- State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Integrative Microbiology Research Centre, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, South China Agricultural University, Guangzhou, China
Paraquat herbicide has served over five decades to control annual and perennial weeds. Despite agricultural benefits, its toxicity to terrestrial and aquatic environments raises serious concerns. Paraquat cannot rapidly degrade in the environment and is adsorbed in clay lattices that require urgent environmental remediation. Advanced oxidation processes (AOPs) and bioaugmentation techniques have been developed for this purpose. Among various techniques, bioremediation is a cost-effective and eco-friendly approach for pesticide-polluted soils. Though several paraquat-degrading microorganisms have been isolated and characterized, studies about degradation pathways, related functional enzymes and genes are indispensable. This review encircles paraquat removal from contaminated environments through adsorption, photocatalyst degradation, AOPs and microbial degradation. To provide in-depth knowledge, the potential role of paraquat degrading microorganisms in contaminated environments is described as well.
Introduction
Paraquat or methyl violet(1,1′-dimethyl-4,4′-bipyridinium dichloride) is a broad-spectrum cationic contact herbicide that is widely used in more than 100 countries (Rashidipour et al., 2019). Paraquat is applied against annual or perennial weeds of cotton, rice, soybean, and cocoa (Paraquat Information Center, 2018). However, because of high environmental and human toxicity, it was banned in some countries including Austria, South Korea and the European Union (Cha et al., 2016; Bang et al., 2017; Verssimo et al., 2017). China banned paraquat aqueous solution in 2016 but the pesticide is still marketed under other formulations.
Paraquat was synthesized in 1882 but its role as a weedicide was discovered in 1955 and commercialized in 1962 by Imperial Chemical Industries (ICI or Syngenta) (Alexander, 1999). Low-cost, efficient weed elimination and a unique mechanism made it popular for massive applications. Paraquat deviates electron flow from photosystem that inhibits reduction of oxidized nicotinamide adenine dinucleotide phosphate (NADP+) during photosynthesis to produce PQ+ (Setif, 2015). Paraquat specifically targets photosynthesizing green plant parts where PQ+ is re-oxidized by the O2 produced in chloroplasts. During the re-oxidization, lethal superoxide radical (O+) is generated and its subsequent oxidation results in cell death (Reczek et al., 2017).
Paraquat is comparatively safe for soil microorganisms and plant roots, but its long-term exposure results in harmful biomagnification in humans and mammals (Frimpong et al., 2018). Extensive paraquat applications lead to widespread residues in soil surface and aquatic environments that ultimately enter the food chain (Pateiro-Moure et al., 2009).
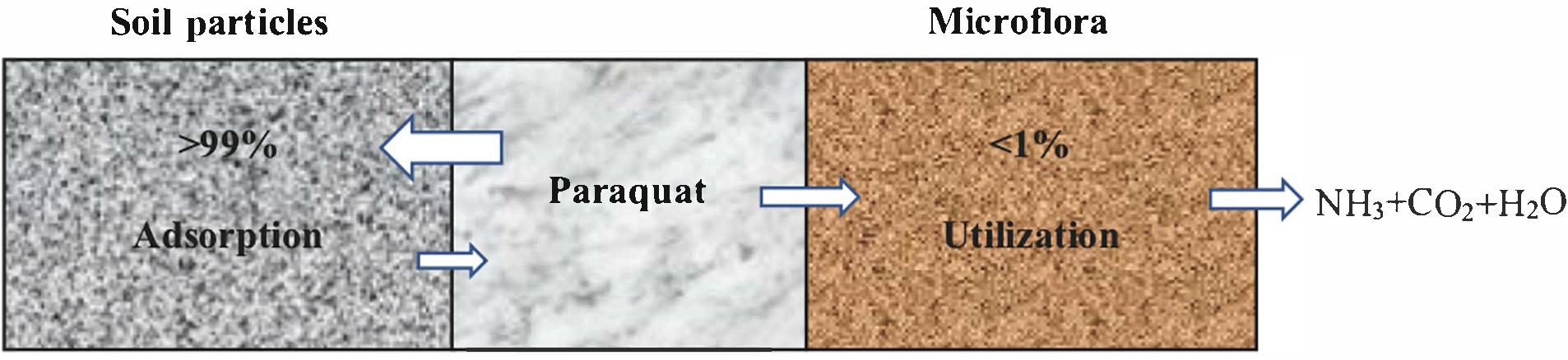
Depending upon the texture and composition, soil particles immediately adsorb paraquat (Singh and Singh, 2016). Rashidzadeh et al. (2017) described better paraquat adsorption in montmorillonite as compared to clinoptilolite clay and adsorption in clay is stronger than sandy soil (Amondham et al., 2006). Microorganisms can only utilize and degrade less than 1% of paraquat in soil particles (Figure 1) (Roberts et al., 2002), and its half-life can be up to 3∼6.6 years (Hance et al., 1980; Pateiro-Moure et al., 2009). Alexander (1999) concluded that microorganisms could completely degrade soil paraquat in 6 years. Such a prolonged half-life causes serious impact on humans and other mammals.
