Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives
- 1Laboratory of Photobiology and Molecular Microbiology, Centre of Advanced Study in Botany, Institute of Science, Banaras Hindu University, Varanasi, India
- 2Centre of Advanced Study in Botany, Institute of Science, Banaras Hindu University, Varanasi, India
- 3Retired, Möhrendorf, Germany
Sustainable supply of food and energy without posing any threat to environment is the current demand of our society in view of continuous increase in global human population and depletion of natural resources of energy. Cyanobacteria have recently emerged as potential candidates who can fulfill abovementioned needs due to their ability to efficiently harvest solar energy and convert it into biomass by simple utilization of CO2, water and nutrients. During conversion of radiant energy into chemical energy, these biological systems produce oxygen as a by-product. Cyanobacterial biomass can be used for the production of food, energy, biofertilizers, secondary metabolites of nutritional, cosmetics, and medicinal importance. Therefore, cyanobacterial farming is proposed as environment friendly sustainable agricultural practice which can produce biomass of very high value. Additionally, cyanobacterial farming helps in decreasing the level of greenhouse gas, i.e., CO2, and it can be also used for removing various contaminants from wastewater and soil. However, utilization of cyanobacteria for resolving the abovementioned problems is subjected to economic viability. In this review, we provide details on different aspects of cyanobacterial system that can help in developing sustainable agricultural practices. We also describe different large-scale cultivation systems for cyanobacterial farming and discuss their merits and demerits in terms of economic profitability.
Introduction
The human population of our planet is projected to reach ~9.7 billion by 2050, and majority of this increased population would be contributed by developing countries of Asia and Africa (DESA UN, 2015). This increase in global population is associated with increased demand of food security in future. To overcome this challenge, the World Health Organization has suggested a doubling of food production by 2050, while the United Nations have suggested a 50% increase in global food production by 2030. The productivity gains resulted from the “Green Revolution” have essentially reached a plateau, and feeding the increased global population is further challenged by limited availability of agriculture land. The range of non-conventional biotechnological measures involving improvement in CO2 fixation efficiency of crop plants can be used to enhance the food productivity per hectare of agricultural land (Parry and Hawkesford, 2010; Parry et al., 2011). Nevertheless, a production system which has a higher productivity but requires a small land area and time for cultivation would be the requirement of the future agriculture.
Several factors such as haphazard nutrient mining, water limitation, accumulation of noxious xenobiotic compounds in the soil, soil corrosion and climate change have deteriorated the quality and fertility of agricultural land (Singh, 2014). Additionally, soil salinity is a well-known stressor which affects the plant growth and crop production globally (Upadhyay et al., 2011). The social and economic activities have resulted in reduction of the cultivable area, and hence, in addition to conservation of fertile land, enhancement of the coverage of cultivable land should be one of the focused areas to feed the human population (Singh et al., 2016; Pathak et al., 2017). Modern green technologies such as biofertilizers consisting of cyanobacteria, fungi (arbuscular mycorrhizal fungi, AMF) and bacteria (plant growth promoting rhizobacteria, PGPR) could improve and restore the soil fertility and ensure the sustainable agricultural production. Moreover, these microorganisms can reduce the energy demand in the form of synthetic fertilizers, and have the capability to mitigate stressed agroecosystems and wastelands.
Microbial biotechnology plays an important role particularly in secondary metabolites, biofertilizers, bioenergy, bioprocessing, biopesticide production, waste treatment, and bioremediation (Du et al., 2007; Mohammadi and Sohrabi, 2012). The sustainable agriculture involves soil, water and pest management, crop selection, soil preservation and processing. These sustainable agricultural practices together with biotechnology have the potential to increase the productivity by developing new transgenic plants, microbes and animals (Singh, 2000). Over the last decade, biotechnology has significantly contributed to sustainable agriculture by the development of stress resistance in microbes and plants, bioremediation of polluted soils, enhanced nitrogen fixation and increased nutrient uptake efficiency (Singh, 2000). However, the cultivation of genetically modified organisms in an open area is still a matter of debate all over the world.
In recent years, microalgae, including cyanobacteria, have emerged as potential candidates for their application in development of environment friendly and sustainable agricultural practices (Singh et al., 2016, 2017). These oxygen-evolving photosynthetic organisms do not compete for arable land for their cultivation. Cyanobacteria, which can be cultivated using seawater, require residual nutrients for high areal productivity and have high protein and reasonable amount of carbohydrate as well as lipid contents per gram of their biomass (Williams and Laurens, 2010; Milledge, 2011; Hoekman et al., 2012). Globally about 25 Gt a−1 of carbon can be fixed into energy-dense biomass by cyanobacteria using atmospheric CO2 and solar energy (Pisciotta et al., 2010). Therefore, generation of microbiological energy through massive solar energy transformation permits harvesting various forms of eco-friendly energy reserves (Hall et al., 1995; Paumann et al., 2005). The aforementioned characteristics make cyanobacteria potential microorganisms for their application as feedstocks for sustainable production of food and non-food commodities, including valuable chemicals and bioenergy (Wase and Wright, 2008; Sarsekeyeva et al., 2015; Rajneesh et al., 2017). Cyanobacteria produce a number of valuable compounds such as ethanol, butanol, fatty acids, and other organic acids, and therefore, are promising candidates for fulfilling our energy demands in a sustainable manner (Rajneesh et al., 2017). In addition, cyanobacteria have potential applications in agriculture as biofertilizers and in the food industry as food supplements (Figure 1). Recent developments in the techniques of genetic engineering, culturing and screening of cyanobacteria have enabled novel ways to utilize the potentials of available wealth of these ancient microorganisms. Genetic manipulation techniques are well developed for several cyanobacteria, and therefore, a synthetic biology approach provides an opportunity to exploit these organisms as green factories for the production of new lines of commodities. In this paper, we review various ways of cyanobacterial farming that could be utilized in the development of sustainable agriculture practice in an eco-friendly manner (Figure 1).
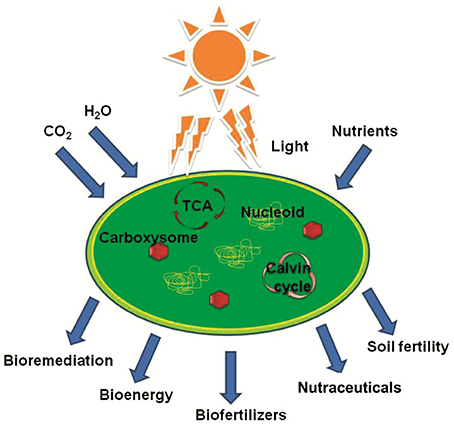
Figure 1. Various outcomes of cyanobacterial growth that can be utilized for the development of sustainable agricultural practices.
Sustainable Agriculture and Microbes
Among various streams of life sciences, agricultural biotechnology could potentially help in developing the sustainable agriculture practices. In modern agriculture, microbial intervention further strengthens the impact of transgenic organisms. Microbes play a vital role in determining the fertility and structure of soils (Vaishampayan et al., 2001; Singh, 2014; Singh et al., 2016), in processing and preservation of crops, and in recycling the residues of crops (Saddler, 1993; Hanson, 1996). Microbial activity in soil fertility, bioremediation and biocontrol requires the introduction of genetically engineered or natural microbial inoculants; however, mere inoculation of microbes in soil is not sufficient. The monitoring of survival and establishment of inoculants in the soil, and their negative or positive interactions with other indigenous microbial population are very crucial (Molin and Molin, 1997; Van Veen et al., 1997). Maintenance of soil fertility with renewable resources is the prime requirement of sustainable agriculture in order to reduce the need for synthetic fertilizers. Among such resources, natural and transgenic N2-fixing microbes are the most promising candidates. In the rhizosphere, the N2-fixing microorganisms can be directly inoculated in soil or can be used as coating on seeds, however, in both cases their survival need to be assured. Several living biosensors have been developed which can be used for monitoring, management and manipulation of interacting microbial consortia (Burlage and Kuo, 1994).
The various metabolites produced by microbes have the potential to aid in the development of sustainable agriculture practice, and the best example is biocompatible microbial polymers which can be used in coating of fertilizers, pesticides, and nutrients. This will help in targeted delivery of fertilizers, pesticides, and nutrients for agriculture by preventing their application in other industry or antisocial activities. Microbial polymers can also be used in food processing units (Vandamme et al., 1996; Sutherland, 1998). One of the sustainable ways to convert wastelands to agricultural practices is bioremediation of contaminated soils and waters. For bioremediation of contaminated soils, natural, or transgenic microbes in mixed culture are used (Atlas, 1995). However, for effective bioremediation of contaminated terrestrial or aquatic ecosystem, the chemical and microbial interactions along with biogeochemical and ecological balances of concerned ecosystem should be considered (Anderson and Lovley, 1997; Tiedje, 1997). Among various beneficial microbes, cyanobacteria can be used in nutrition, energy and agriculture sector because of their ability to fix atmospheric N2 and CO2, and produce energy rich biomass containing myriad of metabolites of economic importance. Furthermore, recent developments in cyanobacterial screening, cultivation, and gene manipulation techniques have enabled new ways to utilize the potential of these photosynthetic microbes to deal with socio-economic problems (Sarsekeyeva et al., 2015).
Cyanobacteria and Sustainable Agriculture
Cyanobacteria contribute significantly to the biogeochemical cycles of carbon, nitrogen and oxygen (Karl et al., 2002; De Ruyter and Fromme, 2008). They have undergone a series of functional and structural modifications during their evolution that permit their current distribution in diverse ecological niches (Olson, 2006). Cyanobacteria can tolerate various stresses such as ultraviolet radiation (UVR; 280–400 nm), desiccation, high or low temperatures and salinity, which contribute to their advantages over different competitors/neighbors in their natural habitats (Gröniger et al., 2000; Herrero and Flores, 2008). For example, Spirulina maxima is known to survive under high alkaline conditions (pH 11) and high salinity which provide advantage and protection from other competitors and grazers (Habib et al., 2008). The nitrogen fixing ability of cyanobacteria aids their successful growth and survival in habitats where no or little combined nitrogen is available. This trait of cyanobacteria makes them agronomically and economically important as biofertilizers (Singh, 1961, 2014; Vaishampayan et al., 2001; Singh et al., 2016).
