Therapeutic Use of Music, Dance, and Rhythmic Auditory Cueing for Patients with Huntington’s Disease: A Systematic Review
Abstract
Background:
Studies have assessed the therapeutic effect of music, dance, and rhythmic auditory cueing for patients with Huntington’s disease (HD). However, the synthesis of evidence in support of their positive impact on symptoms is lacking.
Objective:
We conducted a systematic literature review to evaluate the potential benefits of music, dance, and rhythm on the cognitive, psychiatric and motor function in patients with HD.
Methods:
Two- and three-keyword searches and a manual search identified medical literature published from 1999 through 2019. We considered literature that assessed outcomes of art-based rehabilitation programs or individual modalities for persons with early, middle, or advanced HD. Structured analysis was conducted using data entry tables with categories for patient health status, art methods, and outcomes.
Results:
Seven articles and six abstracts met eligibility criteria, of which nine evaluated art-based rehabilitation programs. Studies mainly assessed cognitive, psychiatric, and motor functions through music, dance, or rhythm modalities. Although results were conflicting, in summary improvements to motor function were dependent on disease severity and more responsive to art therapy programs than rhythm-motor synchronization. Benefits to global cognition that resulted from rhythmic training correlated with microstructural changes. Qualitative data verified a positive impact on language production, chorea, behavior, and quality of life.
Conclusions:
Our review has shown a potential benefit of music, dance, and rhythm for patients with HD, which is particularly important for a disease that has no cure. Art forms seemed to affect cognitive, psychiatric, motor, psychosocial, and neuroanatomical domains. However, evidence is preliminary, warranting further investigation to establish the foundation for this field.
INTRODUCTION
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder characterized by striatal and basal ganglion atrophy [1–4]. Cognitive, psychiatric, and motor dysfunctions progress through early, middle, and advanced phases that correspond to five HD stages on the Shoulson and Fahn Total Functional Capacity (TFC) scale [5–7]. Striatal neuropathology is associated with cognitive and psychiatric functions [8–10] that often precede motor deficits [8–12]. Cognitive impairments are observed in executive function, memory, affective processing, and psychomotor speed [7, 13–15]. Psychiatric symptoms, including irritability, depression, anxiety, apathy, disinhibition, and emotion dysregulation, are common in HD [16–19]. As the clinical hallmark of HD [20], motor impairments initially impact voluntary motor functions such as gait and balance [21] and evolve to involuntary choreiform movements [20]. The triad of HD symptoms are interconnected as a result of cortical loops that coordinate cognitive, emotional, and motor functions [22].
Factors of disease presentation, such as age at onset, rate of decline, and severity of symptoms vary among the HD cohort [7]. However, since there is currently no cure or disease-modifying therapy [23], there is a consistent need for long-term, multidisciplinary care through pharmacological [24, 25] and non-pharmacological [26, 27] interventions. These methods may reduce or stabilize disease progression in other progressive neurodegenerative disorders, such as Parkinson’s disease (PD) [7]. Recent studies have investigated the use of physical therapy for HD patients [28–32]. Multidisciplinary rehabilitation programs are safe and feasible interventions that positively affect verbal learning and memory, anxiety and depression, quality of life, balance, spatial-temporal gait parameters, and gray matter changes [33–36].
In addition to physical exercise, there may be beneficial health effects of using art such as music and dance in patients with neurological diseases [37–39]. Some of these effects could potentially arise from enhanced neuroplasticity; however, empirical data is lacking. Specifically, pathways of emotion are activated in localized brain areas such as the amygdala, cingulate cortex, and hypothalamus [38]. In addition, the use of sensorimotor processing in art interventions may strengthen synaptic connections between auditory and motor cortices and increase localized areas such as the corpus callosum and basal ganglia [37]. Neuroplastic features are specific for the therapeutic modality. For example, listening to music improves global neuroconnectivity and atrophic recovery, while playing a musical instrument, singing, or dancing increases gray and white matter in frontotemporal areas and alters neurochemical levels including endorphins and dopamine [37, 38, 40]. As a result, music (MT) and dance movement therapies (DMT) in conjunction with conventional clinical interventions have a potential to positively affect cognitive, psychiatric, and motor functions for persons with progressive neurodegenerative disease. Studies give a particular focus on PD patients and associated benefits from dance exercise programs that use MT and rhythmic auditory cueing [39, 40]. Findings show improved motor control, gait, spatial memory, functional well-being, and quality of life [41–44]. The rationale for using MT and DMT in rehabilitation programs is the idea that music and dance are specialized auditory and motor forms that induce specific neuroplastic changes [37]. Therefore, the therapeutic outcomes associated with art-based rehabilitation are potentially distinct to that of traditional physiotherapy [37, 45–48].
Given the positive effects of art-based rehabilitation programs for persons with other neurodegenerative diseases [49], there may be similar benefits for patients with HD. However, few studies have examined MT, DMT, or rhythmic auditory cueing in clinical practices specific to HD, rendering us unable to define potential therapeutic outcomes associated with art-based rehabilitation in patients with HD. In the past decade, there has been a slight increase in the number of HD studies investigating MT protocol proposals [50], MT assessment tools [51], clinicians’ experiences of MT [52–54], and music perception abilities [55]. But yet, there lacks a greater focus on MT/DMT outcomes for persons with HD in comparison to other neurodegenerative diseases.
In 2015, a systematic review on the use of MT for patients with HD was published [56]. However, it reviews studies published from 1976 to 2001, including one study published in 2007, and focuses primarily on the history of MT and MT methods. As such, there is a need for an up-to-date systematic review of the literature in the past two decades that includes the cognitive, emotional, and motor outcomes of MT, DMT and rhythmic auditory cueing for HD. We did a systematic review to study the formats and effects reported in the literature on the therapeutic use of music, dance, and rhythmic auditory cueing for patients with HD.
MATERIALS AND METHODS
The systematic review adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement guidelines [57].
Search strategy for study selection
A literature search limited to English-language studies published from 1999 through October 2018 was first performed in November 2018 using PubMed, Scopus, and Web of Science electronic medical databases. Additionally, we conducted a manual search of included studies and systematic reviews in EMBASE. We repeated the literature search in April 2019, which was limited to English-language studies published from 1999 through 2019. This yielded one additional study for inclusion. The search strategy utilized a two- and three-keyword search. The two-keyword search used the following terms: (“Huntington’s [disease]”) AND (“music” OR “music therapy” OR “dance” OR “dance exercise” OR “dance rehabilitation” OR “[rhythm] [auditory] cueing”). The three-keyword search used the following terms: (“Huntington’s [disease]”) AND (“motor [function]” OR “cognitive [function]” OR “psychiatric [function]” OR “quality of life”) AND (“music [therapy]” OR “dance [therapy]” OR “[rhythm] [auditory] cueing”). A total of 278 searches were gathered, of which 184 were duplicates, yielding 94 unique search results.
Eligibility criteria
Included studies met the following criteria: 1) human participants received a genetically or clinically confirmed HD diagnosis 2) the therapeutic effect of art-based rehabilitation was evaluated quantitatively or qualitatively and 3) published research was an article, book chapter, or conference abstract.
This review considered protocols that incorporated MT, DMT, and rhythmic auditory cueing modalities for patients with prodromal, early, middle, or advanced HD and their caregivers. The rationale for including MT, DMT, and rhythmic auditory cueing is that each art intervention incorporates different methodologies. For example, MT and DMT utilize music and dance, respectively, to target domains of function. Conversely, rhythmic auditory cueing is a form of rhythmic training that synchronizes specific functional domains, such as speech and motor movements, with rhythmic patterns.
Studies that were carried out in the community (i.e., public dance studios), patients’ homes, rehabilitation centers, or hospitals were also considered. Quantitative syntheses included cognitive, psychiatric, and motor outcomes. Outcome measures of cognitive function included verbal communication, psychomotor speed, and executive functions. Outcome measures of psychiatric function included emotional regulation and levels of depression, anxiety, apathy, and aggression. Outcome measures of motor function included balance, spatial-temporal gait parameters, and fine motor skills. Studies that reported microstructural outcomes and psychosocial outcomes (i.e., quality of life) were also considered. Qualitative syntheses included patient and/or caregiver feedback regarding the efficacy, safety, and feasibility of the program.
Exclusion criteria were proposals of study designs, assessments of clinical protocols, evaluations of novel measurement tools, and systematic reviews or meta-analyses. Of the 94 unique literature searches, 81 met at least one exclusion criterion, yielding 13 total studies for inclusion in the systematic review (Table 1). The flow chart of the search process and outcome is shown in Fig. 1.
Fig.1
PRISMA flow chart of the selection process of included studies.
