1 | 0 | 8(8) | 6 | 0.5 | 2.5 | MCI(md+) | yes | yes (verbal) | – |
| Case#12 | f | 78 | 8 | R | 29 | 1 | 4 | 3 | 8(8) | 6 | 1 | 3 | Mild dem | – | yes (visual) | – |
| Case#13 | m | 68 | 13 | R | 27 | 6 | 1 | 0 | 5(5) | 6 | 0.5 | 2.5 | MCI(md+) | yes | – | – |
| Case#14 | f | 73 | 18 | R | 25 | 10 | 1 | 1 | 8(8) | 6 | 0.5 | 2.5 | MCI(md-) | – | yes (ver/vis) | – |
| Case#15 | m | 76 | 8 | R | 23 | 24 | 4 | 1 | 5(5) | 6 | 1 | 3 | Mild dem | yes | – | – |
| Case#16 | f | 72 | 5 | R | 19 | 24 | 1 | 4 | 8(8) | 6 | 1 | 3.5 | Mild dem | yes | – | (relatives unspecified, daughter) |
| Case#17 | f | 84 | 13 | R | 27 | 22 | 2 | 1 | 7(8) | 6 | 0.5 | 3 | MCI(md+) | – | – | husband, home |
Edu, education; Han, handedness; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; VRF, vascular risk factors; Hac, Hachinski scale; IADL, instrumental activities of daily living; ADL, activities of daily living; CDR, clinical dementia rating; SOB, sum of boxes; Syndr, syndrome; MCI, Mild cognitive impairment; m, male; f, female; R, right; L, left; (sd+), single domain, amnesic; (md+), multiple domains, amnesic; (md-), multiple domain not amnesic; vis, visual; ver, verbal. §Patient#2 was FM, the patient we studied in a previous case report [1]. °According to IADL standard scoring system, the maximum score was 5 points for male and 8 points for women. +Misidentification emerged at the follow up visits are in brackets.
We reported the level of biomarkers in the CSF and the APOE genotype in Table 2. All patients in the CM-AD (100%) and CA-AD (100%) groups had abnormal low Aβ concentration. Eight CM-ADs (47.1%) and 10 CA-ADs (33.3%) had abnormal high tau concentration. Thirteen CM-ADs (76.5%) and 18 CA-ADs (60%) had abnormal high p-tau concentration. In total, the APOE ɛ4 allele carriers were 12 in the CM-AD group (70.6%) and 13 in the CA-AD group (43.3%). One patient with the CM-phenotype (5.9%) and 4 with the CA-phenotype (13.3%) had ɛ4/ɛ4 genotype.
Table 2
Biomarkers in the CSF and APOE genotype of the CM-AD and CA-AD patients. Concentration threshold for Aβ1 - 42 was set at 640 pg/ml, for tau protein at 580 pg/mL, and for p-tau at 61 pg/mL
| Aβ | tau | p-tau | APOE | |
| CM-AD | ||||
| 1 | 535* | 680* | 45 | ɛ3/ɛ3 |
| 2 | 346* | 575 | 70* | ɛ3/ɛ4# |
| 3 | 260* | 421 | 50 | ɛ3/ɛ4# |
| 4 | 563* | 826* | 109* | ɛ3/ɛ4# |
| 5 | 414* | 419 | 68* | ɛ3/ɛ3 |
| 6 | 226* | 423 | 80* | ɛ2/ɛ4# |
| 7 | 532* | 276 | 73* | ɛ3/ɛ4# |
| 8 | 591* | 815* | 110* | ɛ3/ɛ4# |
| 9 | 288* | 1023* | 66* | ɛ2/ɛ3 |
| 10 | 444* | 349 | 72* | ɛ3/ɛ4# |
| 11 | 515* | 861* | 110* | ɛ3/ɛ4# |
| 12 | 422* | 250 | 62* | ɛ3/ɛ4# |
| 13 | 355* | 873* | 126* | ɛ4/ɛ4## |
| 14 | 588* | 372 | 59 | ɛ2/ɛ3 |
| 15 | 588* | 2589* | 280* | ɛ3/ɛ4# |
| 16 | 343* | 751* | 77* | ɛ3/ɛ4# |
| 17 | 285* | 262 | 42 | ɛ3/ɛ3 |
| CA-AD | ||||
| 1 | 462* | 745* | 64* | ɛ3/ɛ4# |
| 2 | 307* | 609* | Miss | ɛ3/ɛ4# |
| 3 | 393* | 1266* | 70* | ɛ3/ɛ3 |
| 4 | 322* | 208 | 28 | ɛ3/ɛ3 |
| 5 | 291* | 999* | 136* | ɛ3/ɛ3 |
| 6 | 525* | 524 | 76* | ɛ3/ɛ4# |
| 7 | 354* | 567 | 81* | ɛ3/ɛ3 |
| 8 | 445* | 219 | 33 | ɛ3/ɛ4# |
| 9 | 454* | 206 | 22 | ɛ3/ɛ3 |
| 10 | 516* | 110 | 33 | ɛ3/ɛ3 |
| 11 | 492* | 163 | 41 | ɛ3/ɛ3 |
| 12 | 418* | 250 | 26 | ɛ3/ɛ3 |
| 13 | 499* | 153 | 45 | ɛ4/ɛ4## |
| 14 | 449* | 1524* | 155* | ɛ3/ɛ3 |
| 15 | 546* | 491 | 101* | ɛ3/ɛ3 |
| 16 | 447* | 146 | 77* | ɛ3/ɛ3 |
| 17 | 552* | 660* | 66* | ɛ3/ɛ4# |
| 18 | 509* | 512 | 75* | ɛ3/ɛ4# |
| 19 | 504* | 572 | 52 | ɛ3/ɛ4# |
| 20 | 420* | 526 | 66* | ɛ3/ɛ4# |
| 21 | 410* | 2269* | 140* | ɛ4/ɛ4## |
| 22 | 432* | 454 | 68* | ɛ3/ɛ4# |
| 23 | 319* | 763* | 94* | ɛ3/ɛ3 |
| 24 | 592* | 348 | 58 | ɛ4/ɛ4## |
| 25 | 504* | 567 | 84* | ɛ3/ɛ3 |
| 26 | 481* | 600* | 89* | ɛ3/ɛ3 |
| 27 | 400* | 376 | 67* | ɛ4/ɛ4## |
| 28 | 560* | 395 | 61 | ɛ3/ɛ3 |
| 29 | 417* | 302 | 54 | ɛ3/ɛ3 |
| 30 | 550* | 614* | 84* | ɛ3/ɛ3 |
*Abnormal concentration. #one ɛ4 allele carriers. ## two ɛ4 allele carriers. APOE, apolipoprotein E.
The main cognitive and behavioral features of the CM-phenotype
In Table 3, we reported the frequency of the main cognitive and behavioral features of the possible CM-phenotype detected at baseline by the two assessments (informant report and NPE), and the cumulative frequency of the same features collected at geriatric follow-ups. We reported a more detailed description of the cognitive and behavioral characteristics that emerged in the CM-AD group in the Supplementary Material.
Table 3
Frequency of cognitive and behavioral features of the 17 CM-AD patients emerged at baseline (T0) (absolute frequency) and longitudinal assessment (cumulative frequency)
| Visit/months | T0 | T12 | T24 | T36 | T48 | T60 | T72> | |
| Years from onset | 3 y | 4 y | 5 y | 6 y | 7 y | 8 y | 9 y | |
| Stage (CDR) | 0.5–1 | 0.5–1 | 1 | 1-2 | 2 | 2 | 2-3 | |
| n° patients | 17 | 17 | 11 | 8 | 5 | 3 | 3 | |
| Absolute frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | ||
| N | % | % | % | % | % | % | % | |
| Recent memory deficits | 16 | 94.1 | 100 | |||||
| Temporal disorientation | 15 | 88.2 | ||||||
| Confabulation | 15 | 88.2 | ||||||
| Executive functions deficits | 15 | 88.2 | 88.2 | 88.2 | 94.1 | |||
| Attention deficits | 14 | 82.3 | ||||||
| Reduced Insight | 13 | 76.5 | 76.5 | 82.3 | 88.2 | |||
| Past memory deficits | 11 | 64.7 | ||||||
| Psychomotor slowness | 10 | 58.8 | ||||||
| Topographical disorientation | 7 | 41.2 | 76.5 | 76.5 | 82.3 | 82.3 | 88.2 | |
| Delusion (not-misidentification) | 7 | 41.2 | 41.2 | 41.2 | 70.6 | |||
| Apathy | 7 | 41.2 | 52.9 | 64.7 | 82.3 | |||
| Language deficits | 6 | 35.3 | 52.9 | 58.8 | 70.6 | 76.5 | ||
| Aggression | 6 | 35.3 | 47.1 | 47.1 | 58.8 | 58.8 | 64.7 | |
| Anxiety | 6 | 35.3 | 52.9 | 58.8 | 64.7 | 70.6 | ||
| Logorrhea | 5 | 29.4 | ||||||
| Misidentification | 4 | 23.5 | 23.5 | 41.2 | 47.1 | 47.1 | 52.9 | |
| Irritability | 4 | 23.5 | 41.2 | 58.8 | 70.6 | 76.5 | ||
| Hallucination | 3 | 17.6 | 29.4 | 47.1 | 64.7 | |||
| Fluctuations | 3 | 17.6 | 23.5 | 35.3 | 47.1 | 52.9 | ||
| Visit/months | T0 | T12 | T24 | T36 | T48 | T60 | T72> | |
| Years from onset | 3 y | 4 y | 5 y | 6 y | 7 y | 8 y | 9 y | |
| Stage (CDR) | 0.5–1 | 0.5–1 | 1 | 1-2 | 2 | 2 | 2-3 | |
| n° patients | 17 | 17 | 11 | 8 | 5 | 3 | 3 | |
| Absolute frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | Cumulative frequency | ||
| Social disinhibition | 3 | 17.6 | ||||||
| Euphory/Fatuity | 3 | 17.6 | ||||||
| Insomnia | 3 | 17.6 | 52.9 | 64.7 | 70.6 | |||
| Depression | 3 | 17.6 | 35.3 | 58.8 | 70.6 | |||
| Praxis deficits | 2 | 11.8 | 23.5 | 29.4 | 47.1 | 47.1 | 47.1 | 52.9 |
| Hypersomnia | 2 | 11.8 | ||||||
| Rem Behavior Disorders (RBD) | 2 | 11.8 | ||||||
| Lability | 2 | 11.8 | ||||||
| Abulia | 1 | 5.9 | ||||||
| Obsession- Compulsion | 1 | 5.9 | ||||||
| Hyperactivity/psychomotor agitat. | 1 | 5.9 | 35.3 | 52.9 | 76.5 | |||
| Personality changes | 0 | 0 | ||||||
| Wandering | 0 | 0 | ||||||
| Purposeless activity | 0 | 0 | ||||||
| Impulse control disorders | 0 | 0 | ||||||
| Hyperfagia | 0 | 0 | 17.6 | 29.4 | 47.1 | 64.7 | 70.6 | 76.5 |
| Confusional arousal | 0 | 0 | ||||||
We marked in bold the main features of the CM-phenotype which emerged in more than 50%of the patients at the first visit and follow-ups. In addition, we marked in bold the frequency of a feature (both absolute or cumulative) when it reached the highest value considering the whole period of observation. Finally, we did not report the cumulative frequency of a feature at follow-up either when it never reached 50%or when it had already reached its highest value at the first visit. We displayed cumulative instead of absolute frequencies at the geriatric follow-ups since longitudinal data resulted be quite incomplete, especially later than 24 months from baseline. On the other hand, cumulative frequencies well capture how much a feature was common in the phenotype.
