Decentralizing Cell-Free RNA Sensing With the Use of Low-Cost Cell Extracts
- 1ANID – Millennium Science Initiative Program – Millennium Institute for Integrative Biology (iBio), Santiago, Chile
- 2Schools of Engineering, Institute for Biological and Medical Engineering, Medicine and Biological Sciences, Pontificia Universidad Católica de Chile, Santiago, Chile
- 3Departamento de Genética Molecular y Microbiología, Pontificia Universidad Católica de Chile, Santiago, Chile
- 4Department of Plant Sciences, University of Cambridge, Cambridge, United Kingdom
- 5Department of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge, United Kingdom
- 6Microsoft Research, Cambridge, United Kingdom
- 7Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON, Canada
- 8FONDAP Center for Genome Regulation, Santiago, Chile
Cell-free gene expression systems have emerged as a promising platform for field-deployed biosensing and diagnostics. When combined with programmable toehold switch-based RNA sensors, these systems can be used to detect arbitrary RNAs and freeze-dried for room temperature transport to the point-of-need. These sensors, however, have been mainly implemented using reconstituted PURE cell-free protein expression systems that are difficult to source in the Global South due to their high commercial cost and cold-chain shipping requirements. Based on preliminary demonstrations of toehold sensors working on lysates, we describe the fast prototyping of RNA toehold switch-based sensors that can be produced locally and reduce the cost of sensors by two orders of magnitude. We demonstrate that these in-house cell lysates provide sensor performance comparable to commercial PURE cell-free systems. We further optimize these lysates with a CRISPRi strategy to enhance the stability of linear DNAs by knocking-down genes responsible for linear DNA degradation. This enables the direct use of PCR products for fast screening of new designs. As a proof-of-concept, we develop novel toehold sensors for the plant pathogen Potato Virus Y (PVY), which dramatically reduces the yield of this important staple crop. The local implementation of low-cost cell-free toehold sensors could enable biosensing capacity at the regional level and lead to more decentralized models for global surveillance of infectious disease.
Introduction
Cell-free sensors have emerged as a promising technology for fast and field-deployable detection of nucleic acids (Pardee et al., 2014, 2016; Ma et al., 2018; Takahashi et al., 2018; Verosloff et al., 2019) and chemical pollutants (Didovyk et al., 2017; Jung et al., 2020; Liu et al., 2020; Silverman et al., 2020; Thavarajah et al., 2020). Cell-free toehold RNA sensors (CFTS) have been shown to detect viruses such as Ebola (Pardee et al., 2014), norovirus (Ma et al., 2018), and Zika (ZIKV) (Pardee et al., 2016) at femtomolar concentrations of viral RNA when combined with isothermal RNA amplification (Pardee et al., 2016). Toehold RNA sensors are de novo designed genetic structures that can be programmed to detect RNAs of arbitrary sequences (Green et al., 2014). A toehold switch relies on the formation of a hairpin loop at the 5′-end of a reporter gene that structurally prohibits an interaction between the ribosome binding site (RBS) and ribosomes, thereby preventing translation. This structure is released via toehold-mediated strand displacement by an unstructured RNA “trigger” with sequence-specific complementarity to the switch (Green et al., 2014), thus enabling ribosomal-binding and subsequent protein translation. These CFTS can be lyophilized and transported at room temperature for in-field detection of viral RNAs (Pardee et al., 2014, 2016; Ma et al., 2018).
CFTS are implemented in a reconstituted cell-free expression system, known commercially as PURExpress, which consists of a mixture of 34 individually purified components involved in transcription, translation and energy generation (Shimizu et al., 2001). Despite the potential of CFTS for point-of-care deployment, constraints in the accessibility to PURE systems in South America impose significant barriers to locally engineer de novo sensors. PURE systems are expensive and proprietary (Laohakunakorn et al., 2020). Furthermore, they need to be imported from the Northern hemisphere under cold-chain shipping, which increases their costs and the risk of activity loss. PURE systems can also be prepared in the laboratory by purifying the components individually or as a single step from a bacterial consortium of strains (Shimizu et al., 2001; Horiya et al., 2017; Villarreal et al., 2018; Lavickova and Maerkl, 2019). Alternatively, cell lysate-based protein expression systems, made from E. coli crude extract that do not require purification of particular components, can also be used as a substrate for CFTS (Silverman et al., 2019). Lehr et al. have shown the implementation of CFTS in commercially available cell-free transcription-translation systems (TX-TL) based on cell extracts (Lehr et al., 2019), which are also sourced from the US and shipped under cold chain. Encouragingly, Silverman et al. (2019), McNerney et al. (2019) and Pieters et al. (2021) have recently shown the successful implementation of CFTS in home-made cell extracts, offering a path for local prototyping de novo sensors of interest (McNerney et al., 2019; Silverman et al., 2019; Pieters et al., 2021).
Cell extracts are cell lysate-based protein expression systems that have been instrumental to molecular biology and biotechnology for decades (Nirenberg and Matthaei, 1961). Cell extracts have also become a versatile tool for engineering complex biomolecular systems outside living cells (Garenne and Noireaux, 2019; Laohakunakorn et al., 2020). Diverse genetic systems have been implemented in cell extracts, e.g. for sensing small molecules (Salehi et al., 2018; Jung et al., 2020; Silverman et al., 2020; Thavarajah et al., 2020), engineering metabolic pathways (Dudley et al., 2016; Grubbe et al., 2020; Swartz, 2020), producing virus-like particles (Bundy et al., 2008; Rustad et al., 2018), and prototyping genetic devices (Siegal-Gaskins et al., 2014; Moore et al., 2017), including RNA circuits (Takahashi et al., 2015), transcriptional cascades (Shin and Noireaux, 2012; Garamella et al., 2016) and oscillators (Niederholtmeyer et al., 2015; Tayar et al., 2017; Yelleswarapu et al., 2018). Various protocols have been crafted to simplify extract preparation and optimize lysate performance, including methods based on cell homogenization (Kim et al., 2006), sonication (Shrestha et al., 2012; Kwon and Jewett, 2015), bead-beating (Sun et al., 2013), enzymatic lysis or flash freezing (Didovyk et al., 2017).
With the goal of making cell-free toehold sensor technology more accessible and affordable, here, we describe the implementation of CFTS in cell extracts supplemented with maltodextrin as an energy source. These CFTS can detect RNA at similar sensitivity to the PURExpress-based CFTS but for a fraction of the cost. Furthermore, using a CRISPRi strategy for silencing nucleases before harvesting cells, we increased DNA stability, enabling cell-free protein synthesis from linear, PCR-derived templates. These optimized extracts were suitable for fast prototyping of novel toehold sensors directly from linear PCR products using a lacZ gene template. As a proof-of-concept demonstration, we applied our framework to engineer cost-effective CFTS for PVY virus detection, which poses a significant economic threat to local potato farmers and producers of potato seeds.