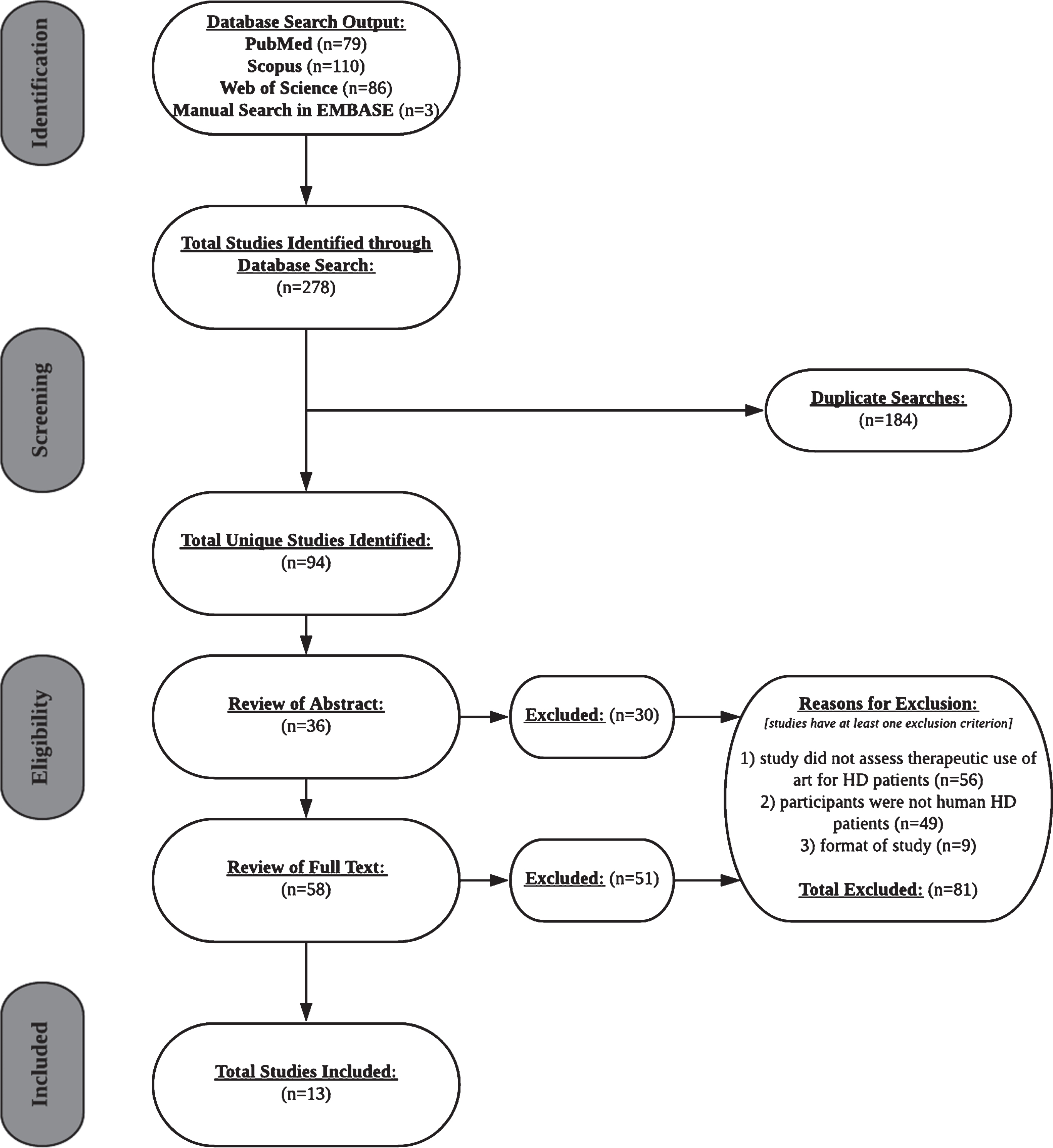
Table 1
Design and study population of seven articles and six abstracts on the therapeutic use of art
| Source (first author, year publication reference number) | Descriptive characteristics | Literature format | Country | Setting | Study design | Outcome measures and assessment method | Participant information | Sample (sample size); age and gender | HD stage |
| Brandt M., 2016 [58] | Conference abstract | Netherlands | Case report | Outcomes: | HD patients | Patients were able to speak but had verbal communication deficits (speech unintelligible); had intact language comprehension; were motivated and not easily distracted; had affinity for music | |||
| 1) Cognitive: language processing | |||||||||
| 2) Psychiatric: levels of anxiety or fear pertaining to the daily use of speech | |||||||||
| 3) Motor: components of language production (i.e., articulation, fluency, use of voice, speaking volume, breathing) | |||||||||
| 4) Psychosocial: quality of life as a result of daily use of speech | |||||||||
| Tools: Subjective assessment of program efficacy and perceived benefits through patient feedback | |||||||||
| Delval A., 2008 [59] | Article | France | Cross-sectional study | Outcomes: | 1) HD patients (n = 15); mean age = 43.9±9.8 years; sex ratio = 0.52 | Patients could perform dual-motor and motor-cognitive tasks | |||
| 1) Motor: spatial kinematic gait parameters (i.e., stride length, velocity); temporal kinematic gait parameters (i.e., cadence, ratio of single-limb support time to double-limb support time); kinematic angle parameters (i.e., sagittal plane excursion angles for hip, knee, and ankle joints); frequency of the center of mass of knee and ankle joint trajectories; time between heel strikes and metronomic cue | 2) Healthy controls matched by age and gender (n = 15); mean age = 40.5±10.5 years; sex ratio = 0.52 | ||||||||
| Tools: Video motion system: (VICON video system, Oxford Metrics, Oxford UK) | |||||||||
| Hyson C., 2005 [60] | Conference abstract | United States of America (USA) | Non-randomized 6-week pilot clinical trial | Outcomes: | HD patients (n = 5); 4 male | Patients could adhere to and participate in program | |||
| 1) Cognitive: memory and attention | |||||||||
| 2) Psychiatric: mood | |||||||||
| 3) Motor: balance and posture, fine motor skills, vocalization | |||||||||
| Tools: Subjective assessment of MT and program tolerability through survey | |||||||||
| Cognitive, Psychiatric, Motor: UHDRS | |||||||||
| Johnson K.A., 2000 [61] | Article | Australia | Cross-sectional study | Outcomes: | 1) HD patients (n = 12); mean age = 53.7±8.23 years; 9 male | Patients were in stages I, II, or II (TFC scale) | |||
| 1) Motor: bimanual in-phase and anti-phase hand movement parameters (i.e., variation in and accuracy of co-ordination pattern and velocity) | 2) Healthy controls (n = 12); mean age = 54.2±7.82 years; 9 male | ||||||||
| Tools: Bimanual cranks were used to perform rotary hand movements; automatic algorithms used displacement data gathered by laptop computer to determine hand movement parameters | |||||||||
| Kloos A.D., 2013 [62] | Article | USA | Patients’ homes | Cross-over, controlled, single-blinded, six-week trial | Outcomes: | HD patients (n = 18); mean age = 50.7±14.7 years; 7 male | Ambulatory patients represented “a wide range of disease severity”; patients could walk 10 m without assistance | ||
| 1) Psychiatric: balance confidence | |||||||||
| 2) Motor: spatiotemporal gait parameters (i.e., speed, maneuverability, forward and backward variability); balance and mobility | |||||||||
| 3) Psychosocial: quality of life | |||||||||
| 4) Other: program feasibility, adherence, acceptability, safety | |||||||||
| Tools: | |||||||||
| Psychiatric: Activities-Specific Balance Confidence Scale | |||||||||
| Motor: GAITRite walkway system (CIR Systems, Inc, Haverton, PA), FSST and TT | |||||||||
| Psychosocial: WHO-QoL | |||||||||
| Other: subjective assessment of program acceptability through patient feedback; physiological parameters assessed program safety | |||||||||
| Lagesen S., 2011 [63] | Conference Abstract | Norway | NKS Olaviken Hospital (specialized nursing home for persons with advanced HD) | Case report | Outcomes: | HD patients (n = 2) | Patients were in advanced phase | ||
| Case 1: | |||||||||
| 1) Psychiatric: behavioral control | |||||||||
| Case 2: | |||||||||
| 2) Motor: Speech and articulation, choreiform movements | |||||||||
| Tools: Subjective assessment of program efficacy and perceived benefits through clinician feedback | |||||||||
| Metzler-Baddeley C., 2014 [64] | Article | United Kingdom | Patients’ homes and the Cardiff University Brain Research Imaging Centre | Non-randomized 8-week pilot clinical trial | Outcomes: | HD patients (n = 10); age range = 24–64 years; 5 male | Patients had prodromal HD (n = 1) or early to advanced HD (n = 9) | ||
| 1) Cognitive: Executive functions (i.e., multi-tasking, attention switching and inhibition, verbal working memory, verbal and category fluency, processing speed) | |||||||||
| 2) Neuroanatomical: Subcortical basal ganglia volume | |||||||||
| Tools: | |||||||||
| Cognitive: standard dual task, TMT, Stroop task, DSST, Delis and Kaplan executive function battery | |||||||||
| Neuroanatomy: Signa HDx3.0TTM MRI system (GE Medical Systems, Milwaukee) | |||||||||
| Muto K., 2005 [65] | Conference Abstract | Japan | Pilot observation study | Outcomes: | Workshop groups included patients with HD, persons at risk for HD, and their family members (n = 13–15 per group); age range = 3–70 years | Patients were able to adhere to workshop protocol and provide verbal feedback | |||
| 1) Psychiatric: levels of tension and fear related to developing or having HD motor symptoms; caregivers’ awareness (empathy) of patients’ emotional wellbeing | |||||||||
| 2) Psychosocial: participant satisfaction | |||||||||
| Tools: Subjective assessment of participant satisfaction through survey and perceived benefits through structured discussions and clinicians’ observations | |||||||||
| Orejas M.E., 2008 [66] | Conference Abstract | Spain | Patients’ homes (treatment group) or research centre (placebo group) | 6-month RCT | Outcomes: | 1) HD patients in treatment group (n = 7) 2) HD patients in placebo group | Patients were able to adhere to exercise programs and provide feedback | ||
| 1) Psychiatric: UHDRS-behavioral scale functions (outcomes unspecified) | |||||||||
| 2) Motor: UHDRS-motor scale functions (outcomes unspecified) | |||||||||
| 3) Psychosocial: life quality satisfaction, functional ability | |||||||||
| Tools: | |||||||||
| subjective assessment of satisfaction through questionnaire | |||||||||
| Psychosocial: PDQ-39, NHQ, FIM and Barthel scales | |||||||||
| Salgues J., 2016 [67] | Conference Abstract | France | Patients’ homes, Salpêtrière Hospital, or dance studios | Pilot observation study | Outcomes: | Workshops included HD patients and their relatives or caregivers | |||
| 1) Cognitive: development of consciousness | |||||||||
| 2) Psychiatric: level of relaxation | |||||||||
| Tools: Subjective assessment of program efficacy and perceived benefits through instructor’s observations | |||||||||
| Thaut M.H., 1999 [68] | Article | USA | Gait training area of Neurologic Rehabilitation Centre | Cross-sectional study | Outcomes: | HD patients (n = 27); mean age = 47±10.7 years; 13 male | Patients had prodromal HD (n = 4) or HD of various phases (n = 23) (Shoulson-Fahn scores of degree of chorea and functional disability ranged from 0–2.5 on a 0–3 scale) | ||
| 1) Motor: uncued and cued gait patterns (i.e., modulation of gait velocity as a function of step rates and stride length, synchronization between step cadence and rhythmic cue) | |||||||||
| Tools: Stride analysis system (Infotronic CDG, Eindhoven, The Netherlands) assessed stride parameters (i.e., gait velocity, cadence, stride length swing symmetry) | |||||||||
| Trinkler I., 2019 [69] | Article | France | Contemporary Dance Studio | Randomized Controlled pilot study | Outcomes: | 1) HD patients (n = 19); age range 43–78 years; eight male 2) Healthy age- and education matched controls (n = 12); age range 44–72 years; four male | Patients had mild to moderate HD (TFC range 7–13, UHDRS motor score range 3–58) | ||
| 1) Cognitive: UHDRS cognitive score (i.e., literal fluency) | |||||||||
| 2) Psychiatric: Apathy and Problem behavior | |||||||||
| 3) Motor: UHDRS motor score | |||||||||
| 4) Neuroanatomical: brain volume and structural changes | |||||||||
| 5) Pyschosocial: quality of life, patient experiences of DMT | |||||||||
| Tools: | |||||||||
| Cognitive: Stroop task, Symbol Digit Code, MDRS, TMT | |||||||||
| Psychiatric: LARS, PBA-s | |||||||||
| Neuroanatomy: sMRI and VBM | |||||||||
| Psychosocial: QLI, semi-structured interview | |||||||||
| Van Bruggen-Rufi M.C., 2017 [70] | Article | Netherlands | Long-term care facilities | Multi-center RCT | Outcomes: | 1) HD patients in intervention group (n = 32); mean age = 54.5 years; 10 male | Patients had advanced HD (n = 63) | ||
| 1) Cognitive: social-cognitive functioning (i.e., communicative and expressive skills) | |||||||||
| 2) Psychiatric: behavioral problems (i.e., mental rigidity, aggression) | 2) HD patients in control group (n = 31); mean age = 54.3 years; 10 male | ||||||||
| Tools: | |||||||||
| Cognitive: BOSH | |||||||||
| Psychiatric: PBA-s and BOSH |
Data extraction
After reviewing 36 abstracts and 58 full texts, 30 and 51 studies were excluded, respectively. 56 excluded studies neglected to examine therapeutic effects of art. Participants of 49 excluded studies were either clinicians, persons with other neurodegenerative diseases, or HD animal models. 9 studies were excluded due to literature format (i.e., study protocol proposal, systematic review). Data was extracted from published conference abstracts as a result of the lack of literature.