Major differences between the CM- and CA-phenotype of AD
Primary outcomes
Informant report and NPE at baseline. We reported the cognitive and behavioral features that emerged by clinical assessment (informant report and NPE) of the CM-AD group compared to those of the CA-AD, and HC groups in Table 4, Figs. 2, and 3. In addition, we presented the cognitive and behavioral features that emerged by each method (informant report and NPE) separately in the Supplementary Material (see Supplementary Results and Supplementary Tables 4 and 5). The CM-AD group showed more temporal disorientation (88.2%versus 53.3%, p < 0.02), confabulation (88.2%versus 0%, p < 0.001), delusion not-misidentification (41.2%versus 3.3%, p = 0.002), logorrhea (29.4%versus 0%, p = 0.004), and misidentification (23.5%versus 0%, p = 0.013) than the CA-AD group. In addition, a similar difference with tendency to significance for the comparison between the CM-AD and CA-AD group emerged also for attention deficits (82.3%versus 56.7%, p = 0.074), reduced insight (76.5%versus 43.3%, p = 0.028), social disinhibition (17.6%versus 0%, p = 0.042), and euphory/fatuity (17.6%versus 0%, p = 0.042). Finally, a similar difference with a tendency to significance emerged also for executive function deficits (64.7%versus 36.7%, p = 0.064) considering the outcomes from informant report (see Supplementary Table 4), and for both anterograde (52.9%versus 23.3%, p = 0.040) and retrograde (64.7%versus 36.7%, p = 0.064) amnesia considering the outcomes from the NPE (see Supplementary Table 5).
Fig. 2
Frequency of cognitive features detected by clinical assessment (informant report and/or NPE) in the CM-AD group of patients compared to those in the CA-AD and HC group. *indicates a statistically significant difference CM > CA at p < 0.05. **indicates a statistically significant difference CM > CA at p < 0.01. ***indicates a statistically significant difference CM > CA at p < 0.001. (*) indicates a difference CM > CA with tendency to significance.
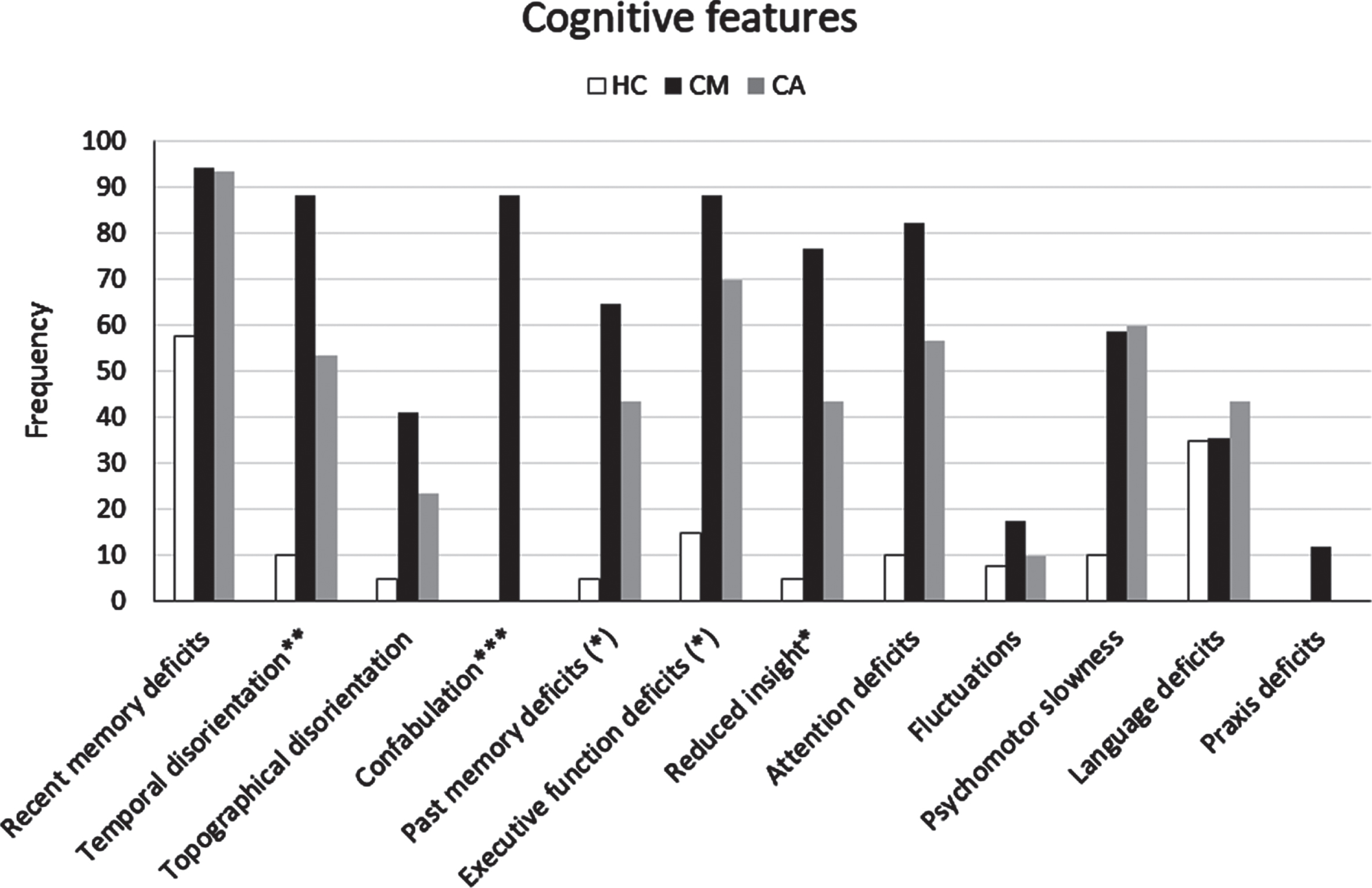
Fig. 3
Frequency of behavioral features detected by clinical assessment (informant report and/or NPE) in the CM-AD group of patients compared to those in the CA-AD and HC group. *indicates a statistically significant difference CM > CA at p < 0.05. **indicates a statistically significant difference CM > CA at p < 0.01. (*) indicates a difference CM > CA with tendency to significance.
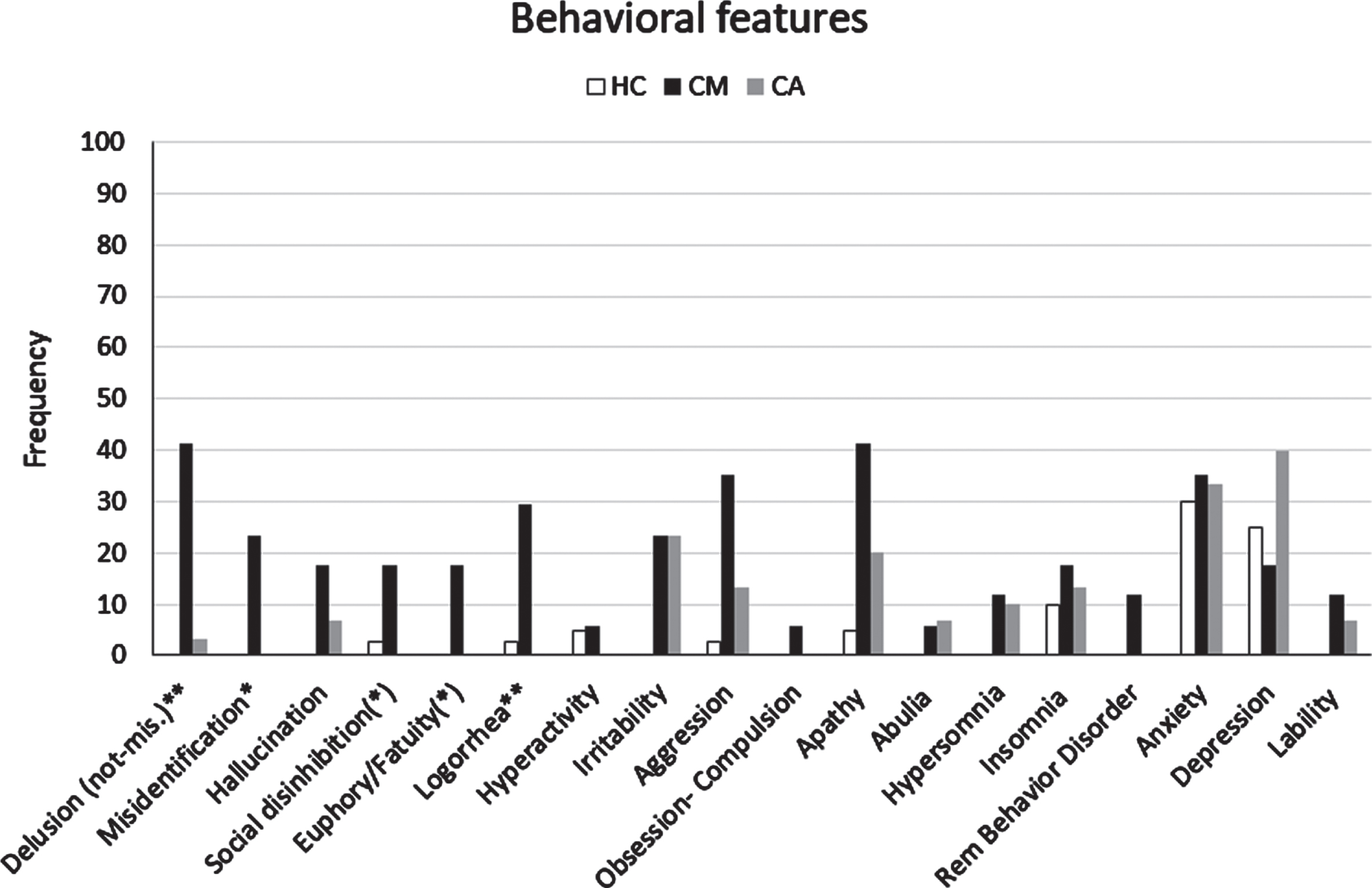
Table 4
Comparison of cognitive and behavioral features among the three groups of participants: HCs, AD patients with the CM-phenotype (CM-AD), and AD patients with the CA-phenotype (CA-ADs). (Statistically significant comparisons are in bold)
| HC | CM | CA | |||||||||||||
| report | NPE | TOT | TOT | report | NPE | TOT | TOT | report | NPE | TOT | TOT | HC-CM | HC-CA | CM-CA | |
| n | n | N | % | n | n | N | % | n | n | N | % | p | p | p | |
| Recent memory deficits | 23 | 0 | 23 | 57.5 | 16 | 9 | 16 | 94.1 | 28 | 7 | 28 | 93.3 | p < 0.01 | p = 0.002 | p = 1.000* |
| Temporal disorientation | 0 | 4 | 4 | 10 | 6 | 15 | 15 | 88.2 | 2 | 16 | 16 | 53.3 | p < 0.0001 | p < 0.0001 | p < 0.02 |
| Confabulation | 0 | 0 | 0 | 0 | 7 | 10 | 15 | 88.2 | 0 | 0 | 0 | 0 | p < 0.001* | nv | p < 0.001* |
| Executive functions deficits | 6 | 1 | 6 | 15 | 11 | 11 | 15 | 88.2 | 11 | 21 | 21 | 70.0 | p < 0.001 | p < 0.001 | p = 0.156 |
| Attention deficits | 4 | 0 | 4 | 10 | 9 | 8 | 14 | 82.3 | 17 | 8 | 17 | 56.7 | p < 0.001 | p < 0.0001 | p = 0.074 |
| Reduced Insight | 1 | 1 | 2 | 5 | 8 | 12 | 13 | 76.5 | 6 | 12 | 13 | 43.3 | p < 0.001* | p < 0.001 | p = 0.028 |
| Past memory deficits | 2 | 0 | 2 | 5 | 3 | 11 | 11 | 64.7 | 7 | 11 | 13 | 43.3 | p < 0.001* | p < 0.001 | p = 0.159 |
| Psychomotor slowness | – | 4 | 4 | 10 | 0 | 10 | 10 | 58.8 | 0 | 18 | 18 | 60.0 | p < 0.0001 | p < 0.0001 | p = 0.937 |
| Topographical disorientation | 2 | 0 | 2 | 5 | 7 | 0 | 7 | 41.2 | 7 | 0 | 7 | 23.3 | p < 0.01 | p < 0.05 | p = 0.199 |
| Delusion( not-misidentification) | 0 | 0 | 0 | 0 | 7 | 0 | 7 | 41.2 | 1 | 0 | 1 | 3.3 | p < 0.0001 | p = 0.429* | p = 0.002* |
| Apathy | 2 | 0 | 2 | 5 | 7 | 1 | 7 | 41.2 | 6 | 0 | 6 | 20.0 | p < 0.005 | p = 0.066* | p = 0.176* |
| Language deficits | 13 | 2 | 14 | 35 | 4 | 4 | 6 | 35.3 | 13 | 0 | 13 | 43.3 | p = 0.983 | p = 0.478 | p = 0.589 |
| Aggression | 1 | 0 | 1 | 2.5 | 6 | 0 | 6 | 35.3 | 4 | 0 | 4 | 13.3 | p < 0.005 | p = 0.157* | p = 0.136* |
| Anxiety | 12 | 0 | 12 | 30 | 6 | 0 | 6 | 35.3 | 10 | 0 | 10 | 33.3 | p = 0.694 | p = 0.766 | p = 0.862 |
| Logorrhea | 0 | 1 | 1 | 2.5 | 0 | 5 | 5 | 29.4 | 0 | 0 | 0 | 0 | p = 0.007* | p = 1.000* | p = 0.004* |
| Misidentification | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 23.5 | 0 | 0 | 0 | 0 | p < 0.01 | nv | p = 0.013* |
| Irritability | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 23.5 | 7 | 0 | 7 | 23.3 | p < 0.01 | p < 0.005 | p = 1.000* |
| Hallucination | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 17.6 | 2 | 0 | 2 | 6.7 | p < 0.05 | p = 0.180* | p = 0.336* |
| Fluctuations | 3 | 0 | 3 | 7.5 | 3 | 0 | 3 | 17.6 | 3 | 1 | 3 | 10.0 | p = 0.349* | p = 1.000* | p = 0.653* |
| Social disinhibition | 1 | 0 | 1 | 2.5 | 3 | 1 | 3 | 17.6 | 0 | 0 | 0 | 0 | p = 0.075* | p = 1.000* | p = 0.042* |
| Euphory/Fatuity | 0 | 0 | 0 | 0 | 2 | 3 | 3 | 17.6 | 0 | 0 | 0 | 0 | p = 0.023* | nv | p = 0.042* |
| Insomnia | 4 | – | 4 | 10 | 3 | – | 3 | 17.6 | 4 | – | 4 | 13.3 | p = 0.415* | p = 0.717* | p = 0.692* |
| Depression | 10 | 0 | 10 | 25 | 3 | 0 | 3 | 17.6 | 12 | 0 | 12 | 40.0 | p = 0.734* | p = 0.181 | p = 0.114 |
| Praxis deficits | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 11.8 | 0 | 0 | 0 | 0 | p = 0.085* | nv | p = 0.126* |
| Hypersomnia | 0 | – | 0 | 0 | 2 | – | 2 | 11.8 | 3 | – | 3 | 10.0 | p = 0.085* | p = 0.074* | p = 1.000* |
| Rem Behavior Disorders (RBD) | 0 | – | 0 | 0 | 2 | – | 2 | 11.8 | 0 | – | 0 | 0 | p = 0.085* | nv | p = 0.126* |