Materials and Methods
Crude Extract Preparation
BL21 DE3 Star cells (Invitrogen, C601003), BL21dLacGold cells (Didovyk et al., 2017) (Agilent, 230,132), or the CRISPR+, CRISPR-derivatives from this work were grown overnight at 37°C on 2xYT agar plates with the appropriate antibiotics. A single colony was grown in a 5 ml 2xYT culture overnight at 37°C. The next day, a 50 ml culture was started with a 1:1,000 dilution overnight at 37°C. On the fourth day, a total of 2 L of culture was started and split in 5 2-L Erlenmeyer, at an initial OD600 0.05 in 2xYT supplemented with 40 mM phosphate dibasic (Sigma, 94,046), 22 mM phosphate monobasic (Sigma, 74,092) and 20 g/L D-glucose. This culture was grown at 37°C with vigorous agitation (230 rpm) until OD600 reached 0.6–0.8 (approximately 2.5 h) and cells were induced with 1 mM IPTG for another 3 h at 37°C before harvesting by centrifugation at 5,000 g and 4°C. Pellets were washed twice with cold S30B buffer (5 mM tris acetate pH 8.2, 14 mM magnesium acetate, 60 mM potassium glutamate, and 2 mM dithiothreitol) (Sun et al., 2013). The pellet was then weighted, and a relationship of 0.9 ml of S30B and 5 g of 0.1 mm diameter silica beads (Biospec, 11079101) were added per Gram of pellet obtained. 2.5 ml bead-beating tubes and cups were filled with the viscous solution composed of cells and glass beads, without generating bubbles, sealed and beaten for 30 s using a homogenizer (MP Biomedicals, FastPrep-24 5G). To remove the beads from extracts, processed samples were placed on the top of a micro-chromatography column (Biorad, 7326204), which was placed into an empty bead-beating tube. The bead-beaten tube attached to the micro-chromatography column and the empty recipient tube was placed into a 15 ml Falcon tube and centrifuged at 6,000 g for 5 min at 4°C. Properly beat extracts should appear clear, and two distinct layers should be observed. The supernatant from all tubes was pooled and agitated at 37°C inside a 5 ml unsealed tube for 1 hour for runoff. After the run-off reaction, the tubes were placed on ice and re-centrifuged at 6,000 g for 10 min at 4°C to pull down the debris generated during the run-off reaction. The supernatant from this centrifugation, i.e., the crude extract, was aliquoted, frozen, and stored at −80°C until use. A link to the full protocol of crude extract and cell-free reaction preparation can be found here: https://www.protocols.io/view/preparation-of-cell-free-rnapt7-reactions-kz2cx8e.
Cell-Free Transcription-Translation Reaction
The in-house cell-free reactions were composed of 45% crude lysate and 40% reaction buffer. The remaining 15% included DNA input solution chlorophenol red-β-D-galactopyranoside (Sigma, 59,767 at final concentration of 0.6 mg/ml), and in the case of the RNA sensing reactions, trigger RNA from in vitro transcription. A typical reaction is composed of 50 mM HEPES pH 8, 1.5 mM ATP and GTP, 1.4 mM CTP and UTP as triphosphate ribonucleotides, 0.2 mg/ml tRNA (Roche, 01109541001), 0.26 mM Coenzyme A, 0.33 mM NAD, 0.756 mM cAMP, 0.0068 mM folinic acid, 0.112 mg/ml spermidine, 2% PEG-8000, 3.4 mM of each of the 20 amino acids (glutamate is also added in excess in potassium and magnesium salts), and 12 mg/ml maltodextrin and 0.6 mg/ml sodium hexametaphosphate (Sigma, 305,553) or 30 mM 3-PGA as an energy source. Cell-free reactions were mounted on ice in a final volume of 10 µL in 1.5 ml Eppendorf tubes and vortexed for 30 s before sampling 5 µL that were loaded in V-bottom 96 well plates (Corning, CLS3957) or rounded 384 well-plates (Corning, CLS3540). All the reactions were incubated at 29°C in a Synergy HTX plate reader (BioTek).
Input DNA Preparation
All plasmids used in this work correspond to single transcriptional units (Level 1 plasmids) that were prepared using Golden Gate assembly from Level 0 parts (T7 Promoter, T7 terminator, Toehold Sensors, Triggers, etc.) (Pollak et al., 2019). The level 0 parts were previously prepared by Gibson assembly (Gibson et al., 2009) from PCR linear DNA amplified from gblocks (IDT). All plasmids were commercially sequenced before use. Plasmid DNA input was produced by midi prepping an overnight culture of 200 ml LB with the appropriate strain (Promega, A2492) and cleaned again using PCR cleanup (Promega, A6754). Plasmid DNA inputs were used in a set of concentrations ranging from 0.5 to 10 nM. Input linear DNA was produced by PCR and cleaned with PCR cleanup kit (Promega, A6754), quantified and diluted to desired concentrations using ultra-pure water. A list of all the primers and plasmids used in this work is shown in Supplementary Tables S1, 2, respectively.
Trigger RNA Preparation
PCR products containing the trigger T7 transcriptional unit were cleaned by PCR cleanup kit (Promega, A6754) and 250 ng were processed by in vitro transcription using the HiScribe T7 kit (Promega, E2040S) and incubated at 37°C overnight. The products were then treated with DNAseI for 1 hour at 37°C and the enzyme was inactivated by heat at 70°C for 10 min. Next, standard RNA clean up steps were performed according to the RNeasy MinElute kit (Qiagen, 74,204) before quantification.
Isothermal NASBA Amplification
We used the NASBA lyophilized kit from Life Sciences following the manufacturer’s instructions. Negative controls and RNA dilutions in the range 27 mM to 0.2 fM were prepared in ultra-pure water just before use. The lyophilized reaction buffer containing nucleotide mix (Life Sciences, LRB-10) was reconstituted with DRB-10 diluent (Life Sciences, DRB-10), heated at 41°C for 5 min and kept at room temperature. The initial mixture, consisting of 10 µL of the reconstituted reaction buffer, 1 µL RNAsin (Promega, N2111), 380 mM of each DNA primer, 2 µL ultra-pure water and a 1 µL RNA amplicon dilution, were assembled at 4°C and incubated at 65°C for 2 min, followed by a 10 min annealing at 41°C. During annealing, the lyophilized enzyme mix, consistent of AMV RT, RNaseH, T7 RNAP, and high molecular weight sugar mix (Life Sciences, LEM-10), was reconstituted with cold D-PDG (Life Sciences, D-PDG-10) and placed on ice. Immediately after annealing, 5 µL of the dissolved enzyme mix was added to the reaction, to a final volume of 20 µL, and the mixture was incubated at 41°C for 2 h with thermocycler lid at 98°C. A 10 min incubation at 70°C was performed for enzyme inactivation. The amplified product was subsequently cleaned up with the RNeasy MinElute kit (Qiagen, 74,204).
Lyophilization and Storage of Cell-free Reactions
Lyophilization was performed by assembling 9 µL of cell extracts and 8 µL of reaction buffer in a PCR tube. In a separate tube, 3 µL of the corresponding plasmid were mixed with 1 µL of substrate (9 mg/ml), when LacZ reporter was used. Tubes containing the assembled reactions were closed with adhesive aluminum film and punctured with a 16-gauge needle to create a hole. The tubes were placed in a FreeZone 2.5 L Triad Benchtop Freeze Dryer (Labconco), previously pre-chilled at −75°C (shelf temperature), for overnight freeze-drying of two segments. The first segment was at −75°C (shelf temperature) with a temperature collector of −80°C for 12 h and 0.04 mbar of pressure. The second segment was performed at −20°C for 4 h with the same pressure. Samples were stored at room temperature in open plastic bags over 30 g of silica inside a closed Tupperware. Rehydration of cell-free reactions was done with 20 µL of a plasmid solution in ultra-pure water. pT7:sfGFP was used at 6 nM or 8 nM final concentration whereas ZIKV Toehold 27 was used at 10 nM final concentration. Trigger 27 RNA was added at a final concentration of 300 nM.
Design of Novel Toehold Sensors
With the aim of designing novel toehold sensors, we implemented an algorithm developed by Green et al. (Green et al., 2014), as an in silico tool called NupackSensors (http://www.github.com/elanibal/NupackSensors). To identify possible triggers from a target sequence, the algorithm first determines all contiguous 36 nucleotide sub-sequences. Each possible trigger defines one specific toehold sensor. After filtering out the sensors that contained stop codons, potential structural defects of each design were calculated, along with a set of thermodynamic parameters (Supplementary Figure S1; Supplementary Tables S3, 4). The following score function was implemented, and one score value was assigned to each of the possible designs (Ma et al., 2018):
Here,
For the PVY toehold sensor design, two RNA target sequences were evaluated in NupackSensors, corresponding to the long and short sequences found to be conserved in several PVY strains (Supplementary Tables S5, 6). For each of them, four of the highest scores were picked for experimental evaluation. Linear DNA encoding the PVY toeholds were prepared by PCR using specific primers that add the T7 promoter and each toehold sensor sequence on their 5’ tails along with the UNSXR reverse primer. LacZ full length was used as template (Supplementary Table S1 for primer sequences).
sgRNA Design
Sequences of each operon of the genes that are part of the RecBCD complex and the EndI endonuclease were identified in E.coli K12 strain genome using the web platform RegulonDB (Salgado et al., 2013). sgRNAs were designed according to (Larson et al., 2013), following these steps: all available 5'->3' PAM sites (CCN) within the nucleotide sequence of the gene of interest were marked, identifying contiguous sites as target candidates; the target candidates were analyzed and selected as the 18–22 bp contiguous to the PAM sequence that are within the first ∼156 nt of the CDS (i.e., counting from the ATG); sequences that end in C or T were selected so that the sgRNA begins with A or G (corresponding to nucleotide +1 of the sgRNA transcript) since this increases the transcription of the promoter used (Larson et al., 2013; Qi et al., 2013). The designed sgRNA corresponded to the reverse complementary sequences of the chosen target. These steps were performed in ApE open source software (https://jorgensen.biology.utah.edu/wayned/ape/). We designed three sgRNAs that targeted the cistron of recB and recD, recC and endA genes, respectively.