Several cyanobacteria are known to establish symbiotic associations, and this ability could be exploited in developing the consortia of microorganisms for their application in bioremediation of affected soils or aquatic systems (Rai et al., 2000; Subashchandrabose et al., 2011; Hamouda et al., 2016). Strains of cyanobacteria which are native and adapted to local climatic conditions have a capacity to survive in wet soils. This significantly affects the nutritional status, structural stability and crop productivity of such soils (Nisha et al., 2007). Twenty five percent of the total biomass of cyanobacteria is contributed by the exopolysaccharides (EPS) (Nisha et al., 2007). The upper crust of the soil is the site of cyanobacterial activities and the EPS act as a gluing agent on soil particles. The EPS can hold soil particles together which leads to soil aggregation, organic content accumulation and increase in water holding capacity of the top layer of soil (Malamlssa et al., 2001). This increase in soil moisture and organic content can support the survival and growth of plant-growth promoting rhizobacteria (PGPR). Thus, cyanobacterial growth positively alters the chemical and physical property of soils, and PGPRs along with EPS-producing cyanobacteria may contribute to an improvement and reclamation of infertile soils (Flaibani et al., 1989; Verrecchia et al., 1995; Zulpa et al., 2003; Paul and Nair, 2008). The consortium of PGPR and cyanobacteria increases the plant growth by improving the soil fertility and nutrient utilization. In additions, this consortium also enhances the tolerance of plants against environmental stresses such as drought and salinity (Singh et al., 2011; Prasanna et al., 2012; Singh, 2014). However, community structure and diversity of cyanobacteria should be studied in depth particularly in reference to environmental conditions and ecosystem functions before devising application of a consortium under field conditions. In following sections, different properties of cyanobacteria are presented that can be utilized for the development of sustainable agriculture practice in environment friendly manner.
Cyanobacteria as Bioremediators
Cyanobacteria can be used for the bioremediation of several contaminants such as heavy metals, pesticides, crude oil, phenanthrene, naphthalene, phenol, catechol, and xenobiotics (Kesaano and Sims, 2014; Hamouda et al., 2016; Kumar et al., 2016; Singh et al., 2016). Synechococcus elongatus, Microcystis aeruginosa and Anacystics nidulans have shown potentials to remove organo-chlorine and organo-phosphorus insecticides (Vijayakumar, 2012). Cyanobacterial genera such as Oscillatoria, Synechococcus, Nodularia, Nostoc, Anabaena, and Microcystis break down lindane residues (El-Bestawy et al., 2007). Anabaena sp., Lyngya sp., Microcystis sp., Nostoc sp. and Spirulina sp. can utilize glyphosate as the source of phosphorus, and therefore, helps in removal of this herbicide from contaminated soil (Forlani et al., 2008; Lipok et al., 2009). Thus, an above mentioned cyanobacterial strains are potential candidates for their application in developing sustainable agricultural practice where they can be used for removing various contaminants from soil as well as water.
The consortia of cyanobacteria and chemotrophic bacteria have been effectively used to bioremediate wastewaters and oil-contaminated sediments (Abed and Köster, 2005). Cyanobacteria can oxidize oil components as well as other complex organic compounds such as herbicides and surfactants (Mansy and El-Bestway, 2002; Subashchandrabose et al., 2013). The consortium of Oscillatoria-Gammaproteobacteria can degrade phenanthrene, dibenzothiophene, pristine and n-octadecane (Abed and Köster, 2005). Similarly, consortia of Microcoleus chthonoplastes with organotrophic bacteria can fix atmospheric nitrogen and degrade aliphatic heterocyclic organo-sulfur compounds and hydrocarbons such as alkylated monocyclic and polycyclic aromatic compounds (Sánchez et al., 2005). An artificially designed biofilm consortium of hydrocarbon-degrading bacteria and cyanobacteria on gravel particles and glass plates has been developed that can be used for cleaning up crude-oil contamination of sea water samples (Al-Awadhi et al., 2003). The consortium of Anabaena oryzae and Chlorella kessleri can be used to biodegrade crude oil under mixotrophic condition (Hamouda et al., 2016). Cyanobacteria are also capable of removing heavy metals from water bodies and can reduce the excess of nitrate and phosphate from agricultural fields (Kesaano and Sims, 2014; Kumar et al., 2016). Consortia of cyanobacteria and bacteria have shown positive results in wastewater treatment. Cultures of Aphanocapsa sp. BDU 16, Oscillatoria sp. BDU 30501 and Halobacterium US 101 can be utilized for minimizing the amount of calcium and chloride in wastewater to a level that can support the survival of fishes (Uma and Subramanian, 1990). Similarly, Phormidium valderianum BDU 30501 and Oscillatoria boryana BDU 92181 can be utilized to remove phenol and melanoidin, respectively, from the effluents of distillery (Shashirekha et al., 1997; Kalavathi et al., 2001). Desertification is another challenge for sustainable agriculture practices which can be reversed by the application of cyanobacterial inoculums. Cyanobacteria together with bacteria, mosses, algae, lichens and fungi forms biological soil crusts (BSCs) in semiarid and arid areas of various geographical regions (Rossi et al., 2017). These BSCs play an important role in stabilization and primary colonization of desert soil surface by increasing the nutrient and moisture contents (Rossi et al., 2017). Thus, cyanobacteria can be ideally utilized for removing the various contaminants. However, for bioremediation of polluted sites, focus should be given to utilization of indigenous consortia that could potentially reduce the need of adding new microorganisms or fertilizers to the target site. Further improvement in bioremediation property can be achieved by genetic engineering of parent strains which can help in developing a maintenance-free and economical remediation technology while producing the biomass of high value for other purposes (Kuritz and Wolk, 1995; Cuellar-Bermudez et al., 2017).
Cyanobacteria as Bioenergy Resource
First and second generations of biofuels have utilized feedstocks such as rapeseed, soybean, sunflower, wheat, switchgrass, peanuts, and sesame seeds. These raw materials have been used to produce different energy sources such as ethanol, propanol, butanol, and vegetable oils (Quintana et al., 2011). However, energy crops, which are used in first and second generations of biofuels production, compete with conventional food sources for water, nutrients and fertile land. Therefore, the third generation of biofuel production using microalgae has emerged as an alternative to avoid the competition between food crops and energy crops for available resources, and furthermore, cyanobacteria are one of the most promising feedstocks for the production of third generation biofuels (Quintana et al., 2011; Al-Haj et al., 2016; Rajneesh et al., 2017). Rapid growth rate and cultivation in suitable in-house bioreactors and/or on non-arable land gives cyanobacteria an advantage over plants (Singh, 2014; Sarma et al., 2016). Furthermore, cyanobacteria show higher photosynthetic efficiency (~10%), as compared to land plants (~3–4% maximum efficiency) (Lewis and Nocera, 2006; Melis, 2009). Cyanobacteria can be easier genetically manipulated than other algae, and hence, serve as a better candidate in comparison to eukaryotic algae for the production of targeted chemicals and fuels. The genome size of cyanobacteria is relatively small and till date genomes of several genera have been sequenced (Rajneesh et al., 2017). Therefore, cyanobacteria provide an exceptional opportunity to conduct genetic and metabolic engineering studies for improved biomass production which is comparatively difficult to do with eukaryotic algae (Rittmann, 2008). Cyanobacteria contain considerable amounts of lipids; primarily located in the thylakoid and plasma membranes, and show higher rates of growth and photosynthesis. Enhanced production of biofuel from cyanobacteria utilizing genetic engineering has been attempted mainly on Synechocystis sp. PCC 6803 and S. elongatus PCC 7942, whose genomes are fully sequenced and molecular techniques are well-established (Kaneko et al., 1996). Genetic engineering can be used for the production of various fuels such as 2,3-butanediol, acetone 1-butanol, ethylene, ethanol, fatty acids, isobutyraldehyde, isobutanol, 2-methyl-1-butanol, and isoprene in cyanobacteria. Therefore, genetically modified cyanobacteria might play a crucial role in reduction of crude oil dependency and CO2 emissions, owing to direct photosynthetic fixation of CO2 to biofuel and other valuable secondary metabolites (Ducat et al., 2011; Oliver et al., 2016).
However, cyanobacteria have some limitations for their application in biofuel production. The production of valuable chemicals in photoautotrophic cyanobacteria is always less than sugar-utilizing systems such as S. cerevisiae and E. coli (Savakis and Hellingwerf, 2014). Generally, photoautotrophic cyanobacterial chassis can produce only ~100 mg of biochemicals per liter of cell culture (Gao et al., 2016), which is far too low for any commercially viable application. Theoretical yields under heterotrophic and autotrophic growth conditions for production of several chemicals have been computed for cyanobacterial chasis to find out the limiting factors in cyanobacterial metabolic network (Gudmundsson and Nogales, 2015). However, study suggests that low yield is not due to the topology of the photosynthetic metabolic networks in cyanobacteria. Therefore, it is important to optimize the inherent biological chassis for enhancing the yield of biochemicals from cyanobacteria. In recent years, several groups have emphasized on construction, designing, and expression of the biosynthetic pathways along with development of the toolboxes for metabolic engineering in cyanobacteria which could lead to economic profitability by increasing the production of existing and novel chemicals and biofuels (Wang et al., 2012; Berla et al., 2013; Desai and Atsumi, 2013; Oliver and Atsumi, 2014; Gudmundsson and Nogales, 2015; Markley et al., 2015).
