- 1Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, AL, United States
- 2Graduate Biomedical Sciences Program, University of Alabama at Birmingham, Birmingham, AL, United States
- 3Department of Dermatology, University of Alabama at Birmingham, Birmingham, AL, United States
- 4Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL, United States
- 5The O’Neal Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL, United States
Regulatory T-cells (Tregs) are important for maintaining self-tolerance and tissue homeostasis. The functional plasticity of Tregs is a key feature of this lineage, as it allows them to adapt to different microenvironments, adopt transcriptional programs reflective of their environments and tailor their suppressive capacity in a context-dependent fashion. Tregs, particularly effector Tregs (eTregs), are abundant in many types of tumors. However, the functional and transcriptional plasticity of eTregs in tumors remain largely to be explored. Although depletion or inhibition of systemic Tregs can enhance anti-tumor responses, autoimmune sequelae have diminished the enthusiasm for such approaches. A more effective approach should specifically target intratumoral Tregs or subvert local Treg-mediated suppression. This mini-review will discuss the reported mechanisms by which the stability and suppressive function of tumoral Tregs are modulated, with the focus on eTregs and a subset of eTregs, follicular regulatory T (TFR) cells, and how to harness this knowledge for the future development of new effective cancer immunotherapies that selectively target the tumor local response while sparing the systemic side effects.
Introduction
An effective immune system must be capable of maintaining self-tolerance while generating robust responses to foreign antigens. Tregs are important components participating in such immune regulation (1, 2). In both human and mice, Tregs are characterized by their high expression of both the IL-2 receptor α-chain (CD25) and the transcription factor Foxp3, which are essential for their development, suppressive activity and stability (3–8). Foxp3+ Tregs comprise both central Treg (cTreg) and eTreg subsets (9, 10). Accumulation of Tregs, particularly eTregs, within the tumor represents a major obstacle to the development of effective anti-tumor immunity (11–13). The frequency of Tregs among tumor-infiltrating lymphocytes (TIL) is often associated with poor prognosis of patients with many types of cancer (14), although Tregs can also be beneficial during early stages of inflammation-related cancers, such as colorectal cancer, and correlate with better prognosis (15–18). Substantial reviews have discussed the homeostatic regulation of Tregs and their suppressive function, including the most recent one centering on tumoral Tregs (19). This review will cover Treg stability with a focus on eTregs and TFR cells, and how their stability affects cancer progression and how it can be targeted for therapy.
Treg and eTreg Biology
Tregs mediate suppression through various mechanisms including obstructing CD80/CD86 co-stimulation via the surface receptor CTLA-4, limiting IL-2 availability for effector T-cells (Teff) and secreting inhibitory molecules IL-10, IL-35 or TGF-β (20). However, Tregs are phenotypically and functionally diverse. Based on the developmental origin, Tregs are defined as either thymic or peripheral Tregs. Thymic Tregs (tTregs) begin as CD4 single positive thymocytes with TCRs displaying high affinity for self-antigens. Peripheral Tregs (pTregs) develop from naïve CD4+ T-cells in the periphery that experience antigen and receive specific environmental stimuli, such as TGF-β and IL-2 (21, 22). Although the definitive markers distinguishing tTregs from pTregs remain obscure, all Tregs in the periphery reside in multiple lymphoid and non-lymphoid tissues to maintain tolerance or suppress ongoing inflammatory responses. In the circulation and lymphoid organs, the majority of Tregs that express the homing receptors CD62L and CCR7, but low level of CD44, are cTregs and are largely IL-2-dependent (9). In contrast, a large population of Tregs in the non-lymphoid tissues that have a CD44hiCD62LloCCR7lo surface phenotype resembling activated or effector conventional T-cells are eTregs (9, 23). In the presence of TCR, CD28 and IL-2 signaling, cTregs differentiate into eTregs accompanying the upregulation of IRF4 and Blimp1 (23, 24). eTregs can further undergo stimulus-specific differentiation that is regulated by signals and transcription factors typically associated with the differentiation of conventional T-helper (TH) cells. This polarization allows Tregs to regulate specific immune responses mediated by their analogous effector CD4+ T-cells in addition to their generic suppressive capacity (23). In addition to the high level of CD44, eTregs express effector markers, including ICOS and GITR (10, 24). Analogous subsets also exist for human Tregs, including resting FOXP3loCD45RA+ and effector FOXP3hiCD45RA– suppressive subsets, while FOXP3loCD45RA– cells are non-suppressive cytokine-secreting subsets (25). Importantly, CD15s has been identified as a biomarker for most suppressive human FOXP3hi eTregs (26). Although eTregs are predominantly found in non-lymphoid tissues, B-cell follicles in the lymphoid or lymphoid-like organs contain a subset of eTreg, known as TFR cells, which are responsible for regulating the follicular helper T (TFH)–B-cell interaction in the germinal center (GC), and thus the production of high-affinity antibody (27–30).
TFR cell Biology
TFR cells share many features with TFH cells, but they express Foxp3 and belong to eTregs. Like TFH cells, TFR cells express high levels of PD-1 and CXCR5, which allows them to traffic to B-cell follicles following the chemokine CXCL13 gradients (27–30). Both TFR and TFH cells require ICOS and CD28 signaling for their development and maintenance and are dependent of antigen presenting cells and B-cells in the GC (27–31). TFH and TFR cells express high levels of Bcl6, however, unlike TFH cells, TFR cells also co-express Blimp1, which antagonizes Bcl6. While Bcl6 is critical for the development of TFR cells as depletion of Bcl6 results in an almost complete loss of TFR cells, Blimp1 is important for the regulation of TFR suppressive function (31–36). Additionally, PD-1 and IL-2 signals are critical for TFR cells. Mice deficient in PD-1 or its ligand PD-L1 have increased TFR cell abundance with enhanced suppressive activity (37), while high IL-2 concentrations at the peak of influenza infection prevent TFR cell development (38). However, the maintenance of developed TFR cell stability appears to require the IL-2 signaling that is regulated by Blimp1 (34).
While TFR cells are capable of regulating a variety of immune responses similar to conventional Tregs, they are uniquely known for their ability to regulate GC response and antibody production (27–30). Despite the low frequency, the importance of TFR cells has been re-emphasized in a recent study in which a mouse model with a selective depletion of TFR cells displays a profound alteration of immune responses, including increased self-reactive antibody (39). Several mechanisms for TFR-mediated suppression have been reported, including the one mediated by CTLA-4. Genetic deletion or blockade of CTLA-4 impairs TFR cell development and function, leading to spontaneous TFH differentiation and GC expansion (40, 41). TFR cells are also shown to inhibit specific effector molecules, central metabolic and anabolic pathways in both TFH and GC B-cells, but retain their transcriptional signature (42). This type of suppression appears durable and persists in their absence, and can be overcome by IL-21 signals (42). However, it remains unclear if TFR cells directly target TFH and/or B-cells during GC responses, and whether TFR cells can regulate memory B-cells or plasma cells directly.