Outputs for keyword combinations in each database were recorded in structured tables during the search process. Data entry forms had the following categories: research setting, aims, study design, procedure, participant sample (sample size), participant age and gender (mean±SD), art form, measurement tools, outcome measures, and results.
Data synthesis and analysis
Due to the limited number of studies that assessed the cognitive, psychiatric, and motor outcomes of MT, DMT, or rhythmic auditory cueing for HD patients, findings from included studies were synthesized and summarized as descriptive results.
RESULTS
This systematic review included seven research articles published between 1999 and 2019 in English-language journals (Table 1). Data was collected from six conference abstracts (see Tables 1–3) but missing information led to its exclusion from the primary synthesis.
Descriptive characteristics of included studies
Studies originated from North American, European, and Australian countries. Research settings included participants’ homes (n = 2), a dance studio (n = 1), long-term care facilities (n = 1), and rehabilitation centers (n = 1). Two studies did not specify research setting. Cross-sectional studies [59, 61, 68], randomized controlled trials (RCT) [69, 70], cross-over [62], and non-randomized [64] pilot clinical trials were included. Studies assessed a combination of cognitive (n = 3), psychiatric (n = 3), motor (n = 5), psychosocial (n = 2), and microstructural (n = 2) outcomes. One study evaluated program feasibility and safety [62]. Outcomes were quantitatively [59, 61, 62, 64, 68–70] and/or qualitatively [62, 69] obtained.
Participants
Studies included patients with HD [62, 64, 68] or compared patients to age-matched healthy control subjects [59, 61, 69, 70]. Early, middle, and advanced phases of HD were represented [61, 64, 68–70]. Two studies did not specify disease phase but provided information on patient functionality. Participants included both males and females and were in early-to-middle adulthood (Table 1).
Research aims and procedures
The effect of MT, DMT, and rhythmic auditory cueing for patients with HD was examined by evaluating cognitive [64, 69, 70], psychiatric [62, 69, 70], motor [59, 61, 62, 68, 69], psychosocial [62, 69], and/or neuroanatomical [64, 69] changes in response to art-based clinical programs [62, 64, 69, 70] or external rhythmic cueing [59, 61, 68] (Tables 2 and 3).
Functional outcomes of two programs were compared to control treatments without art [62, 69, 70]. All studies that independently assessed rhythm compared gait parameters [59, 68] or bimanual hand coordination [61] across cueing conditions and health status.
Features of MT, DMT, and rhythmic auditory cueing
Art modalities, genres, and instruments
Studies evaluated a combination of music (n = 4), dance (n = 1), and rhythm (n = 5) modalities (Table 2). Rhythmic patterns included Brazilian samba, Spanish rumba, West-African kuku, and Cuban son [64] as well as folk music [68] and contemporary dance [69]. All rhythmic patterns were external auditory cues since they required the use of an external source, such as an instrument or metronome, as opposed to an internal source, such as singing. One study allowed participants to select their preferred music genres [62]. Studies used instruments such as bongo drums [64], metronomes [59, 61, 68], or computerized melodies [62]. These instrumental features distinguished the use of musical from non-musical stimuli, which are considered to be independent forms of auditory information [37]. In addition, the use of instruments to create live music or computerized music may influence the emotional salience of auditory input [37]. One study lacked this information [70].
Table 2
Characteristics of art forms in seven articles and six abstracts
| Source (first author, year of publication reference number) | Art modality | Art genres | Instruments | Methods | |||||
| Music | Dance | Rhythm auditory cueing | Structured direction OR improvisation | Individual OR group | Instructor and participant roles | ||||
| Brandt M., 2016 [58] | X | Piano | Structured Direction: participants dictated phrases in coordination with repetitive rhythmic melodies | Individual | Instructor: music therapist performed piano melodies while speech therapist dictated phrases for patient to verbalize | ||||
| Participant: patient produced speech by following music therapist and speech therapist | |||||||||
| Delval A., 2008 [59] | X | Metronome | Structured Direction: participants synchronized gait to set rhythmic tempos | Individual | Instructor: investigators provided rhythmic cues to participants | ||||
| Participant: patients synchronized gait to cues while performing motor and cognitive tasks; selected comfortable tempo for cues through their baseline pace | |||||||||
| Hyson C., 2005 [60] | X | X | X | Musical instruments (details unspecified) | Structured Direction: RAS used to synchronize gait to external auditory cues | Individual and Group | Instructor: led rhythmic auditory cueing exercises and supervised PSE and TIMP | ||
| Improvisation: PSE technique applied acoustical properties of music to non-rhythmic body movements; TIMP allowed participants to engage in the free-form playing of musical instruments | Participant: synchronized gait to rhythmic cues and played music instruments | ||||||||
| Johnson K.A., 2000 [61] | X | Metronome | Structured Direction: participants synchronized hand movements to set rhythmic tempos | Individual | Instructor: investigators provided rhythmic cues to participants | ||||
| Participant: synchronized hand movements to cues | |||||||||
| Kloos A.D., 2013 [62] | X | X | X | Computerized melodies on video game software; footpad with arrows were used to coordinate dance movements with arrow visual cues on screen | Structured Direction: dance movements were directed by arrow visual cues and auditory rhythmic cues | Individual | Instructor: investigators assisted in selecting level of difficulty by helping participants choose song speeds | ||
| Participant: chose preferred songs and song speeds, followed visual and auditory cues while dancing | |||||||||
| Lagesen S., 2011 [63] | X | X | Voice (details unspecified) | Structured Direction: patients listened to familiar songs and completed MIT, which connected their speech to melodic features | Individual | Instructor: led clinical MT according to patients’ specific needs | |||
| Improvisation: program included clinical improvisation and song writing | Participant: patients listen to, composed, and performed music; patients had agency to select songs and use artistic creativity | ||||||||
| Metzler-Baddeley C., 2014 [64] | X | X | Brazilian samba, Spanish rumba, West-African kuku, Cuban son | Bongo drums | Structured Direction: through a pre-recorded training program, participants followed specific rhythmic exercises | Individual (patients’ caregivers or relatives were involved when it was necessary) | Instructor: led rhythmic exercises through pre-recorded CD | ||
| Participant: played musical instrument as they followed instructions on CD | |||||||||
| Muto K., 2005 [65] | X | Buto dance | Structured Direction: participants followed specific dance movements | Group | Instructor: led workshop by providing Buto dance imagery for participants | ||||
| Participant: recreated imagery through dance | |||||||||
| Orejas M.E., 2008 [66] | X | X | Ballroom dance | (details unspecified) | Group | (details unspecified) | |||
| Salgues J., 2016 [67] | X | X | Contemporary dance | (details unspecified) | Individual and Group | Instructor: manipulated participants’ body posture | |||
| Participant: followed instructors’ dance movements | |||||||||
| Thaut M.H., 1999 [68] | X | X | Folk music | Computerized music; metronome | Structured Direction: participants synchronized gait to set rhythmic tempos | Individual | Instructor: administered auditory cues to participants | ||
| Participant: synchronized gait to cues; selected comfortable tempo of cues | |||||||||
| Trinkler I., 2019 [69] | X | X | Contemporary dance | Structured Direction: there were four features of workshops that were consistent throughout participation | Group | Instructor: led and supervised structured dance classes | |||
| Improvisation: participants improvised dance movements for individual body parts and in collaboration with other participants | Participant: used artistic creativity through improvisation of dance movements; had the choice to participate in at least one of three dance class options per week | ||||||||
| Van Bruggen-Rufi M.C., 2017 [70] | X | X | Structure Direction: instructors used an established MT protocol | Individual and Group | Instructor: led program by “loosely” adhering to MT protocol | ||||
| Improvisation: content of music therapy program was based on instructors’ and patients’ current goals; patients engaged in “expressive musical interaction” | Participant: had the agency to dictate content of each MT session |
Table 3
Aims, interventions, and outcomes in seven studies and six abstracts on the therapeutic use of art
| Source (first author, year publication reference number) | Research aim | Procedure | Art form methods | Data analyses and outcomes |
| Brandt M., 2016 [58] | To evaluate the efficacy of combining MT and speech therapy methods as Huntington Speech Music Therapy (HSMT) for HD patients based on speech production and perception outcomes | Speech therapist assisted patients with verbal production of automatic sequences (i.e., greetings, alphabet etc.), patient-related words and sentences (i.e., names of family and friends, personal hobbies etc.) and conversations while music therapist accompanied patient with repetitive melodic sequences on the piano | HSMT used repetitive, short piano melodies to accompany patients as they produced words and sentences | HSMT effectively structured incoming and outgoing verbal information, and therefore, facilitated ease for patients when engaging in daily complex conversations; HSMT stimulated patients’ use of speech |
| Conclusion: HSMT improved speech production of automatic sequences, patient-related words and sentences, and of general conversation. | ||||
| Delval A., 2008 [59] | To assess the impact of rhythmic cues on gait parameters during a walking task (free gait) and dual task paradigms involving motor (gait+motor task) and cognitive (gait+cognitive task) domains | Task 1: Participants walked at their baseline cadence (free gait) without rhythmic cues and then walked with cues set at 120% of their baseline cadence. Three trials were completed | During gait tasks, rhythmic cues from a metronome were set a three frequencies: subjects’ baseline cadence, 100% of subjects’ baseline cadence, 120% of subjects’ baseline cadence | Task 1: Healthy controls increased their gait speed and cadence but not stride length when cues were set at 120% of their baseline cadence (p < 0.05). No observed differences in gait parameters with added cues for HD patients. Step synchronization was more impaired for HD patients |
| Task 2: Participants walked at their baseline cadence while carrying a tray of filled glasses (gait+motor task) and then walked with cues set at 100% and 120% of their baseline cadence. Three trials were completed | Tasks 2 and 3: Healthy controls increased their gait speed and cadence but not stride length when cues were set at 120% of their baseline cadence (p < 0.05) compared to when cues were set at 100% of their cadence. No observed differences in gait parameters with added cues of various frequencies for HD patients. Step synchronizations was more impaired for HD patients | |||
| Task 3: Participants walked at their baseline cadence while counting backwards (gait+cognitive task) and then walked with cues set at 100% and 120% of their baseline cadence. Three trials were completed | ||||