| Lability | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 11.8 | 2 | 0 | 2 | 6.7 | p = 0.085* | p = 0.180* | p = 0,613* |
| Abulia | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 5.9 | 2 | 0 | 2 | 6.7 | p = 0.298* | p = 0.180* | p = 1.000* |
| Obsession- Compulsion | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 5.9 | 0 | 0 | 0 | 0 | p = 0.298* | nv | p = 0.362* |
| Hyperactivity/psychomotor agit. | 1 | 2 | 2 | 5 | 1 | 0 | 1 | 5.9 | 0 | 0 | 0 | 0 | p = 1.000* | p = 503* | p = 0.362* |
| Personality changes | 0 | – | 0 | 0 | 0 | – | 0 | 0 | 0 | – | 0 | 0 | nv | nv | nv |
| Wandering | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | nv | nv | nv |
| Purposeless activity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | nv | nv | nv |
| Impulse control disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | nv | nv | nv |
| Hyperfagia | 0 | – | 0 | 0 | 0 | – | 0 | 0 | 0 | – | 0 | 0 | nv | nv | nv |
| Confusional arousal | 0 | – | 0 | 0 | 0 | – | 0 | 0 | 0 | – | 0 | 0 | nv | nv | nv |
nv, no variability in the data. *Fisher’s exact test.
Geriatric follow-up visits. We reported the cognitive and behavioral features that emerged at geriatric follow-ups during the entire observation period in the Supplementary Material (see Supplementary Tables 2 and 3 , respectively). The results of simple non-parametric (Chi square) comparisons between the two groups of AD patients showed that CM-ADs had more hallucinations (41.2%versus 11.1%, p = 0.030), social disinhibition (23.5%versus 0%, p = 0.018), and reduced insight (58.8%versus 25.9%, p < 0.029) than CA-ADs at 12 months from baseline (Table 5). In addition, confabulation was also more frequent in the CM-AD than in the CA-AD group at 12 months, with the difference between the two groups that tends to significance (23.5%versus 3.7%, p = 0.065). No statistically significant difference emerged between the two groups at 24 months from baseline. However, many raw differences between the two groups on additional cognitive and behavioral features at both 12 and 24 months were in the same direction as those that emerged at baseline (see the Supplementary Material). Moreover, in a supplemental analysis, we took into account that the two most salient features of the possible CM-phenotype, namely confabulation and misidentification, never emerged at geriatric follow-ups for 20 out of 29 (68.9%) CA-AD (CA-AD negative) patients (see Supplementary Table 7). Moreover, the 9 CA-ADs who developed confabulation or misidentification during follow-up (CA-AD positive) began to show these features at later stages of dementia.
Table 5
Comparison of cognitive and behavioral features reported at geriatric follow-up visits at 12 and 24 months between patients with the CM and CA phenotype (Statistically significant differences are in bold)
| T12 | T24 | |||||||||
| CM | CA | CM | CA | |||||||
| N = 17 | % | N = 27 | % | p | N = 11 | % | N = 19 | % | p | |
| Cognitive features | ||||||||||
| Fluctuations | 3 | 17.6% | 1 | 3.7% | 0.282* | 2 | 18.2% | 4 | 21.1% | 1.000* |
| Sundowning | 1 | 5.9% | 3 | 11.1% | 1.000* | 0 | 0 | 1 | 5.3% | 1.000* |
| Psychomotor slowness | 1 | 5.9% | 1 | 3.7% | 1.000* | 1 | 9.1% | 0 | 0 | 0.367* |
| Confusional episodes | 0 | 0 | 2 | 7.4% | 0.515* | 1 | 9.1% | 0 | 0 | 0.367* |
| Recent memory deficits | 15 | 88.2% | 23 | 85.2% | 1.000* | 5 | 45.4% | 12 | 63.2% | 0.454* |
| Retrograde memory deficits | 2 | 11.8% | 8 | 29.6% | 0.271* | 0 | 0 | 1 | 5.3% | 1.000* |
| Confabulation | 4 | 23.5% | 1 | 3.7% | 0.065* | 0 | 0 | 0 | 0 | nv |
| Temporal disorientation | 6 | 35.3% | 7 | 25.9% | 0.507 | 2 | 18.2% | 2 | 10.5% | 0.611* |
| Topographical disorientation | 11 | 64.7% | 12 | 44.4% | 0.190 | 3 | 27.3% | 6 | 31.6% | 1.000* |
| Attention deficits | 5 | 29.4% | 14 | 51.8% | 0.143 | 1 | 9.1% | 1 | 5.3% | 1.000* |
| Executive function deficits | 7 | 41.2% | 9 | 33.3% | 0.598 | 3 | 27.3% | 2 | 10.5% | 0.327* |
| Reduction of insight | 10 | 58.8% | 7 | 25.9% | 0.029 | 3 | 27.3% | 1 | 5.3% | 0.126* |
| Language deficits | 7 | 41.2% | 17 | 63% | 0.158 | 3 | 27.3% | 5 | 26.3% | 1.000* |
| Praxis deficits | 5 | 29.4% | 7 | 25.9% | 1.000* | 4 | 36.4% | 2 | 10.5% | 0.156* |
| Behavioral features | ||||||||||
| Misidentification | 4 | 23.5% | 2 | 7.4% | 0.186* | 3 | 27.3% | 2 | 10.5% | 0.327* |
| Delusion not-misidentification | 7 | 41.2% | 7 | 25.9% | 0.290 | 5 | 45.4% | 7 | 36.8% | 0.712* |
| Hallucination | 7 | 41.2% | 3 | 11.1% | 0.030* | 3 | 27.3% | 3 | 15.8% | 0.641* |
| Apathy | 8 | 47.1% | 9 | 33.3% | 0.363 | 4 | 36.4% | 2 | 10.5% | 0.156* |
| Abulia | 1 | 5.9% | 5 | 18.5% | 0.380* | 2 | 18.2% | 1 | 5.3% | 0.537* |
| Irritability | 8 | 47.1% | 14 | 51.8% | 0.757 | 5 | 45.4% | 9 | 47.4% | 0.919 |
| Aggression | 6 | 35.3% | 8 | 29.6% | 0.694 | 5 | 45.4% | 6 | 31.6% | 0.696* |
| Social disinhibition | 4 | 23.5% | 0 | 0 | 0.018* | 2 | 18.2% | 0 | 0 | 0.126* |
| Impulse control disorder | 0 | 0 | 1 | 3.7% | 1.000* | 0 | 0 | 0 | 0 | nv |
| Obsession-compulsion | 1 | 5.9% | 1 | 3.7% | 1.000* | 0 | 0 | 0 | 0 | nv |
| Hyperactivity (psychomotor agit.) | 5 | 29.4% | 7 | 25.9% | 1.000* | 5 | 45.4% | 5 | 26.3% | 0.425* |
| Wandering | 3 | 17.6% | 3 | 11.1% | 0.662* | 0 | 0 | 2 | 10.5% | 0.520* |
| Purposeless activity | 4 | 23.5% | 8 | 29.6% | 0.739* | 3 | 27.3% | 9 | 47.4% | 0.442* |
| Hyperphagia | 3 | 17.6% | 3 | 11.1% | 0.662* | 2 | 18.2% | 0 | 0 | 0.126* |
| Euphory/fatuity | 1 | 5.9% | 1 | 3.7% | 1.000* | 0 | 0 | 0 | 0 | nv |
| Insomnia | 7 | 41.2% | 10 | 37% | 0.784 | 4 | 36.4% | 6 | 31.6% | 1.000* |
| Hypersomnia | 2 | 11.8% | 5 | 18.5% | 0.689* | 4 | 36.4% | 2 | 10.5% | 0.156* |
| Rem behavior disorder (RBD) | 3 | 17.6% | 1 | 3.7% | 0.282* | 1 | 9.1% | 2 | 10.5% | 1.000* |
| Confusional arousal | 2 | 11.8% | 0 | 0 | 0.144* | 0 | 0 | 0 | 0 | nv |
| Lability | 2 | 11.8% | 4 | 14.8% | 1.000* | 2 | 18.2% | 1 | 5.3% | 0.537* |
| Anxiety | 6 | 35.3% | 13 | 48.1% | 0.402 | 4 | 36.4% | 4 | 21.1% | 0.417* |
| Depression | 9 | 52.9% | 17 | 63% | 0.510 | 6 | 54.5% | 6 | 31.6% | 0.266* |
nv, no variability in the data. *Fisher’s exact test.