Treg/TFR Stability
Tregs must maintain their anergic phenotype and suppressive activity during ongoing inflammatory responses (43–45). This functional stability reflects a lack of effector activity by Tregs (i.e., expression of pro-inflammatory cytokines) and may or may not require maintenance of Foxp3 expression (44–46). Loss of Foxp3 (even a slight reduction) often results in the generation of ex-Tregs (47), while conversion into effector T-cells with unaltered Foxp3 expression is referred as Treg “fragility” (48). Several factors appear to be important for Treg stability/fragility, including CD25/STAT5 signals (43), PTEN/Akt/Foxo1/3a pathway (49–51), CARMA1–BCL10–MALT1 (CBM) signalosome complex (52), autophagy (53), Ezh2 (54, 55), Helios (56), Eos (57) and Nrp1 (48, 58). While the former 6 pathways regulate Foxp3, ablation of the latter 2 factors does not affect Foxp3 expression. Many of these pathways implicated in the context of tumor will be discussed in Treg/TFR Stability in the TME. Here we focus on the CD25/STAT5/Foxp3-dependent regulation of Treg stability and function.
Foxp3-Dependent Treg Stability
Foxp3 is crucial for maintaining Treg identity. Loss of Foxp3 results in Treg instability, dysfunction, and potential life-threatening autoimmune diseases (59–62). At steady state, Foxp3 expression and tTregs are incredibly stable (63). However, Tregs often become unstable under inflammatory conditions. Treatment of Tregs in vitro with proinflammatory cytokines like IL-4 and IL-6 results in the downregulation of Foxp3 and the upregulation of effector cytokines such as IFNγ (43, 64). Adoptive transfer of Foxp3+ Tregs into lymphodepleted mice also results in the loss of Foxp3 expression by a substantial population of Tregs, which appears to be limited to the CD25loFoxp3+ subset as the majority of CD25hiFoxp3+ cells retain Foxp3 expression (65–67). While a portion of the Foxp3– population, ex-Tregs, acquires Teff function, others are capable of reacquiring Foxp3 expression upon activation (66), suggesting the heterogeneity of Tregs and their ability to accommodate their function by adapting to environmental stimuli. These ex-Tregs are consistently reported to be autoreactive and pathogenic, causing autoimmune diseases upon adoptive transfer (35, 67–69).
Mechanisms for Foxp3-Dependent Treg Stability
Mechanisms to reinforce Foxp3 expression and Treg stability have been extensively studied. TCR stimulation, along with the recruitment of transcription factors, such as NFAT, Foxo1 and Foxo3, to the Foxp3 promoter, is the primary step in triggering Foxp3 gene transcription (70–73). Additionally, the conserved non-coding sequence (CNS) elements at the Foxp3 locus are important for Treg fate determination and lineage stability (74–76). The pioneer element CNS3 facilitates Foxp3 induction and increases the generation of both tTregs and pTregs. While tTregs do not rely on CNS1 for Foxp3 induction, CNS1 is indispensable for pTreg generation as it contains a TGF-β-NFAT response element and is dependent of TGF-β signaling to induce histone acetylation in the Foxp3 enhancer region (76–78). CNS2, which contains the Treg specific demethylation region (TSDR), is crucial for the maintenance of Foxp3 expression in dividing Tregs (43, 76). CNS2, the CpG-rich region, is fully methylated in conventional T-cells, but largely demethylated in tTregs and partially methylated in pTregs. Upon TSDR demethylation, Foxp3, along with STAT5, NFAT and Cbfβ-Runx1, binds to CNS2, stabilizing Foxp3 expression through positive feedback mechanisms (62, 79–83). The availability of IL-2 and activation status of CD25/STAT5 signals that are modulated by several factors, including Helios and Blimp1 (34, 56), are essential for CNS2 to sustain Foxp3 expression, preventing Treg differentiation into Teff by counteracting proinflammatory cytokine signaling (43), which explains why CD25hiFoxp3+ cells are more stable than CD25loFoxp3+ cells.
Blimp1-Mediated Regulation of Treg/TFR Stability
eTregs are marked by the expression of Blimp1 (10), however, its role in eTregs have been largely restricted to its regulation of IL-10 expression until recent findings from our group and others showing that it is important for Treg lineage stability and suppressive activity (34, 35). Consistent with the finding that expression of Blimp1 in the thymus is very low and Blimp1 unlikely regulates early T-cell development (84), mice with a Treg-specific deletion of Blimp1 do not show overt autoimmune phenotype (34, 35). However, Tregs from these mice are unstable with reduced Foxp3 expression and produce inflammatory cytokines after immunization, and these mice develop severe experimental autoimmune encephalitis (EAE) (34, 35, 68). At the peak of EAE, the presence of IL-6 activates the DNA methylating enzyme Dnmt3a, resulting in CNS2 methylation. Blimp1 is able to inhibit Dnmt3a upregulation and CNS2 methylation, thereby preventing the acquisition of a Teff phenotype (35). Additionally, Blimp1 can repress IL-23R-STAT3 signaling while retaining the CD25-STAT5 pathway in eTregs to sustain Foxp3 expression (34). Blimp1 is also critical for both TFR lineage stability and their proper entry into the GC (34). Blimp1-deficient TFR cells display an impaired suppressive phenotype in vivo with reduced Foxp3 and CTLA-4 expression, while increasing proinflammatory cytokines like IL-17A and IFNγ. These unstable TFR cells prematurely migrate into the GC and differentiate into TFH-like cells, resulting in TFH and GC B-cell expansion along with increased antibody and autoantibody production. Furthermore, adoptive transfer of Blimp1-deficient TFR cells can promote pathogenesis associated with dysregulated GC responses (34, 68). Taken together, these studies have revealed Blimp1 as a new and central regulator of eTreg and TFR lineage stability and suppressive capacity.
Treg/TFR Stability in the TME
Tregs are often recruited to the tumor microenvironment (TME) via various chemokines, such as CCL20, where they become highly activated and suppressive (11–13, 19, 85–87). Many pathways have been implicated in the regulation of TIL Treg stability.