| Conclusion: Rhythmic cues were ineffective for improving kinematic gait parameters of HD patients. Although there was an observed “trend towards improvement” in gait parameters, significance was not achieved. Attentional deficits associated with HD may explain synchronization deficits. | ||||
| Hyson C., 2005 [60] | To evaluate whether a novel MT program is accepted by HD patients and effectively impacts mood and motor symptoms | Patients participated in a six-week MT program that involved one individual session and one group session each week. Patients were evaluated with the UHDRS at baseline and post-MT and completed a post-MT feedback questionnaire | MT program combined methods of RAS, PSE, and TIMP | Tolerability of MT program was achieved (100% adherence, 98% attendance). The majority of HD patients perceived a positive benefit of the use of MT. Improvements in UHDRS scores were observed (i.e., finger tapping, protonation/supination, Luria), though not significant |
| Conclusion: MT is a tolerated clinical method with positive perceived benefits for HD patients. Preliminary evidence suggested a positive impact of MT on UHDRS motor score. | ||||
| Johnson K.A., 2000 [61] | To determine whether rhythmic cues facilitate improved coordination of bimanual in-phase and anti-phase hand movements | Participants performed bimanual in-phase and anti-phase hand movements at fast and slow speeds. A bimanual crank was used to perform circular hand motions. In the “cue on” trials, participants tried to synchronize in-phase and anti-phase movements with rhythmic cues (i.e., one hand rotation per beat). In the “cue off” trials, participants tried to remember the beat frequencies after exposure as they completed hand movements | During in-phase and anti-phase hand movement tasks, rhythmic cues from a metronome were set at two frequencies: fast (1.5 Hz) and slow (0.5) Hz | Fast and slow in-phase hand movements were unimpaired for controls and HD patients. HD patients’ bimanual coordination was less accurate [F(1,22) = 6.58, P < 0.018] and more variable [F(1,11) = 14.044, P < 0.003] at fast speeds. Performance was not influenced by the use of rhythmic cues |
| Fast and slow anti-phase movements were unimpaired for controls but impaired for HD patients [F(1,22) = 12.39, P < 0.002]. Both controls and HD patients had high coordination variability [F(1,22) = 7.72, P < 0.011] at fast speeds. Performance was not influenced by the use of rhythmic cues | ||||
| Conclusion: While in-phase hand movements are less impaired than anti-phase hand movements, rhythmic cueing is ineffective for improving both forms of coordination patterns for HD patients. This finding is inconsistent with that of other patient populations such as PD, which may be due to HD ganglion atrophy. | ||||
| Kloos A.D., 2013 [62] | To evaluate the feasibility, acceptability, and safety of a DDR video game exercise program and to determine whether it affects gait, psychiatric, and psychosocial outcomes | Patients completed either six weeks of DDR or a handheld video game (i.e., Bingo, Blackjack, Solitaire) (45 minute sessions, two days a week). DDR exercise sessions increased in difficulty as patients improved synchronization accuracy. Handheld video games worked to stimulate cognitive networks. After six weeks, patients completed the opposing exercise intervention for another six weeks. Outcome measures were assessed at baseline and post-treatment for each intervention | DDR required patients to synchronize their dance movements guided by visual cues (arrows) and auditory cues (melodic beats). Patients and instructors controlled song selection and tempo | DDR demonstrated: |
| 1) Feasibility: patients could increase level of difficulty of video game while maintaining accuracy scores | ||||
| 2) Adherence: 100% participation for all participants | ||||
| 3) Acceptance: patient feedback showed high motivation to participate in program | ||||
| 4) Safety: normal physiological parameters, no adverse events or injuries reported | ||||
| For all patients, certain gait parameters significantly improved after completing DDR: double support percentage (2.54% reduction, p = 0.03), backward walking (4.18% reduction, p = 0.01). For patients with minor motor impairments, significant improvements in forward heel-to-heel base of support | ||||
| No significant difference in Activities-Specific Balance Confidence Scale, TT, or WHO-QoL scores between treatment groups | ||||
| Conclusion: DDR, which combined music and dance into one cohesive exercise program, benefited gait and was a feasible and safe method of physical exercise. | ||||
| Lagesen S., 2011 [63] | To determine the clinical benefits of MT for two patients with advanced HD | Case 1: a patient who presented with difficult behaviors during morning-care procedures was provided four weeks of MT | MT included improvisation, song writing, listening to familiar songs, and modified MIT. MT features were altered for each patient, based on symptoms and specific goals | Case 1: clinicians’ observations showed that MT reduced patient’s challenging behaviors during morning-care procedures |
| Case 2: a patient who presented with speech and articulation deficits and chorea was provided six weeks of MT | Case 2: clinicians’ observations showed that MT improved speech and articulation and reduced choreiform movements. MT additionally benefited patient’s relationship with clinical staff | |||
| Conclusion: The clinical use of MT may individually benefit patients with advanced HD. | ||||
| Metzler-Baddeley C., 2014 [64] | To assess the efficacy of a rhythmic drumming exercise program according to changes in executive functioning and microstructure of cortical areas | Patients were provided with rhythmic drumming exercises on CD (22 different exercises, 15 minutes each), bongo drums, and a training diary. Patients exercised 15 minutes/day five days/week for eight weeks by following drumming exercises on CD and recording each session in diary | Rhythm exercise patterns varied in genre (i.e., Brazilian samba, Spanish rumba, West-African kuku, Cuban son). Although drumming exercises progressed in speed and rhythmic complexity, patients were able to progress through levels of difficulty at their own pace | Significant improvements were observed in cognition (t(4) = 3.30, p≤0.03): |
| 1) Dual task mean(SD) accuracy scores (baseline: 9.4(3.1), post-baseline: 14.0(3.7)) | ||||
| 2) DSST mean(SD) scores (baseline: 42.4(15.4), post-baseline: 43.6(13.6)) | ||||
| 3) TMT mean(SD) response times in seconds (baseline: 39.0(18.9), post-baseline: 31.4(9.4)) | ||||
| 4) Stroop task mean(SD) response times in seconds (baseline: 111.0(20.1), post-baseline: 93.8(24.1)) | ||||
| 5) Verbal fluency mean(SD) accuracy scores (baseline: 28.0(10.4), post-baseline: 39.8(8.8)) | ||||
| 6) Category fluency mean(SD) accuracy scores (baseline: 30.4(11.3), post-baseline: 39.2(15.4)) | ||||
| Significant microstructural changes in fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD) indices were observed in white matter: | ||||
| 1) segment 1 of corpus callosum: significant increase [FA [t(4) = 5.2, p = 0.006]; AD [t(4) = 5.1, p = 0.007]]; significant decrease [RD[t(4) = –4.6, p = 0.01]] | ||||
| 2) segment 2 of corpus callosum: significant increase [AD[t(4) = 3.03, p = 0.04]] | ||||
| 3) cortico-spinal tract: significant increase [FA [t(4) = 3.3, p = 0.03]; AD [t(4) = 2.9, p = 0.04]]; significant decrease [RD [t(4) = –4.2, p = 0.014]] | ||||
| 4) anterior thalamic radiation: significant increase [FA [t(4) = 3.7, p = 0.02]; AD [t(4) = 4.04, p = 0.016]] | ||||
| Significant positive correlations were obtained for changes in cognition and microstructure of corpus callosal segments. | ||||
| Conclusion: A two-month drumming training program improved executive function and changed white matter microstructure in HD patients. Improvements in executive function resulted from microstructural changes in the corpus callosum, suggesting that long-term training induced neuroplasticity. | ||||
| Muto K., 2005 [65] | To assess the satisfaction level of a dance workshop for patients, persons at risk for HD, and their caregivers. To determine whether dance effectively targets psychiatric issues related to the HD diagnosis and creates mutual understanding amongst patients and caregivers on the illness experience | Participants and instructors engaged in an initial friendly discussion before dance workshop. Participants subsequently danced for two hours while following instructors’ Buto dance movements. After the workshop, participants completed a satisfaction survey | Buto dance incorporated “dark, slow, and contorted” imagery as an artistic representation of one’s “inner unconscious space.” | Patients’ fears and tensions related to hiding motor impairments were diminished while dancing. Dancing allowed persons at risk for HD to let go of their fears about developing motor impairments. For caregivers, dance allowed them to better relate to the HD illness experience |
| Conclusion: HD patients, individuals at risk for HD, and their caregivers were satisfied with a Buto dance workshop as a way to improve mood and emotional issues. Participation of caregivers or individuals without HD may additionally result in an empathic understanding of the HD experience. | ||||
| Orejas M.E., 2008 [66] | To evaluate the therapeutic effect of ballroom dancing for HD patients according to motor, functional capacity, behavioral, and quality of life outcomes | Treatment group: HD patients completed weekly one hour sessions of DMT for six months (details unspecified) | DMT used classical ballroom dancing (details unspecified) | Results of quantitative measurements not available. Patients in treatment group showed high levels of motivation to participate and compliance compared to patients in control group. Patients in treatment group reported feeling better overall. |
| Control group: HD patients completed a home-based exercise program without music or dance (details unspecified | Conclusion: DMT effectively motivated patients to engage in exercise and while complying to protocol. Dance is a feasible intervention in HD rehabilitation programs and may target feelings of apathy that make it difficult for patients to achieve long-term and consistent participation in exercise. | |||
| All patients were assessed at baseline and post-treatment with motor, functional, behavioral, and quality of life scales. All patients completed a post-treatment satisfaction questionnaire | ||||
| Salgues J., 2016 [67] | To examine the therapeutic effect of a contemporary dance workshop for patients and persons indirectly affected by HD | Dance instructors led creative dance exercises that required participants with and without HD to experiment with different body movements. Participants learned how body posture, regardless of chorea, directly reflects their consciousness | Contemporary dance workshops combined artistic methods (i.e., imagination, poeticism, invention, multiple sensory modalities) with therapeutic approaches that target the development of consciousness and empathy through manipulation of body posture | Observations showed that dance workshops helped HD patients feel less isolated as a result of their diagnosis (details unspecified) |
| Conclusion: There is a positive impact of DMT on patients, their relatives, and their caregivers because dance served as a medium for understanding the illness experience (details unspecified). | ||||
| Thaut M.H., 1999 [68] | To evaluate the impact of external rhythmic cues on HD patients’ ability to modulate gait velocity at faster and slower speeds | Patients walked on a 26-meter walkway without rhythmic cues for four trials with the following pacing conditions: | Rhythmic auditory cueing utilized metronomic beats and musical beat patterns | Modulation of gait velocities was achieved with and without rhythmic cueing (p < 0.05) but not with music. A carry-over effect was observed after cued trials, as unpaced gait velocity maintained a significantly greater value than baseline velocity (p < 0.05). A carry-over effect was not observed for trials without rhythmic cues |