Stability of PCR-Derived Linear DNA in Cell-free Reactions
Linear DNA fragments of 1.5 kb and 500 bp were prepared via PCR for its use as linear DNA input and standards, respectively. Those were purified by wizard gel and PCR clean-up kit (Promega), achieving a final concentration of 200 ng/μL 600 ng of the 1.5 kb linear DNA were added to 20 μL cell-free reactions and incubated at 29°C for 0, 30, 60 or 120 min and quickly submerged in liquid nitrogen to stop the reaction. Frozen samples were put on ice; and immediately, a volume of binding buffer from PCR clean-up kit (Promega, A9282) was added followed by 2 µL (300 ng) of the 500 bp linear DNA that served as an internal standard for the purification. The samples were then purified following standard supplier’s protocol and eluted with 30 µL of ultra-pure water. Next, 0.3 volumes of loading buffer were added to the purified samples and 10 µL were run on a 1% agarose gel electrophoresis containing 1X SYBR-Safe for DNA visualization. 1.5 kb and 500 bp DNA fragments were placed in two independent lines along with another line for 1 kb Ladder (NEB, N232). Once bands were clearly identifiable, the gel was photographed on a blue LED light transilluminator with a blue-light filter. The intensity of the 1.5 kb bands in each sample was integrated using ImageJ and normalized with the intensity of the 500 bp band of the same lane.
Results
Implementation of an In-House Cell-free Gene Expression System
We first sought to implement a low-cost and simple protocol for cell-free gene expression. To do this, we combined the use of S12 crude extracts–a simple post lysate processing which does not require ultracentrifugation nor dialysis (Kim et al., 2008)—with a highly concentrated amino acid mixture (Caschera and Noireaux, 2015b) and a cost-effective energy source. This energy source is based on maltodextrin and polyphosphates, which do not require cold-chain transportation (Caschera and Noireaux, 2015a). We characterized our system by measuring the dynamics or endpoints of the pT7:sfGFP constitutive expression (Figure 1A). Initially, we prepared four different batches and tested optimum magnesium concentration in the reaction buffer for the cell-free expression of sfGFP. Optimal values ranged between 8.5 and 10 nM for all extracts (Supplementary Figure S2). With the goal of reducing the cost of lysate preparation, we compared maltodextrin and polyphosphates (HMP) to the conventional and more expensive 3-phosphoglyceric acid (3-PGA) energy source. In a side-by-side comparison with 3-PGA, maltodextrin produced a similar initial rate in sfGFP fluorescence (Supplementary Figure S3). Moreover, the endpoint measurements were significantly higher for the cost-effective maltodextrin-based energy source in each batch tested (Figure 1B). Next, we measured the dynamics of constitutive pT7:sfGFP (Figure 1A) expression in these extracts compared to two RNAP T7-based commercial kits. In comparison to these commercial cell-free systems, our in-house cell lysates generated higher endpoint fluorescent values, although variability was observed between different batches of cell-free extracts (Figure 1C).
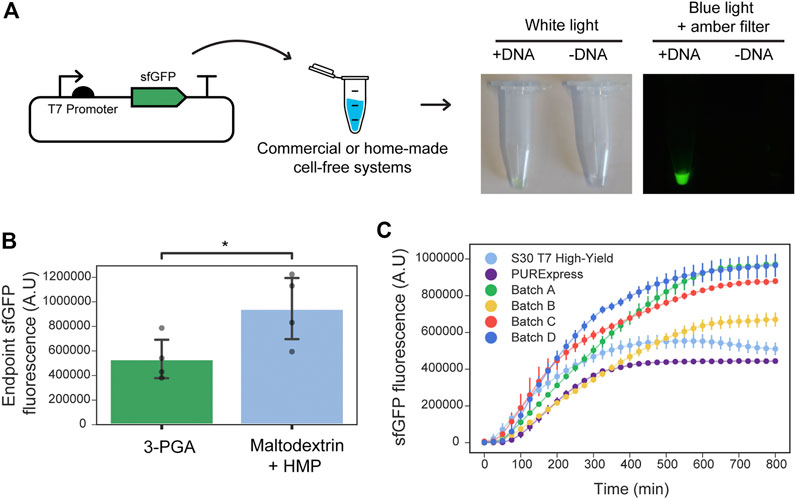
FIGURE 1. Optimized in-house cell-free reactions compared to commercial alternatives. (A) Left: Schematic representation of testing the performance of home-made or commercial cell-free systems using the sfGFP constitutive reporter. Right: Example of the endpoint sfGFP reaction and negative control (without input DNA) in a home-made cell-free system supplemented with maltodextrin energy source. Tubes were photographed under white light or blue light plus an amber filter that allows visualizing the sfGFP fluorescence. (B) Endpoint sfGFP fluorescence (plasmid DNA at 9 nM final concentration) was measured in four different cell extracts (Batch A, B, C, D) supplemented with either maltodextrin and polyphosphates (light blue) or 3-PGA (green) as energy source. Grey dots represent the arithmetic mean of three measurements performed on each batch, and error bars represent standard deviations of the means of the four batches tested (N = 4). t-test for paired measurements was performed and statistically significance was found between the two groups (p-value = 0.03, shown by *). Assumptions of the paired t-test were verified using the Shapiro-Wilk test for normality of the differences between energy sources for a given batch (p-value = 0.97), and Levene test for homoscedasticity of the 3-PGA and maltodextrin data sets (p-value = 0.25). (C) sfGFP production dynamics from plasmid DNA (9 nM final concentration) in reactions performed at 29°C using NEB PURExpress and Promega S30 T7 High Yield commercial kits along with four optimized in-house cell-free reactions (Batch A, B, C, D) using maltodextrin and polyphosphates as the energy source. Error bars represent the standard deviations of three independent replicates, dots are centered at the arithmetic mean for each time point.
A cost-breakdown analysis of lab-scale extract production of this optimized cell-free lysate system was performed, comparing reagent prices available to the collaborating institutions in Chile and the United Kingdom (Supplementary Tables S7–13). The cost per reaction (5 µL) in Chile was $0.069 USD for the maltodextrin-based preparation in comparison to $7.80 USD for the locally purchased commercial PURExpress, representing a reduction of two orders of magnitude. In comparison, the home-made preparations were about two times less expensive in the United Kingdom than in Chile (Supplementary Figure S4; Supplementary Table S7). Maltodextrin-based reactions were approximately 20% cheaper than the 3-PGA alternative.
Implementation of CFTS in Low-Cost Cell-free Reactions
To investigate whether RNA toehold sensors would be functional in the in-house prepared cell extracts, we used Zika virus (ZIKV) toehold sensors already shown to work well in PURExpress (Pardee et al., 2016). We selected ZIKV toehold sensors 8 and 27 and their respective triggers due to their high dynamic range and orthogonality (Pardee et al., 2016). These toehold sensors, controlling full-length LacZ expression, were incubated with the corresponding RNA trigger (400 nM) independently (Figure 2A). For each sensor, we chose the highest DNA concentration that ensures low-background in PURExpress and used this concentration for comparison with in-house cell-free reactions. Sensor 8 (at 0.8 nM plasmid DNA) behaved similarly in both reaction conditions, achieving a near-maximal response about 120 min after induction while maintaining a low signal in the absence of the trigger (Figure 2B). Sensor 27 (2 nM input DNA), also displayed comparable performance and sequence-specific activation in both systems used (Figures 2B,C). We also compared the performance of the full-length LacZ with LacZ-alpha reporters supplemented with the previously expressed LacZ-omega peptide in commercial and in-house cell-free extracts (Supplementary Figure S5). Full-length LacZ reporters exhibited higher ON/OFF endpoint absorbance values in both systems tested (Supplementary Figure S6, right). This can be explained by the slightly higher OFF state observed on both toehold sensors when using LacZ-alpha as a reporter (Supplementary Figure S6).