During the past few decades, paraquat poisoning has been reported on a global scale. According to the World Health Organization (WHO), the minimum lethal dose of concentrated paraquat in humans is 35 mg/kg (Tsai, 2013). Paraquat can cause neurological damage and dysfunctional kidneys and liver in humans and animals. In severe cases, fatalities can occur due to irreversible pulmonary fibrosis, inflammation, and respiratory failure (Blanco-Ayala et al., 2014; Shadnia et al., 2018). The paraquat toxicity mechanism is based on redox cycle and intercellular oxidative stress (Dinis-Oliveira et al., 2008). Flechel et al. (2018) revealed that out of 26 patients who ingested paraquat at a median intake of 103 mg/kg, only six survived after 36 h of emergency treatment. A study by Elenga et al. (2018) revealed higher adult mortality (65%) as compared to children (22%) due to difference in ingested amounts. In addition, paraquat is also associated with Parkinson’s disease (Bastias-Candia et al., 2019; Tamano et al., 2019). These studies indicate the severe toxicity of paraquat and its potential damage to mammalian cells.
Considering the hazards of paraquat residues on the environment and humans, it is necessary to study paraquat-degrading microorganisms. Microbial degradation is a significant pathway for paraquat breakdown (Mercurio et al., 2014; Wang et al., 2016) and various microorganisms including fungi, bacteria and yeast, have been reported for effective paraquat degradation (Wu et al., 2013; Bai et al., 2014). Anti-oxidative enzyme superoxide dismutase (SOD) contributes toward paraquat tolerance by removing superoxides from living cells, produced during paraquat toxicity (Dos Santos and Silva, 2015). Hoshina et al. (2018) indicated that catalase (CAT) could reduce paraquat cytotoxicity by increasing 4-phenylbutyrate. However, the literature lacks reports on microbial degradation pathways of paraquat, and no study has been reported about any functional gene of paraquat degradation. Here we aim to summarize microbial and physicochemical paraquat degradation methods and pathways, and analyze the potential of bioremediation in paraquat-contaminated environments. This review will increase the understanding about paraquat-contaminated sites and possible solutions through microbial applications.
Physicochemical Methods for Paraquat Degradation
Paraquat herbicide is widely used in agriculture and silviculture; however, increasing attention is being paid to its soil residues. Currently, adsorption and degradation are the two main methods to remove/reduce paraquat from aquatic environment. Previous studies concluded that the adsorption of paraquat mainly depends on activated carbon (Sieliechi and Thue, 2015), activated bleaching soil (Tsai et al., 2004), modified zeolite (Pukcothanung et al., 2018), montmorillonite (Gu et al., 2015) and organoclay (Guegan et al., 2015; Keawkumay et al., 2017). On the other hand, physicochemical paraquat degradation methods depend on titanium dioxide, ozone, ultraviolet radiation and various advanced oxidation processes (AOPs) (Hamad et al., 2016; Gao et al., 2017; Javier et al., 2017).
A photocatalyst titanium dioxide has emerged as a promising degradation pathway for pesticide pollution treatment because of its low price, high efficiency and non-toxic properties (EI Madani et al., 2015; Phuinthiang and Kajitvichyanukul, 2019). To the best of our knowledge, the wavelength of incident radiation required to activate photocatalysis is related to bandgap energy of semiconductor materials, and larger bandgap energy requires shorter radiation wavelength (Liu et al., 2010). Titanium dioxide has a bandgap energy of 3.2 eV that requires ultraviolet light for activation (De Souza and Corio, 2013). Cantavenera et al. (2007) showed that the complete photocatalytic mineralization of paraquat (20 mg/L) was achieved after 3 h of irradiation by 0.4 g/L TiO2 at pH 5.8. According to Badli et al. (2016), similar conditions resulted in only 9.08% paraquat removal in the absence of photocatalysis and increased to 84.41% after the addition of ZrO2/TiO2 (20:80) at 0.3 g/L. This indicates that paraquat degradation necessarily requires oxygen, catalyst and UV-light.