Secondary outcomes
Clinical variables. Some differences emerged between the two AD groups even when considering some secondary outcomes. In particular, CM-ADs had higher NPI scores than CA-ADs (mean±SD: 12.2±8.6 versus 5.9±5.6, p = 0.012) at baseline (Table 6). In addition, in both phenotypes, the most frequent first cognitive symptom was recent memory impairment, but it was associated with confabulation exclusively in one CM-AD patient, and misidentification was the first symptom exclusively in another CM-AD patient. Furthermore, the first symptom was unspecified cognitive impairment only in the CA-AD compared with the CM-AD group (26.7%versus 0%, p = 0.038). By contrast, no difference emerged when considering additional clinical variables and neurological examination (see the Supplementary Material). Finally, the two groups of AD patients were homogeneous at baseline considering all possible confounders (Table 6).
Table 6
Comparison of demographic variables, major clinical variables, biomarkers in the CSF, and APOE genotype collected at baseline multidimensional assessment, among the three groups of cross-sectional study participants (Statistically significant differences are in bold)
| HC | CM-AD | CA-AD | HC versus | HC versus | CM-AD versus | |
| N = 40 | N = 17 | N = 30 | CM-AD | CA-AD | CA-AD | |
| Gender - Count F (%) | 28 (70) | 10 (58.8) | 19 (63.3) | p = 0.413 | p = 0.557 | p = 0.760 |
| Age - Mean±SD | 78±4.7 | 75.8±4.6 | 75.8±4.4 | p = 0.117 | p = 0.060 | p = 0.975 |
| Education - Mean±SD | 10.5±3.8 | 9.9±4.4 | 9±4.9 | p = 0.593 | p = 0.154 | p = 0.543 |
| Handedness - Count Right (%) | 40 (100) | 16 (94.1) | 28 (93.3) | p = 0.298* | p = 0.180* | p = 1.000* |
| MMSE - Mean±SD | 28.9±1.1 | 24.7±2.6 | 24.4±3 | p < 0.0001 | p < 0.0001 | p = 0.841 |
| ADL - Median (IQR) | 6 (1) | 6 (1) | 6 (1) | p = 0.789 | p = 0.994 | p = 0.798 |
| IADL - Median (IQR) | 8 (0) | 7 (3) | 7 (3) | p < 0.0001 | p < 0.0001 | p = 1.000 |
| CDR - Median (IQR) | 0 (0) | 1 (0.5) | 1 (0.5) | p < 0.0001 | p < 0.0001 | p = 0.491 |
| CDR sum of boxes Median (IQR) | 0 (0) | 3.5 (1.3) | 4 (2.5) | p < 0.0001 | p < 0.0001 | p = 0.695 |
| MCI/mild Dementia –Count (N) | – | 8/9 | 11/19 | p = 0.485 | ||
| Months from onset - Mean±SD | – | 33.4±17.8 | 30.5±19.9 | p = 0.539 | ||
| Cardiovascular risk factors-Mean±SD | 1.8±1 | 2.7±1.7 | 2.8±1.2 | p = 0.111 | p = 0.002 | p = 0.668 |
| Hachinsky scale score - Mean±SD | 1.5±1.1 | 1.9±1.2 | 1.6±1.2 | p = 0.352 | p = 0.917 | p = 0.399 |
| Familiarity for dementia - Count (%) | 8 (20) | 5 (29.4) | 9 (30) | p = 0.499* | p = 0.334 | p = 0.966 |
| Past delirium - Count (%) | 0 | 0 | 0 | nv | nv | nv |
| Drugs at first visit - Count (%) | ||||||
| Benzodiazepines | 5 (29.4) | 3 (10) | p = 0.118* | |||
| Antidepressants | – | 1 (5.9) | 2 (6.7) | p = 1.000* | ||
| Antipsychotics | – | 1 (5.9) | 0 (0) | p = 0.362* | ||
| Biomarkers in the CSF –Mean±SD | ||||||
| Aβ | – | 429.1±126.1 | 452.3±79.9 | p = 0.443 | ||
| tau | – | 692.1±548.5 | 571.3±454.1 | p = 0.421 | ||
| p-tau | – | 88.2±55.1 | 70.5±32.8 | p = 0.179 | ||
| tau/Aβ | – | 1.6±1.0 | 1.4±1.2 | p = 0.394 | ||
| APOE Genotype - Count (%) | ||||||
| ɛ2/ɛ3 | – | 2 (11.8) | 0 (0) | |||
| ɛ3/ɛ3 | – | 3 (17.6) | 17 (56.7) | |||
| ɛ2/ɛ4 | – | 1 (5.9) | 0 (0) | |||
| ɛ3/ɛ4 | – | 10 (58.8) | 9 (30) | |||
| ɛ4/ɛ4 | – | 1 (5.9) | 4 (13.3) | |||
| ɛ4 carriers | – | 12 (70.6) | 13 (43.3) | p = 0.072 | ||
| Type of onset - Count (%): | ||||||
| Insidious | – | 16 (94.1%) | 27 (90%) | p = 1.000* | ||
| Abrupt | – | 1 (5.9%) | 1 (3.3%) | p = 1.000* | ||
| Not reported | – | 0 | 2 | |||
| First cognitive symptom - Count (%): | ||||||
| Unspecified cognitive impairment | – | 0 | 8 (26.7%) | p = 0.038* | ||
| Recent memory | – | 7 (41.2%) | 9 (30%) | p = 0.437 | ||
| Recent memory + executive | – | 1 (5.9%) | 3 (10%) | p = 1.000* | ||
| Recent memory + language | – | 0 | 2 (6.7%) | p = 0.528* | ||
| Recent memory + attention | – | 0 | 1 (3.3%) | p = 1.000* | ||
| Recent memory + confabulation | – | 1 (5.9%) | 0 | p = 0.362* | ||
| Recent memory + past memory | – | 1 (5.9%) | 0 | p = 0.362* | ||
| Recent memory + temporal dis. | – | 1 (5.9%) | 0 | p = 0.362* | ||
| Recent memory + topograph. dis. | – | 1 (5.9%) | 0 | p = 0.362* | ||
| Language | – | 1 (5.9%) | 2 (6.7%) | p = 1.000* | ||
| Misidentification | – | 1 (5.9%) | 0 | p = 0.362* | ||
| Not reported | – | 3 | 5 | |||
| Course - Count (%): | ||||||
| Gradual worsening | – | 15 (88.2%) | 23 (76.7%) | p = 0.455* | ||
| Stable | – | 2 (11.8%) | 5 (29.4%) | p = 1.000* | ||
| Stepwise | – | 0 | 1(3.3%) | p = 1.000* | ||
| Not-reported | – | 0 | 1 | |||
| Fluctuations of cognition - Count (%) | 2 (5,0) | 1 (5,9) | 3 (10,0) | p = 1.000* | p = 0.645* | p = 1.000* |
| Fluctuations of attention - Count (%) | 1 (2,5) | 2 (11,8) | 1 (3,3) | p = 0.209* | p = 1.000* | p = 0.283* |
| Sundowning - Count (%) | 0 | 0 | 1 (3,3) | nv | p = 0.429* | p = 1.000* |
| NPI total score –Mean±SD | 3.2±3.2 | 12.2±8.6 | 5.9±5.6 | p < 0.0001 | p = 0.077 | p = 0.012 |
MMSE, Mini-Mental State Examination; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; CDR, Clinical Dementia Rating; APOE, apolipoprotein E; MCI, mild cognitive impairment; NPI, Neuropsychiatric Inventory; nv, no variability in the data. *Fisher’s exact test.
Cognitive testing. As expected, the HCs showed a better performance in almost all tests in the battery compared to both clinical groups of AD patients (see Supplementary Table 8). Instead, no statistically significant difference emerged between CM-ADs and CA-ADs at the baseline cognitive testing.
Visual rating of vascular burden and atrophy on MRI TI images. MRI scan at baseline was available for 7 CM-AD (41.2%) and 13 CA-AD (43.3%) patients (Table 7). The subgroup of the CM-ADs (5 females, 71.43%) had 75.71±5.12 years of age and 8±3.74 years of education. Their mean MMSE score was 22.29±5.85. The subgroup of the CA-ADs (9 females, 69.23%) had 76.62±3.48 years of age and 10.15±4.56 years of education. Their mean MMSE score was 24.54±2.70. The two groups were comparable for age (p = 0.64) education (p = 0.27) and MMSE score (p = 0.36). The sample of 15 HCs (7 females, 46.7%) had comparable mean age (69.5±6.52) and education (7.78). Their mean MMSE score was 29.25±0.96 [32].