Pathways to Regulate Foxp3-Dependent TIL Treg Stability
A significant portion of TIL Tregs express PTEN and Foxo3a. The PTEN/Akt/Foxo3a pathway is important for the suppression of responses to apoptotic cells, including apoptotic tumor cells (49). Disruption of the PTEN/Akt/Foxo3a pathway through inhibition of PTEN results in Treg instability and the transitioning of suppressive Foxp3+ Tregs to proinflammatory ex-Tregs, leading to a more immunogenic microenvironment and substantial tumor regression (49–51). Disruption of the CBM signalosome complex also results in the acquisition of an anti-tumor effector phenotype by TIL Tregs, i.e., production of IFNγ, and reduced tumor growth. Increased IFNγ activates macrophages and upregulates PD-L1 by tumor cells. Accordingly, PD-1 blockade therapy along with CARMA-1 or MALT1 disruption eradicates tumors that do not respond to anti-PD-1 monotherapy, suggesting that induction of Treg instability confers the sensitivity to checkpoint inhibitor (52). Similarly, disruption of Ezh2 activity or depletion of Helios in Tregs leads to Foxp3 instability with an increased expression of effector cytokines like IFNγ and TNFα, enhanced anti-tumor immunity, and decreased tumor growth and progression (54, 55, 88). Importantly, colorectal cancers with abundant infiltration of FOXP3lo non-suppressive T-cells display better prognosis than those infiltrated mainly with FOXP3hi Tregs (18).
Pathways to Regulate Foxp3-Independent TIL Treg Stability
Tregs can become unstable with an intact Foxp3 expression. The transcription factor Eos functions as a Foxp3 co-repressor to inhibit downstream target genes and to maintain Treg suppressive phenotype (89). In response to proinflammatory cytokines like IL-6, Eos but not Foxp3 is downregulated, leading to Treg reprogramming and the acquisition of a TH phenotype with the upregulation of CD40L, IL-2, and IL-17A (57, 90). Co-transfer of “Eos-labile” Tregs results in more robust anti-tumor responses and better tumor control compared to transfer of Eos-stable Tregs. Moreover, reprogrammed Tregs upregulate CD40L and are able to facilitate DC cross-presentation to activate CD8+ T-cell anti-tumor response after vaccination with an tumor antigen (91).The Nrp1-Sema4a pathway is another mechanism for reinforcing TIL Treg function and limiting anti-tumor immune responses, while it is dispensable for the suppression of autoimmunity and the maintenance of immune homeostasis by Tregs. Ligation of Nrp1 on Tregs by Sema4a increases Treg survival and potentiates stable suppression with the increased production of IL-10 and IL-35, due to diminished Akt activation via the recruitment of PTEN (58, 92). Interestingly, loss of Nrp1 in Tregs results in high expression of IFNγ that drives the instability of surrounding wild-type Tregs. Consequently, mice with Nrp1-deficient Tregs display enhanced anti-tumor immunity and tumor clearance, prolonged survival and increased responsiveness to anti-PD-1 therapy without autoimmune abnormalities (48).
Metabolic Pathways to Regulate TIL Treg Stability
Unlike Teff, Tregs favor oxidative phosphorylation but keep glycolysis under strict control, which plays an important role in shaping Treg identity and function (93, 94). The TME creates a low-glucose and high lactate environment that often promotes Treg suppressive function (95–99). Tregs may couple the survival mechanism, like autophagy to metabolic homeostasis by limiting glycolysis and reducing PI3K/Akt/Myc activation to ensure their integrity in the hostile TME (53). A most recent study has further elucidated that high-glucose conditions impair the function and stability of Tregs (100). However interestingly, Tregs have evolved to benefit from the symbiosis with tumors by utilizing the glycolytic by-product lactic acid to proliferate and prevent the destabilization effects of high glucose. This alternative pathway appears to be exclusively important for the stability and suppressive identity of tumoral but not peripheral Tregs. Similarly, limiting lipid uptake or metabolism by genetic or pharmacologic inhibition of FABP5 disrupts mitochondrial respiration, but also enhances Treg suppression by increasing IL-10 expression, suggesting another layer of complexity for the regulation of TIL Tregs (101).
New Pathways to Regulate TIL Treg and TFR Stability
Our recent study has revealed the importance of Blimp1 in the regulation of eTreg/TFR stability and suppressive function under immune and autoimmune conditions (34, 68). However, the specific impact of Blimp1+ eTregs on, and mechanisms of action within, tumors are not yet explored. Since a majority of TIL Tregs express Blimp1 in some tumor models (102), and Blimp1 is suggested to be used for outcome prediction of cancer patients (103), loss of Blimp1 in eTregs may reprogram these cells into Teff, and potentially lead to increased anti-tumor immunity and decreased tumor progression, although this awaits further investigation. Importantly, these effects are likely restricted to TIL Tregs, since Blimp1 is expressed at low levels by Tregs at steady state (24). Despite a few reports showing that TFR cells are significantly increased in cancer patients compared to healthy controls (104, 105), their mechanisms of action in the tumor are unclear. The increased TIL TFH and B-cells, as likely observed in mice with the Treg-specific deletion of Blimp1, and tertiary lymphoid structure formation are associated with favorable outcomes in certain types of cancer and better responses to immunotherapy (106–112). Thus, it is important to define the contribution of TFR cells to tumor progression and the impact of Blimp1 on TFR function in the tumor.
Therapeutic Approaches Targeting Treg Stability
Current cancer immunotherapy, particularly checkpoint inhibitor and CAR T-cell transfer, have shown great promise in some types of cancer. However, the success rates remain suboptimal (113–115), and some of these approaches are complicated with systemic immune-related adverse effects (116–118). Since Tregs, particularly eTregs, are one of major suppressive immune components in many cancers, most of these approaches are complicated with negative outcomes from Tregs in addition to positive effects on anti-tumor effector cells. For example, IL-2 can potently activate both T-cells and nature killer cells, and is potentially applicable for tumor control. However, IL-2 has the propensity to amplify Tregs, representing a major barrier for IL-2-based cancer therapy. The next generation of IL-2 that specifically targets tumor and preferentially boosts CD8+ T-cell response without inducing Treg responses appears to be promising (119). Similarly, high PD-1 expression is deleterious to Treg and TFR suppression; anti-PD-1 may promote CD8+ T-cell anti-tumor response while inducing potent Treg/TFR-mediated suppression (37, 120). Therefore, the PD-1 expression balance between Teff and Tregs can predict the clinical efficacy of PD-1 blockade therapy, and needs to be considered when anti-PD-1 or anti-PD-L1-based therapy is applied (121). Interestingly, another checkpoint inhibitor, CTLA-4 blockade, has been recently shown to drive Treg instability in glycolysis-low tumors (122), a new mechanism beyond the conventional role of anti-CTLA-4 therapy in inducing Treg depletion.
Depletion of Tregs has been demonstrated to enhance anti-tumor responses, however, this ablation also results in lethal autoimmunity (60–62, 123). Studies from us and others suggest that a more effective approach would entail the specific reprogramming of TIL Tregs and reshaping the TME by employing the features of Treg instability, while not altering the stability of Tregs in the periphery (44, 45) (Figure 1). Disruption of the CBM signalosome complex or targeting Helios or Nrp1 or ligation of GITR in Tregs is shown to be effective for tumor control without peripheral autoimmune effects reported (48, 52, 88, 124). Based on the profound effect of Blimp1 depletion on the stability and suppressive ability of eTreg and TFR cells, our findings suggest that targeting Blimp1+ eTreg may generate similar anti-tumor effects while limiting systemic toxicity. In addition to inducing eTreg destabilization (34), targeting Blimp1+ eTregs may also induce potent anti-tumor humoral responses, thus achieving multifaceted anti-tumor effects.