Sorolla et al. (2012) reported 71% paraquat degradation by 2 wt.% Cu-TiO2/SBA-15 under visible light in 8 h that decreased to 67% at 5 wt.% Cu-TiO2/SBA-15. Scattering effect in suspension by excessive photocatalyst hinders light photons from entering the reaction mixture and reduces its paraquat degradation capability (Bensaadi et al., 2014). Kanchanatip et al. (2011) demonstrated that TiO2 along with vanadium and fullerene (C60) degraded 70% of paraquat under visible light after 4 h. Liu et al. (2014) evaluated photocatalytic degradation activities of HPW/MCM-48 against paraquat by loading the photocatalyst phosphotungstic acid H3PW12O40 (HPW) to molecular sieve MCM-48 through impregnation method under UV radiation (365 nm). Results showed that 63.79% paraquat (50 mL, 10 mg/L) was degraded by 20 mg HPW/MCM-48 catalyst after 14 h of UV irradiation whereas only 5% paraquat degradation was noted in the blank group.
In addition to the above-mentioned paraquat degradation techniques, other methods have also been reported. Fernandes et al. (2017) described new magnetic nanosorbents, composed of magnetite cores functionalized with bio-hybrid siliceous shells that can be used to uptake paraquat from water. Biopolymer k-carrageenan induction into the siliceous shells significantly increased its paraquat adsorption capacity at 257 mg/g. Desipio et al. (2018) proposed carbon nitride system as a catalyst to remove paraquat from water. Photocatalytic decomposition of paraquat solely by carbon nitride under visible light was negligible, but the addition of hydrogen peroxide in small amounts remarkably enhanced its paraquat degrading efficiency (70%) within 10 h.
Recently, various AOPs have emerged for the treatment of industrial or agricultural paraquat-contaminated wastewater. The principal mechanism of contaminant degradation through AOPs is based on the release of a highly reactive non-specific oxidant hydroxyl radical (OH) that dissociates organic molecules in water (Vilhunen and Sillanpaa, 2010; Rosman et al., 2018). Hydroxyl radical breaks larger molecules into smaller fragments that eventually mineralize to harmless products (Ong et al., 2014). Hydroxyl radical acts as a nucleophile during paraquat degradation. Treatment of wastewater by AOPs include UV/H2O2, ultraviolet, Fenton, photoelectro-Fenton, ozonation, photochemical, and electrochemical oxidation (Khongthon et al., 2016; Khataee et al., 2017). Dhaouadi and Adhoum (2009) reported photoelectric-Fenton and electro-Fenton as the most efficient treatments to remove paraquat from aqueous acidic solution at pH 3.0. Addition of 0.2 mM Fe2+ to the water containing 20 mg/L paraquat decreases its oxygen requirement by 97 and 94%, respectively. Similarly, Ye and Lemley (2008) investigated paraquat degradation in clay slurry through AOPs and found that strong adsorption of paraquat in clay interlayers protects the herbicide from hydroxyl radicals. The major disadvantage of physicochemical methods is their failure to control photo-catalysis conditions in situ remediation and that they are not cost-effective (Arora et al., 2018; Zhan et al., 2018a).
