Mammal Diversity in Oil Palm Plantations and Forest Fragments in a Highly Modified Landscape in Southern Mexico
- 1Department of Biology, Wheaton College, Norton, MA, United States
- 2División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, Villahermosa, Mexico
Oil palm (Elaeis guineensis) plantations are one of the most rapidly expanding agroecosystems in the tropics, including Latin America. While many studies have demonstrated that large oil palm monocultures (>100 ha) are detrimental to biodiversity, including mammals, little is known about the impact of small-scale oil palm plantations, especially in the Neotropics. Here we used a camera trapping survey to compare species richness, community structure, and relative abundances of mid to large-bodied terrestrial mammals in small-scale oil palm plantations (<100 ha) and secondary forest fragments within a highly modified landscape mosaic in the southeastern lowlands of Tabasco, Mexico. Contrary to our expectations, we found no differences in the overall mammal communities between the oil palm and forest fragments, including species richness or mean relative abundance. Individual species showed some apparent differences in their total detections between the two habitats, with 11 having greater detections in forest than oil palm, and only two with greater detections in oil palm. Further, oil palm sites were more similar to one another in terms of mammal community structure than the secondary forest fragments. We found that shorter distance to forest patches was related to higher mammal species richness in both forest fragments and oil palm plantations. Twelve terrestrial mammal species known to occur in forested areas in the state of Tabasco were never detected in either vegetation type in our surveys, highlighting the fact that the mammal community in this landscape had already been reduced to those species most resilient to human disturbance. Our findings suggest that small-scale oil palm plantations in this region are used at least to some degree by most mammals that are also found in the remaining secondary forest fragments in this landscape, but that access to nearby forest is important for these species. In order to recover more of the original mammal community of the region and prevent further reductions in biodiversity, conservation priorities should center around reducing hunting pressure, allowing forest regeneration and increasing connectivity between protected areas and along waterways.
Introduction
Latin America, like most tropical regions, has experienced dramatic losses of native forest cover to agriculture over the last two centuries (Aide et al., 2013), particularly since the Green Revolution of the 1960s (Graesser et al., 2015). As agriculture expanded, the value of such new agroecosystems for wildlife and finding ways to integrate efficient agricultural production with biodiversity conservation have become increasingly important areas of research (Foley et al., 2007; Tscharntke et al., 2012; Railsback and Johnson, 2014). Some ecologists have gone so far as to suggest that the long-term future of most terrestrial biodiversity may depend largely on the answers to these questions (Balvanera et al., 2001; Rosenzweig, 2003; Armsworth et al., 2007). The landscape context (composition and configuration) of agroecosystems and native habitat is now understood to be one of the main determinants of species persistence at a local scale (Daily et al., 2003; Fischer et al., 2006; Lindenmayer et al., 2007; Begotti et al., 2018). However, there is still some controversy surrounding the best landscape management practices for maintaining biodiversity and ecosystem services; for example, the “land-sparing vs. land-sharing” debate (Fischer et al., 2014; Jiren et al., 2018).
Oil palm (Elaeis guineensis) plantations are one of the most rapidly expanding agroecosystems in the tropics, with more than 18 million ha already converted (Meijaard et al., 2018). Palm oil is now the world's most widely consumed vegetable oil, and is used in a wide array of processed foods, cosmetics and cleaning supplies, along with its potential as a biofuel (Furumo and Aide, 2017). While the majority of oil palm is currently grown in Southeast Asia, Latin America quickly caught on to this profitable crop, and palm oil output has doubled in the region since 2001 (Furumo and Aide, 2017). Further, Latin America has the largest remaining forested area in the world suitable for oil palm cultivation (Furumo and Aide, 2017). Some authors have suggested that conversion to oil palm may be among the major drivers of the next wave of habitat change in the Neotropical lowlands (Gutiérrez-Vélez and DeFries, 2013; Lees and Vieira, 2013). While the majority of studies on the impacts of oil palm plantations on biodiversity have taken place in Southeast Asia, recent work in Latin America shows similar patterns to those found in Asia in terms of major reductions in bird, mammal, amphibian and invertebrate species in oil palm compared to native forest (Gallmetzer and Schulze, 2015; Lees et al., 2015; Juen et al., 2016; Mendes-Oliveira et al., 2017; Pardo et al., 2018a). Further, oil palm plantations appear to be a barrier to movement for some species of forest birds, bats, and bees (Freudmann et al., 2015; Knowlton et al., 2017; Brito et al., 2018). By decreasing landscape permeability and population connectivity even for these highly mobile animals, oil palm may consequently diminish crucial ecosystem services (Freudmann et al., 2015).
The impacts of oil palm plantations on biodiversity in Latin America have mostly been studied in large-scale (>100 ha) plantations in the South American countries of Brazil (e.g., Lees et al., 2015; Mendes-Oliveira et al., 2017) and Colombia (e.g., Pardo et al., 2018a), while none to our knowledge have examined the smaller-scale expansion of oil palm in Mexico. Conversion of forest to oil palm plantation is likely to exacerbate the already severe impacts of habitat modification and clearance in Mexico, where 72% of primary vegetation coverage was lost or degraded by 2011. For instance, the state of Tabasco in the southeastern lowlands has lost all but a few tiny fragments of what were once extensive areas of rainforest and wetlands (Arriaga-Weiss et al., 2008). So far, natural habitats such as forests have largely been cleared for pasture and other types of plantation agriculture, driven by a combination of federal government policy and population growth (Tudela, 1989; Uribe Iniesta, 2016). As a consequence, mammal species that depend on forest or other woody vegetation have declined (Briones-Salas et al., 2015). Nevertheless, the state of Tabasco contains some of the most biodiverse ecosystems in this megadiverse country (Peterson and Navarro-Sigüenza, 2016). Beginning in the late-1980s, a combination of global demand and government incentives spurred many farmers to begin planting oil palm, primarily in areas that had previously been cleared for cattle or other row crops. This expansion of oil palm was driven by federal bioenergy and rural development policies (Aguilar-Gallegos et al., 2015) and feedstock demand for export to the global north (Pischke et al., 2018). The pattern of oil palm replacing pasture is importantly different from most Asian oil palm expansion, which has occurred in areas recently cleared of native primary and secondary forest (Koh and Wilcove, 2008; Furumo and Aide, 2017). Today, oil palm plantations continue to expand rapidly in southern Mexico, with approximately 76,000 ha already planted (SAGARPA, 2016) and close to 2.5 million ha deemed suitable for oil palm cultivation (INIFAP, 2006), which could supply roughly 30% of the total demand for transportation energy in the country via palm oil biodiesel (Lozada et al., 2010).
Mammals play important roles in food webs and provide many ecosystem services, including seed dispersal, pollination, predation, and decomposition (Pardo et al., 2018a). Only a few studies have examined the impact of oil palm plantations on mammals in Latin America (Mendes-Oliveira et al., 2017; Pardo et al., 2018a), and none to our knowledge has taken place in small-scale plantations or anywhere in Mexico. In this study, we used a camera trapping survey to examine how terrestrial mid to large-bodied mammals respond to a highly modified landscape mosaic in the southeastern lowlands of Tabasco, Mexico, including small (<100 ha) oil palm plantations and secondary forest fragments. We compared species composition and relative abundance of mammals between the oil palm plantations and the forest fragments to determine whether the plantations impact even the relatively adaptable species that persist in this highly disturbed landscape. We predicted that because of its different vegetation structure, reduced leaf litter, lack of understory vegetation and increased human presence, oil palm plantations would have fewer species and a different mammalian community composition than the secondary forest fragments. We also examined the influence of landscape structure on mammal species richness and abundance, in order to determine the best strategies for conserving mammals in this region. Finally, we discuss the implications of continued expansion of small-scale oil palm plantations for Neotropical mammals.
