Microbes, Clinical trials, Drug Discovery, and Vaccine Development: The Current Perspectives
Abstract
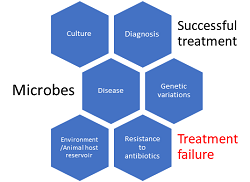
Because of the frequent emergence of novel microbial species and the re-emergence of genetic variants of hitherto known microbes, the global healthcare system, and human health has been thrown into jeopardy. Also, certain microbes that possess the ability to develop multi-drug resistance (MDR) have limited the treatment options in cases of serious infections, and increased hospital and treatment costs, and associated morbidity and mortality. The recent discovery of the novel Coronavirus (n-CoV), the Severe Acute Respiratory Syndrome CoV-2 (SARS-CoV-2) that is causing the CoV Disease-19 (COVID-19) has resulted in severe morbidity and mortality throughout the world affecting normal human lives. The major concern with the current pandemic is the non-availability of specific drugs and an incomplete understanding of the pathobiology of the virus. It is therefore important for pharmaceutical establishments to envisage the discovery of therapeutic interventions and potential vaccines against the novel and MDR microbes. Therefore, this review is attempted to update and explore the current perspectives in microbes, clinical research, drug discovery, and vaccine development to effectively combat the emerging novel and re-emerging genetic variants of microbes.
Full text article
References
2. Pal M, Berhanu G, Desalegn C, Kandi V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus. 2020;12(3):e7423. doi:10.7759/cureus.7423
3. Mahal A, Duan M, Zinad DS, Mohapatra RK, Obaidullah AJ, Wei X, et al. Recent progress in chemical approaches for the development of novel neuraminidase inhibitors. RSC Adv. 2021;11(3):1804-40. doi:10.1039/D0RA07283D
4. Mohapatra RK, Pintilie L, Kandi V, Sarangi AK, Das D, Sahu R, et al. The recent challenges of highly contagious COVID-19, causing respiratory infections: Symptoms, diagnosis, transmission, possible vaccines, animal models, and immunotherapy. Chem Biol Drug Des. 2020;96(5):1187-208. doi:10.1111/cbdd.13761
5. Gubler DJ, Vasilakis N, Musso D. History and Emergence of Zika Virus. J Infect Dis. 2017;216(Suppl_10):S860-S867. doi:10.1093/infdis/jix451
6. Bhatti AB, Usman M, Kandi V. Current Scenario of HIV/AIDS, Treatment Options, and Major Challenges with Compliance to Antiretroviral Therapy. Cureus. 2016;8(3):e515. doi:10.7759/cureus.515
7. Ramana KV, Prakash GK. Mystery behind emergence and re-emergence of Chikungunya virus. Ann Trop Med Public Health. 2009;2(1):1-3.
8. Ramana KV. Dengue Viral Infection: Focus on Epidemiology, Laboratory Diagnosis, Management and Control Measures. J Appl Environ Microbiol. 2014;2(5):249-52. doi:10.12691/jaem-2-5-8
9. Bottone EJ. Bacillus cereus, a Volatile Human Pathogen. Clin Microbiol Rev. 2010;23(2):382-98. doi:10.1128/CMR.00073-09
10. Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645-58. doi:10.2147/IDR.S173867
11. Kandi V. Tungiasis Presenting as Onychomycosis: Probably the First Report of Flea Infestation of the Nail Observed Using Modified Potassium Hydroxide Mount Technique. Cureus. 2018;10(3):e2278. doi:10.7759/cureus.2278
12. Kandi V, Vaish R, Palange P, Bhoomagiri MR. Chronic Pulmonary Histoplasmosis and its Clinical Significance: an Under-reported Systemic Fungal Disease. Cureus. 2016;8(8):e751. doi:10.7759/cureus.751
13. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309-18. doi:10.1179/2047773215Y.0000000030
14. Vadakedath S, Kandi V, Mohapatra RK, Pinnelli VBK, Yegurla RR, Shahapur PR, et al. Immunological aspects and gender bias during respiratory viral infections including novel Coronavirus disease-19 (COVID-19): A scoping review. J Med Virol. 2021;93(9):5295-309. doi:10.1002/jmv.27081
15. Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281-6. doi:10.1007/s12098-020-03263-6
16. Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509-17. doi:10.1038/ni.2039
17. Svastalog AL, Doney D, Kristoffersen NJ, Gajovic S. Concepts and definitions of health and health-related values in the knowledge landscapes of the digital society. Croat Med J. 2017;58(6):431-5. doi:10.3325/cmj.2017.58.431
18. Hochberg ME. An ecosystem framework for understanding and treating disease. Evol Med Public Health. 2018;2018(1):270-86. doi:10.1093/emph/eoy032