Table 7
Study of atrophy and vascular burden on T1 and FLAIR MRI images using visual rating scales and classification of [18F]-FDG-PET scan results reported in reports. (Statistically significant differences are in bold)
| HC# | CM-AD | CA-AD | HC versus | HC versus | CM-AD versus | ||
| N = 15 | N = 7 | N = 13 | CM-AD | CA-AD | CA-AD | ||
| Range | p | p | p | ||||
| MRI-imaging | |||||||
| Exams - count (%) | 15 (100%) | 7 (41.2%) | 13 (43.3%) | ||||
| Vascularity (Fazekas scale) - Mean (SD) | |||||||
| Periventricular (PV) | 0–3 | 0.27 (0.46) | 1.57 (1.27) | 1.38 (1.19) | 0.032 | 0.019 | 0.943 |
| White matter hyperintensities (WHM) | 0–3 | 0.53 (0.74) | 1.71 (1.11) | 1.38 (1.04) | 0.043 | 0.063 | 0.789 |
| Visual scales of atrophy - Mean (SD) | |||||||
| Orbitofrontal right (OFR) | 0–3 | 0.27 (0.46) | 1.00 (0.82) | 0.92 (0.86) | 0.068 | 0.075 | 0.976 |
| Orbitofrontal left (OFL) | 0–3 | 0.27 (0.46) | 1.00 (0.82) | 0.85 (0.9) | 0.068 | 0.162 | 0.907 |
| Anterior cingulated right (ACR) | 0–3 | 0.67 (0.72) | 0.86 (0.9) | 1.23 (0.72) | 0.891 | 0.126 | 0.595 |
| Anterior cingulated left (ACL) | 0–3 | 0.67 (0.62) | 1.14 (0.7) | 0.85 (0.55) | 0.276 | 0.669 | 0.558 |
| Anterior temporal right (ATR) | 0–4 | 0.33 (0.62) | 1.43 (0.79) | 1.23 (1.01) | 0.006 | 0.0033 | 0.941 |
| Anterior temporal left (ATL) | 0–4 | 0.2 (0.41) | 1.43 (0.79) | 1.62 (1.04) | 0.001 | <0.001 | 0.869 |
| Frontoinsular right (FIR) | 0–3 | 1.20 (0.56) | 2.00 (0.58) | 1.69 (0.63) | 0.023 | 0.112 | 0.519 |
| Frontoinsular left (FIL) | 0–3 | 0.87 (0.64) | 2.00 (0.82) | 1.77 (0.83) | 0.015 | 0.012 | 0.864 |
| Medial temporal atrophy right (MTAR) | 0–4 | 0.4 (0.51) | 1.29 (0.76) | 1.31 (0.95) | 0.030 | 0.021 | 0.999 |
| Medial temporal atrophy left (MTAL) | 0–4 | 0.27 (0.46) | 1.57 (0.79) | 1.54 (1.05) | 0.004 | 0.002 | 0.976 |
| Parietal atrophy right (PAR) | 0–3 | 0.93 (0.8) | 1.86 (0.9) | 1.85 (0.99) | 0.065 | 0.049 | 0.996 |
| Parietal atrophy left (PAL) | 0–3 | 1.13 (0.74) | 1.71 (0.95) | 2.00 (0.91) | 0.272 | 0.054 | 0.851 |
| Dorsal parietal right (DPR) | 0–3 | 0.87 (0.91) | 1.57 (0.98) | 1.92 (0.86) | 0.278 | 0.019 | 0.669 |
| Dorsal parietal left (DPL) | 0–3 | 1.00 (0.84) | 1.71 (1.11) | 2.00 (0.82) | 0.304 | 0.013 | 0.821 |
| Posterior cingulate right (PCSR) | 0–3 | 1.33 (0.82) | 1.86 (0.9) | 2.00 (0.91) | 0.269 | 0.109 | 0.935 |
| Posterior cingulate left (PCSL) | 0–3 | 1.33 (1.05) | 1.86 (1.07) | 2.08 (0.82) | 0.492 | 0.129 | 0925 |
| Precuneus right (PRER) | 0–3 | 0.67 (0.72) | 1.29 (0.76) | 1.54 (0.88) | 0.192 | 0.030 | 0.866 |
| Precuneus left (PREL) | 0–3 | 0.8 (0.68) | 1.57 (0.98) | 1.77 (0.83) | 0.144 | 0.012 | 0.940 |
| Parieto-occipital right (POSR) | 0–3 | 1.13 (0.74) | 2.14 (1.07) | 1.77 (1.09) | 0.053 | 0.190 | 0.710 |
| Parieto-occipital left (POSL) | 0–3 | 1.07 (0.88) | 1.86 (1.07) | 2 (1.22) | 0.195 | 0.078 | 0.869 |
| [18F]-FDG-PET-imaging –count (%) | |||||||
| Exams | – | 10 (58.8%) | 13 (43.3%) | 0.307 | |||
| Positive/negative | – | 10/0 | 12/1 | ||||
| Temporal + Parietal | – | 4 (40%) | 5 (38.5%) | 1.000* | |||
| Focal Temporal | – | 3 (30%) | 4 (30.8%) | 1.000* | |||
| Temporal + Parietal + Occipital | – | 1 (10%) | 2 (15.4%) | 1.000* | |||
| Temporal + Frontal | – | 1 (10%) | 0 | 0.435* | |||
| Parietal + cingulum | – | 0 | 1 (7.7%) | 1.000* | |||
| Mild diffuse | – | 1 (10%) | 0 | 0.435* | |||
| Total medial temporal | – | 5 (50%) | 4 (30.8%) | 0.417* | |||
| Total Temporal | – | 9 (90%) | 11 (84.6%) | 1.000* | |||
| Total Parietal | – | 5 (50%) | 8 (61.5%) | 0.685* | |||
| Total Occipital | – | 1 (10%) | 2 (15.4%) | 1.000* | |||
| Total Frontal | – | 1 (10%) | 2 (15.4%) | 1.000* | |||
| Bilateral | – | 4 (40%) | 1 (7.7%) | 0.127* | |||
| right > left | – | 4 (40%) | 5 (38.5%) | 1.000* | |||
| left > right | – | 2 (20%) | 6 (46.1%) | 0.379* |
#The sample of healthy people was recruited in the Neurologic Unit of the same Hospital and it has been described in a previous article (reference [26] in the Supplementary Material). *Fisher’s exact test.
Both CM-ADs and CA-ADs showed more vascular burden than HCs by the periventricular Fazekas scale (PV) (p = 0.032 and 0.019 respectively) on Flair images. In addition, both the groups showed more vascular burden at the Fazekas scale for WMH, but the difference from HCs did not reach significance in the CA-ADs (p = 0.043 and 0.063 respectively). Instead, no differences emerged between CM-ADs and CA-ADs in vascular burden at both the Fazekas PV and WMH scales.
The neurologist rated more medial temporal (right: p = 0.030; left, p = 0.004), anterior temporal (right, p = 0.006; left, p = 0.001) and fronto-insular atrophy (right, p = 0.023; left, p = 0.015) in the CM-AD group compared to the HCs on T1 images. Likewise, CA-ADs showed more atrophy than the HCs at the medial temporal (right: p = 0.0021; left, p = 0.002), anterior temporal (right, p = 0.0033; left, p < 0.001) and left fronto-insular scales (right, p = ns; left, p = 0.012). In addition, CA-ADs presented more atrophy than HCs also at the dorsal parietal (right: p = 0.019; left, p = 0.013) and precuneus (right: p = 0.030; left, p = 0.012) scales. Instead, no statistically significant differences emerged between the two CM-AD and CA-AD groups.
Analysis of the [18F]-FDG PET scan outcomes reported in the charts. Ten CM-ADs (58.8%) and 13 CA-ADs (43.3%) underwent [18F]-FDG PET scan (Table 7). Substantial cortical glucose hypo-metabolism emerged in all the CM-ADs and in 12/13 of the CA-ADs. A similar pattern of cortical involvement, mainly temporal-parietal and focal temporal, including medial temporal, emerged in both groups. Moreover, some data suggested that hypo-metabolism in the CM-ADs might be more frequently bilateral compared to the CA-ADs (40%versus 7.7%, p = 0.127). Accordingly, more left than right hypo-metabolism emerged more frequently in CA-ADs compared to CM-ADs (46.1%versus 20%, p = 0.379). Unfortunately, these last comparisons did not reach statistical significance.
DISCUSSION
We described a small group of patients diagnosed with late-onset AD supported by positive biomarkers in the CSF who presented an amnesic phenotype quite different from the CA-AD. In particular, anterograde amnesia dominated both CM-AD and the CA-AD phenotypes, but in the present case series of CM-AD patients, it is frequently associated with more confabulation, disorientation, delusion not-misidentification, misidentification, and logorrhea even at early disease stages. Further common cognitive features were retrograde amnesia, reduction of insight, attention deficits, and executive impairment. Among these symptoms, the most salient cognitive feature of the new phenotype was confabulation. It emerged early and often included spontaneous confabulation other than provoked confabulation at testing. Interestingly, spontaneous confabulation was the first symptom in one patient. Another distinguishing characteristic of the new phenotype was misidentification. Misidentification often emerged at intermediate stages, but in some cases, it was early, and in one patient it was the first symptom. In addition, misidentification was often severe and involved multiple manifestations such as close relatives, the patient’s home, and TV celebrities in the same patient. Delusion not-misidentification was quite early and common as well, but it was ever infrequent and unstructured compared to misidentification. Similarly, misinterpretation and hallucination were present but were ever brief and sporadic. Further distinctive characteristics of the new phenotype were more early and frequent behavioral disturbances compared to the CA-phenotype. We recognized early soft signs of the hyperactive-disinhibition syndrome, which included mild logorrhea in a subset of patients, verbal distractibility, euphoria/fatuity, and social disinhibition. More severe signs of the hyperactive-disinhibition syndrome with psychomotor agitation, aggression, and hyperphagia, became common features including data from later stages. In parallel, signs of the a-dynamic syndrome emerged with apathy, sleep disturbances, especially central insomnia associated with nocturnal hyperactivity and wandering, and affective symptoms with more anxiety than depression in the early stages. By contrast, CA-ADs seemed to show more language deficits and depression in the early stages than CM-ADs. Some preliminary results of the longitudinal study suggested that the CM-phenotype was enduring. Indeed, follow-up data confirmed that some distinctive features of the CM-phenotype were still more frequent in the CM-ADs than CA-ADs at 12 months follow-up. Moreover, raw differences in the frequency of many cognitive and behavioral features between CM-ADs and CA-ADs appeared to persist concordantly at 24 months after baseline.