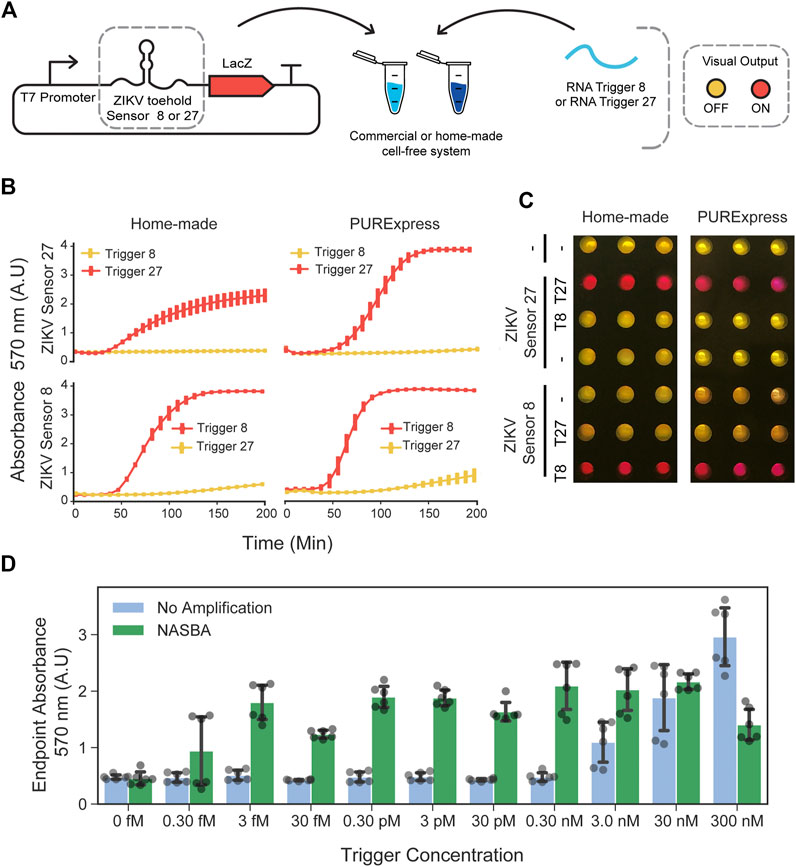
FIGURE 2. Performance of ZIKV toehold sensors in low-cost cell-free lysate reactions. (A) Schematic representation of toehold-mediated RNA sensing. (B) Dynamics of the RNA sensing reactions performed with ZIKV toehold sensor 8 (0.7 nM plasmid DNA) and 27 (2 nM plasmid DNA), regulating the expression of the full-length LacZ in home-made cell extracts and PURExpress cell-free reactions. Error bars represent the standard deviations of three independent experiments, dots are centered at the arithmetic mean for each time point. (C) Example of the endpoint visualization of the experiments after 4 hours of incubation at 29°C. (D) Endpoint measurement of RNA sensing reactions performed with ZIKV sensor 27 and trigger 27 in a range of concentrations with and without NASBA isothermal amplification. Gray dots represent data from six independent measurements performed from two independent NASBA amplifications performed on different days. Black error bars correspond to standard deviations of these six measurements.
Next, we studied the effect of plasmid DNA input concentration on the leaky expression and saturation of sensing reactions. We tested three ZIKV toehold sensors controlling full-length LacZ expression (ZIKV Sensors 8, 27, and 32) (Pardee et al., 2016) in concentrations ranging from 0.5 to 10 nM. In all sensors, OFF state signal increased as a function of the input DNA concentration (Supplementary Figure S7). These results indicate that input DNA concentration can affect the dynamic range of RNA toehold sensors and that optimal concentrations may be different for each sensor.
To increase the sensitivity of CFTS in home-made cell-free preparations, we performed NASBA amplification of the target RNA before adding it to the reactions containing the sensors (Pardee et al., 2016). While unamplified RNA was detected at concentrations no lower than 3 nM, the NASBA amplification of target RNAs upstream in the workflow permitted the detection of RNA at concentrations as low as three fM (Figure 2D), representing a sensitivity increment of six orders of magnitude.
Lyophilization and Shelf-Stability of In-House Cell-free Reactions
Deployment of RNA sensors in point-of-care settings is facilitated by the lyophilization of cell-free reactions, enabling room temperature transportation and use upon rehydration (Pardee et al., 2016). In order to test whether home-made CFTS can also be freeze-dried and stored at room-temperature, we lyophilized reactions supplemented with pT7:sfGFP plasmid. These reactions were sealed immediately after lyophilization and kept at room temperature for up to 90 days in a closed tupperware containing desiccant and, upon rehydration, compared to a fresh sample for reference. We observed more than 60% recovery of the endpoint sfGFP expression 1 week after lyophilization, which decreased to 17% after 90 days at room temperature (Supplementary Figure S8). The ZIKV Sensor 27 was also tested in home-made cell extracts after one and 7 days of lyophilization. Besides the evident decrease in dynamic range, especially after 7 days, these reactions retained the ability to detect its target trigger, while maintaining target specificity (Supplementary Figure S9). Taken together, these results indicate that the home-made cell-free system is compatible with lyophilization both for constitutive protein expression and for toehold RNA sensing reactions. The efficiency loss during storage at room temperature indicates that the preservation conditions should be addressed for further optimization and the use of cryoprotectants (Karig et al., 2017).
Increasing PCR-Product Stability in Home-Made Cell-free Reactions
The use of linear DNA for CFTS offers several advantages compared to plasmid DNA that has to be generated by cloning and then amplified using bacterial cultures prior to use (Yang et al., 1980; Bassett and Rawson, 1983; Hoffmann et al., 2001; Michel-Reydellet et al., 2005; Seki et al., 2008; Shrestha et al., 2012; Sun et al., 2014; Marshall et al., 2017). Linear DNA can be produced by PCR amplification from a lacZ gene template, enabling the design of toehold structures as primer tails for rapid screening of CFTS libraries. The use of linear dsDNA in cell-free, however, is affected by nuclease-mediated DNA degradation in crude cell extracts. This effect is primarily due to the action of exonuclease V (Yang et al., 1980; Bassett and Rawson, 1983) (encoded in the RecBCD operon) (Dillingham and Kowalczykowski, 2008) and endonuclease I (encoded by endA), which are the dominant sources of endonuclease activity in E. coli (I. R. Lehman et al., 1962). Previous studies have shown an increase in the efficiency of cell-free protein synthesis from PCR products using several approaches, such as supplementing with exonuclease V inhibitors (Sitaraman et al., 2004; Sun et al., 2014), using modified nucleotides downstream the PCR reaction (Hoffmann et al., 2001), incorporating competitive DNA strands containing χ-sites (Marshall et al., 2017), adding dsDNA binding proteins (Zhu et al., 2020), depleting exonuclease V in the crude extracts (Seki et al., 2008), deleting RecBCD with the lambda phage red recombinase (Michel-Reydellet et al., 2005), and generating strains lacking endA (Michel-Reydellet et al., 2005; Hong et al., 2015).
To increase the stability of PCR-derived linear DNA, we explored a CRISPRi-based strategy (Larson et al., 2013) to control the genes encoding exonuclease V and endonuclease I in cells before harvesting. We designed sgRNAs targeting the 5’ ends of these genes and an IPTG-inducible dCas9 (Supplementary Figure S10A–C) and transformed E coli strain BL21 DE3 STAR (hereafter named CRISPRi+). As a control, an off-target sgRNA that does not have a known target in the E.coli genome (Nuñez et al., 2017) was transformed into E coli strain BL21 DE3 STAR and tested in parallel (hereafter named CRISPRi-). A growth comparison was performed between the CRISPRi+, CRISPRi- and the basal genotype strain BL21 DE3 STAR, finding a slightly lower growth rate for the CRISPRi+ (Supplementary Figure S10D).