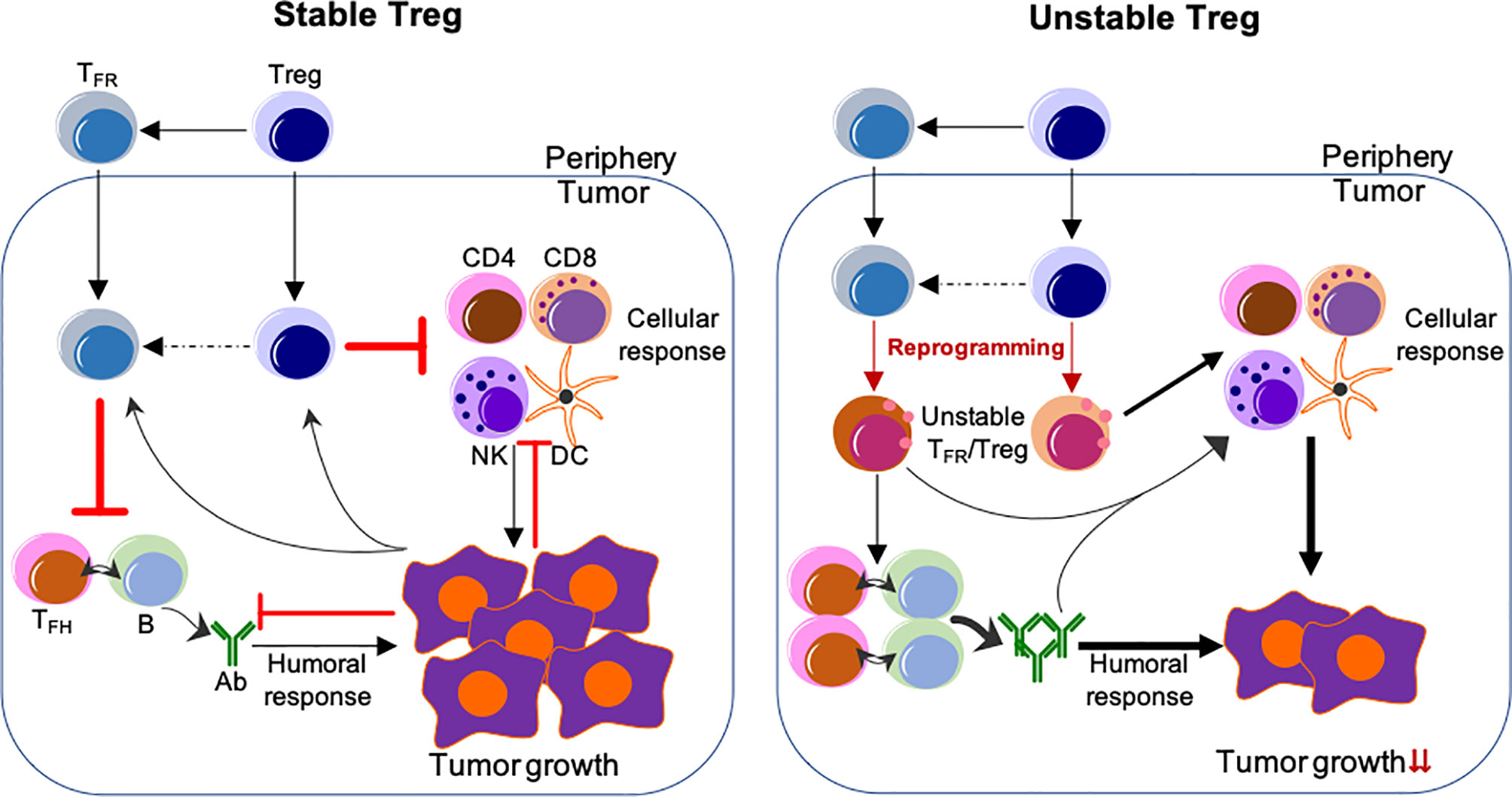
Figure 1 Reprogramming of TIL Tregs to control tumor by targeting their stability. Left, Stable Treg. Treg and TFR cells mainly suppress the cellular and humoral anti-tumor immune responses, respectively. Conversely, tumor cells impose suppression on both cellular and humoral immune responses, but foster the immune suppression by Treg and TFR cells. Right, Unstable Treg. Factors or approaches destabilize or reprogram Treg and TFR cells into effector-like cells, which display impaired suppressive activity, but instead cooperate with both cellular and humoral anti-tumor components to control tumor growth and progression. The peripheral events are not depicted, but strategies used to selectively reprogram TIL Tregs, but not Tregs in the periphery, are expected to be most effective without systemic adverse effects. The unclear events are indicated by dashed lines. Not depicted: Peripheral TFH and B-cells and their migration into the tumor; expansion of Treg/TFR cells and anti-tumor effector cells; other cells regulating anti-tumor responses (e.g., myeloid-derived suppressor cells and macrophages, etc.).
Conclusion/Perspective
It is important to recognize that Treg stability can be manipulated to induce changes of immune responses, achieving the therapeutic benefit. Notably, loss of TIL eTreg stability in various tumors leads to remodeling of the TME from a suppressive state to an effective anti-tumor state and decreased tumor progression. Current and future challenges include the ability to selectively induce these changes in specific subsets of Tregs and in the TME but not systemically. As the field of cancer immunology progresses, understanding factors that regulate Tregs specifically in the tumor, yet have limited impact on Tregs in the periphery, is highly desirable and important for treating nearly every cancer patient, particularly any patient treated with immunotherapy, as it will direct the development of effective, targeted immunotherapies with reduced adverse events. This represents a new direction for how to manipulate Treg activity for cancer treatment.
Author Contributions
MLD, JDL, and JWL drafted the manuscript and revised it critically. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was supported by the University of Alabama at Birmingham faculty start-up funds to JWL. MLD is supported by NIH pre-doctoral training program (T32 AI007051). JWL is also supported by DoD W81XWH-18-1-0315 and NIH grant R01AI148711. Due to the limited space, the authors regret that this minireview article cannot include all interesting studies in the field.