Considering the neuroanatomical substrate of the possible CM-phenotype, we found that a subgroup of CM-ADs and CA-ADs showed a similar pattern of cortical hypometabolism, mainly temporal-parietal and focal temporal, including medial temporal, on [18F]-FDG PET scanning. However, preliminary data suggest that CM-ADs may have more symmetrical involvement than CA-ADs, which more often showed asymmetrical hypometabolism on the left side. The study of vascular burden and atrophy by visual rating scales on FLAIR and T1 MRI images, respectively, found no significant differences between the two subgroups of CM and CA-AD patients. In particular, both subgroups had greater perivascular and white matter hyperintensities than HCs. In addition, both groups showed greater anterior temporal, medial temporal, and left fronto-insular atrophy than HCs. However, the CM-AD subgroup also showed greater right fronto-insular atrophy than HCs, while the CA-AD subgroup also showed greater dorsal parietal, precuneus, and right parietal atrophy than HCs. These results suggest that CM-ADs may have somewhat more anterior and especially less posterior atrophy than CA-ADs.
Taken together, all the findings reported above lead us to hypothesize that the CM-phenotype would be a currently unrecognized distinct presentation of AD. The following data seem preliminarily support this hypothesis (Table 8).
Table 8
Several sources of evidence supporting the view of the CM-phenotype as a distinct presentation of AD
| Confabulation-Misidentification | Classical Amnesic | |
| (CM-) phenotype | (CA-) phenotype | |
| Results of cross-sectional study | -more confabulation, disorientation, delusion not-misidentification, misidentification and logorrhea than CA-AD | -more language and depression at early stages than CM-AD* |
| -more early and frequent behavioral disturbances than CA-AD (NPI) | ||
| -more right frontoinsular atrophy than HCs at MRI scan | -more right parietal, dorsal parietal, and precuneus atrophy than HCs at MRI scan | |
| -more symmetric temporal hypometabolism at FDG-PET scan compared to CA-AD* | -more left-sided temporal hypometabolism at FDG-PET scan compared to CM-AD* | |
| Rate and onset time of confabulation and misidentification | -frequent confabulators; total amount of confabulation is higher and earlier than in the CA-ADs | -mild confabulators; the amount of confabulation increases with the severity of cognitive decline |
| -spontaneous confabulation more frequent and early | -spontaneous confabulation is an infrequent phenomenon, especially at early stages | |
| -higher prevalence of misidentification | -misidentification less frequent | |
| Features presenting associated | -greater tendency to confabulate in AD patients with delusions and/or aggression and in those with delusions alone | |
| -factor analysis of AD psychosis symptoms identified a “misidentification” subtype, consisting of misperceptions, misidentification, and visual or auditory hallucinations | -factor and cluster analysis of AD psychosis symptoms identified a nonpsychotic and a paranoid subtype distinct from the misidentification subtype | |
| Similarity to Korsakoff syndrome | -The CM-phenotype is similar to Korsakoff syndrome (KS) which is considered the prototype of diencephalic amnesia | -the classical phenotype of AD is hippocampal amnesia |
| Resemblance with Presbyophrenia | -The CM-phenotype seems to coincide with presbyophrenia | |
| -presbyophrenia has been recognized as similar to KS | -the classical phenotype of AD is hippocampal amnesia |
*differences not statistically significant.
First, the cross-sectional study confirmed that many features that we recognized as distinctive in the possible CM-phenotype in the case-series study were indeed more frequent in the CM-AD group than in the CA-AD group. This fact supported a distinction between the CM- and CA-phenotype, against the fact that the two groups of AD patients were homogeneous for many possible confounders.
Second, the two most salient features of the possible CM-phenotype, namely confabulation and misidentification, are usually reported to be less common and early in AD. In this regard, previous studies have found that AD patients are mild confabulators [38, 39], and the amount of confabulation increases with the severity of cognitive decline [40]. Accordingly, provoked confabulations [41] on testing, often simple intrusions [42], have been reported in the early stages of AD [40, 43, 44], whereas overt and more elaborate confabulations appear to be associated with severe AD [38, 45]. Furthermore, spontaneous confabulation [41] is thought to be an infrequent phenomenon in AD, especially in its early stages [46]. By contrast, AD patients in the present case-series were in some cases frequent overt confabulators who resembled patients with focal brain damage. Moreover, they showed spontaneous confabulation at a very early stage of dementia corresponding to MCI or mild dementia. Similarly, previous studies have reported a median prevalence of 25.6%(range: 3.6–38.9%) of misidentification phenomena in AD [47–52]. Furthermore, misidentification increases in prevalence as the disease progresses [50, 53] and has been associated with greater global cognitive deficits and advanced limbic pathology [47]. By contrast, misidentification in the current case-series reached 52.9%, and emerged at very early stages in some patients. Interestingly, misidentifications are commonly reported in patients with dementia with Lewy bodies [54–56]. Furthermore, in a study that included autopsy-confirmed cases of LBD, AD, and AD with amygdala-dominant Lewy bodies (AD-ALB), the authors found that family misidentification was present in all three groups but was more common in the AD-ALB group (93%). However, as the authors pointed out, patients in the AD-ALB group had a longer estimated disease duration, a higher number of limbic tau neurofibrillary tangles, and were at an advanced stage of the disease. Therefore, it was unclear whether amygdala Lewy bodies played a specific role in misidentification or whether the high presence in this AD subgroup was a reflection of general advanced pathology [57]. The possible contribution of concomitant Lewy body pathology on the expression of misidentification and other features in CM-AD remains an important question for future research.
Third, previous studies found that some features among those characterizing the possible CM-phenotype were indeed associated with some AD patients. For example, a greater tendency to confabulate about personal/autobiographical events has been reported in AD patients with delusions and/or aggression [58] and in those with delusions alone [59, 60]. In addition, factor analysis of AD psychosis symptoms identified a “misidentification” subtype, consisting of misperceptions, misidentification, and visual or auditory hallucinations [47, 61].
Fourth, multiple features of the possible CM-phenotype overlapped with key features of diencephalic amnesia, the prototype of which is the Korsakoff syndrome (KS) [62, 63], rather than the typical hippocampal amnesic syndrome of AD. Indeed, KS is characterized by anterograde and retrograde amnesia, executive dysfunction, confabulation, and apathy, as well as affective and social-cognitive impairment [62, 63]. KS alcoholic patients have been described as emotionally flat and affectively detached [62–65], but also affectively unstable in the early stages, often being irritable or euphoric [64–67]. In addition, suspicion and intense emotions are easily provoked [64–67]. The similarity between the CM-phenotype and the KS, which is recognized to have a neural-anatomical substrate distinct from that of CA-AD, not only gives some anatomical foundation to the CM-phenotype, but also further supports the hypothesis that it is a distinct clinical manifestation of AD.
Fifth, the complex phenotype we recognized in the case-series patients has already been reported. Indeed, there is a striking overlap between the CM-phenotype and the historical description of presbyophrenia. This now-forgotten term was introduced by Kahlbaum in 1863 [68] to describe an age-related form of paraphrenic psychosis found in the elderly and characterized by disorientation, amnesia, delusional misidentification, and confabulation [68]. Then, starting from early in the twentieth century the concept of presbyophrenia was used to refer to a subtype of dementia characterized by memory deficits, disorientation, confabulation, hyperactivity, euphoric or irritable mood, and preservation of social façade [2, 3, 68]. In addition, delusional misidentification may be a part of it, and a more frankly manic presentation may emerge [68]. Unfortunately, as reported by Berrios [3], the concept of presbyophrenia began to decline in the 1920 s as dementia was redefined in terms of impaired cognition, cortical symptomatology, and specific neuropathological alterations. However, there have been sporadic more recent reports of presbyophrenia. Specifically, Berrios described a cohort of 15 cases in 1985 [2], and Zervas and colleagues reported three cases in 1993 [68]. Interestingly, a striking clinical similarity between presbyophrenia and the amnestic syndrome described by Korsakoff was noted early in the last century [3]. Accordingly, some authors have described presbyophrenia as “a chronic Korsakoff-like picture with no history of alcoholism and no polyneuritic signs” [68]. Not only that, early data linked presbyophrenia to AD. Oskar Fisher in the same year as Alzheimer’s seminal article published a clinicopathologic study of 16 cases of dementia, 12 of which had neuritic plaques [69, 70]. He studied whether clinical symptoms distinguished the 12 cases from the other four and found that the cases with neuritic plaques had features compatible with a clinical diagnosis of presbyophrenia. Our study confirmed the association between a presbyophrenic-like syndrome, which we renamed the CM-phenotype, and AD, because the CM-AD patients in our study had positive biomarkers for AD in the CSF. Moreover, our results were in line with Berrios’ conclusion about the unfortunate fate of the concept of presbyophrenia. Indeed, we (re)found a clear presbyophrenic phenotype exclusively by detailed clinical evaluation, while common cognitive testing, routine imaging, and CSF biomarkers could not discriminate between CA- and CM-AD groups.
This study has some limitations. First, the longitudinal data about cognitive and behavioral features were limited to brief reports by informants and some clinical observations from geriatricians. Second, there was a lack of geriatric follow-up for many patients. Thus, we have no valid longitudinal data on the course of the CM-phenotype over time, especially later than 24 months after baseline. Closely related, the description of the possible CM-phenotype has focused primarily on the first presentation at baseline. Third, because of the retrospective nature of the study and the incompleteness of the longitudinal data collected, we cannot completely rule out misclassification, especially for patients presenting with a CA-phenotype at baseline. We partially compensate for this limitation by reporting in a supplementary analysis that CA-ADs who developed confabulation and/or misidentification at follow-up (positive-CA) were few and were in advanced stages of dementia when the core features of the CM-phenotype appeared. Fourth, we cannot exclude that the frequency of the possible CM-phenotype among other AD presentations might be overestimated in our outpatient registry. Indeed, due to the advanced age of outpatients, we look for AD biomarkers in the CSF in a minority of patients only in our Geriatric Unit, forcing the execution of the rachicentesis, especially for those patients who presented peculiar features at the first visit, as confabulation and misidentification. As a consequence, the frequency of patients with the possible CM-phenotype in our registry of AD patients could not represent the true frequency of this peculiar clinical manifestation. Finally, our imaging data were limited to the assessment of atrophy using visual rating scales in a subset of participants. Indeed, we cannot perform more advanced analyses of whole-brain atrophy because of the limited number of examinations available, the technical differences between examinations, and the limited number of slices provided in routine clinical imaging. Therefore, we did not perform any statistical imaging analysis, such as voxel-based morphometry, and this was another limitation of our study.