This CRISPRi strategy is cost-effective as it does not require the supplementation with extra components, or in vitro modifications of the PCR product. To test this approach, our crude extracts of each genotype (BL21 DE3 STAR/CRISPRi+, BL21 DE3 STAR/CRISPRi- and BL21 DE3 STAR) were prepared and the stability of a 1.5 Kb linear DNA was quantified over a 2-h time course using agarose gel electrophoresis (Supplementary Figure S11A). The following exponential degradation model was fitted to the data:
where
Plasmid-derived endpoint expression was also measured in the three genotypes. No statistical differences were found between these values (Supplementary Figure S12B). The linear/plasmid ratio of end-point sfGFP fluorescence was also calculated for each batch, revealing statistical differences between CRISPRi+ and each of the other two genotypes (Supplementary Figure S12C). Taken together, these results indicate that CRISPRi + strain enhances stability of linear DNA, and increases expression yields of sfGFP from PCR-derived linear templates.
We observed a small increase in variability in the PCR-derived linear DNA expression of sfGFP with respect to the expression from plasmid DNA. This was observed across the three genotypes, with coefficients of variability of 0.89 and 1.29 for the expression of sfGFP in CRISPRi+ from plasmid and linear DNA, respectively (Supplementary Table S14).
Fast Prototyping de-novo Designed Sensors
We prepared CRISPRi-optimized extracts using the BL21-Gold-dLac strain and tested the RNA sensing capability of ZIKV toehold sensors expressed directly from PCR linear products. Using these CRISPRi extracts, both ZIKV sensors, 8 and 27, were able to detect RNA in a sequence-specific manner and behaved similarly to the plasmid derived sensors (Supplementary Figure S13). Subsequently, we used these CRISPRi-optimized crude extracts to prototype novel RNA sensors for Potato Virus Y (PVY), a virus affecting local agriculture in Chile. First, we selected a conserved sequence from a wide range of PVY strains (Supplementary Figure S14), which was subsequently generated as a synthetic fragment, transcribed in vitro and used for screening potential toehold sensors (Figure 3; Supplementary Table S5, 6).
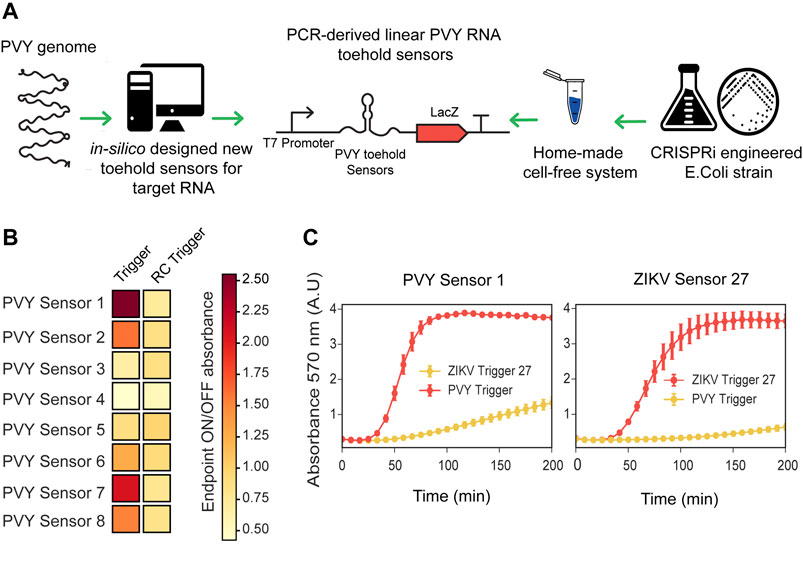
FIGURE 3. Fast prototyping de-novo designed sensors in low-cost optimized cell-free systems. (A) Scheme representation of fast prototyping de-novo designed RNA toeholds sensors against synthetic fragments of PVY virus. (B) PCR-purified transcriptional units (at 10 nM final concentration) encoding for PVY RNA toehold sensors were incubated with synthetic RNA direct or RNA reverse complementary (RC) (at 300 nM final concentration) and absorbance at 570 nm was measured in plate reader. Endpoint ON/OFF absorbance was calculated at 200 min with respect to an untriggered control. Heatmap values correspond to the average of six experimental replicates from two PCR amplifications performed on different days. (C) PVY Sensor 1 and ZIKV Sensor 27 encoded in plasmids (at 3.5 nM final concentration) were incubated with ZIKV Trigger 27 RNA or with PVY Trigger RNA (at 480 nM final concentration). Plotted values correspond to arithmetic mean and standard deviation of two independent experiments.
The majority of published toehold sensors have been designed with NUPACK, a software tool for modeling the thermodynamics properties of DNA and RNA molecules (Green et al., 2014; Pardee et al., 2016; Ma et al., 2018). In this work, we have implemented an algorithm that leverages NUPACK and can be generally applied to the analysis and design of novel toehold sensors according to ideal designs (Supplementary Figure S1). Using NupackSensors (see Methods), we analyzed the consensus sequence identified previously and selected eight different toehold RNA sensors for experimental screening. Transcriptional units encoding for each PVY sensor were prepared by PCR amplification and incubated with synthetic RNA direct or reverse complementary (RC) to test their ability to detect RNA in a sequence-specific manner (Figure 3A; Supplementary Figure S15). A control without RNA was used as a baseline in the ON/OFF endpoint calculations. Among the eight sensors tested, two PVY sensors had ON/OFF endpoint measurements greater than two and background level lower than 1.2 (Figure 3B).
In order to validate the fast characterization of PVY toehold sensors using PCR-derived linear DNA, the best scored sensors (PVY Sensor one and PVY Sensor 7) were cloned in plasmids and compared side by side to the plasmid version of ZIKV Sensor 27 in a gradient of input DNA concentrations ranging from 1 to 4 nM (Supplementary Figure S16). Consistent with previous observations (Supplementary Figure S7), input DNA concentrations affect the performance of the toehold sensors. Some sensors (e.g. PVY Sensor 7) showed significant background activation (i.e., being active without a trigger) (Supplementary Figure S16). This background activation is proportional to input DNA concentration. The best performance of PVY Sensor seven was found at a lower concentration (1–2 nM) than for PVY Sensor 1, whose optimal concentration was between 2 and 4 nM (Supplementary Figure S16). Once a good working concentration was identified, PVY Sensor one and ZIKV Sensor 27 were incubated in home-made cell-free reactions (at 3.5 nM final concentration) and supplemented with ZIKV Trigger 27 or PVY RNA (at 480 nM final concentration). PVY Sensor one displayed sequence-specific activation comparable with ZIKV Sensor 27 (Figure 3C). These results showed that the fast-prototyping pipeline presented here, followed by an optimization of input DNA concentration, is a suitable strategy for identifying novel RNA toehold sensors of good performance.
Discussion
The increasing frequency of infectious disease outbreaks and the long-standing impact of viral pathogens on agriculture and livestock highlight the need for more efficient, cost-effective and ubiquitous surveillance of viruses. Cell-free toehold sensors have emerged as a promising technology to address this challenge (Pardee et al., 2014; Pardee et al., 2016; Ma et al., 2018). However, in Latin America the high cost and shipping constraints of the commercial in vitro transcription and translation systems has limited the local production and distributed development of new sensors. These constraints could lead to centralized production models and fragile technological dependencies prone to failure, as evidenced recently by the disrupted supply chain of diagnostic reagents for SARS-CoV-2 testing (The COVID-19 testing debacle, 2020; Nkengasong, 2020). These shortages highlight the relevance of biotechnological tools that can be shared, produced and deployed locally at a lower cost and without the restrictions of intellectual property (Kellner et al., 2020; Mascuch et al., 2020; Thomas G.W. et al., 2020; JOGL (Just One Gigant Lab)-Open COVID19 Initiative, 2020; Reclone, 2020). In-house prepared cell-free toehold sensors, implemented in cell lysates or home-made PURE systems (Shimizu et al., 2001; Villarreal et al., 2018; Lavickova and Maerkl, 2019), could contribute to the decentralization of this technology and its adaptation to local needs.