| (1) normal speed (pre-test baseline), (2) slower than baseline, (3) faster than baseline, (4) normal speed (post-test baseline). | While severity of HD did not impact modulation, greater disability and chorea scores significantly lowered gait velocity and impaired synchronization of movements to cues | |||
| In the next four trials, patients walked with rhythmic cues set at the following pacing conditions: | Conclusion: HD patients can modulate gait velocity with and without external rhythmic cues, though not with music. However, external rhythmic cues were ineffective for achieving gait synchronization, regardless of disease severity. | |||
| (1) metronome 10% slower than baseline, (2) metronome 20% faster than baseline, (3) music tempo 20% faster than baseline (4) normal speed (post-test baseline) Gait parameters were collected for each trial | ||||
| Trinkler I., 2019 [69] | To assess the impact of contemporary dance on motor, neuropsychiatric and cognitive functions, including apathy, quality of life, and brain structure for patients with HD | Group 1: five months of contemporary dance, participated in at least one two-hour class per week. Evaluation of outcomes occurred after five months of treatment | Four features of contemporary dance workshops: | Patients’ UHDRS motor, cognitive, (i.e., MDRS, Stroop, TMTA), and neuropsychiatric scores (i.e., “irritability” and “lack of initiative” subscales on PBA) were significantly worse than healthy controls. Apathy and quality of life scores were not significantly different amongst patient and control groups |
| Group 2: five months of usual care. Evaluation of outcomes occurred after five months of treatment. Participants continued with contemporary dance treatment for another five months (at least one two-hour session per week). Evaluation of outcomes occurred after DMT | 1.) warm-up session incorporated relaxation and body consciousness exercises | Motor impairments (median[IQR]) decreased in contemporary dance group from 28 [6–51] to 27 [7–33]. Motor impairments increased in those who received usual care from 19 [13–35] to 25 [14–42] (Z = –2.44, p = 0.015). Significant differences in cognitive and behavioral scores were not observed across treatment groups | ||
| 2.) participants improvised movements with and without music based on themes (i.e., “machines,” “ocean,” “neck,” etc.) | Increase in medial superior parietal and paracentral lobule volume post-DMT | |||
| 3.) group improvisation of dance movements based on same themes | Conclusion: Contemporary dance workshops that incorporate “spatial and bodily representations” were accepted and adhered to by patients. They significantly improved motor functions and induced neuroplastic effects that reflected intact compensatory mechanisms and aligned with positive patient feedback. | |||
| 4.) massage exercises on the floor | ||||
| Van Bruggen-Rufi M.C., 2017 [70] | To compare the therapeutic effect of MT and recreational therapy on quality of life based on communication and behavior outcomes | Treatment Group (MT): Group sessions followed MT protocol with the aim of “improving and stimulating communication and self-expression” (one-hr/week, 16 weeks) | MT was partially structured, as each session started and ended with the same songs. However, overall content of each session depended on the goals of patients. Patients’ musical experiences varied (level of experience did not impact ability to participate in MT) | Significant difference in social-cognitive subscale scores between treatment and control groups (p = 0.042), with a positive effect observed for control group |
| Control Group (Recreational therapy): Group activities involved communication between patients (i.e., arts-and-crafts, puzzles, reading, cooking). Musical activities were excluded | An additional benefit of MT group sessions on communication and behavior compared to recreational therapy was not observed. No significant difference in mental rigidity/aggression mean scores on BOSH (p = 0.125) was observed | |||
| Communication and behavior outcomes were gathered at baseline and after 8, 16 and 28 weeks | Conclusion: RCT research design and communication outcomes were not optimal features of clinical trials that aimed to evaluate the impact of art-based interventions on quality of life for HD patients, especially for studies that included patients with limited communication at the advanced phase of disease. |
Neurologic art therapy techniques
Included studies lacked information on the use of art therapy techniques. However, specific MT protocols were identified in abstracts [58, 60, 63]. Of these studies, two used MT for speech and language rehabilitation via melodic intonation therapy (MIT) [63, 71] and modified-MIT in the form of the Speech Music Therapy for Aphasia (SMTA) program [58, 72]. MT was additionally used for sensorimotor rehabilitation by combining rhythmic auditory stimulation (RAS), patterned sensory enhancement (PSE), and therapeutic instrumental music performance (TIMP) techniques into one clinical program [60, 73, 74].
Methods (structured direction or improvisation)
All studies required participants to follow structured directions administered by an in-person instructor [69, 70], a pre-recorded tape [62, 64], or set rhythmic patterns for motor synchronization [59, 61, 68]. Two clinical programs combined structured direction and improvisation techniques by using a structured protocol that allowed participants to engage in artistic creativity through dance [69] and music [70].
Methods (individual or group sessions)
Studies that evaluated rhythmic auditory cueing independently from a clinical program administered protocol individually to participants [59, 61, 68]. In studies that assessed the efficacy of a DMT program, individual [62] and group sessions [69] were utilized. Two of the included studies chose individual or group sessions based on patients’ needs [64, 70].
Methods (instructor and participant roles)
Instructor roles ranged from dictating rhythmic patterns for participants to follow through movement [59, 61, 68] and MT [64] to guiding program safety [62] and structure [69, 70] as participants took the lead. Participants coordinated movements to external cues [59, 61, 68], danced [62, 69], listened to music [70], or played instruments [64].
Cognitive function outcomes
One non-randomized pilot clinical trial evaluated changes to verbal working memory and attention, multi-tasking, verbal/category fluency, and processing speed that resulted from participation in a rhythmic drumming exercise program [64] (Table 1). Measurement tools included the standard dual task [75], Trails Making Test (TMT) [75], Stroop task [76], Digit Symbol Substitution Test (DSST) [77], and Delis and Kaplan executive function battery [78]. Findings showed significant improvements to memory and attention, verbal/category fluency, and DSST post-baseline accuracy scores as well as TMT and Stroop task response times [64] (Table 3). Another multi-center RCT compared MT to recreational non-MT therapy by assessing changes to social-cognitive functioning [70] on the Behavior Observation Scale Huntington (BOSH) [79] (Table 1). Improvements to social-cognitive subscale scores achieved significance for participants of recreational therapy but not MT (Table 3). In another RCT, a contemporary dance workshop was evaluated by observing changes to UHDRS cognitive scores including Stroop task [76], Symbol Digit Code [6], Mattis Dementia Rating Scale (MDRS), a measure for global cognitive functioning [80], and TMT [75]. Changes to cognitive scores were insignificant.
Cognitive outcomes were additionally examined in conference abstracts, which included language processing [58], memory and attention [60], and development of consciousness [67]. Positive patient feedback was collected on a novel MT program for speech rehabilitation [58].
Psychiatric function outcomes
A cross-over pilot clinical trial [62] evaluated the efficacy of a novel video-game DMT program by assessing balance confidence with the Activities-Specific Balance Confidence Scale [81] (Table 1). Changes to baseline scores did not reach significance. Another multi-center RCT used the Problem Behavior Assessment (PBA-s) [82] and BOSH [79] to compare improvements to mental rigidity, aggression, and behavior severity across MT and recreational therapy programs [70]. Improvements to individual estimated mean scores were not significantly different across treatment groups (Table 3). Another RCT that used the PBA-s [82] and Lille Apathy Rating Scale (LARS) [83] to evaluate the efficacy of contemporary dance workshops did not find significant improvements to behavior.
Other psychiatric outcomes were represented in conference abstracts, which included overall mood [60, 67], fear and anxiety [58, 65] and behavioral control [63, 65]. Preliminary qualitative findings showed that MT facilitated ease of speech production [58], increased patients’ motivation [60], and reduced challenging behaviors of an in-patient during morning-care procedures [63]. Additionally, DMT reduced fears and tensions associated with HD progression and increased caregivers’ empathic understanding of the illness experience [65].
Motor function outcomes
Two cross-sectional studies compared gait parameters [59] and modulation of gait velocity [68] across external rhythmic cueing and no cueing conditions (Table 1). A significant benefit of auditory cues for kinematic gait parameters and velocity modulation was not observed [59, 68] (Table 3). Although one study found a trend towards improvement in gait, significance was not achieved [59]. In fact, in one study that included musical cues, music, but not rhythmic patterns, impeded gait velocity modulation [68]. In another study that evaluated the effect of contemporary dance workshops on motor function using the UHDRS [5], findings showed a decrease in motor impairments post-DMT and an increase in motor impairments after usual care that did not involve art forms [69]. Another clinical trial [62] evaluated the efficacy of a novel video-game DMT program by assessing changes to spatiotemporal gait parameters through the GAITRite walkway system [84]. Significant improvements to certain gait parameters were dependent on the severity of HD, such as forward heel-to-heel base of support [62].
The clinical trial [62] also compared balance outcomes between DMT and non-DMT treatments through the Four Step Square Test (FSST) [85] and Tinetti Mobility Test (TT) [86] (Table 1). Significant differences in balance scores were not observed across treatment groups. Additionally, a cross-sectional study used bimanual cranks [87] to evaluate fine motor skills by comparing bimanual in-phase and anti-phase hand movements across external rhythmic cueing and no cueing conditions [61]. Findings showed that coordination accuracy and velocity were not influenced by the use of rhythmic cues [61] (Table 3).
Motor outcomes such as language production [58, 60, 63], fine motor skills [60], posture [60], and choreiform movements [63] were also examined in conference abstracts (Table 1). Clinical observations collected in one case report showed improvements to motor control and speech production [63]. Patient feedback from another study was positive for the use of music in speech therapy [58]. Additionally, an MT clinical trial showed an improvement trend in fine motor sub-scores on the Unified Huntington’s Disease Rating Scale (UHDRS) [5]; however, significance was not achieved [60] (Table 3).