AD is clinically heterogeneous, grouping different clinical anatomical syndromes [71]. In particular, some atypical phenotypes (variants) are currently recognized and included in the diagnostic criteria for AD [17] together with the CA-phenotype (i.e., posterior cortical atrophy, logopenic, frontal–executive) [72–74]. Interestingly, the variants accepted in the current nosology seem not to complete the full spectrum of clinical presentations of AD. Indeed, also the corticobasal syndrome can be considered an unusual presentation of AD [75]. In addition, further different phenotypes of AD have been reported anecdotally [76]. An accurate characterization of the clinical–radiological syndromes of AD is crucial to improve clinical decisions (e.g., early diagnosis, symptoms management, counseling to caregivers, planning proper cognitive rehabilitation) and support the development of ad hoc interventions. Moreover, a better knowledge of phenotypic diversity in AD seems to be an essential step toward a broader understanding of dementia [77]. In this context, our results could contribute to further refining the full spectrum of the clinical presentations of AD. In particular, we (re)found a possible amnestic AD phenotype, which is different from the CA phenotype from a clinical point of view. Unfortunately, our imaging study suggested that the differentiation between CM-AD and CA-AD might be rather difficult from a neuroanatomical point of view. This fact was not unexpected for two reasons. First, unlike the other recognized phenotypic variants of AD, which primarily affect a region other than the medial temporal lobe (MTL) and impair a cognitive faculty other than memory, the possible CM-phenotype is an amnesic phenotype that affects the MTL like CA-AD. However, our preliminary findings of a more symmetrical, more anterior (fronto-insular), and less posterior (dorsal parietal and precuneus) atrophy in CM-ADs might be promising starting points to study the neuroanatomical differences between the two amnestic phenotypes of AD. Second, we believe that it might be difficult to show early damage to some of the brain regions involved in the possible CM-phenotype. Indeed, we hypothesized what the neuroanatomical substrate of the CM phenotype might be, considering the results of previous studies on the substrate of KS [62, 63] and spontaneous confabulation [78, 79], as well as evidence of misidentification in AD [47, 48, 80]. Data on clinical anatomical correlations found in the literature seem to converge on a possible common limbic-diencephalic network [81] involving limbic structures (e.g., thalamic nuclei, mammillary bodies) and the frontal regions directly connected with them (e.g., medial and posterior orbitofrontal cortex), the hippocampus with its projection areas (e.g., parahippocampal gyrus and transentorhinal cortex) and other possible related structures (e.g., amygdala). At this point, it is worth noting that, for example, neuroimaging in humans has provided little or no evidence regarding the locations and nature of thalamic lesions at the nuclear level, as conventional structural MRI sequences in the whole brain do not provide high-resolution contrast between different thalamic nuclei [82].
Our study was on a small group of patients. It will be necessary to replicate our observations in a larger number of patients with late- and early-onset AD to generalize our findings. In addition, a valid longitudinal study of the course of the possible CM-phenotype over time is lacking. In addition, it will be important to plan adequate imaging studies to better characterize the neural substrate of the possible CM-phenotype, possibly keeping in mind the hypothesis of a limbic-diencephalic network. The ultimate goal will be to define a clinical-anatomical syndrome that supports the view of a new (yet old) diencephalic variant of AD.
ACKNOWLEDGMENTS
This study was supported and funded by the Italian Ministry of Health - Ricerca Corrente.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0919r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220919.
REFERENCES
[1] | Abbate C , Trimarchi PD , Rotondo E , Inglese S , Nicolini P , Rossi PD , Arosio B , Mari D ((2016) ) Spontaneous confabulations in amnestic-mild cognitive impairment due to Alzheimer’s disease: A new (yet old) atypical variant? Neurocase 22: , 451–460. |
[2] | Berrios GE ((1985) ) Presbyophrenia: Clinical aspects. BJPsych 147: , 76–79. |
[3] | Berrios GE ((1986) ) Presbyophrenia: The rise and fall of a concept. Psychol Med 16: , 267–275. |
[4] | Nedjam Z , Dalla Barba G , Pillon B ((2000) ) Confabulation in a patient with frontotemporal dementia and a patient with Alzheimer’s disease. Cortex 36: , 561–577. |
[5] | Moulin CJA ((2013) ) Disordered recognition memory: Recollective confabulation. Cortex 49: , 1541–1552. |
[6] | Belli E , Nicoletti V , Radicchi C , Bonaccorsi J , Cintoli S , Ceravolo R , Tognoni G ((2020) ) Confabulations in cases of dementia: Atypical early sign of Alzheimer’s disease or misleading feature in dementia diagnosis? Front Psychol 11: , 553886. |
[7] | Abbate C , Trimarchi PD ((2013) ) Clinical neuropsychologists need a standard preliminary observational examination of cognitive functions. Front Psychol 4: , 314. |
[8] | Abbate C , Trimarchi PD , Inglese S , Tomasini E , Bagarolo R , Giunco F , Cesari M ((2021) ) Signs and symptoms method in neuropsychology: Apreliminary investigation of a standardized clinical interview forassessment of cognitive decline in dementia. Appl NeuropsycholAdult 28: , 282–296. |
[9] | Folstein MF , Folstein SE , McHugh PR ((1975) ) Mini-Mental State. J Psychiatr Res 12: , 189–198. |
[10] | Katz S , Downs TD , Cash HR , Grotz RC ((1970) ) Progress in development of the Index of ADL. Gerontologist 10: , 20–30. |
[11] | Lawton MP , Brody EM ((1969) ) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: , 179–186. |
[12] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2308. |
[13] | Hughes CP , Berg L , Danziger WL , Coben LA , Martin RL ((1982) ) A new clinical scale for the staging of dementia. BJPsych 140: , 566–72. |
[14] | Hachinski VC , Iliff LD , Zilhka E , Du Boulay GH , McAllister VL , Marshall J , Ross Russell RW , Symon L ((1975) ) Cerebral blood flow in dementia. Arch Neurol 32: , 632–7. |
[15] | Hachinski V , Oveisgharan S , Romney AK , Shankle WR ((2012) ) Optimizing the Hachinski Ischemic Scale. Arch Neurol 69: , 169–75. |
[16] | Albert MS , Dekosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[17] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[18] | Basso A , Capitani E , Laiacona M ((1987) ) Raven’s colored progressive matrices: Normative values on 305 adult normal controls. Funct Neurol 2: , 189–194. |
[19] | Giovagnoli AR , Del Pesce M , Mascheroni M , Simoncelli M , Laiacona M , Capitani E ((1996) ) Trail Making Test: Normative values from 287 normal adult controls. Ital J Neurol Sci 17: , 305–309. |
[20] | Novelli G , Papagno C , Capitani E , Laiacona M ((1986) ) Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatr 47: , 477–506. |
[21] | Monaco M , Costa A , Caltagirone C , Carlesimo G ((2013) ) Forward and backward span for verbal and visuospatial data: Standardization and normative data from an Italian adult population. Neurol Sci 34: , 749–754. |
[22] | Orsini A , Grossi D , Capitani E , Laiacona M , Papagno C , Vallar G ((1987) ) Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8: , 537–548. |
[23] | Caffarra P , Vezzadini G , Dieci F , Zonato F , Venneri A ((2002) ) Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol Sci 22: , 443–447. |
[24] | Spinnler H , Tognoni G ((1987) ) Italian Group on the Neuropsychological Study of Ageing: Italian standardization and classification of neuropsychological tests. Ital J Neurol Sci 6: (suppl 8), 1–120. |
[25] | Gauthier L , Dehaut F , Joanette Y ((1989) ) The bells test: A quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol 11: , 49–54. |
[26] | Vallar G , Rusconi ML , Fontana S , Musicco M ((1994) ) Tre test di esplorazione visuo-spaziale: Taratura su 212 soggetti normali. Arch Psicol Neurol Psichiatr 55: , 827–841. |
[27] | Laiacona M , Barbarotto R , Trivelli C , Capitani E ((1993) ) Dissociazioni semantiche intercategoriali: Descrizione di una batteria standardizzata e dati normativi. Arch Psicol Neurol Psichiatr 54: , 209–248. |
[28] | De Renzi E ((1985) ) Methods of limb apraxia examination and their bearing on the interpretation of the disorder. In Neuropsychological studies of apraxia and related disorders, Roy EA, ed . North Holland, Amsterdam, pp. 45–64. |
[29] | Fumagalli GG , Basilico P , Arighi A , Bocchetta M , Dick KM , Cash DM , Harding S , Mercurio M , Fenoglio C , Pietroboni AM , Ghezzi L , van Swieten J , Borroni B , de Mendonça A , Masellis M , Tartaglia MC , Rowe JB , Graff C , Tagliavini F , Frisoni GB , Laforce R Jr , Finger E , Sorbi S , Scarpini E , Rohrer JD , Galimberti D ((2018) ) Distinct patterns of brain atrophy in Genetic Frontotemporal Dementia Initiative (GENFI) cohort revealed by visual rating scales. Alzheimers Res Ther 10: , 46. |
[30] | Harper L , Fumagalli GG , Barkhof F , Scheltens P , O’Brien JT , Bouwman F , Burton EJ , Rohrer JD , Fox NC , Ridgway GR , Schott JM ((2016) ) MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain 139: , 1211–1225. |
[31] | Rorden C , Karnath HO , Bonilha L ((2007) ) Improving lesion-symptom mapping. J Cogn Neurosci 19: , 1081–1088. |
[32] | Fumagalli GG , Basilico P , Arighi A , Mercurio M , Scarioni M , Carandini T , Colombi A , Pietroboni AM , Sacchi L , Conte G , Scola E , Triulzi F , Scarpini E , Galimberti D ((2020) ) Parieto-occipital sulcus widening differentiates posterior cortical atrophy from typical Alzheimer disease. Neuroimage Clin 28: , 102453. |
[33] | Carandini T , Arighi A , Sacchi L , Fumagalli GG , Pietroboni AM , Ghezzi L , Colombi A , Scarioni M , Fenoglio C , De Riz MA , Marotta G , Scarpini E , Galimberti D ((2019) ) Testing the 2018 NIA-AA research framework in a retrospective large cohort of patients with cognitive impairment: From biological biomarkers to clinical syndromes. Alzheimers Res Ther 11: , 84. |
[34] | De Meyer G , Shapiro F , Vanderstichele H , Vanmechelen E , Engelborghs S , De Deyn PP , Coart E , Hansson O , Minthon L , Zetterberg H , Blennow K , Shaw L , Trojanowski JQ ((2010) ) Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67: , 949–956. |