Cyanobacteria as Biofertilizers
It is very expensive to produce inorganic nitrogen fertilizers due to the requirement of large amount of fossil-fuel energy. This necessitated the development of alternate, sustainable and cost-effective biologically available nitrogen resources which can fulfill the nitrogen demand of agriculture in sustainable manner (Mahanty et al., 2017). For this purpose biological systems have been identified which can fix atmospheric dinitrogen (Hegde et al., 1999; Vaishampayan et al., 2001). Biological nitrogen fixation contributes ~2 × 102 Mt of nitrogen annually (Guerrero et al., 1981). According to Metting (1988), the total nitrogen fixation can be ~90 kg N ha−1 y−1. Symbiotic and free-living eubacteria, including cyanobacteria, are two groups of nitrogen-fixing organisms. The free-living cyanobacteria fix <10 kg of N ha−1y−1, however, annually ~10–30 kg of N ha−1 is fixed by dense mats of cyanobacteria (Aiyer et al., 1972; Watanabe et al., 1977). Therefore, cyanobacteria constitute an important component of naturally available biofertilizers (Vaishampayan et al., 2001; Prasanna et al., 2013). Rice production in tropical countries mainly depends on biological N2 fixation by cyanobacteria which are a natural component of paddy fields (Vaishampayan et al., 2001). In these cultivated agriculture systems, annually ~32 Tg of nitrogen is fixed by biological nitrogen fixers (Singh et al., 2016), and cyanobacteria add about 20–30 kg fixed nitrogen ha−1 along with organic matter to the paddy fields (Subramanian and Sundaram, 1986; Issa et al., 2014). Cyanobacteria also make symbiotic associations with different photosynthetic and non-photosynthetic organisms such as algae, fungi, diatoms, bryophytes, hornworts, liverworts, mosses, pteridophytes, gymnosperms, and angiosperms (Rai et al., 2000; Sarma et al., 2016).
Several heterocystous cyanobacterial genera such as Nostoc, Anabaena, Nodularia, Scytonema, Cylindrospermum, Mastigocladus, Calothrix, Anabaenopsis, Aulosira, Tolypothrix, Haplosiphon, Camptylonema, Stigonema, Fischerella, Gloeotrichia, Chlorogloeopsis, Rivularia, Nostochopsis, Westiellopsis, Wollea, Westiella, Chlorogloea, and Schytonematopsis have been shown to be efficient N2 fixers (Venkataraman, 1993). Table 1 contains a list of potential cyanobacteria which can be used as biofertilizers in agricultural fields (Vaishampayan et al., 2001). For the first time, Fritsch (1907) studied the abundance and importance of cyanobacteria with respect to maintenance of soil fertility of paddy fields through biological nitrogen fixation, which was afterwards recognized by several other workers (Singh, 1950; Fogg and Stewart, 1968; Holm-Hanson, 1968). Generally, for algalization of the rice fields, mixed cyanobacterial cultures of free-living forms are used (Venkataraman, 1972; Roger and Kulasooriya, 1980). The water fern Azolla harbors Anabaena azollae in its fronds and the cyanobacterium releases ammonium into the water when paddy fields are inoculated with foam-immobilized A. azollae strains (Kannaiyan et al., 1997). Significant increase in grain yield, biomass and nutritive value of rice can be achieved by inoculating Anabaena doliolum and A. fertilissima in paddy fields with or without urea (Dubey and Rai, 1995). Several cyanobacterial species such as Anabaena iyengarii var. tenuis, A. fertilissima, Nostoc commune, N. ellipsosporum, N. linckia, and Gloeotrichia natans are known to contribute to the productivity of rice fields in Chile (Pereira et al., 2009). Generally, application of 12.5 kg ha−1 of cyanobacterial biofertilizer has been recommended for quantitative and qualitative improvements in rice production (Dubey and Rai, 1995).
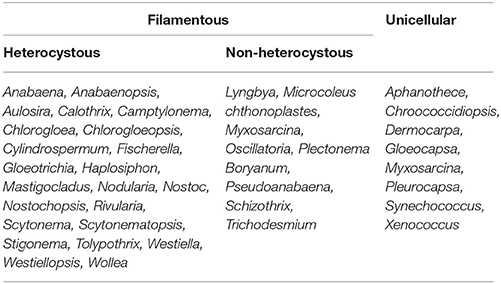
Table 1. List of nitrogen-fixing cyanobacteria important for their application in biofertilizer industry (adapted from Vaishampayan et al., 2001).
In addition to rice crop, cyanobacterial biofertilizers can also enhance the yield, shoot/root length, and dry weight of wheat crops (Spiller and Gunasekaran, 1990; Obreht et al., 1993; Karthikeyan et al., 2009). Inoculation of soil with various cyanobacterial strains like Nostoc carneum, N. piscinale, Anabaena doliolum and A. torulosa results in significantly higher acetylene reducing activity (Prasanna et al., 2013). Additionally, the acetylene reducing activity is highest at harvest stage when wheat fields are inoculated with an Anabaena-Serratia biofilm along with rock phosphate (Swarnalakshmi et al., 2013). The cyanobacteria based biofertilizers are cost-effective as they cost one third to that of chemical fertilizers (Prasanna et al., 2013). In addition to nitrogen fixation, cyanobacteria also contribute to mobilization of inorganic phosphates through excretion of organic acids and extracellular phosphatases (Bose and Nagpal, 1971; Rai and Sharma, 2006). Cyanobacteria solubilize and mobilize the insoluble organic phosphates and improve the availability of phosphorus to the crop (Dorich et al., 1985; Cameron and Julian, 1988). The humus content generated after death and decay of cyanobacteria creates strong reducing condition in soil which improve the soil structure and fertility (Abdel-Raouf et al., 2012). Different cyanobacterial strains are known to produce plant growth hormones and siderophores, and therefore, cyanobacteria can affect the development and productivity of crops (Rodriguez et al., 2006; Rastogi and Sinha, 2009). The EPS secreted by cyanobacteria induces aggregation of soil particles which improve the soil structure and fertility by enhancing the accumulation of organic content and water accumulation. These findings collectively support the importance of cyanobacteria as biofertilizers, and methods have been developed for their cultivation and utilization in fertilizer industry (Brouers et al., 1987; Shi and Hall, 1988; Vaishampayan et al., 2000).
Cyanobacteria in Nutrition and Health Sector
Cyanobacteria are a well-known non-conventional source of protein, healthy lipids, minerals, antioxidants, and vitamins (Pulz and Gross, 2004; Singh et al., 2005; Gantar and Svircev, 2008; Rosenberg et al., 2008). The cyanobacterium Spirulina (Arthrospira) has been used by the Aztec population as food supplement for a long time (Pulz and Gross, 2004). The various strains of Nostoc, Spirulina, and Anabaena are used as food supplements in Peru, Mexico, Philippines, and Chile. Cyanobacteria generally have low lipid content but 6–13% lipid on dry weight basis has been reported in Spirulina (Cohen, 1997). Spirulina is a good source of rare polyunsaturated fatty acid, i.e., gamma-linolenic acid (GLA), which has several pharmaceutical properties. GLA is 170 times more effective than linoleic acid, which is the main constituent of majority of polyunsaturated oils (Huang et al., 1982).
Arthrospira platensis is a best example of non-conventional source of food supplement which is mass cultivated in outdoor ponds or bioreactors, and commercially available in the form of flakes, powder, capsules or pills. It is very nutrient rich, containing more than 60% of proteins. Arthrospira is a rich source of thiamine, riboflavin, beta-carotene, and it is also considered as an excellent whole-food source of vitamin E and vitamin B12 (Abed et al., 2009). Arthrospira (~20 g) can provide the required regular doses of vitamin B12 and 50% of vitamin B2 (riboflavin), 70% of B1 (thiamine) and 12% of B3 (niacin) in humans (Switzer, 1981). It also contains a fair amount of tocopherol (vitamin E), which is almost three times that of pure wheat germ (Hudson and Karis, 1974). The native population of the lake Chad region of Africa uses Spirulina (Arthrospira) as hardened, sun dried mat (Dihé) as a food supplement rich in proteins, minerals, carotenoids, vitamin E, folate and lipids containing essential unsaturated fatty acids (Carcea et al., 2015). Spirulina (Arthrospira) is generally termed as “magic agent” because of its wide applications as feeds, food, anticancer agent and cosmetics (Vonshak, 1997). However, Spirulina proteins are low in methionine, lysine and cysteine contents, and therefore, inferior to milk or meat protein but it is superior to plant proteins such as legumes (Ciferri, 1983). Thus, Spirulina could be utilized as a source of protein and other nutrients in countries where starvation and malnutrition is the main problem for human civilization. Spirulina can also be used for feeding hens because it stimulates egg production with intense yolk color, while Spirulina-fed fish develop a pink yellow meat of high commercial value (Sharma et al., 2011). Tilapia fish shows high growth rates in outdoor and indoor cultures when fed with marine cyanobacteria (Mitsui et al., 1983). P. valderianum is non-toxic and possesses high nutritional value, and therefore, it can be used as a complete aquaculture feeds (Steinberg et al., 1998). Thus, cyanobacteria can be used as a quality food in aquaculture and poultry farming which increases the quality and quantity of products.
Several cyanobacteria such as Anabaena flos-aquae, A. hassali, Nostoc punctiforme, Phormidium bijugatum, and Microcystis pulverana are good source of vitamin B-complex, pentothene and nicotinic acid (Robbins et al., 1951; Koptera, 1970). Similarly, N. commune is a rich sources of fibers and proteins (Abed et al., 2009). Additionally, cyanobacteria are source of pigments such as phycobiliproteins, chlorophyll a, and carotenoids. Cyanobacteria synthesize UV screening compounds such as mycosporine-like amino acids and scytonemins which have their applications in cosmetic and pharmaceutical industry (Rajneesh et al., 2017). However, several species of cyanobacteria are known to produce various cyanotoxins such as hepatotoxins, neurotoxins, cytotoxins, and dermatotoxins, and therefore, care should be taken when toxin producing cyanobacteria are used for the production of edible chemicals (Rajneesh et al., 2017). Cyanobacteria-based diet can be also used in reducing the cholesterol levels; cyanobacterial diet has been found to reduce the lipoproteins and cholesterol levels in blood serum of rats (Nakaya et al., 1988; Hori et al., 1994). Detailed studies suggest that cyanobacterial feeding increases the lipase enzyme which helps in lowering of triglycerides (Iwata et al., 1990). The consumption of cyanobacterium A. flos-aquae decrease the blood cholesterol level, stimulate liver functioning and accelerate the recovery from mild traumatic brain injury (Vlad et al., 1995; Valencia and Walker, 1999).