Glossary
References
1. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell (2008) 133(5):775–87. doi: 10.1016/j.cell.2008.05.009
2. Yuan X, Cheng G, Malek TR. The Importance of Regulatory T-Cell Heterogeneity in Maintaining Self-Tolerance. Immunol Rev (2014) 259(1):103–14. doi: 10.1111/imr.12163
3. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat Immunol (2003) 4(4):330–6. doi: 10.1038/ni904
4. Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-Dependent Programme of Regulatory T-Cell Differentiation. Nature (2007) 445(7129):771–5. doi: 10.1038/nature05543
5. Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science (2003) 299(5609):1057–61. doi: 10.1126/science.1079490
6. Cheng G, Yu A, Dee MJ, Malek TR. IL-2R Signaling is Essential for Functional Maturation of Regulatory T Cells During Thymic Development. J Immunol (2013) 190(4):1567–75. doi: 10.4049/jimmunol.1201218
7. Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, et al. Foxp3 Occupancy and Regulation of Key Target Genes During T-Cell Stimulation. Nature (2007) 445(7130):931–5. doi: 10.1038/nature05478
8. Williams LM, Rudensky AY. Maintenance of the Foxp3-Dependent Developmental Program in Mature Regulatory T Cells Requires Continued Expression of Foxp3. Nat Immunol (2007) 8(3):277–84. doi: 10.1038/ni1437
9. Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, et al. CCR7 Provides Localized Access to IL-2 and Defines Homeostatically Distinct Regulatory T Cell Subsets. J Exp Med (2014) 211(1):121–36. doi: 10.1084/jem.20131142
10. Cretney E, Kallies A, Nutt SL. Differentiation and Function of Foxp3(+) Effector Regulatory T Cells. Trends Immunol (2013) 34(2):74–80. doi: 10.1016/j.it.2012.11.002
11. Nishikawa H, Sakaguchi S. Regulatory T Cells in Tumor Immunity. Int J Cancer (2010) 127(4):759–67. doi: 10.1002/ijc.25429
12. Chao JL, Savage PA. Unlocking the Complexities of Tumor-Associated Regulatory T Cells. J Immunol (2018) 200(2):415–21. doi: 10.4049/jimmunol.1701188
13. Nishikawa H, Sakaguchi S. Regulatory T Cells in Cancer Immunotherapy. Curr Opin Immunol (2014) 27:1–7. doi: 10.1016/j.coi.2013.12.005
14. Tanaka A, Sakaguchi S. Regulatory T Cells in Cancer Immunotherapy. Cell Res (2017) 27(1):109–18. doi: 10.1038/cr.2016.151
15. Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic Value of Tumor-Infiltrating Foxp3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci Rep (2015) 5:15179. doi: 10.1038/srep15179
16. Zhang X, Kelaria S, Kerstetter J, Wang J. The Functional and Prognostic Implications of Regulatory T Cells in Colorectal Carcinoma. J Gastrointest Oncol (2015) 6(3):307–13. doi: 10.3978/j.issn.2078-6891.2015.017
17. Quandt J, Arnovitz S, Haghi L, Woehlk J, Mohsin A, Okoreeh M, et al. Wnt-Beta-Catenin Activation Epigenetically Reprograms Treg Cells in Inflammatory Bowel Disease and Dysplastic Progression. Nat Immunol (2021) 22(4):471–84. doi: 10.1038/s41590-021-00889-2
18. Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T Cell Subpopulations Distinctly Control the Prognosis of Colorectal Cancers. Nat Med (2016) 22(6):679–84. doi: 10.1038/nm.4086
19. Glasner A, Plitas G. Tumor Resident Regulatory T Cells. Semin Immunol (2021), 101476. doi: 10.1016/j.smim.2021.101476
20. Sojka DK, Huang YH, Fowell DJ. Mechanisms of Regulatory T-Cell Suppression - a Diverse Arsenal for a Moving Target. Immunology (2008) 124(1):13–22. doi: 10.1111/j.1365-2567.2008.02813.x
21. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu Rev Immunol (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
22. Plitas G, Rudensky AY. Regulatory T Cells: Differentiation and Function. Cancer Immunol Res (2016) 4(9):721–5. doi: 10.1158/2326-6066.Cir-16-0193
23. Liston A, Gray DH. Homeostatic Control of Regulatory T Cell Diversity. Nat Rev Immunol (2014) 14(3):154–65. doi: 10.1038/nri3605
24. Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, et al. The Transcription Factors Blimp-1 and IRF4 Jointly Control the Differentiation and Function of Effector Regulatory T Cells. Nat Immunol (2011) 12(4):304–11. doi: 10.1038/ni.2006
25. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the Foxp3 Transcription Factor. Immunity (2009) 30(6):899–911. doi: 10.1016/j.immuni.2009.03.019
26. Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, et al. Sialyl Lewis X (CD15s) Identifies Highly Differentiated and Most Suppressive FOXP3high Regulatory T Cells in Humans. Proc Natl Acad Sci USA (2015) 112(23):7225–30. doi: 10.1073/pnas.1508224112
27. Lim HW, Hillsamer P, Kim CH. Regulatory T Cells can Migrate to Follicles Upon T Cell Activation and Suppress GC-Th Cells and GC-Th Cell-Driven B Cell Responses. J Clin Invest (2004) 114(11):1640–9. doi: 10.1172/jci22325
28. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ Follicular Regulatory T Cells Control the Germinal Center Response. Nat Med (2011) 17(8):975–82. doi: 10.1038/nm.2425
29. Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the Germinal Center Reaction by Foxp3+ Follicular Regulatory T Cells. J Immunol (2011) 187(9):4553–60. doi: 10.4049/jimmunol.1101328
30. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular Regulatory T Cells Expressing Foxp3 and Bcl-6 Suppress Germinal Center Reactions. Nat Med (2011) 17(8):983–8. doi: 10.1038/nm.2426
31. Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A P85alpha-Osteopontin Axis Couples the Receptor ICOS to Sustained Bcl-6 Expression by Follicular Helper and Regulatory T Cells. Nat Immunol (2015) 16(1):96–106. doi: 10.1038/ni.3050