Here, we demonstrate the implementation and rapid prototyping of novel sensors using low-cost, locally produced cell-free lysates. As our cost analysis showed, we estimate that this approach could enable the manufacture of bespoke diagnostic tests to meet local needs (for instance in Chile the cost was $0.069 USD/test, considering a 5 µL reaction). While the RNA amplification step still requires commercial inputs, such reactions can also be made in-house (Compton, 1991; Aufdembrink et al., 2020). Future work will focus on the in-house preparation of NASBA from locally purified, and IP-free RNAseH, T7 RNAP, pyrophosphatase, and reverse transcriptase enzymes (Aufdembrink et al., 2020; Reclone, 2020; Matute et al., 2021).
Future lines of research will also address the cost efficiency of crude extract preparation. 45–60% of the maltodextrin-based reaction cost relates to growing, inducing, and processing cells, where IPTG (used for induction) and the micro-chromatography columns (used for separation of the cell extract from the glass beads) add up to more than one third of the cost per reaction (Supplementary Figure S4). Therefore, sonication (Shrestha et al., 2012), or other column-free methods for disrupting the cells should be evaluated, along with the use of alternatives to the relatively expensive IPTG such as use of the autolysis strain (Didovyk et al., 2017) or the autoinduction media formulation (Levine et al., 2019).
A key aspect of cell-free biology is the possibility of freeze-drying genetic systems into pellets or paper-based reactions that can be transported at room temperature and deployed in the field. Here, we have tested the performance of toehold sensors in lyophilized cell extracts stored at room temperature for up to 7 days. The decay of the activity after lyophilization highlights the need for further optimization of the storage conditions. Modifications of the storage atmosphere conditions either by replacing air with an inert gas, such as nitrogen or argon, in addition to the use of vacuum sealing, oxygen absorbers, and desiccants (Jung et al., 2020), remains to be explored.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author. All the data collected in this work is publicly available at: https://zenodo.org/record/4988741#.YM0pzWhKhPY.
Author Contributions
FF conceived the project, supervised the research, assisted in preparing the figures and writing the manuscript. AA designed and performed the experiments, analyzed the results, prepared the figures and wrote the first draft of the manuscript. ND assisted the coding writing, data analysis and statistics. JP, FC, CG, and TM conceived and performed experiments and analyzed the results. KP, JM, JH, and ND supervised the research and assisted in writing the manuscript.
Funding
AA was founded by CONICYT scholarship No. 21140714. FF, AA and TM were funded by ANID―Millennium Science Initiative Program―ICN17_022, Fondo de Desarrollo de Áreas Prioritarias―Center for Genome Regulation ANID/FONDAP/15090007 and ICGEB CRP/CHL19-01. FF, AA, and JP were funded by Proyecto Investigación Interdisciplinaria 2018 VRI. FC was supported by EPSRC EP/R014000/1 under the project LCVD: Low-cost Cell-extract Viral Diagnostics.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jonathan Dhalin (Technical University of Denmark) and Vincent Noireaux (University of Minnesota) for providing positive controls and examples for cell-free protein synthesis reactions at early stages of the project. We thank the Pardee lab (University of Toronto) for welcoming Anibal during his internship. We thank members of the lab de tecnología libre (Pontificia Universidad Católica de Chile) for comments and feedback on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.727584/full#supplementary-material
References
Anonymous, (2020). The COVID-19 testing debacle (2020). Nat. Biotechnol. 38, 653. doi:10.1038/s41587-020-0575-3
Aufdembrink, Lauren. M., Khan, Pavana, J Gaut, Nathaniel, Adamala, Katarzyna. P., and Aaron, E. (2020). Highly specific, multiplexed isothermal pathogen detection with fluorescent aptamer readout. RNA 26, 1283–1290. doi:10.1261/rna.075192.120
Bassett, C. L., and Rawson, J. R. (1983). In vitro coupled transcription-translation of linear DNA fragments in a lysate derived from a recB rna pnp strain of Escherichia coli. J. Bacteriol. 156, 1359–1362. doi:10.1128/JB.156.3.1359-1362.1983
Bundy, B. C., Franciszkowicz, M. J., and Swartz, J. R. (2008). Escherichia coli-based cell-free synthesis of virus-like particles. Biotechnol. Bioeng. 100, 28–37. doi:10.1002/bit.21716
Caschera, F., and Noireaux, V. (2015a). A cost-effective polyphosphate-based metabolism fuels an all E. coli cell-free expression system. Metab. Eng. 27, 29–37. doi:10.1016/j.ymben.2014.10.007
Caschera, F., and Noireaux, V. (2015b). Preparation of amino acid mixtures for cell-free expression systems. BioTechniques 58 , 40–43. doi:10.2144/000114249
Compton, J. (1991). Nucleic acid sequence-based amplification. Nature 350, 91–92. doi:10.1038/350091a0
Didovyk, A., Tonooka, T., Tsimring, L., and Hasty, J. (2017). Rapid and Scalable Preparation of Bacterial Lysates for Cell-free Gene Expression. ACS Synth. Biol. 6, 2198–2208. doi:10.1021/acssynbio.7b00253
Dillingham, M. S., and Kowalczykowski, S. C. (2008). RecBCD Enzyme and the Repair of Double-Stranded DNA Breaks. Microbiol. Mol. Biol. Rev. 72, 642–671. doi:10.1128/MMBR.00020-08
Dudley, Q. M., Anderson, K. C., and Jewett, M. C. (2016). Cell-Free Mixing of Escherichia coli Crude Extracts to Prototype and Rationally Engineer High-Titer Mevalonate Synthesis. ACS Synth. Biol. 5, 1578–1588. doi:10.1021/acssynbio.6b00154
Garamella, J., Marshall, R., Rustad, M., and Noireaux, V. (2016). The All E. coli TX-TL Toolbox 2.0: A Platform for Cell-free Synthetic Biology. ACS Synth. Biol. 5, 344–355. doi:10.1021/acssynbio.5b00296
Garenne, D., and Noireaux, V. (2019). Cell-free transcription–translation: engineering biology from the nanometer to the millimeter scale. Curr. Opin. Biotechnol. 58, 19–27. doi:10.1016/j.copbio.2018.10.007
Gibson, D. G., Young, L., Chuang, R.-Y., Venter, J. C., Hutchison, C. A., and Smith, H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345. doi:10.1038/nmeth.1318
Green, A. A., Silver, P. A., Collins, J. J., and Yin, P. (2014). Toehold Switches: De-novo-designed Regulators of Gene Expression. Cell 159, 925–939. doi:10.1016/j.cell.2014.10.002
Grubbe, W. S., Rasor, B. J., Krüger, A., Jewett, M. C., and Karim, A. S. (2020). Cell-free styrene biosynthesis at high titers. Metab. Eng. 61, 89–95. doi:10.1016/j.ymben.2020.05.009
Hoffmann, Thomas., Nemetz, Cordula., Schweizer, Regina., Mutter, Wolfgang., and Watzele, Mandred. (2001). “High-Level Cell-free Protein Expression from PCR-Generated DNA Templates,” in Cell-Free Translation Systems. Editor A. S. Spirin (Berlin, Heidelberg: Springer).
Hong, S. H., Kwon, Y.-C., Martin, R. W., Des Soye, B. J., de Paz, A. M., Swonger, K. N., et al. (2015). Improving Cell-free Protein Synthesis through Genome Engineering of Escherichia coli Lacking Release Factor 1. ChemBioChem 16, 844–853. doi:10.1002/cbic.201402708
Horiya, S., Bailey, J. K., and Krauss, I. J. (2017). “Directed Evolution of Glycopeptides Using mRNA Display,” in Methods in Enzymology (Amsterdam, Netherlands: Elsevier), 83–141. doi:10.1016/bs.mie.2017.06.029
JOGL (Just One Gigant Lab)-Open COVID19 Initiative (2020). JOGL-open COVID19 Initiat. Available at: https://app.jogl.io/program/opencovid19.