[35] | Palmqvist S , Zetterberg H , Mattsson N , Johansson P , Minthon L , Blennow K , Olsson M , Hansson O ((2015) ) Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 85: , 1240–1249. |
[36] | Mo Y , Stromswold J , Wilson K , Holder D , Sur C , Laterza O , Savage MJ , Struyk A , Scheltens P , Teunissen CE , Burke J , Macaulay SL , Brathen G , Sando SB , White LR , Weiss C , Cowes A , Bush MM , DeSilva G , Darby DG , Rainey-Smith SR , Surls J , Sagini E , Tanen M , Altman A , Luthman J , Egan MF ((2017) ) A multinational study distinguishing Alzheimer’s and healthy patients using cerebrospinal fluid tau/Aβ42 cutoff with concordance to amyloid positron emission tomography imaging. Alzheimers Dement 6: , 201–209. |
[37] | Ferri E , Gussago C , Casati M , Mari D , Rossi PD , Ciccone S , Cesari M , Arosio B ((2019) ) Apolipoprotein E gene in physiological and pathological aging. Mech Ageing Dev 178: , 41–45. |
[38] | Dalla Barba G , Nedjam Z , Dubois B ((1999) ) Confabulation, executive functions, and source memory in Alzheimer’s disease. Cogn Neuropsychol 16: , 385–398. |
[39] | La Corte V , Serra M , Attali E , Boissé M-F , Dalla Barba G ((2010) ) Confabulation in Alzheimer’s disease and amnesia: A qualitative account and a new taxonomy. J Int Neuropsychol Soc 16: , 967–974. |
[40] | Talberg I-M , Almkvist O ((2001) ) Confabulation and memory in patients with Alzheimer’s disease. J Clin Exp Neuropsychol 23: , 172–184. |
[41] | Kopelman MD ((2010) ) Varieties of confabulation and delusion. Cogn Neuropsychiatry 15: , 14–37. |
[42] | Attali E , De Anna F , Dubois B , Dalla Barba G ((2009) ) Confabulation inAlzheimer’s disease: Poor encoding and retrieval of over-learnedinformation. Brain 132: , 204–212. |
[43] | Pelati O , Castiglioni S , Isella V , Zuffi M , de Rino F , Mossali I , Franceschi M , Pelati O ((2011) ) When Rey-Osterrieth’s complex figure becomes a Church. Prevalence and correlates of graphic confabulations in dementia. Dement Geriatr Cogn Dis Extra 1: , 372–380. |
[44] | van der Linde R , Stephan BCM , Matthews FE , Brayne C , Savva GM ((2010) ) Behavioral and psychological symptoms in the older population without dementia–relationship with socio-demographics, health and cognition. BMC Geriatrics 10: , 87. |
[45] | Cooper JM , Shanks MF , Venneri A ((2006) ) Provoked confabulations in Alzheimer’s disease. Neuropsychologia 44: , 1697–1707. |
[46] | El Haj M , Larøi F ((2017) ) Provoked and spontaneous confabulations in Alzheimer’s disease: A n examination of their prevalence and relation with general cognitive and executive functioning. Psychiatry Clin Neurosci 71: , 61–69. |
[47] | Reeves SJ , Gould RL , Powell JF , Howard RJ ((2012) ) Origins of delusions in Alzheimer’s disease. Neurosci Biobehav Rev 36: , 2274–2287. |
[48] | Ropacki SA , Jeste DV ((2005) ) Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am J Psychiatry 162: , 2022–2030. |
[49] | Harciarek M , Kertesz A ((2008) ) The prevalence of misidentification syndromes in neurodegenerative diseases. Alzheimer Dis Assoc Disord 22: , 163–169. |
[50] | Savva GM , Zaccai J , Matthews FE , Davidson JE , McKeith I , Brayne C ((2009) ) Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. BJPsych 194: , 212–219. |
[51] | Steinberg M , Shao H , Zandi P , Lyketsos CG , Welsh-Bohmer KA , Norton MC , Breitner JCS , Steffens DC , Tschanz JoAT , Cache County Investigators ((2008) ) Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. Int J Geriatr Psychiatry 23: , 170–177. |
[52] | Sweet RA , Bennett DA , Graff-Radford NR , Mayeux R ((2010) ) Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain 133: , 1155–1162. |
[53] | Devanand DP , Jacobs DM , Tang M-X , Del Castillo-Castaneda C , Sano M , Marder K , Bell K , Bylsma FW , Brandt J , Albert M , Stern Y ((1997) ) The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry 54: , 257–263. |
[54] | Perini G , Carlini A , Pomati S , Alberoni M , Mariani C , Nemni R , Farina E ((2016) ) Misidentification delusions. Alzheimer Dis Assoc Disord 30: , 331–337. |
[55] | Nagahama Y , Fukui T , Akutagawa H , Ohtaki H , Okabe M , Ito T , Suga H , Fujishiro H ((2020) ) Prevalence and clinical implications of the mirror and TV signs in advanced Alzheimer’s disease and dementia with Lewy bodies. Dement Geriatr Cogn Dis Extra 10: (1), 56–62. |
[56] | Naasan G , Shdo SM , Rodriguez EM , Spina S , Grinberg L , Lopez L , Karydas A , Seeley WW , Bruce L Miller BL , Rankin KP ((2021) ) Psychosis in neurodegenerative disease: Differential patterns of hallucination and delusion symptoms. Brain 144: , 999–1012. |
[57] | Ferman TJ , Arvanitakis Z , Fujishiro H , Duara R , Parfitt F , Purdy M , Waters C , Barker W , Graff-Radford NR , Dickson DW ((2013) ) Pathology and temporal onset of visual hallucinations, misperceptions and family misidentification distinguishes dementia with Lewy bodies from Alzheimer’s disease. Parkinsonism Relat Disord 19: , 227–231. |
[58] | Lee E , Akanuma K , Meguro M , Ishii H , Yamaguchi S , Meguro K ((2007) ) Confabulations in remembering past and planning future are associated with psychiatric symptoms in Alzheimer’s disease. Arch Clin Neuropsychol 22: , 949–956. |
[59] | Lee E , Kinomura S , Meguro K , Akanuma K , Meguro M , Fukuda H ((2009) ) Confabulations on episodic and semantic memory questions are associated with different neurologic backgrounds in Alzheimer disease. Cogn Behav Neurol 22: , 81–88. |
[60] | Lee E , Meguro K , Hashimoto R , Meguro M , Ishii H , Yamaguchi S , Mori E ((2007) ) Confabulations in episodic memory are associated with delusions in Alzheimer’s disease. J Geriatr Psychiatry Neurol 20: , 34–40. |
[61] | Cook SE , Miyahara S , Bacanu S-A , Perez-Madrinan G , Lopez OL , Kaufer DI , Nimgaonkar VL , Wisniewski SR , DeKosky ST , Sweet RA ((2003) ) Psychotic symptoms in Alzheimer disease: Evidence for subtypes. Am J Geriatr Psychiatry 11: , 406–413. |
[62] | Kopelman MD , Thomson AD , Guerrini I , Marshall EJ ((2009) ) The Korsakoff syndrome: Clinical aspects, psychology and treatment. Alcohol Alcohol 44: , 148–154. |
[63] | Arts NJ , Walvoort SJ , Kessels RP ((2017) ) Korsakoff’s syndrome: A critical review. Neuropsychiatr Dis Treat 13: , 2875–2890. |
[64] | Korsakoff SS ((1889) ) Mental disturbances in combination with polyneuritis (psychosis polyneuritica, s. cerebropathia psychica toxaemica). Medizinskoje Obozr 32: , 3–18. |
[65] | Korsakoff SS ((1889) ) Some cases of individual cerebropathy in polyneuritis (cerebropathia psychica toxaemica). Ezhenedelnaja Klin Gaz 9: , 85–92. |
[66] | Talland G ((1965) ) Deranged Memory: A Psychonomic Study of the Amnesic Syndrome. Academic Press, New York. |
[67] | Bonhoeffer K (1901) Die Akuten Geisteskrankheiten Der Gewohnheitstrinker. Fischer, Jena. |
[68] | Zervas IM , Fliesser JM , Woznicki M , Fricchione GL ((1993) ) Presbyophrenia: A possible subtype of dementia. J Geriatr Psychiatry Neurol 6: , 25–28. |
[69] | Goedert M ((2009) ) Oskar Fischer and the study of dementia. Brain 132: , 1102–1111. |
[70] | Fischer O ((1907) ) Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmassige Veranderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiat Neurol 22: , 361–72. |
[71] | Lam B , Masellis M , Freedman M , Stuss DT , Black SE ((2013) ) Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther 5: , 1. |
[72] | Crutch SJ , Schott JM , Rabinovici GD , Murray M , Snowden JS , van der Flier WM , Dickerson BC , Vandenberghe R , Ahmed S , Bak TH , Boeve BF , Butler C , Cappa SF , Ceccaldi M , Cruz de Souza L , Dubois B , Felician O , Galasko D , Graff-Radford J , Graff-Radford NR , Hof PR , Krolak-Salmon P , Lehmann M , Magnin E , Mendez MF , Nestor PJ , Onyike CU , Pelak VS , Pijnenburg Y , Primativo S , Rossor MN , Ryan NS , Scheltens P , Shakespeare TJ , Suarez Gonzalez A , Tang-Wai DF , Yong KXX , Carrillo M , Fox NC ((2017) ) Consensus classification of posterior cortical atrophy. Alzheimers Dement 13: , 870–884. |
[73] | Gorno-Tempini ML , Brambati SM , Ginex V , Ogar J , Dronkers NF , Marcone A , Perani D , Garibotto V , Cappa SF , Miller BL ((2008) ) The logopenic/phonological variant of primary progressive aphasia. Neurology 71: , 1227–1234. |
[74] | Ossenkoppele R , Pijnenburg YAL , Perry DC , Cohn-Sheehy BI , Scheltens NME , Vogel JW , Kramer JH , van der Vlies AE , La Joie R , Rosen HJ , van der Flier WM , Grinberg LT , Rozemuller AJ , Huang EJ , van Berckel BNM , Miller BL , Barkhof F , Jagust WJ , Scheltens P , Seeley WW , Rabinovici GD ((2015) ) The behavioral/dysexecutive variant of Alzheimer’s disease: Clinical, neuroimaging and pathological features. Brain 138: , 2732–2749. |
[75] | Armstrong MJ , Litvan I , Lang AE , Bak TH , Bhatia KP , Borroni B , Boxer AL , Dickson DW , Grossman M , Hallett M , Josephs KA , Kertesz A , Lee SE , Miller BL , Reich SG , Riley DE , Tolosa E , Tr oster AI , Vidailhet M , Weiner WJ ((2013) ) Criteria for the diagnosis of corticobasal degeneration. Neurology 80: , 496–503. |
[76] | Snowden JS , Stopford CL , Julien CL , Thompson JC , Davidson Y , Gibbons L , Pritchard A , Lendon CL , Richardson AM , Varma A , Neary D , Mann DMA ((2007) ) Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex 43: , 835–845. |
[77] | Warren JD , Fletcher PD , Golden HL ((2012) ) The paradox of syndromic diversity in Alzheimer’s disease. Nat Rev Neurol 8: , 451. |
[78] | Schnider A ((2003) ) Spontaneous confabulation and the adaptation of thought to ongoing reality. Nat Rev Neurosci 4: , 662–671. |
[79] | Gilboa A , Moscovitch M ((2002) ) The cognitive neuroscience of confabulation. A review and a model. In The Handbook of Memory Disorders, Baddeley A, Kopelman M, Wilson B, eds. John Wiley & Sons, Chichester, pp. 315–342. |
[80] | Sultzer DL , Brown CV , Mandelkern MA , Mahler ME , Mendez MF , Chen ST , Cummings JL ((2003) ) Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry 160: , 341–349. |
[81] | Catani M , Dell’Acqua F , Thiebaut de Schotten M ((2013) ) A revised limbic system model for memory, emotion and behavior. Neurosci Biobehav Rev 37: , 1724–1737. |
[82] | Segobi S , Laniepce A , Ritz L , Lannuzel C , Boudehent C , Cabe N , Urso L , Vabret F , Eustache F , Beaunieux H , Pitel AL ((2019) ) Dissociating thalamic alterations in alcohol use disorder defines specificity of Korsakoff’s syndrome. Brain 142: , 1458–1470. |