Materials and Methods
Study Area
We conducted this study in the southern part of the Mexican state of Tabasco, close to the border with Chiapas, in the municipalities of Teapa, Tacotalpa and Jalapa (17°13'40”N, 92°51'05” W; Figure 1). This area is called the Sierra Region, and the climate is hot, humid, and rainy, with the average temperature and precipitation ranging from 24 to 28°C and 2,500–4,000 mm, respectively (INEGI, 2015). The three main seasons are the cold fronts (November—February), dry (March–June), and wet (July–October). The landscape is composed of fragments of high and medium subperennifolia tropical forest, secondary vegetation, annual crops, and forest plantations and pastures, with the latter being the dominant matrix (Salazar Conde et al., 2004; Sánchez Munguía, 2005). In fact, more than 65% of the territory of the state of Tabasco is being used for agriculture or livestock (Briones-Salas et al., 2015), and currently has more than 20,000 ha planted with oil palm (SIAP, 2017).
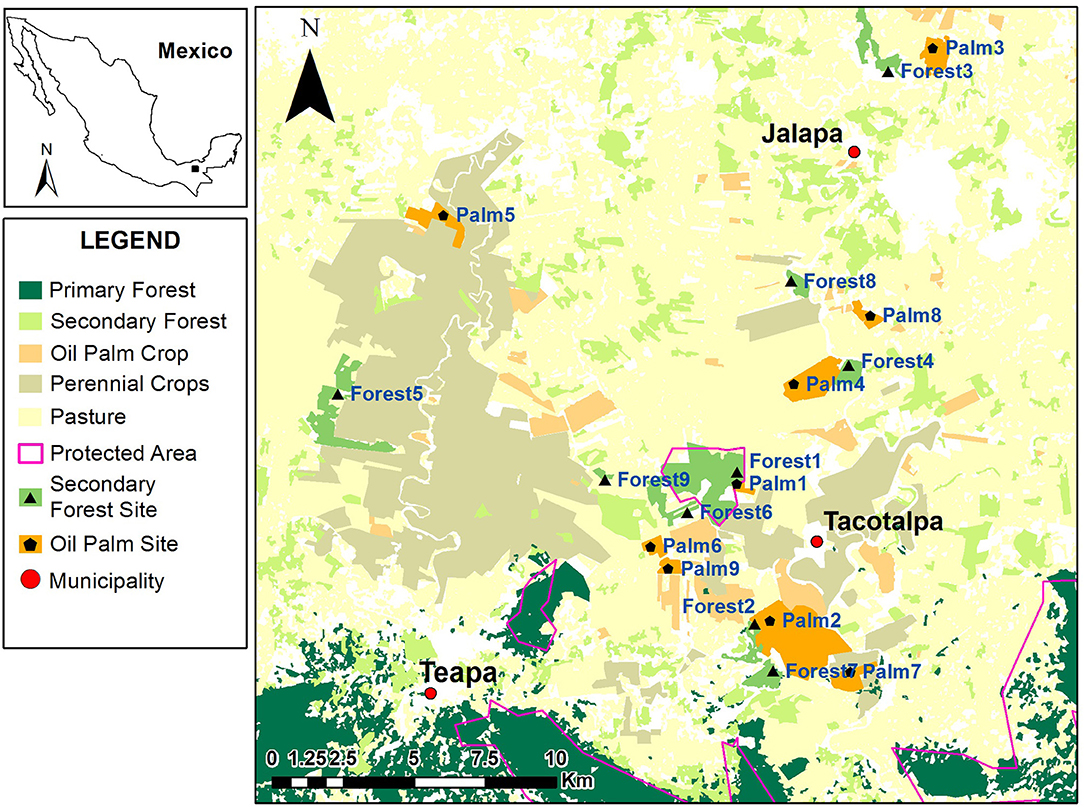
Figure 1. Study area, land use, and location of the nine secondary forest fragments and nine oil palm plantations where camera traps were installed to census the mammal communities.
Sampling Design
With the help of satellite images (2016) we identified and selected nine secondary forest fragments and nine mature oil palm plantations (>10 years old) for the mammal surveys (Figure 1). Each site was ≥1 km from any other. Within each of these sites we placed four camera traps (Browning Strike Force™ HD PRO, United States) ≥200 m from one another at a height of 0.5 m from the ground and secured with a cable lock. All cameras were configured to medium sensitivity and one-second intervals between consecutive photographs (four per trigger). Since we had a limited number of cameras, all sites were not sampled simultaneously, but rather surveys were organized sequentially in different sessions to cover the three main seasons of the region: cold fronts, dry, and wet (see above). The first sampling session took place from 15 December 2017 to 15 February 2018; the second from 19 April to 3 July 2018, and the third from 19 October to 17 December 2018. During each sampling session 24 cameras were active within six different sites: three oil palm plantations and three forest fragments. We believe detection probabilities were similar in both habitats because we were careful to place all cameras only in areas where there was an open view 3 m in front of the camera. The owners of the property and the oil palm plantations at each site gave us permission to conduct this research on their land.
To organize and extract the photograph metadata we used the package camtrapR in program R (R Development Core, 2014; Niedballa et al., 2016). Camera trap photographs were defined as independent if consecutive photos recorded one or more individuals of different species or one or more individuals of the same species over a minimum time interval of 30 min (Pardo et al., 2018a). Using these criteria, all photos defined as non-independent were excluded from subsequent analyses. We did not include any non-mammalian species in our analyses. We pooled the data derived from the four cameras at each site into a single sample, which was used as our sample unit for estimating species richness, composition, and relative abundance. We identified mammal species following Reid (2009) and using the most recent taxonomic classification of Mexican mammals (Ramírez-Pulido et al., 2014). This study did not involve the handling or manipulation of any animal species.
Landscape Variables
We used ArcGIS (10.1, ArcGIS, 2012) to quantify five landscape covariates that have been shown to affect mammal communities (Harvey et al., 2006): (1) Distance between each site (both oil palm and forest fragments sites) to the nearest forest fragment (m); (2) Size of each site (ha) (oil palm plantation or forest fragment); (3) Type of site (oil palm or forest fragment); (4) Distance between each site and the nearest road (m); (5) Distance between each site and the nearest town (m); and (6) Normalized Difference Vegetation Index (NDVI) of each site. All distances were calculated as the average Euclidean distance (m) to the nearest forest fragment, road, or town for all cameras within the site.
Statistical Analyses
We used capture frequencies of individual species as a proxy for relative abundance, calculated as the number of independent photographs divided by the sampling effort x 100 (Pardo et al., 2018a). Camera trap sampling effort is usually defined as the sum of the number of days that the cameras were active within each site (camera days) (e.g., Pardo et al., 2018a). The relative abundance (or habitat use) index is appropriate when the identification of individuals is impossible (Magurran and Henderson, 2011). However, this measure cannot be used as a direct measure of species' abundance or density (Wearn and Glover-Kapfer, 2017). We estimated (modeled) species richness of mammals in secondary forest fragments and oil palm plantations using the Chao 2 estimator (Magurran, 2010). We also calculated the effective number of species in each land use type to better compare the magnitude of difference between them (Moreno et al., 2011). To test for significant differences in observed total mammal species richness and relative abundance between forest fragments and oil palm plantations we used Mann-Whitney U-tests, since the data were not normally distributed. We calculated overall Shannon diversity and evenness values for mammals in forest fragments and oil palm plantations using the diversity function in the vegan package of program R (R Development Core, 2014; Oksanen et al., 2017). The evenness values were calculated as the Pielou index (J'), which is derived from the Shannon index. J' values range from 0.0 to 1.0, with higher values representing more even species distributions.