Jung, J. K., Alam, K. K., Verosloff, M. S., Capdevila, D. A., Desmau, M., Clauer, P. R., et al. (2020). Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 38, 1451–1459. doi:10.1038/s41587-020-0571-7
Karig, D. K., Bessling, S., Thielen, P., Zhang, S., and Wolfe, J. (2017). Preservation of protein expression systems at elevated temperatures for portable therapeutic production. J. R. Soc. Interf. 14, 20161039. doi:10.1098/rsif.2016.1039
Kellner, M. J., Ross, J. J., Schnabl, J., Dekens, M. P. S., Heinen, R., Grishkovskaya, I., et al. (2020). A rapid, highly sensitive and open-access SARS-CoV-2 detection assay for laboratory and home testing. Mol. Biol. doi:10.1101/2020.06.23.166397
Kim, T.-W., Keum, J.-W., Oh, I.-S., Choi, C.-Y., Park, C.-G., and Kim, D.-M. (2006). Simple procedures for the construction of a robust and cost-effective cell-free protein synthesis system. J. Biotechnol. 126, 554–561. doi:10.1016/j.jbiotec.2006.05.014
Kim, T.-W., Kim, H.-C., Oh, I.-S., and Kim, D.-M. (2008). A highly efficient and economical cell-free protein synthesis system using the S12 extract of Escherichia coli. Biotechnol. Bioproc. Eng. 13, 464–469. doi:10.1007/s12257-008-0139-8
Kwon, Y.-C., and Jewett, M. C. (2015). High-throughput preparation methods of crude extract for robust cell-free protein synthesis. Sci. Rep. 5, 8663. doi:10.1038/srep08663
Laohakunakorn, N., Grasemann, L., Lavickova, B., Michielin, G., Shahein, A., Swank, Z., et al. (2020). Bottom-Up Construction of Complex Biomolecular Systems With Cell-free Synthetic Biology. Front. Bioeng. Biotechnol. 8, 213. doi:10.3389/fbioe.2020.00213
Larson, M. H., Gilbert, L. A., Wang, X., Lim, W. A., Weissman, J. S., and Qi, L. S. (2013). CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 8, 2180–2196. doi:10.1038/nprot.2013.132
Lavickova, B., and Maerkl, S. J. (2019). A Simple, Robust, and Low-Cost Method to Produce the PURE Cell-free System. ACS Synth. Biol. 8, 455–462. doi:10.1021/acssynbio.8b00427
Lehman, I. R., Roussos, G. G., and Pratt, E. A. (1962). The Deoxyribonucleases of Escherichia coli. Int. J. Biol. Chem. 233.
Lehr, F.-X., Hanst, M., Vogel, M., Kremer, J., Göringer, H. U., Suess, B., et al. (2019). Cell-Free Prototyping of AND-Logic Gates Based on Heterogeneous RNA Activators. ACS Synth. Biol. 8, 2163–2173. doi:10.1021/acssynbio.9b00238
Levine, M. Z., So, B., Mullin, A. C., Watts, K. R., and Oza, J. P. (2019). Redesigned upstream processing enables a 24-hour workflow from E. coli cells to cell-free protein synthesis. Synth. Biol.. doi:10.1101/729699
Liu, X., Silverman, A. D., Alam, K. K., Iverson, E., Lucks, J. B., Jewett, M. C., et al. (2020). Design of a Transcriptional Biosensor for the Portable, On-Demand Detection of Cyanuric Acid. ACS Synth. Biol. 9, 84–94. doi:10.1021/acssynbio.9b00348
Ma, D., Shen, L., Wu, K., Diehnelt, C. W., and Green, A. A. (2018). Low-cost detection of norovirus using paper-based cell-free systems and synbody-based viral enrichment. Synth. Biol. 3 , ysy018. doi:10.1093/synbio/ysy018
Marshall, R., Maxwell, C. S., Collins, S. P., Beisel, C. L., and Noireaux, V. (2017). Short DNA containing χ sites enhances DNA stability and gene expression in E. coli cell-free transcription-translation systems: Enhancing TXTL-Based Expression With χ-Site DNA. Biotechnol. Bioeng. 114, 2137–2141. doi:10.1002/bit.26333
Mascuch, S. J., Fakhretaha-Aval, S., Bowman, J. C., Ma, M. T. H., Thomas, G., Bommarius, B., et al. (2020). A blueprint for academic labs to produce SARS-CoV-2 RT-qPCR test kits. J. Biol. Chem., 015434. doi:10.1074/jbc.RA120.015434
Matute, T., Nuñez, I., Rivera, M., Reyes, J., Blázquez-Sánchez, P., Arce, A., et al. (2021). Homebrew reagents for low cost RT-LAMP. Public Glob. Health. doi:10.1101/2021.05.08.21256891
McNerney, M. P., Zhang, Y., Steppe, P., Silverman, A. D., Jewett, M. C., and Styczynski, M. P. (2019). Point-of-care biomarker Quantification enabled by sample-specific calibration. Sci. Adv. 5, eaax4473. doi:10.1126/sciadv.aax4473
Michel-Reydellet, N., Woodrow, K., and Swartz, J. (2005). Increasing PCR Fragment Stability and Protein Yields in a Cell-free System with Genetically Modified Escherichia coli Extracts. J. Mol. Microbiol. Biotechnol. 9, 26–34. doi:10.1159/000088143
Moore, S. J., MacDonald, J. T., and Freemont, P. S. (2017). Cell-free synthetic biology for in vitro prototype engineering. Biochem. Soc. Trans. 45, 785–791. doi:10.1042/BST20170011
Niederholtmeyer, H., Sun, Z. Z., Hori, Y., Yeung, E., Verpoorte, A., Murray, R. M., et al. (2015). Rapid cell-free forward engineering of novel genetic ring oscillators. eLife 4, e09771. doi:10.7554/eLife.09771
Nirenberg, M. W., and Matthaei, J. H. (1961). The Dependence of Cell- Free Protein Synthesis in E. coli upon Naturally Occurring or Synthetic Polyribonucleotides. Proc. Natl. Acad. Sci. U. S. A. 47, 1588–1602.
Nkengasong, John. (2020). Let Africa into the market for COVID-19 diagnostics. Nature 580, 556. doi:10.1038/d41586-020-01265-0
Nuñez, I. N., Matute, T. F., Del Valle, I. D., Kan, A., Choksi, A., Endy, D., et al. (2017). Artificial Symmetry-Breaking for Morphogenetic Engineering Bacterial Colonies. ACS Synth. Biol. 6, 256–265. doi:10.1021/acssynbio.6b00149
Pardee, K., Green, A. A., Ferrante, T., Cameron, D. E., DaleyKeyser, A., Yin, P., et al. (2014). Paper-Based Synthetic Gene Networks. Cell 159, 940–954. doi:10.1016/j.cell.2014.10.004
Pardee, K., Green, A. A., Takahashi, M. K., Braff, D., Lambert, G., Lee, J. W., et al. (2016). Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 165, 1255–1266. doi:10.1016/j.cell.2016.04.059
Pieters, P. A., Nathalia, B. L., van der Linden, A. J., Yin, P., Kim, J., Huck, W. T. S., et al. (2021). Cell-Free Characterization of Coherent Feed-Forward Loop-Based Synthetic Genetic Circuits. ACS Synth. Biol. 10, 1406–1416. doi:10.1021/acssynbio.1c00024
Pollak, B., Matute, T., Nuňez, I., Cerda, A., Lopez, C., Vargas, V., et al. (2020). Universal Loop assembly (uLoop): open, efficient, and species-agnostic DNA fabrication. Synth. Biol. 5, ysaa001. doi:10.1101/744854
Qi, L. S., Larson, M. H., Gilbert, L. A., Doudna, J. A., Weissman, J. S., Arkin, A. P., et al. (2013). Repurposing CRISPR as an RNA-Guided Platform for Sequence-specific Control of Gene Expression. Cell 152, 1173–1183. doi:10.1016/j.cell.2013.02.022
Reclone, (2020). Recloneorg Glob. Collab. Equitable Access Biotechnol. Available at: https://reclone.org/.