Psychosocial outcomes
Quality of life
One clinical trial examined the impact of DMT compared to non-DMT programs on patients’ quality of life by utilizing the World Health Organization Quality of Life-Bref scale (WHO-QoL) [88, 89]. Significant differences in WHO-QoL post-baseline scores across treatment groups were not observed. Another RCT did not find a significant impact of contemporary dance workshops on quality of life based on the Quality of Life Index (QLI) [90].
Two conference abstracts also evaluated quality of life connected to language production [58] and overall functionality [66]. Assessment methods included the Parkinson’s disease Questionnaire (PDQ-39) [91], the Nottingham Health Questionnaire (NHQ) [92], the Functional Independence Measure (FIM) [93], and Barthel Scales [94]. Preliminary patient feedback confirmed the positive impact music-based speech therapy had in the daily navigation through complex conversations [58].
Participant satisfaction
One RCT evaluated patients’ experiences of DMT through qualitative interviews [69]. Patient feedback suggested that “contemporary dance altered the way [patients] ‘felt and lived in their bodies.”’ Two conference abstracts assessed participant satisfaction of DMT programs through surveys [65, 66]. Preliminary findings suggested high satisfaction levels from patients [65, 66] and their caregivers [65], though specific outcomes were inconclusive.
Neuroanatomy
One non-randomized pilot clinical trial evaluated the effect of a rhythmic drumming exercise program on subcortical basal ganglia volume [64]. Magnetic resonance imaging (MRI) showed significant changes to corpus callosal white matter microstructure, or myelinated nerve fiber bundles that positively correlated with improvements in global cognition. Another RCT evaluated the effect of contemporary dance workshops on brain volume [69]. Structured MRI (sMRI) and voxel-based morphometry (VBM) showed a volumetric increase in medial superior parietal and paracentral lobule structures post-DMT that is associated with compensatory mechanisms for spatial and somatosensory processing.
Program feasibility, adherence, acceptability, and safety
One cross-over clinical trial made comparisons between a video-game DMT program and a non-DMT program based on feasibility, adherence, acceptability, and safety [62]. Feasibility of DMT was confirmed through significant improvements to accuracy scores. High attendance and completion of both DMT and non-DMT indicated the adherence of programs. Additionally, positive feedback from patients suggested high acceptability of DMT and no adverse health events or injuries confirmed its safety. Another RCT found that contemporary dance workshops were adhered to through high attendance [69].
DISCUSSION
To our knowledge, this systematic review is the first to evaluate outcomes of MT, DMT, and rhythmic auditory cueing for HD patients. We synthesized quantitative and qualitative analyses from 7 prospective articles and 6 conference abstracts that assessed the impact of established art-based clinical programs or individual art forms on functioning. There was heterogeneity across all studies in the use of art modalities, methodologies, and therapeutic outcomes. The current review confirmed a potential positive effect of using art forms in conventional HD clinical practices. Half of the included articles found benefits of music, dance, and rhythm to cognitive, motor, and microstructural outcomes. Abstracts confirmed additional benefits to psychiatric symptoms and quality of life. Art forms were determined to be feasible and safe methods for HD patients. However, it is worth mentioning that limited studies led to the extraction of data from abstracts. Therefore, the field is currently underdeveloped, making it difficult to conclusively define the collective and distinctive benefits that result from MT, DMT, and rhythmic auditory cueing.
We found that improvements to cognitive function scores [64, 69] such as memory and attention, verbal and category fluency, and processing speed were significant. These changes were contradicted by insignificant changes to executive function subscales on the UHDRS [60]. Additionally, positive patient feedback of benefits to speech processing [58] conflicted with insignificant changes to social-cognitive baseline scores [70]. Inconsistencies in findings regarding cognitive outcomes may be explained by the comparisons between dissimilar art programs— rhythmic training [64] versus MT [60] and HSMT [58] versus general MT [70]. Additionally, outcome comparisons between dissimilar research designs— case report [58] versus RCT [70]— could inhibit consistencies in the synthesis. Our review may therefore suggest that the therapeutic effects of music, dance, and rhythmic training are unique to the art form, which potentially results from underlying differences in the neuroplastic effects of varying art interventions [37, 38, 40]. For example, HSMT required persons to listen to music, which potentially stimulated global neuroconnectivity and atrophic recovery of speech production [58]. Conversely, social-cognitive scores improved upon persons improvising dance movements [70]. These findings may result from changes to dopamine levels that facilitated dopaminergic signaling in the amygdala, which is essential for social-cognitive functioning [95]. Findings additionally confirmed that a rhythmic drumming exercise program, which required sensorimotor processing, led to an overall volumetric increase in the corpus callosum [64]. These neuroplastic changes positively correlated with significant improvements in cognitive functions [64]. The results concur with findings from previous studies [37, 38], which suggest that there are potential neuroplastic effects of art interventions, specifically in subcortical basal ganglion areas, that positively affect functioning in cognitive domains. Rhythmic drumming may have additionally induced stronger synaptic connections between auditory and motor cortices that facilitated an intact exercise performance. It is worth mentioning that this was a within-study comparison that was not examined in other studies in the review.
We also found consistent patient feedback on perceived benefits for psychiatric functions. Studies demonstrated that both MT and DMT reduced fears and tensions associated with speech and motor dysfunctions, respectively [58, 65]. MT and DMT also motivated patients to continue a consistent exercise regime [60, 62]. These findings may result from the ability of MT and DMT to activate emotional pathways in the amygdala, cingulate cortex, and hypothalamus that improve mood and reduce anxieties related to HD progression [38]. However, qualitative syntheses were inconsistent with changes to balance confidence [62], depression, and apathy scores [69]. We predict that inconsistences in findings manifest from our synthesis of one patient’s feedback in a case report [58] or that of a very small sample size [60] with outcomes that represented larger samples [62, 69]. Additionally, studies’ lack of information on HD phase represented in participant samples potentially led to outcome cross-comparisons amongst patients with varying degrees of disease progression. As functionality of patients changes with severity of HD, so should the goals of art therapy. Therapeutic effects of art for HD patients should therefore be uniquely interpreted for each disease phase. While certain studies in the review indicated a relationship between feelings of fear and anxiety and motor dysfunctions [58, 65], other studies reported mood symptoms of depression and apathy without reference to an underlying cause [69]. There may be value in defining the causal relationship between features of HD pathology and psychiatric symptoms so that informative between-study comparisons can come to fruition.
In addition, the review has shown consistent benefits of art forms for motor function, which was found through positive clinician feedback and significant improvements in UHDRS scores [63, 69]. These findings complimented the consistent positive feedback from clinicians and patients on the use of MT for speech production [58, 63]. We expected to see a positive effect of art on motor control, considering that published studies have validated the evidence-based clinical use of dance to improve motor impairments of PD patients [41–44]. Additionally, we expected to find that improvements in UHDRS motor scores were accompanied by an increase in medial superior parietal and paracentral lobule volume as a result of participating in contemporary DMT [69]. These neuroplastic effects may result from sensorimotor processing, which is integral to dance [45]. Although HD and PD pathologies differ, various art forms provide similar benefits to these patient cohorts. MT, DMT, and rhythmic auditory cueing may therefore be pertinent for persons with other forms of neuromuscular disease. Our review also found positive effects of DMT on gait [62], which was not observed in studies that evaluated rhythmic auditory cueing [59, 68]. Generally, studies in the review found that synchronization of gait and hand movements to external auditory cues was impaired for all patients, yielding comparable outcomes to that of free movement [59, 61, 68]. Therefore, our review has demonstrated a distinctive purpose of DMT for gait function that may be more adaptable to the HD phase.
We did not expect to synthesize psychosocial outcomes from less than a third of the included studies in the review. Considering that the concurrence of conventional and alternative treatment methods aims to promote quality of life and patient satisfaction, we expected to synthesize psychosocial functions across the majority of the included studies. Furthermore, to our surprise, findings showed inconsistencies in the comparison of positive patient feedback [58] to insignificant changes to life quality scores [62, 69]. It is worth mentioning that items on the WHO-QoL and QLI are not specific to the HD illness experience, which may account for observed inconsistences. However, levels of satisfaction were comparable between patients and their caregivers [65, 66], suggesting the potential for art forms to transcend healthcare roles.
In the synthesis of positive outcomes represented in the review, we present probable explanations that are supported by the included studies. First, the therapeutic use of art for persons with HD promotes neuroplastic effects to the brain that may reduce cognitive, psychiatric, and motor impairments or perhaps restore function. Because artistic activities such as music performance and dance require specialized cognitive and motor abilities to that of non-artistic activities, these changes may be distinct from that of conventional HD rehabilitation. Only two studies investigated neuroplastic effects as a result of art-based rehabilitation, so our understanding of this comparison is currently inconclusive. Second, music, dance, and rhythmic auditory cueing are conduits for motivating HD patients to engage in exercise. For a disease in which apathy is a clinical hallmark, it is essential to develop strategies to promote patients’ long-term and consistent engagement in exercise regimes. Our review has shown that art forms can maintain excellent attendance and achieve high satisfaction. Last, we have synthesized studies that represented patients across the entire spectrum of HD severity. As such, art methods work regardless of patients’ functionality, suggesting that it can be a consistent method of treatment that is flexible for change.
It is worth addressing limitations of this review. First, approximately half of the included studies were conference abstracts, limiting our access to vital details on art methodologies, participants, and general outcomes. For example, some conference abstracts provided minimal information on participants’ HD phase and the art therapy designs involved. We included conference abstracts because of the small number of relevant studies currently available. As a result, we had to synthesize studies with potentially few characteristic consistencies, making it difficult to gather informative comparisons. Without informational access, outcome comparisons between different art forms and patients at different HD stages was inevitable. Second, many of the studies we reviewed included small sample sizes, weak protocols, and a general span of investigated outcomes. This also contributed to between-study comparisons that involved unaccounted variability, hindering a collective synthesis. Although these limitations made it difficult to synthesize the therapeutic effects of art for patients with HD, recognizing these confounders allows us to understand the factors that may influence outcomes and therefore, avenues for improvement in future studies. Third, we limited our search to medical databases, excluding sources from artistic databases. Although this may have led to an unawareness of other relevant art-based programs and methodologies, we aimed to focus on the integrity of neurological information and scientific research designs, which is optimally supported by medical resources.