We used Poisson generalized linear models (GLM) with log link using the biodiversity module of program R (R Development Core, 2014) to test the impact of landscape variables on observed species richness and relative abundance. The most parsimonious model was selected by eliminating non-significant variables one by one and verifying the differences between models using ANOVAS with a Chi-squared post-hoc test (Crawley, 2007). These tests were performed in Software R v 3.6.1 (R Development Core, 2014). To examine overall differences in the structure and composition of the mammal communities in forest fragments and oil palm plantations we used a Non-metric Multidimensional Scaling (NMDS) ordination based on a Bray Curtis dissimilarity matrix. The NMDS uses rank orders to evaluate dissimilarities between different communities instead of absolute distances, with “stress” being a measure of the final lack of agreement (McCune and Grace, 2002). We then used an Analysis of Similarity (ANOSIM), a non-parametric test of significant difference between two or more groups (Clarke, 1993), to examine species assemblage similarity between the forest fragments and oil palm plantations. Finally, we used Similarity Percentages Analysis (SIMPER) to determine the contribution of each species to the overall observed similarity between forest fragments and oil palm plantations. These multivariate analyses were conducted using PAST software (Hammer et al., 2001).
Results
Over a sampling period of 4,272 camera days (178 days × 24 cameras), we registered a total of 2,204 independent capture events that included 2,276 mammal sightings of 16 species belonging to seven orders and 12 families (Table 1). Apart from these species, we captured photographs of small rodents, however they were too small to identify and were not included in our analysis. During sampling we also heard Mantled Howler Monkeys (Allouatta palliata) several times, but only in the secondary forest fragments. Since we did not capture any photos of this species it was not included in our results. In agreement with the NORMA Official Mexicana NOM-059-SEMARNAT-2010 of environmental protection of wild native species of flora and fauna of México (SEMARNAT, 2010), five of the registered species are in some type of risk category: Collared Peccary (Dicotyles crassus), Northern Tamandua (Tamandua mexicana), Ocelot (Leopardus pardalis), Jaguarundi (Puma yagouaroundi), and Greater Grison (Galictis vitata); and all but the Greater Grison were observed more frequently in forest fragments than in oil palm (Table 1).
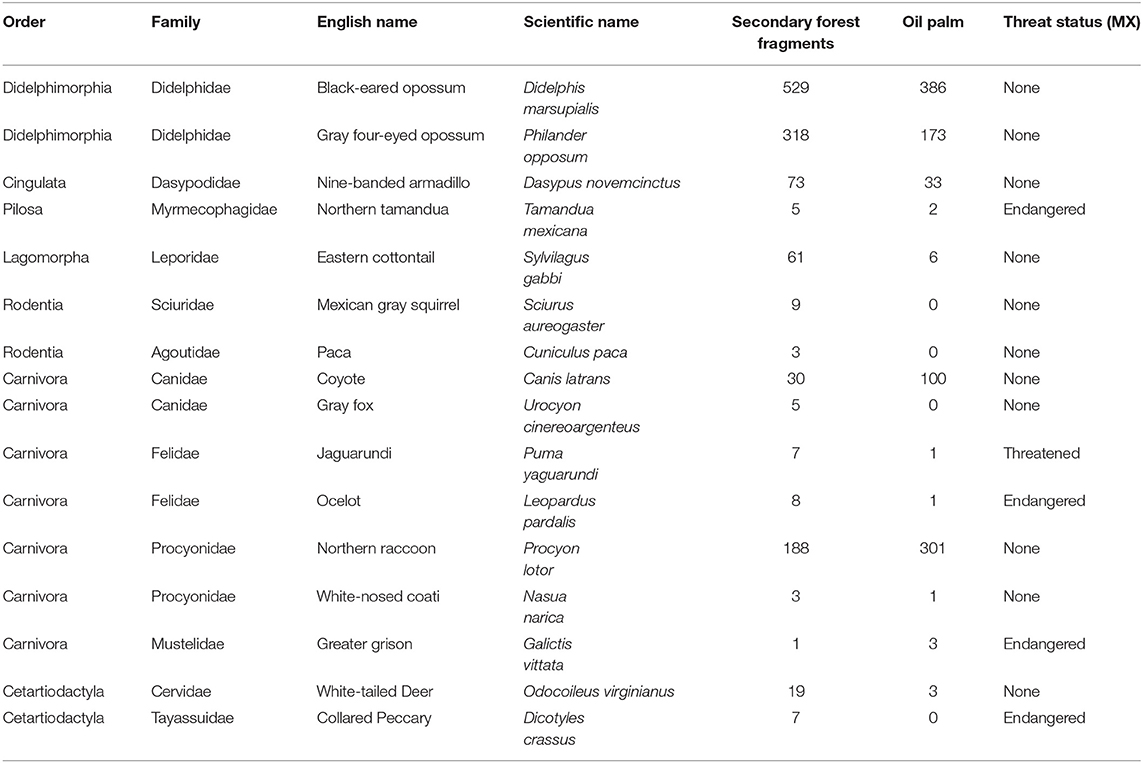
Table 1. Terrestrial mammal species detected by camera trapping surveys (December 2017–December 2018) in secondary forest fragments and oil palm plantations in Tabasco, Mexico.
The overall observed species richness of mammals appeared greater in forest fragments (16 species, mean 8.11 ± 0.63) than in oil palm (12 species, mean 5.56 ± 0.81. However, these differences were not significant (U = 80.5, p = 0.076). Estimated Chao2 species richness was 21.66 for the study region. Shannon diversity and evenness did not differ between the two vegetation types (Figure 2). Of the 16 species recorded in forest fragments, four were not recorded in the oil palm plantations, while there were no species recorded in oil palm that were not also recorded in forest (Table 1). The estimated effective number of species ranged from 2.25 to 7.66 in forest fragments and from 2.19 to 4.49 in oil palm plantations. Detection frequencies were low for most species, and there were no significant differences in mean detection frequencies of any individual species between the secondary forest fragments and the oil palm plantations (Figure 3).
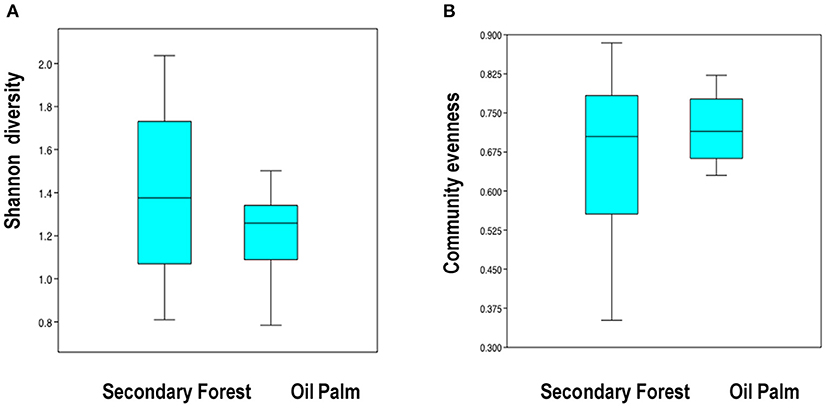
Figure 2. Box plots comparing mammal community Shannon diversity (A) and evenness (B) between the secondary forest fragments and oil palm plantations in Tabasco, Mexico.