The phycobiliproteins produced by cyanobacteria, e.g., phycocyanin, phycoerythrin, and allophycocyanin, have several applications in food, cosmetics, medicine, and diagnostics industry. Phycocyanin from Spirulina has been used in lipsticks, eyeliner and eye shadow (Sharma et al., 2011). In Japan, phycocyanin extracted from A. platensis is marketed as a colorant of cosmetics and food (Prasanna et al., 2007). Phycocyanin is highly stable in the presence of preservatives, high temperature, and light; however, reluctance in consumption of blue foods has limited the interest of industries in utilization of phycocyanin as food colorant (Jespersen et al., 2005; Mishra et al., 2008). Phycocyanin can be also used as a rich source of protein in commercially available dietary supplements. Apart from the nutritional importance, the cyanobacteria also possess anti-inflammatory, antioxidant, anti-viral, and anti-cancer properties (Jensen et al., 2001). Several biologically active compounds have been reported from cyanobacteria (Table 2), which may contribute to the improved health on consumption of cyanobacteria based diet, and furthermore, these compounds can be also used in the development of new drugs (Rajneesh et al., 2017). The consumption of cyanobacterial diet increases the level of IgA in serum, and therefore, cyanobacterial diet can boost our immune system to fight against different allergens (Hayashi et al., 1998). Therefore, cyanobacterial consumption may help in reducing allergies, chronic inflammatory conditions, autoimmune diseases and diseases involving a suppressed immune system.
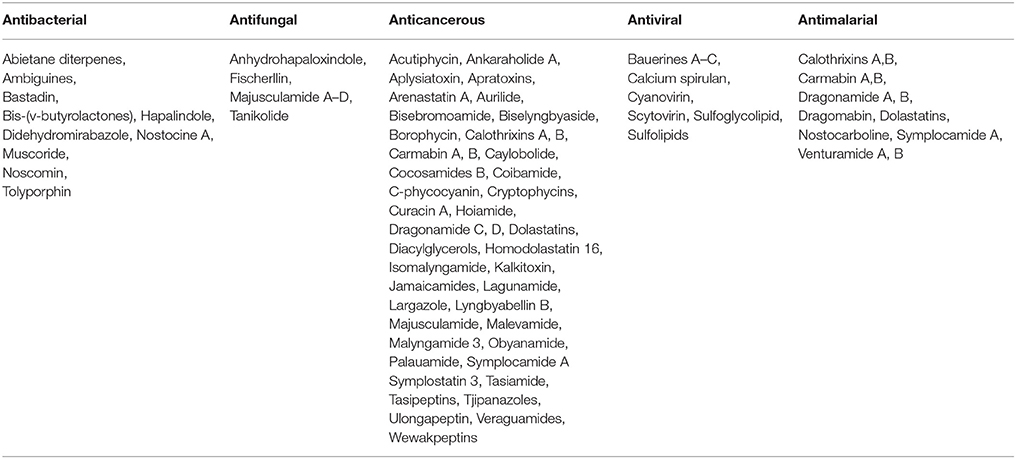
Table 2. Cyanobacterial metabolites of pharmacological and biotechnological importance (Source: Rajneesh et al., 2017).
Mass Cultivation of Cyanobacteria
Cyanobacterial biomass can complement agricultural crops for the production of food, feeds, fuels, and chemicals. However, production of biomass at large scale is required for the application of cyanobacteria in above mentioned purposes. Cyanobacterial cultivation does not compete for resources with agricultural crops, and moreover, they have better aerial productivity than plants (Dismukes et al., 2008; Cañedo and Lizárraga, 2016; Ooms et al., 2016). Genetic modification of cyanobacteria and their usefulness have been studied mainly in the laboratory; however, for commercial production, the process needs to be standardized and applied outdoors. The idea of high-rate (non-nutrient limited) large-scale (>50 L) cultivation of cyanobacteria and microalgae was proposed in early 1950s (Cook, 1951). The recent demand of cyanobacteria and other microalgae in bioenergy, food, and valuable chemicals production sector has necessitated their large-scale cultivation. However, economic sustainability is the most critical factor which determines the success of large scale biomass production and its downstream processing for the production of different commercial products (Figure 2). Light, pH, temperature, carbon dioxide, and nutrient supplements are the five critical abiotic parameters which determine the success of any growth system designed for autotrophic growth of photosynthetic organisms (Pulz, 2001; Flynn et al., 2010). However, tremendous expertise and resources are required to manage all these closely linked factors. Therefore, only few cyanobacteria and microalgae such as Arthrospira, Chlorella, Haematococcus, and Dunaliella have been cultivated on large scale as economically and commercially viable crops (Rosenberg et al., 2008).
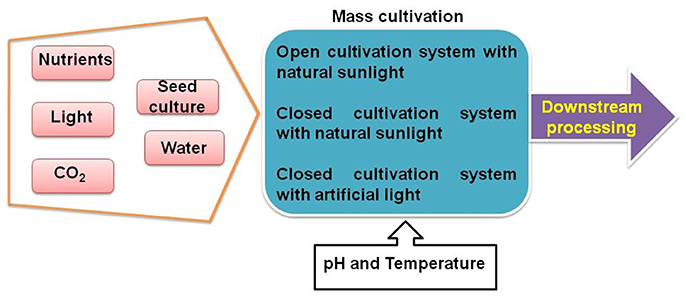
Figure 2. Simplified outline for large-scale cultivation of microalgae. Photosynthetic microorganisms can be cultivated by providing nutrients, light, carbon dioxide, and water using different cultivation systems under controlled pH and temperature conditions. After mass cultivation, biomass is harvested for downstream processing.
Cyanobacterial cultivation requires a source of light, essential nutrients such as C, N, P, S, K, Fe, etc., and water. Commercial production of photosynthetic microorganisms can be achieved in different ways (Figure 3):
(1) Cultivation using sunlight in open systems
(2) Cultivation using sunlight in closed systems
(3) Cultivation using artificial light in closed systems
Figure 3. Different cultivation systems for large-scale production of algal biomass. (A) Open raceway pond system using natural solar light as a source of energy; (B) Closed system using natural solar light as a source of energy; (C) Closed system using artificial light as a source of energy.
The above mentioned systems have both positives and negatives which are discussed in the following section, however, the preference of system depends on the value of the product and the extent of control over various parameters required for the production. Supplying light to culture constitutes a common limitation to all these systems; however, light limitation can be overcome by increasing the surface area of the system to maximize the light absorption.
Cultivation Using Sunlight in Open Systems
Several designs of sunlight dependent open cultivation systems have been proposed and constructed (Oswald, 1988; Chaumont, 1993; Pushparaj et al., 1997). Generally, the raceway or circular type shallow open ponds are used for mass cultivation of cyanobacteria and microalgae (Cañedo and Lizárraga, 2016). These systems are usually large raceways or open ponds where natural light is the source of energy (Figure 3A). The most advantageous aspect of this system is that the source of light, i.e., solar radiation is free of cost. However, several major disadvantages compensate this advantage and limit the overall biomass production. Contamination by algae, grazers and other microorganisms in open systems is unpreventable which compromise the net productivity. However, contamination problems could be avoided for organisms requiring unique growth conditions which generally prevent the growth of other organisms, however, this strategy limits the application of open systems for cultivation of only selected organisms (Costa et al., 2006; De Morais and Costa, 2007; Ugwu et al., 2008).
The outdoor open cultivation systems are also subject to diurnal fluctuation of different environmental conditions such as quality and quantity of light, temperature, evaporation, and rain, which significantly control the productivity and survival of the system. Apart from these limitations, open cultivation systems have been proven successful for the production of specific biomaterials producing tons of dried biomass (Lee, 1997). For example Spirulina has been extensively cultivated in Mexico, USA, China, and Thailand using open system due to its abovementioned unique growth requirement (Li and Qi, 1997; Vonshak, 1997).
Cultivation Using Sunlight in Closed System
Several cultivation systems have been designed which are closed and utilize solar radiation as a source of energy (Qiang and Richmond, 1994; Spektorova et al., 1997; Grima et al., 1999). In these systems, transparent material made up of plastic or glass is used for making vessels which are placed outdoors in the natural light for illumination (Figure 3B) (Khatoon and Pal, 2015). The cost of these systems is increased significantly by application of transparent materials but the closed system helps in preventing contamination of grazers and competitors. Such systems are designed in a manner which provides a higher surface to volume ratio, and therefore, cell densities obtained are often higher than in open systems (Carvalho et al., 2006).
Outdoor systems are subjected to aforementioned abiotic factors that may affect the reproducibility of their outcome. Additionally, the removal of photosynthetically produced oxygen and maintenance of optimum temperature are other factors which need to be critically observed in closed systems. These factors, if not maintained optimally, can cause a sudden crash of cultures. Techniques have been developed to maintain these parameters; however, the associated costs usually offset the cost advantage of using natural sunlight (Khatoon and Pal, 2015).
Cultivation Using Artificial Light in Closed System
Several designs have been developed for closed (indoor) cultivation of photosynthetic microorganisms which utilize artificial light as source of energy (Ratchford and Fallowfield, 1992; Wohlgeschaffen et al., 1992; Lee and Palsson, 1994). These designs/vessels are similar to conventional fermenters in principle and are referred to as photobioreactors; however, these photobioreactors are driven by light unlike the fermenters, which are driven by an organic carbon source (Figure 3C). These photobioreactors empower real-time control and optimization of culture parameters using software, and therefore, could be suitable for the cultivation of various organisms (Ratchford and Fallowfield, 1992).
The cost of photobioreactor-based biomass production increases significantly in comparison to outdoor systems due to utilization of plastic or glass material in making the vessel and power consumption. However, the cost can be offset by the production of high quality and quantity biomass using these systems. Photobioreactors serve as an important tool for the production of high-value products such as stable-isotope-labeled biochemicals (Apt and Behrens, 1999). These systems are also ideal for the cultivation of genetically modified organisms because their release in the environment from closed photobioreactors is limited but not guaranteed. Therefore, analysis of the associated risks of spreading of genetically modified organism into the surrounding is suggested before starting a large-scale production.