19. De Sordi L, Lourenço M, Debarbieux L. The Battle Within: Interactions of Bacteriophages and Bacteria in the Gastrointestinal Tract. Cell Host Microbe. 2019;25(2):210-8. doi:10.1016/j.chom.2019.01.018
20. Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol. 2015;16(6):584-9. doi:10.1038/ni.3152
21. Gray SJ. Timing of Gene Therapy Interventions: The Earlier, the Better. Mol Ther. 2016;24(6):1017-8. doi:10.1038/mt.2016.20
22. von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2016;36:83-102. doi:10.1016/j.bpobgyn.2016.05.005
23. Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current Concepts on the Pathogenesis of Type 1 Diabetes—Considerations for Attempts to Prevent and Reverse the Disease. Diabetes Care. 2015;38(6):979-88. doi:10.2337/dc15-0144
24. Rhoads DD, Sintchenko V, Rauch CA, Pantanowitz L. Clinical Microbiology Informatics. Clin Microbiol Rev. 2014;27(4):1025-47. doi:10.1128/CMR.00049-14
25. Samrot AV, Sean TC, Bhavya KS, Sahithya CS, Chan-Drasekaran S, Palanisamy R, et al. Diagnosis-A Review. Pathogens. 2021;10(2):145. doi:10.3390/pathogens10020145
26. Tanigawa K, Hayashi Y, Hama K, Yamashita A, Yokoyama K, Luo Y, et al. Mycobacterium leprae promotes triacylglycerol de novo synthesis through induction of GPAT3 expression in human premonocytic THP-1 cells. PLoS One. 2021;16(3):e0249184. doi:10.1371/journal.pone.0249184
27. Gaspari E, Malachowski A, Garcia-Morales L, Burgos R, Serrano L, dos Santos VAPM, et al. Model-driven design allows growth of Mycoplasma pneumoniae on serum-free media. NPJ Syst Biol Appl. 2020;6:33. doi:10.1038/s41540-020-00153-7
28. How KY, Song KP, Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol. 2016;7:53. doi:10.3389/fmicb.2016.00053
29. Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2015;79(2):182-94. doi:10.1111/bcp.12403
30. Oliver GF, Carr JM, Smith JR. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: A review. Clin Exp Ophthalmol. 2019;47(3):372-80. doi:10.1111/ceo.13450
31. Raina SK. State of the Globe: Human Nipah Virus Infection needs "One Health". J Glob Infect Dis. 2020;12(1):1-2. doi:10.4103/jgid.jgid_155_19
32. Bos S, Gadea G, Despres P. Dengue: a growing threat requiring vaccine development for disease prevention. Pathog Glob Health. 2018;112(6):294-305. doi:10.1080/20477724.2018.1514136
33. Wolf J, Bruno S, Eichberg M, Jannat R, Ruso S, VanRheenen S, et al. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. NPJ Vaccines. 2020;5:51. doi:10.1038/s41541-020-0204-7
34. Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):e1-e94. doi:10.1093/cid/ciy381
35. Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4(2):15. doi:10.1128/microbiolspec.VMBF-0016-2015
36. Zhang MW, Zhou L, Zhang Y, Chen B, Peng Y, Wang F, et al. Treatment outcomes of patients with multidrug and extensively drug-resistant tuberculosis in Zhejiang, China. Eur J Med Res. 2021;26:31. doi:10.1186/s40001-021-00502-0
37. Kesik‐Brodacka M. Progress in biopharmaceutical development. Biotechnol Appl Biochem. 2018;65(3):306-22. doi:10.1002/bab.1617
38. Taylor D. The Pharmaceutical Industry and the Future of Drug Development. In: Pharmaceuticals in the Environment. London (UK): The Royal Society of Chemistry; 2015. p. 1-33. doi:10.1039/9781782622345-00001
39. Ho CH, Yi J, Wang X. Biocatalytic Continuous Manufacturing of Diabetes Drug: Plantwide Process Modeling, Optimization, and Environmental and Economic Analysis. ACS Sustain Chem Eng. 2019;7(1):1038-51. doi:10.1021/acssuschemeng.8b04673
40. Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323(9):844-53. doi:10.1001/jama.2020.1166
41. Wong CH, Siah KW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273-86. doi:10.1093/biostatistics/kxx069
42. Gurgula O. Strategic Patenting by Pharmaceutical Companies – Should Competition Law Intervene? IIC Int Rev Ind Prop Copyr Law. 2020;51:1062-85. doi:10.1007/s40319-020-00985-0
43. Katiyar C, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: An integrated approach. Ayu. 2012;33(1):10-9. doi:10.4103/0974-8520.100295
44. Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162(6):1239-49. doi:10.1111/j.1476-5381.2010.01127.x