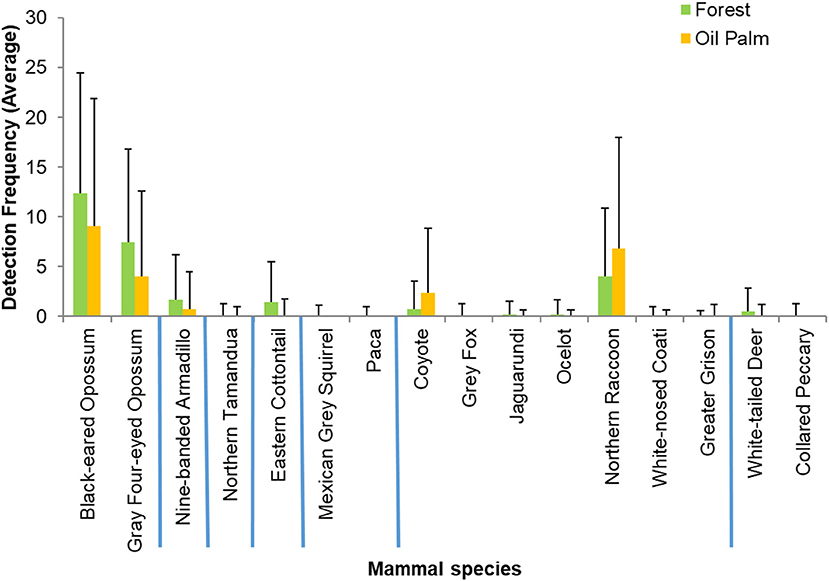
Figure 3. Mean relative abundance of 16 terrestrial mammal species detected in secondary forest fragments and oil palm plantations in Tabasco, Mexico. Bars indicate standard error. Blue lines separate taxonomic orders (from left to right): Didelphimorphia, Cingulata, Pilosa, Lagomorpha, Rodentia, Carnivora, and Cetartiodactyla.
The results of the GLM showed that species richness of mammals was influenced by the distance to the nearest forest, but not any other variables in the model (Table 2). The abundance of mammals was not influenced by any of the variables used in the GLM (Table 2). The NMDS ordination analysis showed nearly complete overlap in the species composition and abundances of mammals between forest fragments and oil palm plantations (Figure 4). Oil palm plantation mammal communities represented a nested subset of the forest fragment communities, with oil palm plantation sites more closely grouped together (i.e., more similar) compared to forest sites (Figure 4). Analysis of Similarity (ANOSIM) indicated no significant difference in the overall mammal community between forest fragments and oil palm plantations (R = 0.061, p = 0.175). Similarity Percentages Analysis (SIMPER) identified three species that contributed most to the difference in the composition of species between forest fragments and oil palm plantations: Black-eared Opossum (Didelphis marsupialis), Northern Racoon (Procyon lotor), and Gray Four-eyed Opossum (Philander opossum) (Table 3).
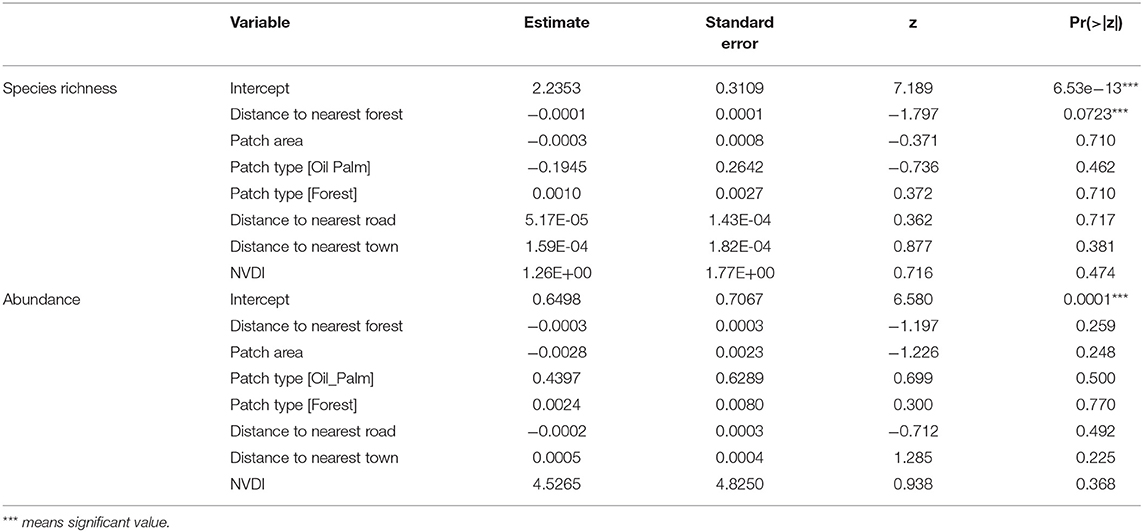
Table 2. Results of Poisson generalized linear models relating mammal species richness and abundance to landscape variables in Tabasco, Mexico.
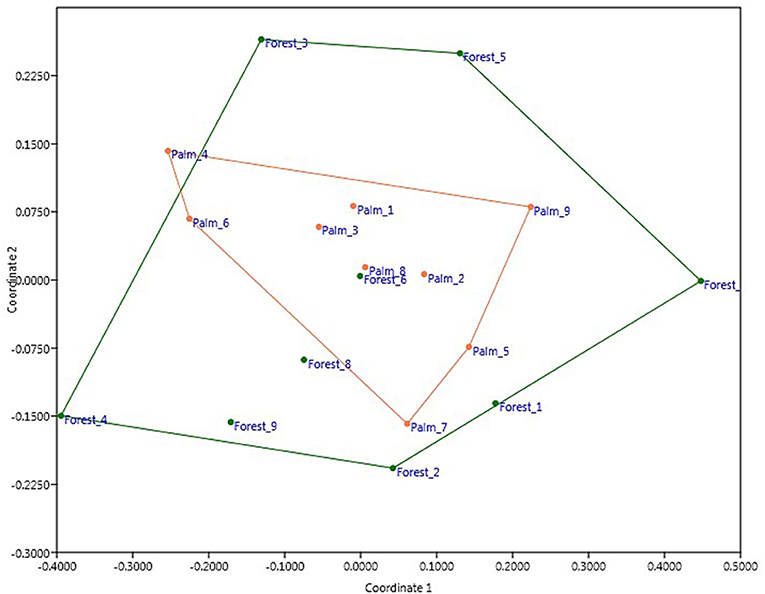
Figure 4. Overall mammal community composition across surveyed sites in oil palm plantations (orange dots) and secondary forest fragments (green dots). Plot is based on capture frequencies of species using Bray-Curtis non-metric multidimensional analysis (NMDS) (stress = 0.17). Polygons connect the vertices of each cover type.
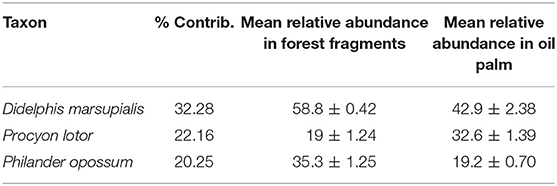
Table 3. Species that contributed most to the difference in the composition of the mammal community between secondary forest fragments and oil palm plantations, based on Similarity Percentages Analysis (SIMPER).