Conclusions and Future Directions
Cyanobacterial farming can help in developing sustainable agricultural practice by the generation of biomass that can be used for the sustainable production of bioenergy, food supplements, nutraceuticals, biofertilizers, and feeds for aquaculture and poultry. In addition, cyanobacterial farming can significantly lower the atmospheric CO2, and therefore, helps in controlling the problems associated with global climate change. The successful utilization of cyanobacterial species or other microalga for the development of sustainable agricultural practices requires their large scale cultivation. The raceway pond and photobioreactors systems have been devised for large scale biomass production, however, cyanobacterial farming using abovementioned systems requires very high amount of investment in the form of capital and operational costs. Therefore, third farming system, i.e., hybrid system, involving both raceway and photobioreactor system, are recommended which significantly reduce the capital and operational costs of farming (Bravo-Fritz et al., 2016). The cost associated with cyanobacterial/microalgal farming is still so high that any venture will have negative net profitability considering the current scenario of capital and operational costs required for the establishment of biorefinery (Bravo-Fritz et al., 2016). Therefore, the cost associated with farming needs to be significantly reduced for net profitability and success of any venture. The economic studies conducted so far have focused only on selected species, and therefore, further economic profitability modeling with a large number of new species is needed. The production cost of photobioreactors is an important area of concern, and attention is required to cut the price to reduce the cost of commodities production.
The reduction in production cost and positive net profitability can be also achieved by production of several commodities and complete utilization of biomass without producing any waste (Bravo-Fritz et al., 2016; Rajneesh et al., 2017). For example, protein extraction before the production of bioenergy has shown the positive net profitability of biorefinery (Bravo-Fritz et al., 2016). Therefore, different aforementioned sustainable services from cyanobacteria can be combined with each other to reduce the waste which could help in reaching a positive net profitability by producing several valuable commodities. The harvesting of organisms from a large volume of water is another challenge that cost enormously to and negatively affect the profitability. The biomass composition of an alga, i.e., percentage of proteins, lipids and carbohydrates, also affects the profitability of the bioenergy business, and therefore, farming of species with high lipid and low protein contents is recommended for the profitability of bioenergy industry (Bravo-Fritz et al., 2016). The requirement of water and nutrients are other major challenges for economic profitability of large scale cyanobacterial farming, however, bioremediation property of cyanobacteria can be exploited to lower the expenditure on water and nutrients. Cyanobacterial farming needs to be optimized using wastewater at small scale before going to large scale cultivation. Outdoor open cultivation of cyanobacteria is challenged by fluctuating environmental conditions and contamination, which collectively result in lower biomass production and pose a threat to economic viability. Abiotic stresses mainly light quality and quantity, temperature, pH, salinity, availability of nutrients, and CO2, and biotic factors such as grazers and other biological contamination controls the biomass production. Therefore, cyanobacterial species need to be identified which can sustain fluctuating environmental conditions and can give consistent and economically viable amounts of biomass throughout the year for various applications. Efforts are also needed to improve the fitness of already known species under various environmental stresses which will enhance the biomass production and profitability of cyanobacterial farming.
Author Contributions
JP: Conceptualized the review, did the literature survey, and wrote the paper; Rajneesh: Helped in collecting literature and making figures; PM: Helped in making figures; SS: Critically reviewed the manuscript and helped in writing paper; D-PH: Provided the valuable suggestions and edited the MS; RS: Gave the concept and thoroughly reviewed the MS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
JP and Rajneesh are thankful to the Council of Scientific and Industrial Research, New Delhi, India (09/013/0515/2013-EMR-I), and Department of Biotechnology, Govt. of India (DBT-JRF/13/AL/143/2158), respectively, for the grants in the form of senior research fellowships. PM is thankful to UGC (3616/NET-DEC2014) for financial support in the form of junior research fellowship. SS acknowledges the UGC for start-up grant and DST-SERB for early career research award.
References
Abdel-Raouf, N., Al-Homaidan, A. A., and Ibraheem, I. B. (2012). Agricultural importance of algae. Afr. J. Biotechnol. 11, 11648–11658. doi: 10.5897/AJB11.3983
Abed, R. M. M., Dobretsov, S., and Sudesh, K. (2009). Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 106, 1–2. doi: 10.1111/j.1365-2672.2008.03918.x
Abed, R. M. M., and Köster, J. (2005). The direct role of aerobic heterotrophic bacteria associated with cyanobacteria in the degradation of oil compounds. Int. Biodeterior. Biodegrad. 55, 29–37. doi: 10.1016/j.ibiod.2004.07.001
Aiyer, R. S., Sulahudean, S., and Venkataraman, G. S. (1972). Long-term algalization field trial with high yielding rice varieties. Indian, J. Agric. Sci. 42, 380–383.
Al-Awadhi, H., Al-Hasan, R. H., Sorkhoh, N. A., Salamah, S., and Radwan, S. S. (2003). Establishing oil-degrading biofilms on gravel particles and glass plates. Int. Biodeterior. Biodegrad. 51, 181–185. doi: 10.1016/S0964-8305(02)00140-3
Al-Haj, L., Lui, Y. T., Abed, R. M. M., Gomaa, M. A., and Purton, S. (2016). Cyanobacteria as chassis for industrial biotechnology: progress and prospects. Life 6:42. doi: 10.3390/life6040042
Anderson, R. T., and Lovley, J. R. (1997). Ecology and biogeochemistry of in situ ground water bioremediation. Adv. Microbial. Ecol. 15, 289–350.
Apt, K. E., and Behrens, P. W. (1999). Commercial developments in microalgal biotechnology. J. Phycol. 3, 215–226. doi: 10.1046/j.1529-8817.1999.3520215.x
Berla, B. M., Saha, R., Immethun, C. M., Maranas, C. D., Moon, T. S., and Pakrasi, H. B. (2013). Synthetic biology of cyanobacteria: unique challenges and opportunities. Front. Microbiol. 4:246. doi: 10.3389/fmicb.2013.00246
Bose, P., and Nagpal, U. S. (1971). Solubilization of tricalcium phosphate by blue-green algae. Curr. Sci. 40, 165–166.
Bravo-Fritz, C. P., Sáez-Navarrete, C. A., Herrera-Zeppelin, L. A., and Varas-Concha, F. (2016). Multi-scenario energy-economic evaluation for a biorefinery based on microalgae biomass with application of anaerobic digestion. Algal Res. 16, 292–307. doi: 10.1016/j.algal.2016.03.028
Brouers, M., De Jong, H., Shi, D. J., Rao, K. K., and Hall, D. O. (1987). Sustained ammonia production by immobilized cyanobacteria. Progr. Photosynth. Res. 2, 645–647. doi: 10.1007/978-94-009-3535-8_153
Burlage, R. S., and Kuo, C. T. (1994). Living biosensors for the management and manipulation of microbial consortia. Ann. Rev. Microbiol. 48, 291–301. doi: 10.1146/annurev.mi.48.100194.001451
Cameron, H. J., and Julian, G. R. (1988). Utilisation of hydroxyapatite by cyanobacteria as their sole source of phosphate and calcium. Plant Soil 109, 123–124. doi: 10.1007/BF02197589
Cañedo, J. C. G., and Lizárraga, G. L. L. (2016). “Considerations for photobioreactor design and operation for mass cultivation of microalgae,” in Algae-Organisms for Imminent Biotechnology, eds N. Thajuddin and D. Dhanasekaran (InTech). doi: 10.5772/63069
Carcea, M., Sorto, M., Batello, C., Narducci, V., Aguzzi, A., Azzini, E., et al. (2015). Nutritional characterization of traditional and improved dihé, alimentary blue-green algae from the lake Chad region in Africa. WT Food Sci. Technol. 62, 753–763. doi: 10.1016/j.lwt.2014.10.039
Carvalho, A. P., Meireles, L. A., and Malcata, F. X. (2006). Microalgal reactors: a review of enclosed system designs and performances. Biotechnol. Prog. 22, 1490–1506. doi: 10.1002/bp060065r
Chaumont, D. (1993). Biotechnology of algal biomass production: a review of systems for outdoor mass culture. J. Appl. Phycol. 5, 593–604. doi: 10.1007/BF02184638
Cohen, Z. (1997). “The chemical of Spirulina,” in Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology, ed A. Vonshak (Philadelphia, PA: Taylor and Francis Inc.), 175–204.
Cook, P. M. (1951). Chemical engineering problems in large scale culture of algae. Ind. Eng. Chem. 43, 2385–2389. doi: 10.1021/ie50502a056
Costa, J. A. V., De Morais, M. G., Dalcanton, F., Reichert, C. C., and Durante, A. J. (2006). Simultaneous cultivation of Spirulina platensis and the toxigenic cyanobacteria Microcystis aeruginosa. Z. Naturforsch. 61, 105–110. doi: 10.1515/znc-2006-1-219
Cuellar-Bermudez, S. P., Aleman-Nava, G. S., Chandra, R., Garcia-Perez, J. S., Contreras-Angulo, J. R., Markou, G., et al. (2017). Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 24, 438–449. doi: 10.1016/j.algal.2016.08.018
De Morais, M. G., and Costa, J. A. V. (2007). Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett. 29, 1349–1352. doi: 10.1007/s10529-007-9394-6
De Ruyter, Y. S., and Fromme, P. (2008). “Molecular structure of the photosynthetic apparatus,” in The Cyanobacteria: Molecular Biology, Genomics and Evolution, eds A. Herrero, and E. Flores (Norfolk: Caister Academic Press), 217–269.
DESA UN (2015). World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. Working Paper No ESA/P/WP. 241, United Nations, Department of Economic and Social Affairs, Population Division, New York, NY.