32. Shen E, Wang Q, Rabe H, Liu W, Cantor H, Leavenworth JW. Chromatin Remodeling by the Nurd Complex Regulates Development of Follicular Helper and Regulatory T Cells. Proc Natl Acad Sci USA (2018) 115(26):6780–5. doi: 10.1073/pnas.1805239115
33. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science (2009) 325(5943):1006–10. doi: 10.1126/science.1175870
34. Shen E, Rabe H, Luo L, Wang L, Wang Q, Yin J, et al. Control of Germinal Center Localization and Lineage Stability of Follicular Regulatory T Cells by the Blimp1 Transcription Factor. Cell Rep (2019) 29(7):1848–61 e6. doi: 10.1016/j.celrep.2019.10.012
35. Garg G, Muschaweckh A, Moreno H, Vasanthakumar A, Floess S, Lepennetier G, et al. Blimp1 Prevents Methylation of Foxp3 and Loss of Regulatory T Cell Identity at Sites of Inflammation. Cell Rep (2019) 26(7):1854–68 e5. doi: 10.1016/j.celrep.2019.01.070
36. Fu W, Liu X, Lin X, Feng H, Sun L, Li S, et al. Deficiency in T Follicular Regulatory Cells Promotes Autoimmunity. J Exp Med (2018) 215(3):815–25. doi: 10.1084/jem.20170901
37. Sage PT, Francisco LM, Carman CV, Sharpe AH. The Receptor PD-1 Controls Follicular Regulatory T Cells in the Lymph Nodes and Blood. Nat Immunol (2013) 14(2):152–61. doi: 10.1038/ni.2496
38. Botta D, Fuller MJ, Marquez-Lago TT, Bachus H, Bradley JE, Weinmann AS, et al. Dynamic Regulation of T Follicular Regulatory Cell Responses by Interleukin 2 During Influenza Infection. Nat Immunol (2017) 18(11):1249–60. doi: 10.1038/ni.3837
39. Clement RL, Daccache J, Mohammed MT, Diallo A, Blazar BR, Kuchroo VK, et al. Follicular Regulatory T Cells Control Humoral and Allergic Immunity by Restraining Early B Cell Responses. Nat Immunol (2019) 20(10):1360–71. doi: 10.1038/s41590-019-0472-4
40. Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The Coinhibitory Receptor CTLA-4 Controls B Cell Responses by Modulating T Follicular Helper, T Follicular Regulatory, and T Regulatory Cells. Immunity (2014) 41(6):1026–39. doi: 10.1016/j.immuni.2014.12.005
41. Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4. Immunity (2014) 41(6):1013–25. doi: 10.1016/j.immuni.2014.12.006
42. Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, et al. Suppression by T(FR) Cells Leads to Durable and Selective Inhibition of B Cell Effector Function. Nat Immunol (2016) 17(12):1436–46. doi: 10.1038/ni.3578
43. Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the Inheritance of Regulatory T Cell Identity by a Cis Element in the Foxp3 Locus. Cell (2014) 158(4):749–63. doi: 10.1016/j.cell.2014.07.031
44. Munn DH, Sharma MD, Johnson TS. Treg Destabilization and Reprogramming: Implications for Cancer Immunotherapy. Cancer Res (2018) 78(18):5191–9. doi: 10.1158/0008-5472.Can-18-1351
45. Overacre-Delgoffe AE, Vignali DAA. Treg Fragility: A Prerequisite for Effective Antitumor Immunity? Cancer Immunol Res (2018) 6(8):882–7. doi: 10.1158/2326-6066.Cir-18-0066
46. Overacre AE, Vignali DA. T(Reg) Stability: To be or Not to be. Curr Opin Immunol (2016) 39:39–43. doi: 10.1016/j.coi.2015.12.009
47. Wan YY, Flavell RA. Regulatory T-Cell Functions are Subverted and Converted Owing to Attenuated Foxp3 Expression. Nature (2007) 445(7129):766–70. doi: 10.1038/nature05479
48. Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-Gamma Drives Treg Fragility to Promote Anti-Tumor Immunity. Cell (2017) 169(6):1130–41 e11. doi: 10.1016/j.cell.2017.05.005
49. Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, et al. The PTEN Pathway in Tregs is a Critical Driver of the Suppressive Tumor Microenvironment. Sci Adv (2015) 1(10):e1500845. doi: 10.1126/sciadv.1500845
50. Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, et al. Control of PI(3) Kinase in Treg Cells Maintains Homeostasis and Lineage Stability. Nat Immunol (2015) 16(2):188–96. doi: 10.1038/ni.3077
51. Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg Cells Require the Phosphatase PTEN to Restrain TH1 and TFH Cell Responses. Nat Immunol (2015) 16(2):178–87. doi: 10.1038/ni.3076
52. Di Pilato M, Kim EY, Cadilha BL, Prüßmann JN, Nasrallah MN, Seruggia D, et al. Targeting the CBM Complex Causes T(Reg) Cells to Prime Tumours for Immune Checkpoint Therapy. Nature (2019) 570(7759):112–6. doi: 10.1038/s41586-019-1215-2
53. Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. Autophagy Enforces Functional Integrity of Regulatory T Cells by Coupling Environmental Cues and Metabolic Homeostasis. Nat Immunol (2016) 17(3):277–85. doi: 10.1038/ni.3365
54. Wang D, Quiros J, Mahuron K, Pai CC, Ranzani V, Young A, et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep (2018) 23(11):3262–74. doi: 10.1016/j.celrep.2018.05.050
55. Goswami S, Apostolou I, Zhang J, Skepner J, Anandhan S, Zhang X, et al. Modulation of EZH2 Expression in T Cells Improves Efficacy of Anti–CTLA-4 Therapy. J Clin Invest (2018) 128(9):3813–8. doi: 10.1172/JCI99760
56. Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, et al. Stable Inhibitory Activity of Regulatory T Cells Requires the Transcription Factor Helios. Science (2015) 350(6258):334–9. doi: 10.1126/science.aad0616
57. Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, et al. An Inherently Bifunctional Subset of Foxp3+ T Helper Cells Is Controlled by the Transcription Factor Eos. Immunity (2013) 38(5):998–1012. doi: 10.1016/j.immuni.2013.01.013
58. Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, et al. Stability and Function of Regulatory T Cells Is Maintained by a Neuropilin-1-Semaphorin-4a Axis. Nature (2013) 501(7466):252–6. doi: 10.1038/nature12428
59. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol (1995) 155(3):1151–64.
60. Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T Cell Development in the Absence of Functional Foxp3. Nat Immunol (2007) 8(4):359–68. doi: 10.1038/ni1445
61. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective Depletion of Foxp3+ Regulatory T Cells Induces a Scurfy-Like Disease. J Exp Med (2007) 204(1):57–63. doi: 10.1084/jem.20061852
62. Kim H-P, Leonard WJ. CREB/ATF-Dependent T Cell Receptor–Induced Foxp3 Gene Expression: A Role for DNA Methylation. J Exp Med (2007) 204(7):1543–51. doi: 10.1084/jem.20070109
63. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the Regulatory T Cell Lineage In Vivo. Science (2010) 329(5999):1667–71. doi: 10.1126/science.1191996
64. Kastner L, Dwyer D, Qin FX-F. Synergistic Effect of IL-6 and IL-4 in Driving Fate Revision of Natural Foxp3+ Regulatory T Cells. J Immunol (2010) 185(10):5778–86. doi: 10.4049/jimmunol.0901948
65. Duarte JH, Zelenay S, Bergman M-L, Martins AC, Demengeot J. Natural Treg Cells Spontaneously Differentiate Into Pathogenic Helper Cells in Lymphopenic Conditions. Eur J Immunol (2009) 39(4):948–55. doi: 10.1002/eji.200839196
66. Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of Natural Foxp3+ T Cells: A Committed Regulatory T-Cell Lineage and an Uncommitted Minor Population Retaining Plasticity. Proc Natl Acad Sci (2009) 106(6):1903–8. doi: 10.1073/pnas.0811556106
67. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the Transcription Factor Foxp3 Leads to the Generation of Pathogenic Memory T Cells In Vivo. Nat Immunol (2009) 10(9):1000–7. doi: 10.1038/ni.1774
68. Luo L, Hu X, Dixon ML, Pope BJ, Leavenworth JD, Raman C, et al. Dysregulated Follicular Regulatory T Cells and Antibody Responses Exacerbate Experimental Autoimmune Encephalomyelitis. J Neuroinflamm (2021) 18(1):27. doi: 10.1186/s12974-021-02076-4
69. Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic Conversion of Foxp3+ T Cells Into TH17 Cells in Autoimmune Arthritis. Nat Med (2014) 20(1):62–8. doi: 10.1038/nm.3432
70. Harada Y, Harada Y, Elly C, Ying G, Paik J-H, DePinho RA, et al. Transcription Factors Foxo3a and Foxo1 Couple the E3 Ligase Cbl-B to the Induction of Foxp3 Expression in Induced Regulatory T Cells. J Exp Med (2010) 207(7):1381–91. doi: 10.1084/jem.20100004
71. Ouyang W, Beckett O, Ma Q, Paik J-H, De Pinho RA, Li MO. Foxo Proteins Cooperatively Control the Differentiation of Foxp3+ Regulatory T Cells. Nat Immunol (2010) 11(7):618–27. doi: 10.1038/ni.1884
72. Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, et al. Novel Foxo1-Dependent Transcriptional Programs Control Treg Cell Function. Nature (2012) 491(7425):554–9. doi: 10.1038/nature11581
73. Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT Cooperate to Induce Foxp3 Expression Through Its Enhancer. Nat Immunol (2008) 9(2):194–202. doi: 10.1038/ni1549
74. Li X, Zheng Y. Regulatory T Cell Identity: Formation and Maintenance. Trends Immunol (2015) 36(6):344–53. doi: 10.1016/j.it.2015.04.006
75. Okada M, Hibino S, Someya K, Yoshmura A. Chapter Eight - Regulation of Regulatory T Cells: Epigenetics and Plasticity. In: Alt FW, editor. Advances in Immunology (2014) 124:249–73. doi: 10.1016/B978-0-12-800147-9.00008-X
76. Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of Conserved Non-Coding DNA Elements in the Foxp3 Gene in Regulatory T-Cell Fate. Nature (2010) 463(7282):808–12. doi: 10.1038/nature08750
77. Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and Negative Transcriptional Regulation of the Foxp3 Gene Is Mediated by Access and Binding of the Smad3 Protein to Enhancer I. Immunity (2010) 33(3):313–25. doi: 10.1016/j.immuni.2010.09.001
78. Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol (2016) 37(11):803–11. doi: 10.1016/j.it.2016.08.012
79. Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 Cis-Element in Protecting Regulatory T Cell Identity. Cell (2014) 158(4):734–48. doi: 10.1016/j.cell.2014.07.030
80. Huehn J, Beyer M. Epigenetic and Transcriptional Control of Foxp3+ Regulatory T Cells. Semin Immunol (2015) 27(1):10–8. doi: 10.1016/j.smim.2015.02.002
81. Morikawa H, Sakaguchi S. Genetic and Epigenetic Basis of Treg Cell Development and Function: From a Foxp3-Centered View to an Epigenome-Defined View of Natural Treg Cells. Immunol Rev (2014) 259(1):192–205. doi: 10.1111/imr.12174
82. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic Control of the Foxp3 Locus in Regulatory T Cells. PloS Biol (2007) 5(2):e38. doi: 10.1371/journal.pbio.0050038
83. Kitagawa Y, Ohkura N, Sakaguchi S. Molecular Determinants of Regulatory T Cell Development: The Essential Roles of Epigenetic Changes. Front Immunol (2013) 4:106. doi: 10.3389/fimmu.2013.00106
84. Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, et al. Transcriptional Repressor Blimp-1 Regulates T Cell Homeostasis and Function. Nat Immunol (2006) 7(5):457–65. doi: 10.1038/ni1320
85. Chaudhary B, Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines (Basel) (2016) 4(3):28. doi: 10.3390/vaccines4030028
86. Facciabene A, Motz GT, Coukos G. T-Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res (2012) 72(9):2162–71. doi: 10.1158/0008-5472.Can-11-3687
87. Magnuson AM, Kiner E, Ergun A, Park JS, Asinovski N, Ortiz-Lopez A, et al. Identification and Validation of a Tumor-Infiltrating Treg Transcriptional Signature Conserved Across Species and Tumor Types. Proc Natl Acad Sci USA (2018) 115(45):E10672–E81. doi: 10.1073/pnas.1810580115
88. Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim H-J. Instability of Helios-Deficient Tregs is Associated With Conversion to a T-Effector Phenotype and Enhanced Antitumor Immunity. Proc Natl Acad Sci (2016) 113(22):6248–53. doi: 10.1073/pnas.1604765113
89. Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, et al. Eos Mediates Foxp3-Dependent Gene Silencing in CD4+ Regulatory T Cells. Science (2009) 325(5944):1142–6. doi: 10.1126/science.1176077
90. Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular Antagonism and Plasticity of Regulatory and Inflammatory T Cell Programs. Immunity (2008) 29(1):44–56. doi: 10.1016/j.immuni.2008.05.007
91. Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed Foxp3(+) Regulatory T Cells Provide Essential Help to Support Cross-Presentation and CD8(+) T Cell Priming in Naive Mice. Immunity (2010) 33(6):942–54. doi: 10.1016/j.immuni.2010.11.022
92. Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T Cell Suppression Is Potentiated by Target T Cells in a Cell Contact, IL-35- and IL-10-Dependent Manner. J Immunol (2009) 182(10):6121–8. doi: 10.4049/jimmunol.0803646
93. Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J Immunol (2011) 186(6):3299–303. doi: 10.4049/jimmunol.1003613
94. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-Dependent Glycolytic Pathway Orchestrates a Metabolic Checkpoint for the Differentiation of TH17 and Treg Cells. J Exp Med (2011) 208(7):1367–76. doi: 10.1084/jem.20110278
95. Shi H, Chi H. Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity. Front Immunol (2019) 10:2716. doi: 10.3389/fimmu.2019.02716
96. Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, et al. TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab (2019) 29(1):103–23 e5. doi: 10.1016/j.cmet.2018.09.020
97. He N, Fan W, Henriquez B, Yu RT, Atkins AR, Liddle C, et al. Metabolic Control of Regulatory T Cell (Treg) Survival and Function by Lkb1. Proc Natl Acad Sci USA (2017) 114(47):12542–7. doi: 10.1073/pnas.1715363114
98. Wang YA, Li XL, Mo YZ, Fan CM, Tang L, Xiong F, et al. Effects of Tumor Metabolic Microenvironment on Regulatory T Cells. Mol Cancer (2018) 17(1):168. doi: 10.1186/s12943-018-0913-y
99. DePeaux K, Delgoffe GM. Metabolic Barriers to Cancer Immunotherapy. Nat Rev Immunol (2021). doi: 10.1038/s41577-021-00541-y
100. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic Support of Tumour-Infiltrating Regulatory T Cells by Lactic Acid. Nature (2021) 591(7851):645–51. doi: 10.1038/s41586-020-03045-2
101. Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab (2020) 31(2):422–37 e5. doi: 10.1016/j.cmet.2019.11.021
102. Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive Plasticity of IL-10(+) and IL-35(+) Treg Cells Cooperatively Promotes Tumor T Cell Exhaustion. Nat Immunol (2019) 20(6):724–35. doi: 10.1038/s41590-019-0346-9
103. Ward-Hartstonge KA, McCall JL, McCulloch TR, Kamps AK, Girardin A, Cretney E, et al. Inclusion of BLIMP-1+ Effector Regulatory T Cells Improves the Immunoscore in a Cohort of New Zealand Colorectal Cancer Patients: A Pilot Study. Cancer Immunol Immunother (2017) 66(4):515–22. doi: 10.1007/s00262-016-1951-1
104. Cha Z, Gu H, Zang Y, Wang Z, Li J, Huang W, et al. The Prevalence and Function of CD4(+)CXCR5(+)Foxp3(+) Follicular Regulatory T Cells in Diffuse Large B Cell Lymphoma. Int Immunopharmacol (2018) 61:132–9. doi: 10.1016/j.intimp.2018.05.025
105. Li L, Ma Y, Xu Y. Follicular Regulatory T Cells Infiltrated the Ovarian Carcinoma and Resulted in CD8 T Cell Dysfunction Dependent on IL-10 Pathway. Int Immunopharmacol (2019) 68:81–7. doi: 10.1016/j.intimp.2018.12.051
106. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity (2013) 39(4):782–95. doi: 10.1016/j.immuni.2013.10.003
107. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) Follicular Helper T Cell Infiltration Predicts Breast Cancer Survival. J Clin Invest (2013) 123(7):2873–92. doi: 10.1172/JCI67428
108. Chu F, Li HS, Liu X, Cao J, Ma W, Ma Y, et al. CXCR5(+)CD8(+) T Cells are a Distinct Functional Subset With an Antitumor Activity. Leukemia (2019) 33(11):2640–53. doi: 10.1038/s41375-019-0464-2
109. Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity (2020) 52(1):183–99 e9. doi: 10.1016/j.immuni.2019.11.014
110. Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B Cells Are Associated With Survival and Immunotherapy Response in Sarcoma. Nature (2020) 577(7791):556–60. doi: 10.1038/s41586-019-1906-8
111. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B Cells and Tertiary Lymphoid Structures Promote Immunotherapy Response. Nature (2020) 577(7791):549–55. doi: 10.1038/s41586-019-1922-8
112. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary Lymphoid Structures Improve Immunotherapy and Survival in Melanoma. Nature (2020) 577(7791):561–5. doi: 10.1038/s41586-019-1914-8
113. Hou AJ, Chen LC, Chen YY. Navigating CAR-T Cells Through the Solid-Tumour Microenvironment. Nat Rev Drug Discov (2021) 20(7):531–50. doi: 10.1038/s41573-021-00189-2
114. Pauken KE, Torchia JA, Chaudhri A, Sharpe AH, Freeman GJ. Emerging Concepts in PD-1 Checkpoint Biology. Semin Immunol (2021) 101480. doi: 10.1016/j.smim.2021.101480
115. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The Next Decade of Immune Checkpoint Therapy. Cancer Discov (2021) 11(4):838–57. doi: 10.1158/2159-8290.CD-20-1680
116. Chhabra N, Kennedy J. A Review of Cancer Immunotherapy Toxicity II: Adoptive Cellular Therapies, Kinase Inhibitors, Monoclonal Antibodies, and Oncolytic Viruses. Med Toxicol (2021) 1–13. doi: 10.1007/s13181-021-00835-6
117. Chhabra N, Kennedy J. A Review of Cancer Immunotherapy Toxicity: Immune Checkpoint Inhibitors. J Med Toxicol (2021). doi: 10.1007/s13181-021-00833-8
118. Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine Release Syndrome and Associated Neurotoxicity in Cancer Immunotherapy. Nat Rev Immunol (2021) 1–12. doi: 10.1038/s41577-021-00547-6
119. Sun Z, Ren Z, Yang K, Liu Z, Cao S, Deng S, et al. A Next-Generation Tumor-Targeting IL-2 Preferentially Promotes Tumor-Infiltrating CD8(+) T-Cell Response and Effective Tumor Control. Nat Commun (2019) 10(1):3874. doi: 10.1038/s41467-019-11782-w
120. Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J, et al. PD-1 Restraint of Regulatory T Cell Suppressive Activity Is Critical for Immune Tolerance. J Exp Med (2021) 218(1):e20182232. doi: 10.1084/jem.20182232
121. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 Expression Balance Between Effector and Regulatory T Cells Predicts the Clinical Efficacy of PD-1 Blockade Therapies. Nat Immunol (2020) 21(11):1346–58. doi: 10.1038/s41590-020-0769-3
122. Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, et al. CTLA-4 Blockade Drives Loss of Treg Stability in Glycolysis-Low Tumours. Nature (2021) 591(7851):652–8. doi: 10.1038/s41586-021-03326-4
123. Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human Foxp3+ Regulatory T Cells in Systemic Autoimmune Diseases. Autoimmun Rev (2011) 10(12):744–55. doi: 10.1016/j.autrev.2011.05.004
Keywords: anti-tumor immunity, effector regulatory T cells, follicular regulatory T cells, Foxp3, Treg lineage stability, humoral antibody response
Citation: Dixon ML, Leavenworth JD and Leavenworth JW (2021) Lineage Reprogramming of Effector Regulatory T Cells in Cancer. Front. Immunol. 12:717421. doi: 10.3389/fimmu.2021.717421
Received: 30 May 2021; Accepted: 14 July 2021;
Published: 28 July 2021.
Edited by:
Khashayarsha Khazaie, Mayo Clinic College of Medicine and Science, United StatesReviewed by:
Salman M. Toor, Hamad bin Khalifa University, QatarArya Biragyn, National Institute on Aging (NIH), United States
Copyright © 2021 Dixon, Leavenworth and Leavenworth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianmei W. Leavenworth, jleavenworth@uabmc.edu
 Michael L. Dixon
Michael L. Dixon Jonathan D. Leavenworth
Jonathan D. Leavenworth Jianmei W. Leavenworth
Jianmei W. Leavenworth