Discussion
In this study we used a camera trapping survey to examine how mid to large-bodied terrestrial mammals responded to a highly modified landscape mosaic in the southeastern lowlands of Tabasco, Mexico, specifically focusing on oil palm plantations and secondary forest fragments. Contrary to our expectations, we found no differences in the overall mammal communities between the oil palm and forest fragments, including species richness or relative abundance. Individual species appeared to show some differences in their total (but not average) detections between the two habitats, with 11 having greater detections in forest than oil palm, and only two with greater detections in oil palm. Further, oil palm sites were more similar to one another in terms of mammal community structure than the secondary forest fragments. The distance of each patch (oil palm or forest fragment) to the nearest forest fragment influenced mammal species richness more than the cover type of the patch. Our findings suggest that small-scale oil palm plantations (<100 ha) in this region are used by a similar mammal community to secondary forest fragments for most species, at least those mammals that had already been able to persist within a highly modified landscape. Because of this, these oil palm plantations are likely a better alternative for the remaining biodiversity of the region than more intensive land uses such as banana or soy plantations (Donald, 2004; Harvey et al., 2006). However, based on our results, maintaining as much forest in the landscape as possible is clearly key to allowing these mammals to persist in the region. These species likely use the oil palm plantations to some degree, but need nearby forest as a refuge or for some components of their life history.
Our results differ somewhat from other studies of oil palm impacts on mammal communities in Latin America, mostly likely due to the differences in plantation sizes. In both Colombia and Brazil, where oil palm plantations are large (>100 ha), species richness and composition of mammals was significantly reduced in oil palm plantations compared to native forests (Mendes-Oliveira et al., 2017; Pardo et al., 2018a). In terms of mammal community structure, in Colombia the riparian fragments had species compositions that were more similar to one another than the oil palm plantations, and there was little overlap in the structure of the two communities (Pardo et al., 2018a). In Brazil there was also very little overlap in the structure of the mammal communities between oil palm plantations and native forest, but this was in part due to the presence of arboreal species in the forest, which we were unable to census in this study (Mendes-Oliveira et al., 2017). Further, in the Brazilian study, 87% of all mammals found in oil palm were never farther than 1.3 km from the forest edge (Mendes-Oliveira et al., 2017). Given that the oil palm plantations in our study were <100 ha, we probably observed some mammal species using the plantations that would not be found in the core of larger plantations. The fact that mammal species richness was positively influenced by nearby forest in our study adds weight to this speculation. The landscape is still a mosaic of forest patches, cattle pastures, and plantations, and our results show how important those remaining forest patches are to the regional mammal community. Our results also underscore the fact that southern Mexico was already highly transformed by agriculture, hunting, and other human activities before the oil palm was planted, and thus the regional mammal community had likely been reduced to species that could adapt to these changes (Prugh et al., 2008; Pardo et al., 2018b). Further, subsistence hunting of mammals still commonly occurs in our study landscape, especially in the secondary forest fragments where access is less restricted than the oil palm plantations, and hunting dogs and feral dogs are common in both land uses. If we had been able to compare mammal communities in the oil palm plantations with a more pristine forest baseline, we would have likely found a much greater contrast in community structure, especially if we were comparing it to industrial-scale (>100 ha) plantations.
Oil palm plantations are less structurally complex than forest, and generally have reduced leaf litter, dead wood, and understory vegetation (Chung et al., 2000; Fayle et al., 2010). This may help to explain why there appeared to be fewer ungulates (White-tailed Deer and Collared Peccary), large rodents (Paca), possums (Black-eared Opossum and Gray Four-eyed Opossum), rabbits (Eastern Cottontail), and armadillos (Nine-banded Armadillo) in oil palm than in secondary forest fragments, even though most of these species are still hunted in the forest fragments by local community members. These results are similar to (but less extreme) than those found in the Amazonian region of Brazil (Mendes-Oliveira et al., 2017) and the Colombian llanos (Pardo et al., 2018a). In our field site, peccary and deer have been implicated in damage to local bean and other subsistence crops, especially those close to forest fragments (Méndez and Bello-Gutiérrez, 2005; Gallegos-Peña et al., 2010), and thus these species are likely persecuted for this reason as well.
Three mesopredator species, which included two felids (Jaguarundi and Ocelot) and a canid (Gray Fox), had more total detections in forest fragments than oil palm; despite oil palm having high densities of small rodents, which consume oil palm fruits (Rajaratnam et al., 2007; Jennings et al., 2015; Mendes-Oliveira et al., 2017). However, detection rates were very low for these species in general, and the differences were not significant. The only species that appeared to have a higher detection in oil palm than in forest in our study were Northern Raccoon and Coyote, and these species both had high detection rates (>100 photos). Northern Racoon abundances explained most of the pattern of community differences between the sites. Northern Raccoon and Coyote are omnivorous mesopredators that are known to be disturbance-tolerant habitat generalists (Pardo et al., 2016). The absence of apex predators (i.e., Jaguar and Puma) in the landscape likely allows for this “mesopredator release,” or high abundance of medium-sized carnivores (Soule et al., 1988), in both oil palm and forest fragments. Coyotes have recently expanded their range south and into more tropical forests, likely aided by forest fragmentation, agriculture, the extirpation of larger predators and hybridization with domestic dogs (Hody and Kays, 2018). Coyote presence could exert unpredictable impacts on local fauna, including native mesopredators and prey, through predation and indirect effects (Cove et al., 2012; Pardo et al., 2016). Overall, our mesopredator results differ from the Colombia and Brazil studies, both of which found similar abundances of mesopredators in oil palm and forest fragments (Mendes-Oliveira et al., 2017; Pardo et al., 2018a). Raccoons and grison were also more abundant or equally abundant in oil palm and forest in Brazil and Colombia (Mendes-Oliveira et al., 2017; Pardo et al., 2018a).
Terrestrial mammal species that have been recorded in the state of Tabasco but were absent from our surveys include the Central American Agouti (Dasyprocta punctata), Margay (Leopardus wiedii), Puma (Puma concolor), Jaguar (Panthera onca), Striped Hog-nosed Skunk (Conepatus semistriatus), Southern Spotted Skunk (Spilogale angustifrons), Water opossum (Chironectes minimus), Long-tailed Weasel (Mustela frenata), Tayra (Eira barbara), White-lipped Peccary (Tayassu pecari), Red Brocket Deer (Mazama temama), and Baird's Tapir (Tapirella bairdii) (Briones-Salas et al., 2015; Hidalgo-Mihart et al., 2017). The absence of these species suggests that even the relatively high density of forest remnants and low intensity of oil palm cultivation in this landscape is insufficient to support them against a backdrop of other human pressures. For instance, agouti, peccary, deer, and tapir are intensely hunted by humans for food and because they damage crops, which probably explains their absence in this landscape. For instance, in Colombia, agouti were never observed in oil palm plantations but were seen in riparian forests (Pardo et al., 2018a), and in Brazil agouti were seen much more often in primary forest than in oil palm plantations (Mendes-Oliveira et al., 2017), perhaps because hunting pressure from humans is lower in those areas. The felids and the tapir have large home ranges and are vulnerable to habitat loss and fragmentation, as well as poaching by humans (Morato et al., 2016; Naranjo, 2018). Diseases transmitted to wild mammals by domestic and feral animals could also be responsible for the decline and absence of many of these species in our study landscape. Pollution, fragmentation, alteration of habitat, introduction of exotic species, and climate change, among other factors, increase the risk of exposure and transmission of infectious diseases to wildlife (Briones-Salas et al., 2015).