Desai, S. H., and Atsumi, S. (2013). Photosynthetic approaches to chemical biotechnology. Curr. Opin. Biotechnol. 24, 1031–1036. doi: 10.1016/j.copbio.2013.03.015
Dismukes, G. C., Carrieri, D., Bennette, N., Ananyev, G. M., and Posewitz, M. C. (2008). Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 19, 235–240. doi: 10.1016/j.copbio.2008.05.007
Dorich, R. A., Nelson, D. W., and Sommers, L. E. (1985). Estimating algal available phosphorus in suspended sediments by chemical extraction. J. Environ. Qual. 14, 400–405. doi: 10.2134/jeq1985.00472425001400030018x
Du, Z., Li, H., and Gu, T. (2007). A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 25, 464–482. doi: 10.1016/j.biotechadv.2007.05.004
Dubey, A. K., and Rai, A. K. (1995). Application of algal biofertilizers (Aulosira fertilissimatenuis and Anabaena doliolum Bhardwaja) for sustained paddy cultivation in Northern India. Isr. J. Plant. Sci. 43, 41–51. doi: 10.1080/07929978.1995.10676589
Ducat, D. C., Way, J. C., and Silver, P. A. (2011). Engineering cyanobacteria to generate high-value products. Trends biotechnol. 29, 95–103. doi: 10.1016/j.tibtech.2010.12.003
El-Bestawy, E. A., Abd El-Salam, A. Z., and Mansy, A. E. R. H. (2007). Potential use of environmental cyanobacterial species in bioremediation on lindane-contaminated effluents. Inter. Biodeterior. Biodegrad. 59, 180–192. doi: 10.1016/j.ibiod.2006.12.005
Flaibani, A., Olsen, Y., and Painter, T. J. (1989). Polysaccharides in desert reclamation: composition of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydrate Res. 190, 235–248. doi: 10.1016/0008-6215(89)84128-X
Flynn, K. J., Greenwell, H. C., Lovitt, R. W., and Shields, R. J. (2010). Selection for fitness at the individual or population levels: modelling effects of genetic modifications in microalgae on productivity and environmental safety. J. Theor. Biol. 263, 269–280. doi: 10.1016/j.jtbi.2009.12.021
Fogg, G. E., and Stewart, W. D. P. (1968). In situ determinations of biological nitrogen fixation in Antarctica. Br. Antarct. Surv. Bull. 15, 39–46.
Forlani, G., Pavan, M., Gramek, M., Kafarski, P., and Lipok, J. (2008). Biochemical basis for a wide spread tolerance of cyanobacteria to the phosphonate herbicide glyphosate. Plant Cell Physiol. 49, 443–456. doi: 10.1093/pcp/pcn021
Fritsch, F. E. (1907). The subaerial and freshwater algal flora of the tropics. Ann. Bot. 30, 235–275. doi: 10.1093/oxfordjournals.aob.a089132
Gantar, M., and Svircev, Z. (2008). Microalgae and cyanobacteria: food for thought. J. Phycol. 44, 260–268. doi: 10.1111/j.1529-8817.2008.00469.x
Gao, X., Sun, T., Pei, G., Chen, L., and Zhang, W. (2016). Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl. Microbiol. Biotechnol. 100, 3401–3413. doi: 10.1007/s00253-016-7374-2
Grima, E. M., Fernández, F. A., Camacho, F. G., and Chisti, Y. (1999). Photobioreactors: light regime, mass transfer, and scale up. J. Biotechnol. 70, 231–247. doi: 10.1016/S0168-1656(99)00078-4
Gröniger, A., Sinha, R. P., Klisch, M., and Häder, D.-P. (2000). Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae-a database. J. Photochem. Photobiol. B Biol. 58, 115–122. doi: 10.1016/S1011-1344(00)00112-3
Gudmundsson, S., and Nogales, J. (2015). Cyanobacteria as photosynthetic biocatalysts: a systems biology perspective. Mol. Biosyst. 11, 60–70. doi: 10.1039/C4MB00335G
Guerrero, M. G., Vega, J. M., and Losada, M. (1981). The assimilatory nitrate-reducing system and its regulation. Annu. Rev. Plant Physiol. 32, 168–204. doi: 10.1146/annurev.pp.32.060181.001125
Habib, M. A. B., Parvin, M., Huntington, T. C., and Hasan, M. R. (2008). A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. Rome: Food and Agriculture Organization of the United Nations.
Hall, D. O., Markov, S. A., Watanabe, Y., and Rao, K. K. (1995). The potential applications of cyanobacterial photosynthesis for clean technologies. Photosynth. Res. 46, 159–167. doi: 10.1007/BF00020426
Hamouda, R. A. E. F., Sorour, N. M., and Yeheia, D. S. (2016). Biodegradation of crude oil by Anabaena oryzae, Chlorella kessleri and its consortium under mixotrophic conditions. Int. Biodeterior. Biodegrad. 112, 128–134. doi: 10.1016/j.ibiod.2016.05.001
Hayashi, O., Hirahashi, T., Katoh, T., Miyajima, H., Hirano, T., and Okuwaki, Y. (1998). Class specific influence of dietary Spirulina platensis on antibody production in mice. J. Nutr. Sci. Vitaminol. 44, 841–851. doi: 10.3177/jnsv.44.841
Hegde, D. M., Dwiwedi, B. S., and Babu, S. N. S. (1999). Biofertilizers for cereal production in India: a review. Indian J. Agric. Sci. 69, 73–83.
Herrero, A., and Flores, E. (2008). The cyanobacteria: Molecular Biology, Genetics and Evolution. Norwich: Horizon Scientific Press.
Hoekman, S. K., Broch, A., Robbins, C., Ceniceros, E., and Natarajan, M. (2012). Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 16, 143–169. doi: 10.1016/j.rser.2011.07.143
Holm-Hanson, O. (1968). Ecology, physiology and biochemistry of blue-green algae. Annual Rev. Microbiol. 22, 47–70. doi: 10.1146/annurev.mi.22.100168.000403
Hori, K. G., Ishibashi, G., and Okita, T. (1994). Hypocholesterolemic effect of blue-green alga, ishikurage (Nostoc commune) in rats fed atherogenic diet. Plant Foods Hum. Nutr. 45, 63–70. doi: 10.1007/BF01091230
Huang, Y. S., Cunnane, S. C., Horrobin, D. F., and Davignon, J. (1982). Most biological effects of zinc deficiency corrected by γ-linolenic acid (18: 3ω6) but not by linoleic acid (18: 2ω6). Atherosclerosis 41, 193–207. doi: 10.1016/0021-9150(82)90185-X
Hudson, B. Z. F., and Karis, I. G. (1974). The lipids of the alga Spirulina. J. Sci. Food. Agric. 25, 759–763. doi: 10.1002/jsfa.2740250703
Issa, A. A., Abd-Alla, M. H., and Ohyama, T. (2014). “Nitrogen fixing cyanobacteria: future prospect,” in Advances in Biology and Ecology of Nitrogen Fixation, ed T. Ohyama (InTech). doi: 10.5772/56995
Iwata, K., Inayama, T., and Kato, T. (1990). Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. J. Nutr. Sci. Vitaminol. 36, 165–171. doi: 10.3177/jnsv.36.165
Jensen, G. S., Ginsberg, D. I., and Drapeau, C. (2001). Blue-green algae as an immuno-enhancer and biomodulator. J. Am. Nutraceut. Ass. 3, 24–30.
Jespersen, L., Strømdahl, L. D., Olsen, K., and Skibsted, L. H. (2005). Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food. Res. Technol. 220, 261–266. doi: 10.1007/s00217-004-1062-7
Kalavathi, F. D., Uma, L., and Subramanian, G. (2001). Degradation and metabolization of the pigment melanoidin in distillery effluent by the marine cyanobacterium Oscillatoria boryana BDU 92181. Enz. Microb. Technol. 29, 249–251. doi: 10.1016/S0141-0229(01)00383-0
Kaneko, T., Sato, S., Kotani, H., Tanaka, A., Asamizu, E., Nakamura, Y., et al. (1996). Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. Res. 3, 109–136.
Kannaiyan, S., Aruna, S. J., Kumari, S. M. P., and Hall, D. O. (1997). Immobilized cyanobacteria as a biofertilizer for rice crops. J. Appl. Phycol. 9, 167–174. doi: 10.1023/A:1007962025662
Karl, D., Michaels, A., Bergman, B., Capone, D., Carpenter, E., Letelier, R., et al. (2002). “Dinitrogen fixation in the World's Oceans,” in The Nitrogen Cycle at Regional to Global Scales, eds E. W. Boyer and R. W. Howarth (Springer), 47–98.
Karthikeyan, N., Prasanna, R., Sood, A., Jaiswal, P., Nayak, S., and Kaushik, B. D. (2009). Physiological characterization and electron microscopic investigations of cyanobacteria associated with wheat rhizosphere. Folia Microbiol. 54, 43–51. doi: 10.1007/s12223-009-0007-8
Kesaano, M., and Sims, R. C. (2014). Algal biofilm based technology for waste water treatment. Algal Res. 5, 231–240. doi: 10.1016/j.algal.2014.02.003
Khatoon, N., and Pal, R. (2015). “Microalgae in biotechnological application: a commercial approach,” in Plant Biology and Biotechnology, eds B. Bahadur, M. V. Rajam, L. Sahijram, and K. V. Krishnamurthy (Springer), 27–47.
Koptera, Z. P. (1970). Biosynthesis of biotin, pyredoxin, nicotinic acid and pantothenic acids by some blue-green algae. Microbiol. Z. 32, 555–560.
Kumar, D., Pandey, L. K., and Gaur, J. P. (2016). Metal sorption by algal biomass: from batch to continuous system. Algal Res. 18, 95–109. doi: 10.1016/j.algal.2016.05.026
Kuritz, T., and Wolk, C. P. (1995). Use of filamentous cyanobacteria for biodegradation of organic pollutants. Appl. Environ. Microbiol. 61, 234–238.
Lee, C.-G., and Palsson, B. O. (1994). High-density algal photobioreactors using light-emitting diodes. Biotechnol. Bioeng. 44, 1161–1167. doi: 10.1002/bit.260441002
Lee, Y. K. (1997). Commercial production of microalgae in the Asia Pacific rim. J. Appl. Phycol. 9, 403–411. doi: 10.1023/A:1007900423275
Lewis, N. S., and Nocera, D. G. (2006). Powering the planet: chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. U.S.A. 103, 15729–15735. doi: 10.1073/pnas.0603395103
Li, D., and Qi, Y. (1997). Spirulina industry in China: present status and future prospects. J. Appl. Phycol. 9, 25–28. doi: 10.1023/A:1007973823532
Lipok, J., Wieczorek, D., Jewginski, M., and Kafarski, P. (2009). Prospects of in vivo 31P NMR method in glyphosate degradation studies in whole cell system. Enzyme Microb. Technol. 44, 11–16. doi: 10.1016/j.enzmictec.2008.09.011
Malamlssa, O. L., Bissonnais, Y., Defarge, C., and Trichet, J. (2001). Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma 101, 15–30. doi: 10.1016/S0016-7061(00)00093-8
Mansy, A. E., and El-Bestway, E. (2002). Toxicity and biodegradation of fluometuron by selected cyanobacterial species. World J. Microbiol. Biotechnol. 18, 125–131. doi: 10.1023/A:1014490811121
Markley, A. L., Begemann, M. B., Clarke, R. E., Gordon, G. C., and Pfleger, B. F. (2015). Synthetic biology toolbox for controlling gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth. Biol. 4, 595–603. doi: 10.1021/sb500260k
Melis, A. (2009). Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 177, 272–280. doi: 10.1016/j.plantsci.2009.06.005
Metting, B. (1988). “Microalgae in agriculture,” in Micro-Algal Biotechnology, eds M. A. Borowitzka and L. J. Borowitzka (Cambridge: Cambridge University Press), 288–304.