45. Mohs RC, Greig NH. Drug discovery and development: Role of basic biological research. Alzheimers Dement. 2017;3(4):651-7. doi:10.1016/j.trci.2017.10.005
46. Kaitin KI. Deconstructing the Drug Development Process: The New Face of Innovation. Clin Pharmacol Ther. 2010;87(3):3556-61. doi:10.1038/clpt.2009.293
47. Dahlin JL, Walters MA. The essential roles of chemistry in high-throughput screening triage. Future Med Chem. 2014;6(11):1265-90. doi:10.4155/fmc.14.60
48. Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26(1):80-93. doi:10.1016/j.drudis.2020.10.010
49. Dugger SA, Platt A, Goldstein DB. Drug development in the era of precision medicine. Nat Rev Drug Discov. 2018;17(3):183-96. doi:10.1038/nrd.2017.226
50. Pradhan S, Sinha C. Sulfonamide derivatives as Mycobacterium tuberculosis inhibitors: in silico approach. In Silico Pharmacol. 2018;6:4. doi:10.1007/s40203-018-0041-9
51. Szymański P, Markowicz M, Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery-toxicological screening tests. Int J Mol Sci. 2012;13(1):427-52. doi:10.3390/ijms13010427
52. Prabhu GRD, Urban PL. The dawn of unmanned analytical laboratories. Trends Anal Chem. 2017;88:41-52. doi:10.1016/j.trac.2016.12.011
53. Lee SL, Saluja B, Garcia-Arieta A, Santos GML, Li Y, Lu S, et al. Regulatory Considerations for Approval of Generic Inhalation Drug Products in the US, EU, Brazil, China, and India. AAPS J. 2015;17(5):1285-304. doi:10.1208/s12248-015-9787-8
54. Vijayanathan A, Nawawi O. The importance of Good Clinical Practice guidelines and its role in clinical trials. Biomed Imaging Interv J. 2008;4(1):e5. doi:10.2349/biij.4.1.e5
55. Das NK, Sil A. Evolution of Ethics in Clinical Research and Ethics Committee. Indian J Dermatol. 2017;62(4):373-9. doi:10.4103/ijd.ijd_271_17
56. Sanmukhani J, Tripathi CB. Ethics in Clinical Research: The Indian Perspective. Indian J Pharm Sci. 2011;73(2):125-30. doi:10.4103/0250-474x.91564
57. Kartoğlu Ü, Siagian RC, Reeves TC. Creating a “Good Clinical Practices Inspection” Authentic Online Learning Environment through Educational Design Research. TechTrends. 2020;64;616-27. doi:10.1007/s11528-020-00509-0
58. Imran M, Samad S, Maaz M, Qadeer A, Najmi AK, Aqil M. Hippocratic oath and conversion of ethico-regulatory aspects onto doctors as a physician, private individual and a clinical investigator. J Midlife Health. 2013;4(4):203-9. doi:10.4103/0976-7800.122232
59. Issa NT, Wathieu H, Ojo A, Byers SW, Dakshanamurthy S. Drug Metabolism in Preclinical Drug Development: A Survey of the Discovery Process, Toxicology, and Computational Tools. Curr Drug Metab. 2017;18(6):556-65. doi:10.2174/1389200218666170316093301
60. Lautié E, Russo O, Ducrot P, Boutin JA. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front Pharmacol. 2020;11:397. doi:10.3389/fphar.2020.00397
61. Aschner M, Autrup HN, Bery SCL, Boobis AR, Cohen SM, Creppy EE, et al. environmental risk assessments and regulations. Toxicology. 2016;371:12-6. doi:10.1016/j.tox.2016.09.005
62. Alshammari TM. Drug safety: The concept, inception and its importance in patients' health. Saudi Pharm J. 2016;24(4):405-12. doi:10.1016/j.jsps.2014.04.008
63. Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
64. Singh K, Mehta S. The clinical development process for a novel preventive vaccine: An overview. J Postgrad Med. 2016;62(1):4-11. doi:10.4103/0022-3859.173187
65. Breithaupt-Groegler K, Coch C, Coenen M, Donath F, Erb-Zohar K, Francke K, et al. Who is a ‘healthy subject’?—consensus results on pivotal eligibility criteria for clinical trials. Eur J Clin Pharmacol. 2017;73:409-16. doi:10.1007/s00228-016-2189-8
66. Burt T, Young G, Lee W, Kusuhara H, Langer O, Rowland M, Sugiyama Y. Phase 0/microdosing approaches: time for mainstream application in drug development? Nat Rev Drug Discov. 2020;19:801-18. doi:10.1038/s41573-020-0080-x
67. Umscheid CA, Margolis DJ, Grossman CE. Key concepts of clinical trials: a narrative review. Postgrad Med. 2011;123(5):194-204. doi:10.3810/pgm.2011.09.2475
68. Katsanis LP, Pitta D. Managing the risk aspects of the product development process at the Upjohn Company. J Prod Brand Manag. 2006;15(4):250-4. doi:10.1108/10610420610679610
69. Narendran R, Narendranathan M. Influence of pharmaceutical marketing on prescription practices of physicians. J Indian Med Assoc. 2013;111(1):47-50.