Several recent studies on the impacts of oil palm plantations on bird and bee communities in the same landscape of Tabasco, Mexico, found that both the diversity and abundance of these groups were greater even in adjacent cattle pasture than in the oil palm plantations (Moreno Jiménez et al., 2017; Moo Culebro et al., n.d.). These results most likely stem from the presence of native tree species within the pastures, including living fences, and their proximity to forests remnants and wetlands (Moo Culebro et al., n.d.). It would be interesting to determine if the same pattern holds for mammals, although it is likely that mammals of this region are more sensitive to forest cover and would come into conflict with humans and livestock in the open pasture areas. However, there might also be a regional pool of open country/grassland species which invade and complement forest species that can survive in pastures—but that oil palm provides a harsher environment which these species cannot utilize. In terms of landscape-level forest cover, many studies estimate that between 25 and 45% of a landscape should remain forested in order to conserve the majority of Neotropical bird and mammal species (Andrén, 1994; Estavillo et al., 2013; Ochoa-Quintero et al., 2015; Pardo et al., 2018b). While our study landscape has this minimum forest coverage, the forest is young and very fragmented, and is under continuous pressure from human hunters and domestic and feral animals. Yet, we found that shorter distances of patches to forest cover had a positive influence on mammal species richness. In order to recover more of the original mammal community and population sizes of the region these human activities must be curbed, and the forest allowed to regenerate. Forested corridors between fragments, ideally along riparian areas, would allow terrestrial mammals (and other species) to move throughout the landscape more safely (Knowlton et al., 2017; Pardo et al., 2018a). The state of Tabasco became part of the Mesoamerican Biological Corridor in 2009, with the aim of connecting different protected areas and increasing conservation, management, and sustainable use of natural resources within them (Briones-Salas et al., 2015). This initiative should include efforts to increase the habitat connectivity of the entire region by specifying a requirement for forested buffers along all watercourses and increasing the enforcement of hunting laws.
Conclusions
In this study we showed that small oil palm plantations and secondary forests are both used to some degree by the mammal communities persisting in the highly-modified landscape of Tabasco, Mexico. We found that minimum distances to forest patches was related to higher mammal species richness in both forest fragments and oil palm plantations. The fact that 12 terrestrial mammal species recorded in the state were never detected in our surveys illustrates that neither secondary forest fragments nor oil palm plantations are appropriate habitat for mammals sensitive to human disturbance. The top predators were absent, and the most abundant species were mid-sized omnivorous habitat generalists that are known to prosper around human settlements. To restore the full complement of species, conservation priorities must center around reducing hunting pressure, allowing forest regeneration, and increasing connectivity between protected areas. Our study has several limitations, most notably the small sample size and low detection rates of most mammal species. In the future the mammal surveys could be supplemented with other techniques, such as nighttime line transects to search for arboreal species and daytime surveys for scat and tracks. Interviews with local community members, hunters and plantation owners could also provide additional information on the mammal species found in each habitat. However, if Mexico were to adopt laws similar to those in Brazil, requiring all landowners to keep a portion of their land as natural habitat and maintain riparian forest buffers, it would likely be of great benefit to mammal and other biodiversity conservation in the region.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
This study was designed by JK, EM, and AR. Field work was conducted by JK, EM, AR, BV-C, and RC-T. EM, BV-C, and RC-T analyzed the data. JK wrote the paper.
Funding
This research was funded by the National Science Foundation Partnerships in International Research and Education (Award No. 124344) and Wheaton College, Massachusetts.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Marco Antonio Torrez, Hilda Díaz-López, Isaac Brindis Santos, Victorio Moreno Jiménez, Elías J. Gordillo-Chavez, Heidi Jiménez-Pérez, and Yareni Itzel López-Bustamante for fieldwork assistance. We also thank Samuel Oporto for help with the data analysis. Universidad Juárez Autónoma de Tabasco provided vehicles.
References
Aguilar-Gallegos, N., Muñoz-Rodríguez, M., Santoyo-Cortés, H., Aguilar-Ávila, J., and Klerkx, L. (2015). Information networks that generate economic value: A study on clusters of adopters of new or improved technologies and practices among oil palm growers in Mexico. Agric. Syst. 135, 122–132. doi: 10.1016/j.agsy.2015.01.003
Aide, T. M., Clark, M. L., Grau, H. R., López-Carr, D., Levy, M. A., Redo, D., et al. (2013). Deforestation and reforestation of latin america and the caribbean (2001–2010). Biotropica 45, 262–271. doi: 10.1111/j.1744-7429.2012.00908.x
Andrén, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355. doi: 10.2307/3545823
ArcGIS [GIS software] (2012). Version 10.1. Redlands, CA: Environmental Systems Research Institute, Inc.
Armsworth, P. R., Chan, K. M. A., Daily, G. C., Ehrlich, P. R., Kremen, C., Ricketts, T. H., et al. (2007). Ecosystem-service science and the way forward for conservation. Conserv. Biol. 21, 1383–1384. doi: 10.1111/j.1523-1739.2007.00821.x
Arriaga-Weiss, S. L., Calmé, S., and Kampichler, C. (2008). Bird communities in rainforest fragments: guild responses to habitat variables in Tabasco, Mexico. Biodivers. Conserv. 17, 173–190. doi: 10.1007/s10531-007-9238-7
Balvanera, P., Daily, G. C., Ehrlich, P. R., Ricketts, T. H., Bailey, S. A., Kark, C., et al. (2001). Conserving biodiversity and ecosystem services. Science 291:2047. doi: 10.1126/science.291.5511.2047
Begotti, R. A., dos Pacífico, E. S., de Ferraz, S. F. B., and Galetti, M. (2018). Landscape context of plantation forests in the conservation of tropical mammals. J. Nat. Conserv. 41, 97–105. doi: 10.1016/j.jnc.2017.11.009
Briones-Salas, M., Hortelano-Moncada, Y., Magaña-Cota, G., Sánchez-Rojas, G., and Sosa-Escalante, J. (2015). Riqueza y conservación de los mamíferos en México a nivel estatal. Asociación Mexicana de Mastozoología A.C. Universidad Nacional Autónoma de México Universidad de Guanajuato 1.
Brito, T. F., Contrera, F. A. L., Phifer, C. C., Knowlton, J. L., Brasil, L. S., Maués, M. M., et al. (2018). Effects of habitat type change on taxonomic and functional composition of orchid bees (Apidae: Euglossini) in the Brazilian Amazon. J. Insect Conserv. 22, 451–463. doi: 10.1007/s10841-018-0073-9
Chung, A. Y. C., Eggleton, P., Speight, M. R., Hammond, P. M., and Chey, V. K. (2000). The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bull. Entomol. Res. 90, 475–496. doi: 10.1017/S0007485300000602
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Cove, M. V., Pardo Valladares, L. E., Spínola, R. M., Jackson, V. L., and Joel, C. (2012). Coyote canis latrans (Carnivora: Canidae) range extension in northeastern costa rica: possible explanations and consequences. Revista Latinoamericana De Conser. 2, 82–86.