Milledge, J. J. (2011). Commercial application of microalgae other than as biofuels: a brief review. Rev. Environ. Sci. Bio-Technol. 10, 31–41. doi: 10.1007/s11157-010-9214-7
Mishra, S. K., Shrivastav, A., and Mishra, S. (2008). Effects of preservatives for food grade C-PC from Spirulina platensis. Process Biochem. 43, 339–345. doi: 10.1016/j.procbio.2007.12.012
Mitsui, M., Enternmann, B., and Gill, K. (1983). “Indoor and outdoor cultivation of Tilapia in Seawater with algae as a sole food source,” in Proceedings of the Second North Pacific Aquaculture System (Tokyo University), 323–340.
Mohammadi, K., and Sohrabi, Y. (2012). Bacterial biofertilizers for sustainable crop production: a review. J. Agric. Biol. Sci. 7, 307–316. doi: 10.1186/1475-2859-13-66
Mahanty, T., Bhattacharjee, S., Goswami, M., Bhattacharyya, P., Das, B., Ghosh, A., et al. (2017). Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 24, 3315–3335.
Molin, J., and Molin, S. (1997). Case: complex adaptive systems ecology. Adv. Microbial Ecol. 15, 27–79. doi: 10.1007/978-1-4757-9074-0_2
Nakaya, N., Homa, Y., and Goto, Y. (1988). Cholesterol lowering effect of Spirulina. Nutr. Rep. Int. 37, 1329–1337.
Nisha, R., Kaushik, A., and Kaushik, C. P. (2007). Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geoderma 138, 49–56. doi: 10.1016/j.geoderma.2006.10.007
Obreht, Z., Kerby, N. W., Gantar, M., and Rowell, P. (1993). Effects of root associated N2-fixing cyanobacteria on the growth and nitrogen content of wheat (Triticum vulgare L.) seedlings. Biol. Fertil. Soils 15, 68–72. doi: 10.1007/BF00336292
Oliver, J. W., and Atsumi, S. (2014). Metabolic design for cyanobacterial chemical synthesis. Photosynth. Res. 120, 249–261. doi: 10.1007/s11120-014-9997-4
Oliver, N. J., Rabinovitch-Deere, C. A., Carroll, A. L., Nozzi, N. E., Case, A. E., and Atsumi, S. (2016). Cyanobacterial metabolic engineering for biofuel and chemical production. Curr. Opin. Chem. Biol. 35, 43–50. doi: 10.1016/j.cbpa.2016.08.023
Olson, J. M. (2006). Photosynthesis in the Archean era. Photosynth. Res. 88, 109–117. doi: 10.1007/s11120-006-9040-5
Ooms, M. D., Dinh, C. T., Sargent, E. H., and Sinton, D. (2016). Photon management for augmented photosynthesis. Nature Comm. 7, 12699. doi: 10.1038/ncomms12699
Oswald, W. J. (1988). “Large scale algal culture systems (engineering aspects),” in Microalgal Biotechnology, edS M. A. Borowitzka and L. J. Borowitzka (Cambridge: Cambridge University Press), 357–410.
Parry, M. A. J., Reynolds, M., Salvucci, M. E., Raines, C., Andralojc, P. J., Zhu, X. G., et al. (2011). Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 62, 453–467. doi: 10.1093/jxb/erq304
Parry, M. A. J., and Hawkesford, M. J. (2010). Food security: increasing yield and improving resource use efficiency. Proc. Nutr. Soc. 69, 592–600. doi: 10.1017/S0029665110003836
Pathak, J., Rajneesh, Pandey, A., Singh, S. P., and Sinha, R.P. (2017). “World agriculture and impact of biotechnology,” in Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production, eds S. K. Dubey, A. Pandey, and R. S. Sangwan (Radarweg; Amsterdam: Elsevier), 1–22.
Paul, D., and Nair, S. (2008). Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 48, 378–384. doi: 10.1002/jobm.200700365
Paumann, M., Regelsberger, G., Obinger, C., and Peschek, G. A. (2005). The bioenergetic role of dioxygen and the terminal oxidase(s) in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 1707, 231–253. doi: 10.1016/j.bbabio.2004.12.007
Pereira, I., Rodrigo, O., Barrientos, L., Moya, M., Reyes, G., and Kramm, V. (2009). Development of a biofertilizer based on filamentous nitrogen-fixing cyanobacteria for rice crops in Chile. J. Appl. Phycol. 21, 135–144. doi: 10.1007/s10811-008-9342-4
Pisciotta, J. M., Zou, Y., and Baskakov, I. V. (2010). Light-dependent electrogenic activity of cyanobacteria. PLoS ONE 5:e10821. doi: 10.1371/journal.pone.0010821
Prasanna, R., Sood, A., Suresh, A., and Kaushik, B. D. (2007). Potentials and applications of algal pigments in biology and industry. Acta. Bot. Hung. 49, 131–156. doi: 10.1556/ABot.49.2007.1-2.14
Prasanna, R., Joshi, M., Rana, A., Shivay, Y. S., and Nain, L. (2012). Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C-N sequestration in soil under rice crop. World J. Microbiol. Biotechnol. 28, 1223–1235. doi: 10.1007/s11274-011-0926-9
Prasanna, R., Sharma, E., Sharma, P., Kumar, A., Kumar, R., Gupta, V., et al. (2013). Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under nonflooded conditions. Paddy Water Environ. 11, 175–183. doi: 10.1007/s10333-011-0302-2
Pulz, O. (2001). Photobioreactors: production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 57, 287–293. doi: 10.1007/s002530100702
Pulz, O., and Gross, W. (2004). Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 65, 635–648. doi: 10.1007/s00253-004-1647-x
Pushparaj, B., Pelosi, E., Tredici, M. R., Pinzani, E., and Materassi, R. (1997). An integrated culture system for outdoor production of microalgae and cyanobacteria. J. Appl. Phycol. 9, 113–119. doi: 10.1023/A:1007988924153
Qiang, H., and Richmond, A. (1994). Optimizing the population density in Isochrysis galbana grown outdoors in a glass column photobioreactor. J. Appl. Phycol. 6, 391–396.
Quintana, N., Van der Kooy, F., Van de Rhee, M. D., Voshol, G. P., and Verpoorte, R. (2011). Renewable energy from cyanobacteria: energy production optimization by metabolic pathway engineering. Appl. Microb. Biotechnol. 91, 471–490. doi: 10.1007/s00253-011-3394-0
Rai, A. K., and Sharma, N. K. (2006). Phosphate metabolism in the cyanobacterium Anabaena doliolum under salt stress. Curr. Microbiol. 52, 6–12. doi: 10.1007/s00284-005-0043-9
Rai, A. N., Söderbäck, E., and Bergman, B. (2000). Cyanobacterium-plant symbioses. New Phytol. 147, 449–481. doi: 10.1046/j.1469-8137.2000.00720.x
Rajneesh, Singh, S. P., Pathak, J., and Sinha, R. P. (2017). Cyanobacterial factories for the production of green energy and value-added products: an integrated approach for economic viability. Renew. Sustain. Energy Rev. 69, 578–595. doi: 10.1016/j.rser.2016.11.110
Rastogi, R. P., and Sinha, R. P. (2009). Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 27, 521–539. doi: 10.1016/j.biotechadv.2009.04.009
Ratchford, I. A., and Fallowfield, H. J. (1992). Performance of a flatplate air-lift reactor for the growth of high biomass algal culture. J. Appl. Phycol. 4, 1–9. doi: 10.1007/BF00003954
Rittmann, B. E. (2008). Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 100, 203–212. doi: 10.1002/bit.21875
Robbins, W. J., Hervey, A., and Stebbins, M. (1951). Further observations on Euglena and B12. Bull. Torrey Bot. Club 86, 367–373.
Rodriguez, A. A., Stella, A. M., Storni, M. M., Zulpa, G., and Zaccaro, M. C. (2006). Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2:7. doi: 10.1186/1746-1448-2-7
Roger, P. A., and Kulasooriya, S. A. (1980). Blue-Green Algae and Rice. Manila: The International Rice Research Institute.
Rosenberg, J. N., Oyler, G. A., Wikinson, L., and Betenbaugh, M. J. (2008). A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 19, 430–436. doi: 10.1016/j.copbio.2008.07.008
Rossi, F., Li, H., Liu, Y., and De Philippis, R. (2017). Cyanobacterial inoculation (cyanobacterisation): perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth Sci. Rev. 171, 28–43. doi: 10.1016/j.earscirev.2017.05.006
Saddler, J. N. (1993). Bioconversion of Forest and Agricultural Plant Residues. Wallingford: CAB International, 394.