We present considerations for future research. First, art-based exercise programs should be tailored to individual phases of HD so that music, dance, and rhythm benefit rather than impede functioning. The development of phase-specific protocols may give patients with HD consistent access to clinical art forms that produce meaningful outcomes. Second, studies should determine the ways in which to effectively incorporate variety within one art method. Utilizing different artistic genres, instruments, and activities, for example, may increase patients’ affinity for art-based programs by catering to their preferences. As a result, programs can maximize benefits by directly targeting apathy. Third, the investigation of the therapeutic use of art should take the form of different study designs. As such, we can establish ideal methodological features to implement for the HD cohort. For example, one study concluded that an RCT was undesirable for evaluating cognitive functioning of patients with advanced HD [70]. Therefore, research designs must be altered to accommodate different disease presentations. Additionally, the investigation of differential outcomes of music, dance, and rhythm through neuroimaging techniques may better inform the future development of art-based rehabilitation programs. Lastly, few studies in the review assessed the impact of art therapy on the quality of life of family members or utilized caregiver feedback to determine the impact of music, dance, and rhythm on patients. Understanding caregiver roles may be particularly useful for characterizing outcomes for patients at middle-to-advanced disease phases as well as the ways in which these outcomes transcend patients to indirectly influence caregiver burden.
In conclusion, the review has provided preliminary support for the clinical use of music, dance, and rhythm for patients with HD, with a particular benefit to gait, global cognitive function, and subcortical plasticity. While it is evident that art forms are feasible and safe methods that garner caregiver and patient satisfaction, limited research has rendered us unable to conclusively determine the manner in which various neurologic art therapy techniques produce therapeutic outcomes. We recommend that future research compares responses of functional domains to art forms by evaluating the mechanistic differences of music, dance, and rhythm that may account for therapeutic outcomes.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work has been possible through a grant from the U.S.-Norway Fulbright Foundation.
REFERENCES
[1] | Raymond LA , Andre VM , Cepeda C , Gladding CM , Milnerwood AJ , Levine MS . Pathophysiology of Huntington’s disease: Time-dependent alteration in synaptic and receptor function. Neuroscience. (2011) ;198: :252–73. |
[2] | Novak MJU , Tabrizi SJ . Huntington’s disease. BMJ. (2010) ;340: :c3109. |
[3] | Zuccato C , Valenza M , Cattaneo E . Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev. (2010) ;90: (3):905–81. |
[4] | Aylward EH , Sparks BF , Field KM , Yallapragada V , Shpritz BD , Rosenblatt A , et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. (2004) ;63: (1):66–72. |
[5] | Shoulson I , Fahn S . Huntington disease: Clinical care and evaluation. Neurology. (1979) ;29: (1):1–3. |
[6] | Huntington Study Group. Unified Huntington’s disease rating scale: Reliability and consistency. Mov Disord. (1996) ;11: (2):136–42. |
[7] | van Walsem MR , Piira A , Mikalsen G , Fossmo HL , Howe EI , Knutsen SF , et al. Cognitive performance after a one-year multidisciplinary intensive rehabilitation program for Huntington’s disease: An observational study. J Huntingtons Dis. (2018) ;7: (4):379–89. |
[8] | Beglinger LJ , O’Rourke JJ , Wang C , Langbehn DR , Duff K , Paulsen JS , et al. Earliest functional declines in Huntington disease. Psychiatry Res. (2010) ;178: (2):414–8. |
[9] | Biglan KM , Zhang Y , Long JD , Geschwind M , Kang GA , Killoran A , et al. Refining the diagnosis of Huntington’s disease: The PREDICT-HD study. Front Aging Neurosci. (2013) ;5: :12. |
[10] | Duff K , Paulsen JS , Beglinger LJ , Langbehn DR , Wang C , Stout JC , et al. “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: Evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. (2010) ;22: (2):196–207. |
[11] | Watkins K , Purks J , Kumar A , Sokas RK , Heller H , Anderson KE . Huntington’s disease and employment: The relative contributions of cognitive and motor decline to the decision to leave work. J Huntingtons Dis. (2018) ;7: (4):367–77. |
[12] | Williams JK , Kim JI , Downing N , Farias S , Harrington DL , Long JD , et al. Everyday cognition in prodromal Huntington disease. Neuropsychology. (2015) ;29: (2):255–67. |
[13] | Paulsen JS . Cognitive impairment in Huntington disease: Diagnosis and treatment. Curr Neurol Neurosci Rep. (2011) ;11: (5):474–83. |
[14] | Philpott AL , Andrews SC , Staios M , Churchyard A , Fisher F . Emotion evaluation and social interference impairments in Huntington’s disease. J Huntingtons Dis. (2016) ;5: (2):175–83. |
[15] | Dumas EM , van den Bogaard SJ , Middelkoop HA , Roos RA . A review of cognition in Huntington’s disease. Front Biosci (Schol Ed). (2013) ;5: :1–18. |
[16] | Diago EB , Martínez-Horta S , Lasaosa SS , Alebesque AV , Pérez-Pérez J , Kulisevsky J , et al. Circadian rhythm, cognition, and mood disorders in Huntington’s disease. J Huntingtons Dis. (2018) ;7: (2):193–8. |
[17] | Mason S , Barker RA . Rating apathy in Huntington’s disease: Patients and companions agree. J Huntingtons Dis. (2015) ;4: (1):49–59. |
[18] | Galvez V , Fernandez-Ruiz J , Bayliss L , Ochoa-Morales A , Hernandez-Castillo CR , Díaz R , et al. Early Huntington’s disease: Impulse control deficits but correct judgment regarding risky situations. J Huntingtons Dis. (2017) ;6: (1):73–8. |
[19] | Petersén A , Gabery S . Hypothalamic and limbic system changes in Huntington’s disease. J Huntingtons Dis. (2012) ;1: (1):5–16. |
[20] | Reilmann R , Schubert R . Motor outcome measures in Huntington disease clinical trials. Handb Clin Neurol. (2017) ;144: :209–25. |
[21] | Grimbergen YAM , Knol MJ , Bloem BR , Kremer BPH , Roos RAC , Munneke M . Falls and gait disturbances in Huntington’s disease. Mov Disord. (2008) ;23: (7):970–6. |
[22] | Ross CA , Pantelyat A , Kogan J , Brandt J . Determinants of functional disability in Huntington’s Disease: Role of cognitive and motor dysfunction. Mov Disord. (2014) ;29: (11):1351–8. |
[23] | Maestre TA , Shannon K . Huntington disease care: From the past to the present, to the future. Parkinsonism Relat Disord. (2017) ;44: :114–8. |
[24] | Bonelli RM , Wenning GK . Pharmacological management of Huntington’s disease: An evidence-based review. Curr Pharm Des. (2006) ;12: (21):2701–20. |
[25] | Anderson KE , van Duijn E , Craufurd D , Drazinic C , Edmondson M , Goodman N , et al. Clinical management of neuropsychiatric symptoms of Huntington disease: Expert-based consensus guidelines on agitation, anxiety, apathy, psychosis and sleep disorders. J Huntingtons Dis. (2018) ;7: (3):355–66. |
[26] | Bilney B , Morris ME , Perry A . Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington’s disease: A systematic review. Neurorehabil Neural Repair. (2003) ;17: (1):12–24. |
[27] | Moorhouse B , Fisher CA . Long-term use of modified diets in Huntington’s disease: A descriptive clinical practice analysis on improving dietary enjoyment. J Huntingtons Dis. (2016) ;5: (1):15–7. |
[28] | Frese S , Petersen JA , Ligon-Auer M , Mueller SM , Mihaylova V , Gehrig SM , et al. Exercise effects in Huntington disease. J Neurol. (2017) ;264: (1):32–9. |
[29] | Fritz NE , Rao AK , Kegelmeyer D , Kloos A , Busse M , Hartel L , et al. Physical therapy and exercise interventions in Huntington’s disease: A mixed methods systematic review. J Huntingtons Dis. (2017) ;6: (3):217–35. |
[30] | Mueller SM , Petersen JA , Jung HH . Exercise in Huntington’s disease: Current state and clinical significance. Tremor Other Hyperkinet Mov (N Y). (2019) ;9: :601. |
[31] | Quinn L , Busse M . The role of rehabilitation therapy in Huntington disease. Handb Clin Neurol. (2017) ;144: :151–165. |
[32] | Quinn L , Busse M , Carrier J , Fritz N , Harden J , Hartel L , et al. Physical therapy and exercise interventions in Huntington’s disease: A mixed methods systematic review protocol. JBI Database System Rev Implement Rep. (2017) ;15: (7):1783–99. |
[33] | Busse M , Quinn L , Debono K , Jones K , Collett J , Playle R , et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J Neurol Phys Ther. (2013) ;37: (4):149–58. |
[34] | Cruickshank TM , Reyes AP , Penailillo LE , Pulverenti T , Bartlett DM , Zaenker P , et al. Effects of multidisciplinary therapy on physical function in Huntington’s disease. Acta Neurol Scand. (2018) ;138: (6):500–7. |
[35] | Piira A , van Walsem MR , Mikalsen G , Nilsen KH , Knutsen S , Frich JC . Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: A prospective intervention study. PLoS Curr. (2013) ;5. |
[36] | Quinn L , Hamana K , Kelson M , Dawes H , Collett J , Townson J , et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat Disord. (2016) ;31: :46–52. |
[37] | Sacks O . Musicophilia: Tales of Music and the Brain. New York: Alfred A. Knopf; 2007. |
[38] | Boso M , Politi P , Barale F , Enzo E . Neurophysiology and neurobiology of the musical experience. Funct Neurol. (2006) ;21: (4):187–91. |
[39] | Sihvonen AJ , Särkämö T , Leo V , Tervaniemi M , Altenmüller E , Soinila S . Music-based interventions in neurological rehabilitation. Lancet Neurol. (2017) ;16: (8):648–60. |
[40] | Rocha PA , Slade SC , McClelland J , Morris ME . Dance is more than therapy: Qualitative analysis on therapeutic dancing classes for Parkinson’s. Complement Ther Med. (2017) ;34: :1–9. |