Daily, G. C., Ceballos, G., Pacheco, J., Suzan, G., and Sanchez-Azofeita, A. (2003). Countryside biogeography of neotropical mammals: conservation opportunities in agricultural landscapes of costa rica. Conserv. Biol. 17, 1814–1826. doi: 10.1111/j.1523-1739.2003.00298.x
Donald, P. F. (2004). Biodiversity impacts of some agricultural commodity production systems. Conserv. Biol. 18, 17–38. doi: 10.1111/j.1523-1739.2004.01803.x
Estavillo, C., Pardini, R., and da Rocha, P. L. B. (2013). Forest loss and the biodiversity threshold: an evaluation considering species habitat requirements and the use of matrix habitats. PLoS ONE 8:e82369. doi: 10.1371/journal.pone.0082369
Fayle, T. M., Turner, E. C., Snaddon, J. L., Chey, V. K., Chung, A. Y. C., Eggleton, P., et al. (2010). Oil palm expansion into rain forest greatly reduces ant biodiversity in canopy, epiphytes and leaf-litter. Basic Appl. Ecol. 11, 337–345. doi: 10.1016/j.baae.2009.12.009
Fischer, J., Abson, D. J., Butsic, V., Chappell, M. J., Ekroos, J., Hanspach, J., et al. (2014). Land sparing versus land sharing: moving forward. Conserv. Lett. 7, 149–157. doi: 10.1111/conl.12084
Fischer, J., Lindenmayer, D. B., and Manning, A. D. (2006). Biodiversity, ecosystem function, and resilience: ten guiding principles for commodity production landscapes. Front. Ecol. Environ. 4, 80–86. doi: 10.1890/1540-9295(2006)004[0080:BEFART]2.0.CO;2
Foley, J. A., Monfreda, C., Ramankutty, N., and Zaks, D. (2007). Our share of the planetary pie. Proc. Natl. Acad. Sci. U.S.A. 104, 12585–12586. doi: 10.1073/pnas.0705190104
Freudmann, A., Mollik, P., Tschapka, M., and Schulze, C. H. (2015). Impacts of oil palm agriculture on phyllostomid bat assemblages. Biodivers. Conserv. 24, 3583–3599. doi: 10.1007/s10531-015-1021-6
Furumo, P. R., and Aide, T. M. (2017). Characterizing commercial oil palm expansion in Latin America: land use change and trade. Environ. Res. Lett. 12:1–12. doi: 10.1088/1748-9326/aa5892
Gallegos-Peña, A., Bello-Gutiérrez, J., and Jesús de la Cruz, A. (2010). “Cuantificación del daño ocasionado por mamíferos terrestres a cultivos de maíz en el ejido Oxolotán del municipio de Tacotalpa, Tabasco,” in Uso y manejo de fauna silvestre en el norte de Mesoamérica, eds M. M. Guerra-Roa, S. Calmé, S. Gallina-Tessaro and E. J. Naranjo-Piñera (Xalapa: Secretaría de Educación del Estado de Veracruz, 297–314.
Gallmetzer, N., and Schulze, C. H. (2015). Impact of oil palm agriculture on understory amphibians and reptiles: a mesoamerican perspective. Global Ecol. Conserv. 4, 95–109. doi: 10.1016/j.gecco.2015.05.008
Graesser, J., Aide, T. M., Grau, H. R., and Ramankutty, N. (2015). Cropland/pastureland dynamics and the slowdown of deforestation in Latin America. Environ. Res. Lett. 10:034017. doi: 10.1088/1748-9326/10/3/034017
Gutiérrez-Vélez, V. H., and DeFries, R. (2013). Annual multi-resolution detection of land cover conversion to oil palm in the Peruvian Amazon. Remote Sens. Environ. 129, 154–167. doi: 10.1016/j.rse.2012.10.033
Hammer, Ø., Harper, D. A. T., and Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9. Available online at: http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Harvey, C. A., Gonzalez, J., and Somarriba, E. (2006). Dung beetle and terrestrial mammal diversity in forests, indigenous agroforestry systems and plantain monocultures in talamanca, costa rica. Biodiver. Conserv. 15:555. doi: 10.1007/s10531-005-2088-2
Hidalgo-Mihart, M. G., Contreras-Moreno, F. M., Jesús-De La Cruz, A., Juárez-López, R., Bravata De La Cruz, Y., Pérez-Solano, L. A., et al. (2017). Inventory of Medium-Sized And Large Mammals in the Wetlands of Laguna de Terminos and Pantanos de Centla. Mexico.
Hody, J. W., and Kays, R. (2018). Mapping the expansion of coyotes (Canis latrans) across North and Central America. ZooKeys 759, 81–97. doi: 10.3897/zookeys.759.15149
INIFAP (2006). Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Available online at: http://www.inifap.gob.mx/ (accessed January 15, 2019).
Jennings, A. P., Naim, M., Advento, A. D., Aryawan, A. A. K., Ps, S., Caliman, J.-P., et al. (2015). Diversity and occupancy of small carnivores within oil palm plantations in central Sumatra, Indonesia. Mam. Res. 60, 181–188. doi: 10.1007/s13364-015-0217-1
Jiren, T. S., Dorresteijn, I., Schultner, J., and Fischer, J. (2018). The governance of land use strategies: Institutional and social dimensions of land sparing and land sharing. Conserv. Lett. 11:e12429. doi: 10.1111/conl.12429
Juen, L., Cunha, E. J., Carvalho, F. G., Ferreira, M. C., Begot, T. O., Andrade, A. L., et al. (2016). Effects of oil palm plantations on the habitat structure and biota of streams in eastern amazon. River Res. Appl. 32, 2081–2094. doi: 10.1002/rra.3050
Knowlton, J. L., Phifer, C. C., Cerqueira, P. V., de Barro, F. C., Oliveira, S. L., Fiser, C. M., et al. (2017). Oil palm plantations affect movement behavior of a key member of mixed-species flocks of forest birds in Amazonia, Brazil. Trop. Conserv. Sci. 10:194008291769280. doi: 10.1177/1940082917692800
Koh, L. P., and Wilcove, D. S. (2008). Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 1, 60–64. doi: 10.1111/j.1755-263X.2008.00011.x
Lees, A. C., Moura, N. G., de Almeida, A. S., and Vieira, I. C. G. (2015). Poor prospects for avian biodiversity in amazonian oil palm. PLoS ONE 10:e0122432. doi: 10.1371/journal.pone.0122432
Lees, A. C., and Vieira, I. C. G. (2013). Forests: oil-palm concerns in Brazilian Amazon. Nature 497, 188–188. doi: 10.1038/497188c
Lindenmayer, D., Hobbs, R. J., Montague-Drake, R., Alexandra, J., Bennett, A., Burgman, M., et al. (2007). A checklist for ecological management of landscapes for conservation. Ecol. Lett. 11, 78–91. doi: 10.1111/j.1461-0248.2007.01114.x
Lozada, I., Islas, J., and Grande, G. (2010). Environmental and economic feasibility of palm oil biodiesel in the Mexican transportation sector. Renew. Sust. Energy Rev. 14, 486–492. doi: 10.1016/j.rser.2009.06.034
Magurran, A., and Henderson, P. (2011). “Commonnes and rarity,” in Biological Diversity: Frontiers in Measurement and Assessment, eds A. E. Magurran and B. J. McGil (Oxford: Oxford University Press, 97–105.
Magurran, A. E. (2010). “Measuring biological diversity in time (and space),” in Biological Diversity: Frontiers in Measurement and Assessment, eds A. E. Magurran and B. J. McGil (Oxford: Oxford University Press).
McCune, B., and Grace, J. (2002). Analysis of Ecological Communities. Gleneden Beach, OR: MjM Software Design.
Meijaard, E., Garcia-Ulloa, J., Sheil, D., Wish, D. A., Carlson, K. M., Juffe-Bignoli, D., et al. (2018). Oil Palm and Biodiversity. Gland: IUCN Oil Palm Task Force International Union For Conservation Of Nature.