Rustad, M., Eastlund, A., Jardine, P., and Noireaux, V. (2018). Cell-free TXTL synthesis of infectious bacteriophage T4 in a single test tube reaction. Synth. Biol. 3, ysy002. doi:10.1093/synbio/ysy002
Salehi, A. S. M., Yang, S. O., Earl, C. C., Shakalli Tang, M. J., Porter Hunt, J., Smith, M. T., et al. (2018). Biosensing estrogenic endocrine disruptors in human blood and urine: A RAPID cell-free protein synthesis approach. Toxicol. Appl. Pharmacol. 345, 19–25. doi:10.1016/j.taap.2018.02.016
Salgado, H., Peralta-Gil, M., Gama-Castro, S., Santos-Zavaleta, A., Muñiz-Rascado, L., García-Sotelo, J. S., et al. (2013). RegulonDB v8.0: omics data sets, evolutionary conservation, regulatory phrases, cross-validated gold standards and more. Nucleic Acids Res. 41, D203–D213. doi:10.1093/nar/gks1201
Seki, E., Matsuda, N., Yokoyama, S., and Kigawa, T. (2008). Cell-free protein synthesis system from Escherichia coli cells cultured at decreased temperatures improves productivity by decreasing DNA template degradation. Anal. Biochem. 377, 156–161. doi:10.1016/j.ab.2008.03.001
Shimizu, Y., Inoue, A., Tomari, Y., Suzuki, T., Yokogawa, T., Nishikawa, K., et al. (2001). Cell-free translation reconstituted with purified components. Nat. Biotechnol. 19, 751–755. doi:10.1038/90802
Shin, J., and Noireaux, V. (2012). An E. coli Cell-free Expression Toolbox: Application to Synthetic Gene Circuits and Artificial Cells. ACS Synth. Biol. 1, 29–41. doi:10.1021/sb200016s
Shrestha, P., Holland, T. M., and Bundy, B. C. (2012). Streamlined extract preparation for Escherichia coli -based cell-free protein synthesis by sonication or bead vortex mixing. BioTechniques 53, 163–174. doi:10.2144/0000113924
Siegal-Gaskins, D., Tuza, Z. A., Kim, J., Noireaux, V., and Murray, R. M. (2014). Gene Circuit Performance Characterization and Resource Usage in a Cell-free “Breadboard. ACS Synth. Biol. 3, 416–425. doi:10.1021/sb400203p
Silverman, A. D., Akova, U., Alam, K. K., Jewett, M. C., and Lucks, J. B. (2020). Design and Optimization of a Cell-free Atrazine Biosensor. ACS Synth. Biol. 9, 671–677. doi:10.1021/acssynbio.9b00388
Silverman, A. D., Kelley-Loughnane, N., Lucks, J. B., and Jewett, M. C. (2019). Deconstructing Cell-free Extract Preparation for in Vitro Activation of Transcriptional Genetic Circuitry. ACS Synth. Biol. 8, 403–414. doi:10.1021/acssynbio.8b00430
Sitaraman, K., Esposito, D., Klarmann, G., Le Grice, S. F., Hartley, J. L., and Chatterjee, D. K. (2004). A novel cell-free protein synthesis system. J. Biotechnol. 110, 257–263. doi:10.1016/j.jbiotec.2004.02.014
Sun, Z. Z., Hayes, C. A., Shin, J., Caschera, F., Murray, R. M., and Noireaux, V. (2013). Protocols for Implementing an Escherichia coli Based TX-TL Cell-free Expression System for Synthetic Biology. J. Vis. Exp., 50762. doi:10.3791/50762
Sun, Z. Z., Yeung, E., Hayes, C. A., Noireaux, V., and Murray, R. M. (2014). Linear DNA for Rapid Prototyping of Synthetic Biological Circuits in an Escherichia coli Based TX-TL Cell-free System. ACS Synth. Biol. 3, 387–397. doi:10.1021/sb400131a
Swartz, J. (2020). Opportunities toward hydrogen production biotechnologies. Curr. Opin. Biotechnol. 62, 248–255. doi:10.1016/j.copbio.2020.03.002
Takahashi, M. K., Chappell, J., Hayes, C. A., Sun, Z. Z., Kim, J., Singhal, V., et al. (2015). Rapidly Characterizing the Fast Dynamics of RNA Genetic Circuitry with Cell-free Transcription–Translation (TX-TL) Systems. ACS Synth. Biol. 4, 503–515. doi:10.1021/sb400206c
Takahashi, M. K., Tan, X., Dy, A. J., Braff, D., Akana, R. T., Furuta, Y., et al. (2018). A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers. Nat. Commun. 9, 3347. doi:10.1038/s41467-018-05864-4
Tayar, A. M., Karzbrun, E., Noireaux, V., and Bar-Ziv, R. H. (2017). Synchrony and pattern formation of coupled genetic oscillators on a chip of artificial cells. Proc. Natl. Acad. Sci. 114, 11609–11614. doi:10.1073/pnas.1710620114
Thavarajah, W., Silverman, A. D., Verosloff, M. S., Kelley-Loughnane, N., Jewett, M. C., and Lucks, J. B. (2020). Point-of-Use Detection of Environmental Fluoride via a Cell-free Riboswitch-Based Biosensor. ACS Synth. Biol. 9, 10–18. doi:10.1021/acssynbio.9b00347
Thomas, G. W., Dailey, Gina. M., and Claire Dugast-Darzacq, M. N. E. (2020). BEARmix: Basic Economical Amplification Reaction one-step RT-qPCR master mix. BEARmix. Available at: https://gitlab.com/tjian-darzacq-lab/bearmix.
Verosloff, M., Chappell, J., Perry, K. L., Thompson, J. R., and Lucks, J. B. (2019). PLANT-dx: A Molecular Diagnostic for Point-of-Use Detection of Plant Pathogens. ACS Synth. Biol. 8, 902–905. doi:10.1021/acssynbio.8b00526
Villarreal, F., Contreras-Llano, L. E., Chavez, M., Ding, Y., Fan, J., Pan, T., et al. (2018). Synthetic microbial consortia enable rapid assembly of pure translation machinery. Nat. Chem. Biol. 14, 29–35. doi:10.1038/nchembio.2514
Yang, H. L., Ivashkiv, L., Chen, H. Z., Zubay, G., and Cashel, M. (1980). Cell-free coupled transcription-translation system for investigation of linear DNA segments. Proc. Natl. Acad. Sci. 77, 7029–7033. doi:10.1073/pnas.77.12.7029
Yelleswarapu, M., van der Linden, A. J., van Sluijs, B., Pieters, P. A., Dubuc, E., de Greef, T. F. A., et al. (2018). Sigma Factor-Mediated Tuning of Bacterial Cell-free Synthetic Genetic Oscillators. ACS Synth. Biol. 7, 2879–2887. doi:10.1021/acssynbio.8b00300
Keywords: cell-free, toehold-sensor, low-cost, diagnostics, decentralization
Citation: Arce A, Guzman Chavez F, Gandini C, Puig J, Matute T, Haseloff J, Dalchau N, Molloy J, Pardee K and Federici F (2021) Decentralizing Cell-Free RNA Sensing With the Use of Low-Cost Cell Extracts. Front. Bioeng. Biotechnol. 9:727584. doi: 10.3389/fbioe.2021.727584
Received: 18 June 2021; Accepted: 06 August 2021;
Published: 23 August 2021.
Edited by:
Simon J. Moore, University of Kent, United KingdomReviewed by:
Nadanai Laohakunakorn, University of Edinburgh, United KingdomAmir Pandi, Max Planck Institute for Terrestrial Microbiology, Germany
Copyright © 2021 Arce, Guzman Chavez, Gandini, Puig, Matute, Haseloff, Dalchau, Molloy, Pardee and Federici. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernán Federici, ffederici@bio.puc.cl
 Anibal Arce
Anibal Arce Fernando Guzman Chavez
Fernando Guzman Chavez Chiara Gandini
Chiara Gandini Juan Puig
Juan Puig Tamara Matute1,2
Tamara Matute1,2  Neil Dalchau
Neil Dalchau Keith Pardee
Keith Pardee Fernán Federici
Fernán Federici