70. Parker RS, Pettijohn CE. Pharmaceutical drug marketing strategies and tactics: a comparative analysis of attitudes held by pharmaceutical representatives and physicians. Health Mark Q. 2005;22(4):27-43. doi:10.1300/j026v22n02_03
71. Murshid MA, Mohaidin Z. A systematic review of the influence of medical representatives and promotional tools on prescribing: A comparison between developed and developing countries. Int J Pharm Healthc Mark. 2017;11(4):361-94. doi:10.1108/IJPHM-09-2016-0047
72. Palcsó B, Zelkó R. Different types, applications and limits of enabling excipients of pharmaceutical dosage forms. Drug Discov Today Technol. 2018;27:21-39. doi:10.1016/j.ddtec.2018.04.002
73. Kumar M, Bishnoi RS, Shukla AK, Jain CP. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev Nutr Food Sci. 2019;24(3):225-34. doi:10.3746/pnf.2019.24.3.225
74. Kandi V, Kandi S. Antimicrobial properties of nanomolecules: potential candidates as antibiotics in the era of multi-drug resistance. Epidemiol Health. 2015;37:e2015020. doi:10.4178/epih/e2015020
75. Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Kain NK, et al. Nanoemulsion: Concepts, development and applications in drug delivery. J Control Release. 2017;252:28-49. doi:10.1016/j.jconrel.2017.03.008
76. Erfle P, Riewe J, Bunjes H, Dietzel A. Stabilized Production of Lipid Nanoparticles of Tunable Size in Taylor Flow Glass Devices with High-Surface-Quality 3D Microchannels. Micromachines. 2019;10(4):220. doi:10.3390/mi10040220
77. Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5(5):442-53. doi:10.1016/j.apsb.2015.07.003
78. Wykes MN. Why haven't we made an efficacious vaccine for malaria? EMBO Rep. 2013;14(8):661. doi:10.1038/embor.2013.103
79. Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445-9. doi:10.1038/nature21060
80. Long CA, Zavala F. Malaria vaccines and human immune responses. Curr Opin Microbiol. 2016;32:96-102. doi:10.1016/j.mib.2016.04.006
81. McCall MBB, Kremsner PG, Mordmüller B. Correlating efficacy and immunogenicity in malaria vaccine trials. Semin Immunol. 2018;39:52-64. doi:10.1016/j.smim.2018.08.002
82. Bliss CM, Drammeh A, Bowyer G, Sanou GS, Jagne YJ, Ouedraogo O, et al. Viral Vector Malaria Vaccines Induce High-Level T Cell and Antibody Responses in West African Children and Infants. Mol Ther. 2017;25(2):547-59. doi:10.1016/j.ymthe.2016.11.003
83. Pallikkuth S, Chaudhury S, Lu P, Pan L, Jongert E, Wille-Reece U, et al. A delayed fractionated dose RTS,S AS01 vaccine regimen mediates protection via improved T follicular helper and B cell responses. eLife. 2020;9:e51889. doi:10.7554/eLife.51889
84. Versteeg L, Almutairi MM, Hotez PJ, Pollet J. Enlisting the mRNA Vaccine Platform to Combat Parasitic Infections. Vaccines. 2019;7(4):122. doi:10.3390/vaccines7040122
85. Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73-82. doi:10.1038/s41577-020-00480-0
86. Vitiello A, Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology. 2021;29(3):645-9. doi:10.1007/s10787-021-00811-0
87. Poveda C, Biter AB, Bottazzi ME, Strych U. Establishing Preferred Product Characterization for the Evaluation of RNA Vaccine Antigens. Vaccines. 2019;7(4):131. doi:10.3390/vaccines7040131
88. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-16. doi:10.1056/NEJMoa2035389
89. Malik JA, Mulla AH, Farooqi T, Pottoo FH, Anwar S, Rengasamy KRR. Targets and strategies for vaccine development against SARS-CoV-2. Biomed Pharmacother. 2021;137:111254. doi:10.1016/j.biopha.2021.111254
90. Wang F, Kream RM, Stefano GB. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med Sci Monit. 2020;26:e924700. doi:10.12659/msm.924700
Authors
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish with this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal, right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles of work (eg, post-institutional repository) or publish it in a book, with acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process, as can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.