Mendes-Oliveira, A. C., Peres, C. A., de Maués, P. C. R., Oliveira, G. L., Mineiro, I. G., Silva de Maria, S. L., et al. (2017). Oil palm monoculture induces drastic erosion of an Amazonian forest mammal fauna. PLoS ONE 12:e0187650. doi: 10.1371/journal.pone.0187650
Méndez, I., and Bello-Gutiérrez, J. (2005). “Impacto de mamíferos silvestres en cultivos de frijol en el ejido Agua Blanca, Tacotalpa, Tabasco, México,”. in Memorias del XXII Simposio de Fauna Silvestre Gral. M. V. Manuel Cabrera Valtierra, eds P. Mejía-Gutiérrez, D. Díaz-Güemez, and P. R. Pescador-Cano (Distrito Federal: Universidad Nacional Autónoma de México, 41–50
Moo Culebro, L. Y., Knowlton, J. L., Flaspohler, D. J., Arriaga Weiss, S. L., and Mata Zayas, E. E. (n.d.). Avifauna Associated With African Palm Plantations, Secondary Vegetation Surrounding Matrix Of The Sierra Tabasqueña. Villahermosa: Ornitologia Neotropical.
Morato, R. G., Stabach, J. A., Fleming, C. H., Calabrese, J. M., De Paula, R. C., Ferraz, K. M. P., et al. (2016). Space use and movement of a neotropical top predator: the endangered jaguar. PLoS ONE 11:e0168176. doi: 10.1371/journal.pone.0168176
Moreno Jiménez, M., Sánchez Soto, S., García López, E., Romero-napoles, J., Knowlton, J. L., Phifer, C., et al. (2017). Diversidad y abundancia de abejas (Hymenoptera: Apoidea) en agroecosistemas de palma aceitera y pastos cultivados. Rev. Nicaraguense de Entomologia N, 115, 1–20.
Moreno, C. E., Barragán, F., Pineda, E., and Pavón, N. P. (2011). Reanalysis of alpha diversity: alternatives to interpret and compare information on ecological communities. Mexican J. Biodiver. 82:1249–1261. doi: 10.22201/ib.20078706e.2011.4.745
Naranjo, E. J. (2018). Baird's tapir ecology and conservation in Mexico Revisited. Trop. Conserv. Sci. 11:194008291879555. doi: 10.1177/1940082918795558
Niedballa, J., Sollmann, R., Courtiol, A., and Wilting, A. (2016). camtrapR : an R package for efficient camera trap data management. Methods Ecol. Evol. 7, 1457–1462. doi: 10.1111/2041-210X.12600
Ochoa-Quintero, J. M., Gardner, T. A., Rosa, I., de Barros Ferraz, S. F., and Sutherland, W. (2015). Thresholds of species loss in Amazonian deforestation frontier landscapes. Conserv. Biol. 29, 440–451. doi: 10.1111/cobi.12446
Oksanen, J., Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2017). Vegan: Community Ecology Package. R package version 2.4–3.
Pardo, L. E., Campbell, M. J., Edwards, W., Clements, G. R., and Laurance, W. F. (2018a). Terrestrial mammal responses to oil palm dominated landscapes in Colombia. PLoS ONE 13:e0197539. doi: 10.1371/journal.pone.0197539
Pardo, L. E., de Roque, F. O., Campbell, M. J., Younes, N., Edwards, W., and Laurance, W. F. (2018b). Identifying critical limits in oil palm cover for the conservation of terrestrial mammals in Colombia. Biol. Conserv. 227, 65–73. doi: 10.1016/j.biocon.2018.08.026
Pardo, L. E. V., Cove, M. V., Spinola, R. M., de la Cruz, J. C., and Saenz, J. C. (2016). Assessing species traits and landscape relationships of the mammalian carnivore community in a neotropical biological corridor. Biodivers. Conserv. 25, 739–752. doi: 10.1007/s10531-016-1089-7
Peterson, A. T., and Navarro-Sigüenza, A. G. (2016). Bird conservation and biodiversity research in Mexico: status and priorities. J. Field Ornithol. 87, 121–132. doi: 10.1111/jofo.12146
Pischke, E. C., Rouleau, M. D., and Halvorsen, K. E. (2018). Public perceptions towards oil palm cultivation in Tabasco, Mexico. Biomass Bioenergy 112, 1–10. doi: 10.1016/j.biombioe.2018.02.010
Prugh, L. R., Hodges, K. E., Sinclair, A. R., and Brashares, J. S. (2008). Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. 105, 20770–20775.
Railsback, S. F., and Johnson, M. D. (2014). Effects of land use on bird populations and pest control services on coffee farms. Proc. Natl. Acad. Sci. U.S.A. 111, 6109–6114. doi: 10.1073/pnas.1320957111
Rajaratnam, R., Sunquist, M., Rajaratnam, L., and Ambu, L. (2007). Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J. Trop. Ecol. 23, 209–217. doi: 10.1017/S0266467406003841
Ramírez-Pulido, J., González-Ruiz, N., Gardner, A. L., and Arroyo-Cabrales, J. (2014). Special Publications List of Recent Land Mammals of Mexico, 2014. Series Editor: R. J. Baker. Lubbock, TX: Museum of Texas Tech University. Number 63, 1–69.
Reid, F. (2009). A Field Guide to the Mammals of Central America and Southeast Mexico, 2nd Edn. New York, NY: Oxford University Press.
Rosenzweig, M. L. (2003). Reconciliation ecology and the future of species diversity. Oryx 37, 194–205. doi: 10.1017/S0030605303000371
Salazar Conde, E., del, C., Zavala Cruz, J., Castillo Acosta, O., and Cámara Artigas, R. (2004). Evaluación espacial y temporal de la vegetación de la Evaluation spatial and temporal of the vegetation. Invest. Geográficas 54, 7–23.
Sánchez Munguía, A. (2005). Uso del suelo agropecuario y desforestación en Tabasco 1950–2000. Villahermosa: Universidad Juárez Autónoma de Tabasco, División Académica de Ciencias Biológicas.
SEMARNAT (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental. Especies Nativas de México de Flora y Fauna Silvestres. Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio. Lista de Especies en Riesgo. Diario Oficial de la Fe.
Soule, M. E., Bolger, D. T., Alberts, A. C., Wrights, J., Sorice, M., and Hill, S. (1988). Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in Urban Habitat Islands. Conserv. Biol. 2, 75–92. doi: 10.1111/j.1523-1739.1988.tb00337.x
Tscharntke, T., Clough, Y., Wanger, T. C., Jackson, L., Motzke, I., Perfecto, I., et al. (2012). Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 151, 53–59. doi: 10.1016/j.biocon.2012.01.068
Uribe Iniesta, R. (2016). Tiempos y procesos en la constitución de un espacio regional: el caso Tabasco / Rodolfo Uribe Iniesta. Cuernavaca : Universidad Nacional Autónoma de México, Centro Regional de Investigaciones Multidisciplinarias. doi: 10.22201/crim.9786070280795e.2016
Keywords: agroecosystem, camera traps, human-dominated landscape, latin america, tabasco
Citation: Knowlton JL, Mata Zayas EE, Ripley AJ, Valenzuela-Cordova B and Collado-Torres R (2019) Mammal Diversity in Oil Palm Plantations and Forest Fragments in a Highly Modified Landscape in Southern Mexico. Front. For. Glob. Change 2:67. doi: 10.3389/ffgc.2019.00067
Received: 22 March 2019; Accepted: 11 October 2019;
Published: 01 November 2019.
Edited by:
Eleanor Slade, Nanyang Technological University, SingaporeReviewed by:
Philip Matthew Chapman, Imperial College London, United KingdomJedediah Brodie, University of Montana, United States
Copyright © 2019 Knowlton, Mata Zayas, Ripley, Valenzuela-Cordova and Collado-Torres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessie L. Knowlton, knowlton_jessie@wheatoncollege.edu
 Jessie L. Knowlton
Jessie L. Knowlton Ena E. Mata Zayas
Ena E. Mata Zayas Andres J. Ripley1
Andres J. Ripley1  Bertha Valenzuela-Cordova
Bertha Valenzuela-Cordova