Sánchez, O., Diestra, E., Esteve, I., and Mas, J. (2005). Molecular characterization of an oil-degrading cyanobacterial consortium. Microb. Ecol. 50, 580–588. doi: 10.1007/s00248-005-5061-4
Sarma, M. K., Kaushik, S., and Goswami, P. (2016). Cyanobacteria: a metabolic power house for harvesting solar energy to produce bio-electricity and biofuels. Biomass. Bioenerg. 90, 187–201. doi: 10.1016/j.biombioe.2016.03.043
Sarsekeyeva, F., Zayadan, B. K., Usserbaeva, A., Bedbenov, V. S., Sinetova, M. A., and Los, D. A. (2015). Cyanofuels: biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 125, 329–340. doi: 10.1007/s11120-015-0103-3
Savakis, P., and Hellingwerf, K. J. (2014). Engineering cyanobacteria for direct biofuel production from CO2. Curr. Opin. Biotechnol. 33C, 8–14. doi: 10.1016/j.copbio.2014.09.007
Sharma, N. K., Tiwari, S. P., Tripathi, K., and Rai, A. K. (2011). Sustainability and cyanobacteria (blue-green algae): facts and challenges. J. Appl. Phycol. 23, 1059–1081. doi: 10.1007/s10811-010-9626-3
Shashirekha, S., Uma, L., and Subramanian, G. (1997). Phenol degradation by the marine cyanobacterium Phormidium valderianum BDU 30501. J. Ind. Microbiol. Biotechnol. 19, 130–133. doi: 10.1038/sj.jim.2900438
Shi, D.-J., and Hall, D. O. (1988). The Azolla-Anabaena association: historical perspective, symbiosis and energy metabolism. Bot. Rev. 54, 253–386. doi: 10.1007/BF02858416
Singh, J. S., Pandey, V. C., and Singh, D. P. (2011). Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Eco. Environ. 140, 339–353. doi: 10.1016/j.agee.2011.01.017
Singh, R. B. (2000). “Biotechnology, biodiversity and sustainable agriculture-a contradiction?” in Regional Conference in Agricultural Biotechnology Proceedings: Biotechnology Research and Policy -Needs and Priorities in the Context of Southeast Asia's Agricultural Activities, ed E. M. T. Mendoza (Bangkok: SEARCA (SEAMEO)/FAO /APSA).
Singh, R. N. (1950). Reclamation of usar soils in India through blue-green algae. Nature 165, 325–326. doi: 10.1038/165325b0
Singh, S., Kate, B. N., and Banerjee, U. C. (2005). Bioactive compounds from cyanobacteria and microalgae: an overview. Crit. Rev. Biotechnol. 25, 73–95. doi: 10.1080/07388550500248498
Singh, J. S. (2014). Cyanobacteria: a vital bio-agent in eco-restoration of degraded lands and sustainable agriculture. Clim. Change Environ. Sustain. 2, 133–137.
Singh, J. S., Kumar, A., Rai, A. N., and Singh, D. P. (2016). Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 7:529. doi: 10.3389/fmicb.2016.00529
Singh, R., Parihar, P., Singh, M., Bajguz, A., Kumar, J., Singh, S., et al. (2017). Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Front. Microbiol. 8:515. doi: 10.3389/fmicb.2017.00515
Singh, R. N. (1961). Role of Blue-Green Algae in Nitrogen Economy of Indian Agriculture. New Delhi: Indian Council Agric. Res.
Spektorova, L., Creswell, R. L., and Vaughan, D. (1997). Closed tubular cultivators. World Aquacult. 6, 39–43.
Spiller, H., and Gunasekaran, M. (1990). Ammonia-excreting mutant strain of the cyanobacterium Anabaena variabilis supports growth of wheat. Appl. Microbiol. Biotechnol. 33, 477–480. doi: 10.1007/BF00176670
Steinberg, C. E. W., Schäfer, H., and Beisker, W. (1998). Do acid-tolerant cyanobacteria exist? Acta Hydrochim. Hydrobiol. 26, 13–19.
Subashchandrabose, S. R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K., and Naidu, R. (2011). Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol. Adv. 29, 896–907. doi: 10.1016/j.biotechadv.2011.07.009
Subashchandrabose, S. R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K., and Naidu, R. (2013). Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 51, 59–72. doi: 10.1016/j.envint.2012.10.007
Subramanian, G., and Sundaram, S. S. (1986). Induced ammonia release by the nitrogen fixing cyanobacterium Anabaena. FEMS Microbiol. Lett. 37, 151–154. doi: 10.1111/j.1574-6968.1986.tb01784.x
Sutherland, I. W. (1998). Novel and established applications of microbial polysaccharides. Trends Biotechnol. 16, 41–46. doi: 10.1016/S0167-7799(97)01139-6
Swarnalakshmi, K., Prasanna, R., Kumar, A., Pattnaik, S., Chakravarty, K., Shivay, Y. S., et al. (2013). Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 55, 107–116. doi: 10.1016/j.ejsobi.2012.12.008
Switzer, L. (1981). Spirulina the Whole Food Revolution. Toronto, ON: Proteus Corporation Bantam Books
Tiedje, J. M. (1997). Environmental biotechnology. Curr. Opin. Biotechnol. 8, 267. doi: 10.1016/S0958-1669(97)80001-8
Ugwu, C. U., Aoyagi, H., and Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresour. Technol. 99, 4021–4028. doi: 10.1016/j.biortech.2007.01.046
Uma, L., and Subramanian, G. (1990). “Effective use of cyanobacteria in effluent treatment,” in Proceedings of the National Symposium on Cyanobacterial N2 Fixation (New Delhi: Indian Agric. Res. Inst.), 437–444.
Upadhyay, S. K., Singh, J. S., and Singh, D. P. (2011). Exopolysaccharide producing plant growth promoting rhizobacteria salinity condition. Pedosphere 21, 214–222. doi: 10.1016/S1002-0160(11)60120-3
Vaishampayan, A., Sinha, R. P., Gupta, A. K., and Häder, D.-P. (2000). A cyanobacterial recombination study, involving an efficient N2-fixing non-heterocystous partner. Microbiol. Res. 155, 1–5. doi: 10.1016/S0944-5013(00)80026-9
Vaishampayan, A., Sinha, R. P., Häder, D.-P., Dey, T., Gupta, A. K., Bhan, U., et al. (2001). Cyanobacterial biofertilizers in rice agriculture. Bot. Rev. 67, 453–516. doi: 10.1007/BF02857893
Valencia, A., and Walker, J. (1999). “A multi-axial treatment paradigm for mild traumatic brain injury to achieve reparative functional metaplasticity,” in 3rd World Congress on Brain Injury (Quebec City, QC: IBIA).
Van Veen, A., Overbeek, L. S., and van Elsas, J. D. (1997). Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 61, 21–135.
Vandamme, E. J., Bruggeman, G., De Baets, S., and Vanhooren, P. T. (1996). Useful polymers of microbial origin. Agro. Food Ind. Hi-Tech. 7, 21–26.
Venkataraman, G. S. (1972). Algal Biofertilizer and Rice Cultivation. New Delhi: Today and tomorrow's Printers and Publishers.
Venkataraman, G. S. (1993). “Blue-green algae (Cyanobacteria)” in Biological Nitrogen Fixation, eds S. N. Tata, A. M. Wadhwani, and M. S. Mehdi (New Delhi: Indian Council Agric. Res.), 45–76.
Verrecchia, E., Yair, A., Kidron, G. J., and Verrecchia, K. (1995). Physical properties of the psammophile cryptogamic crust and their consequences to the water regime of sandy soils, north-western Negev desert, Israel. J. Arid Environ. 29, 427–437. doi: 10.1016/S0140-1963(95)80015-8
Vijayakumar, S. (2012). Potential applications of cyanobacteria in industrial effluents-a review. J. Bioremed. Biodeg. 3, 154. doi: 10.4172/2155-6199.1000154
Vlad, M., Bordas, E., Caseanu, E., Uza, G., Creteanu, E., and Polinicenco, C. (1995). Effect of cuprofilin on experimental atherosclerosis. Biol. Trace Elem. Res. 48, 99–109. doi: 10.1007/BF02789082
Vonshak, A. (ed.). (1997). “Spirulina platensis (Arthrospira),” in Physiology, Cell Biology and Biotechnology (London: Taylor and Francis), 131–158.
Wang, B., Wang, J., Zhang, W., and Meldrum, D. R. (2012). Application of synthetic biology in cyanobacteria and algae. Front. Microbiol. 3:44. doi: 10.3389/fmicb.2012.00344
Watanabe, I., Espianas, C. R., Berja, N. S., and Alimagno, B. V. (1977). Utilization of the Azolla-Anabaena complex as a nitrogen fertilizer for rice. IRRI Res. Paper Ser. 11, 1–15.
Wase, N. V., and Wright, P. C. (2008). Systems biology of cyanobacterial secondary metabolite production and its role in drug discovery. Expert Opin. Drug Discov. 3, 903–929. doi: 10.1517/17460441.3.8.903
Williams, P. J., and Laurens, L. M. (2010). Microalgae as biodiesel and biomass feedstocks: review and analysis of the biochemistry, energetic and economics. Energy Environ. Sci. 3, 554–590. doi: 10.1039/b924978h
Wohlgeschaffen, G. D., SubbaRao, D. V., and Mann, K. H. (1992). Vat incubator with immersion core illumination-A new, inexpensive setup for mass phytoplankton culture. J. Appl. Phycol. 4, 25–29. doi: 10.1007/BF00003957
Keywords: biofertilizers, bioenergy, biotechnology, cyanobacterial farming, nutraceuticals, sustainable agricultural practices
Citation: Pathak J, Rajneesh, Maurya PK, Singh SP, Häder D-P and Sinha RP (2018) Cyanobacterial Farming for Environment Friendly Sustainable Agriculture Practices: Innovations and Perspectives. Front. Environ. Sci. 6:7. doi: 10.3389/fenvs.2018.00007
Received: 15 September 2017; Accepted: 25 January 2018;
Published: 28 February 2018.
Edited by:
Beni Lew, Agricultural Research Organization (Israel), IsraelReviewed by:
Anna De Marco, University of Naples Federico II, ItalyPawan K. Dadheech, Central University of Rajasthan, India
Gaohong Wang, Institute of Hydrobiology (CAS), China
Copyright © 2018 Pathak, Rajneesh, Maurya, Singh, Häder and Sinha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajeshwar P. Sinha, r.p.sinha@gmx.net; rpsinhabhu@gmail.com
 Jainendra Pathak
Jainendra Pathak Rajneesh
Rajneesh Pankaj K. Maurya2
Pankaj K. Maurya2  Shailendra P. Singh
Shailendra P. Singh Donat-P. Häder
Donat-P. Häder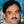 Rajeshwar P. Sinha
Rajeshwar P. Sinha