[41] | Dos Santos Delabary M , Komeroski IG , Monteiro EP , Costa RR , Haas AN . Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: A systematic review with meta-analysis. Aging Clin Exp Res. (2018) ;30: (7):727–35. |
[42] | García-Casares N , Martín-Colom JE , García-Arnés JA . Music therapy in Parkinson’s disease. J Am Med Dir Assoc. (2018) ;19: (12):1054–62. |
[43] | Pereira APS , Marinho V , Gupta D , Magalhães F , Ayres C , Teixeira S . Music therapy and dance as gait rehabilitation in patients with Parkinson’s disease: A review of evidence. J Geriatr Psychiatry Neurol. (2019) ;32: (1):49–56. |
[44] | Raglio A . Music therapy interventions in Parkinson’s disease: The state-of-the-art. Front Neurol. (2015) ;6: :185. |
[45] | Bläsing B , Puttke M , Schack T , editors. The Neurocognition of Dance: Mind, Movement and Motor Skills. Psychology Press, Taylor & Francis Group; (2010) . |
[46] | Kleynen M , Beurskens A , Olijve H , Kamphuis J , Braun S . Application of motor learning in neurorehabilitation: A framework for health-care professionals. Physiother Theory Pract. (2018) ;19: :1–20. |
[47] | Shanahan J , Coman L , Ryan F , Saunders J , O’Sullivan K , Ni Bhriain O , Clifford AM . To dance or not to dance? A comparison of balance, physical fitness and quality of life in older Irish set dancers and age-matched controls. Public Health. (2016) ;141: :56–62. |
[48] | Trevarthen C , Malloch SN . The dance of wellbeing: Defining the musical therapeutic effect. Nord J Music Ther. (2009) ;9: (2):3–17. |
[49] | Patterson KK , Wong JS , Prout EC , Brooks D . Dance for the rehabilitation of balance and gait in adults with neurological conditions other than Parkinson’s disease: A systematic review. Heliyon. (2018) ;4: (3):e00584. |
[50] | van Bruggen-Rufi M , Vink A , Achterberg W , Roos R . Music therapy in Huntington’s disease: A protocol for a multi-center randomized controlled trial. BMC Psychol. (2016) ;4: (1):38. |
[51] | O’Kelly J , Bodak R . Development of the music therapy assessment tool for advanced Huntington’s disease: A pilot validation study. J Music Ther. (2016) ;53: (3):232–56. |
[52] | van Bruggen-Rufi M , Jansen I , van Zwol E . J05 Multi- and interdisciplinary collaboration between the music therapist and other professionals in Huntington’s patient care. J Neurol Neurosurg Psychiatry. (2010) ;81: :A41. |
[53] | van Bruggen-Rufi M , Roos R . Q18 Music therapy as a methods to improve the quality of life of patients with Huntington’s disease: Preliminary findings. J Neurol Neurosurg Psychiatry. (2012) ;83: :A60. |
[54] | van Bruggen-Rufi M , Vink A , Achterberg W , Roos R . Improving quality of life in patients with Huntington’s disease through music therapy: A qualitative explorative study using focus group discussions. Nord J Music Ther. (2018) ;27: (1):44–66. |
[55] | Beste C , Schüttke A , Pfleiderer B , Saft C . Music perception and movement deterioration in Huntington’s disease. PLoS Curr. (2011) ;3: :RRN1252. |
[56] | van Bruggen-Rufi M . The effect of music therapy for patients with Huntington’s disease: A systematic literature review. J Lit Art Stud. (2015) ;5: (1):30–40. |
[57] | Liberati A , Altman DG , Tetzlaff J , Mulrow C , Gøtzsche PC , Ioannidis JPA , et al. The PRIMSA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. (2009) ;6: (7):e1000100. |
[58] | Brandt M , Nieuwkamp M , Kerkdijk E , Verschuur E . Huntington speech music therapy: A therapy based on the principles of SMTA, adjusted for patients with Huntington’s disease. Nord J Music Ther. (2016) ;25: (sup1):121. |
[59] | Delval A , Krystkowiak P , Delliaux M , Blatt JL , Derambure P , Destée A , et al. Effect of external cueing on gait in Huntington’s disease. Mov Disord. (2008) ;23: (10):1446–52. |
[60] | Hyson C , Olivia R , Ladonna KA , Akwaa F , Richards J , Sahler OJ . P010 A pilot study of music therapy in Huntington’s disease. J Neurol Neurosurg Psychiatry. (2005) ;76: (4):A29. |
[61] | Johnson KA , Bennett JE , Georgiou N , Bradshaw JL , Chiu E , Cunnington R , et al. Bimanual co-ordination in Huntington’s disease. Exp Brain Res. (2000) ;134: (4):483–9. |
[62] | Kloos AD , Fritz NE , Kostyk SK , Young GS , Kegelmeyer DA . Video game play (Dance Dance Revolution) as a potential exercise therapy in Huntington’s disease: A controlled clinical trial. Clin Rehabil. (2013) ;27: (11):972–82. |
[63] | Lagesen S . 210 Music therapy as a symptomatic treatment method on patients in advanced stages of Huntington’s disease. Clin Genet. (2011) ;80: (Suppl. 1):14–69. |
[64] | Metzler-Baddeley C , Cantera J , Coulthard E , Rosser A , Jones DK , Baddeley RJ . Improved executive function and callosal white matter microstructure after rhythm exercise in Huntington’s disease. J Huntingtons Dis. (2014) ;3: (3):273–83. |
[65] | Muto K , Chéhère P , Salgues J , Kokado M , Nakai T . P116 Buto dance workshops for Huntington’s disease. J Neurol Neurosurg Pschiatry. (2005) ;76: (suppl 4):A27–54. |
[66] | Orejas Monfort E , Gomez Muniz F , Bascunana Garde M , García de Yébenes J . P2.087 Rehabilitation using dance in Huntington’s disease patients. Parkinsonism Relat Disord. (2008) ;14: :565. |
[67] | Salgues J , Chéhère P . M10 Huntington and dance. J Neurol Neurosurg Psychiatry. (2016) ;87: (Suppl 1):A1–A20. |
[68] | Thaut MH , Miltner R , Lange HW , Hurt CP , Hoemberg V . Velocity modulation and rhythmic synchronization of gait in Huntington’s disease. Mov Disord. (1999) ;14: (5):808–19. |
[69] | Trinkler I , Chéhère P , Salgues J , Monin ML , Tezenas du Montcel S , Khani S , et al. Contemporary dance practice improves motor function and body representation in Huntington’s disease: A pilot study. J Huntingtons Dis. (2019) ;8: (1):97–110. |
[70] | van Bruggen-Rufi MC , Vink AC , Wolterbeek R , Achterberg WP , Roos RA . The effect of music therapy in patients with Huntington’s disease: A randomized controlled trial. J Huntingtons Dis. (2017) ;6: (1):63–72. |
[71] | Sparks R , Helm N , Albert M . Aphasia rehabilitation resulting from melodic intonation therapy. Cortex. (1974) ;10: (4):303–16. |
[72] | Hurkmans J , Jonkers R , de Bruijn M , Boonstra AM , Hartman PP , Arendzen H , et al. The effectiveness of Speech-Music Therapy for Aphasia (SMTA) in five speakers with apraxia of speech and aphasia. Aphasiology. (2015) ;29: (8):939–64. |
[73] | Thaut MH . Rhythm, music and the brain: Scientific foundations and clinical applications. New York: Taylor & Francis; 2005. |
[74] | Bukowska AA , Krężałek P , Mirek E , Bujas P , Marchewka A . eurologic music therapy training for mobility and stability rehabilitation with Parkinson’s disease— a pilot study. Front Hum Neurosci. (2015) ;9: :710. |
[75] | Baddeley A . Exploring the central executive. Q J Exp Psychol. (1996) ;49: (1):5–28. |
[76] | Trenerry MR , Crosson B , DeBoe J , Leber WR . Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; (1989) . |
[77] | Wechsler D . Wechsler Adult Intelligence Scale-3rd UK Edition (WAIS-III UK). Oxford, UK: Psychological Corporation and Pearson Assessment; (1999) . |
[78] | Delis DC , Kaplan E , Kramer JH . Delis-Kaplan Executive Function System (D-KEFS). Oxford, UK: Pearson Assessment; 2001. |
[79] | Timman R , Claus H , Slingerland H , van der Schalk M , Demeulenaere S , Roos RA , et al. Nature and development of Huntington Disease in a nursing home population: The Behavior Observation Scale Huntington (BOSH). Cog Behav Neurol. (2005) ;18: (4):215–22. |
[80] | Schmidt R , Freidl W , Fazekas F , Reinhart B , Grieshofer P , Koch M , et al. The Mattis Dementia Rating Scale: Normative data from 1,001 healthy volunteers. Neurology. (1994) ;44: (5):964–6. |
[81] | Jacobs JV , Horak FB , Van Tran K , Nutt JG . An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol. (2006) ;253: (11):1404–13. |
[82] | Kingma EM , van Duijn E , Timman R , van der Mast RC , Roos RA . Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry. (2008) ;30: (2):155–61. |
[83] | Sockeel P , Dujardin K , Devos D , Denève C , Destée A , Defebvre L . The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. (2006) ;77: (5):579–84. |
[84] | Rao AK , Quinn L , Marder KS . Reliability of spatiotemporal gait outcome measures in Huntington’s disease. Mov Disord. (2005) ;20: (8):1033–7. |
[85] | Dite W , Temple VA . A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehabil. (2002) ;83: (11):1566–71. |
[86] | Kloos A , Kegelmeyer DA , Young GS , Kostyk S . Fall risk assessment using the Tinetti mobility test in individuals with Huntington’s disease. Mov Disord. (2010) ;25: (16):2838–44. |
[87] | Johnson KA , Cunnington R , Bradshaw JL , Phillips JG , Iansek R , Rogers MA . Bimanual co-ordination in Parkinson’s disease. Brain. (1998) ;121: :743–53. |
[88] | McCabe MP , Firth L , O’Connor E . A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. J Clin Psychol Med Settings. (2009) ;16: (4):355–62. |
[89] | Skevington SM , Lotfy M , O’Connell KA ; WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. (2004) ;13: (2):299–310. |
[90] | Ferrans CE , Powers MJ . Psychometric assessment of the Quality of Life Index. Res Nurs Health. (1992) ;15: (1):29–38. |
[91] | Jenkinson C , Fitzpatrick R , Peto V , Greenhall R , Hyman N . The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing. (1997) ;26: (5):353–7. |
[92] | Hunt SM , McKenna SP , McEwen J , Backett EM , Williams J , Papp E . A quantitative approach to perceived health status: A validation study. J Epidemiol Community Health. (1980) ;34: (4):281–6. |
[93] | Stineman MG , Shea JA , Jette A , Tassoni CJ , Ottenbacher KJ , Fiedler R , et al. The Functional Independence Measure: Tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. (1996) ;77: (11):1101–8. |
[94] | Barthel Activities of Daily Living (ADL) Index. Occas Pap R Coll Gen Pract. (1993) ;59:24. |
[95] | Gunaydin LA , Deisseroth K . Dopaminergic dynamics contributing to social behavior. Cold Spring Har Symp Quant. (2014) ;79: :221–7. |