Possible Pathways of Physicochemical Paraquat Degradation
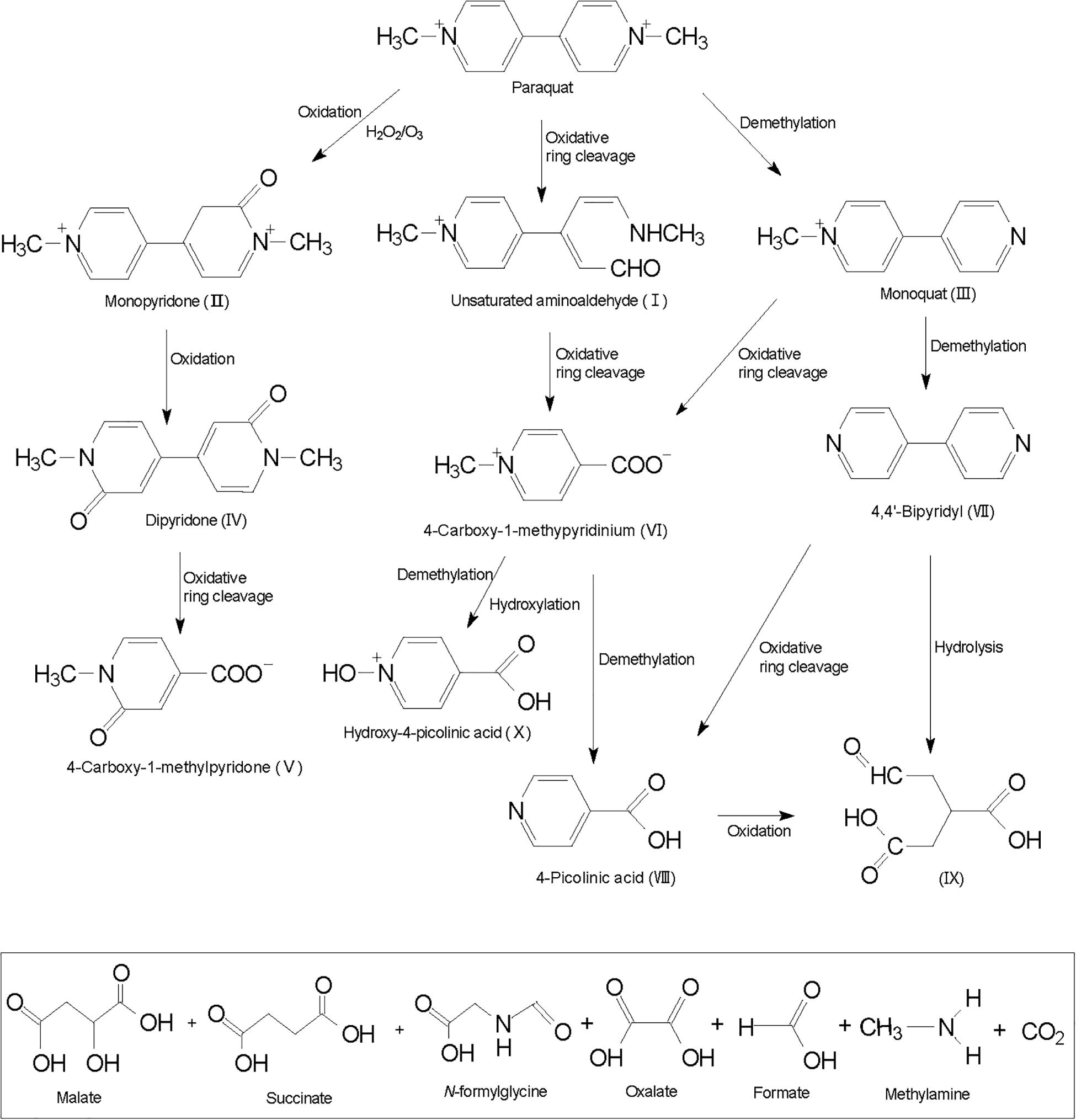
Hitherto, there is no specific literature about the paraquat degradation pathways and its byproducts. Some studies report monoquat and 4-carboxy-1-methylpyridinium as intermediate products of physiochemical paraquat degradation. Slade (1965) proposed that paraquat degradation is initiated by opening the pyridine ring between nitrogen atoms and adjacent carbon atoms, and unsaturated amino aldehyde (I) is generated through the cleavage of oxidation ring (Figure 2). Kearney et al. (1985) reported that when paraquat reacts with a strong oxidant, such as hydrogen peroxide, monopyridone (II) and monoquat (III) are produced as oxidation and demethylation products. Further oxidation of monopyridone forms dipyridone (IV) and opening up the dipyridone oxidative ring leads to the formation of 4-carboxy-1-methylpyridone (V). Oxidative ring cleavage and demethylation of monoquat leads to the formation of 4-carboxy-1-methylpyridinium ion (VI) and 4,4′-bipyridyl (VII). 4-Picolinic acid (VIII) could arise via demethylation of 4-carboxy-1-methylpyridinium or oxidative ring cleavage of 4,4′-bipyridyl in a series of reactions similar to the formation of 4-carboxy-1-methylpyridinium ion from paraquat.
A possible intermediate C6O5H8 (IX), produced from oxidation and further hydrolysis of 4-picolinic acid or 4,4′-bipyridyl, and a demethylated ring product were identified as hydroxy-4-picolinic acid (X) (Florêncio et al., 2004). Intermediate products do not always completely degrade, and some ring fragmentation products have been identified as malate, succinate, N-formylglycine, oxalate, formic, and methylamine (Dhaouadi and Adhoum, 2009). Cantavenera et al. (2007) demonstrated that after TiO2-based paraquat degradation and continuous mineralization, nitrate and ammonium ions gradually accumulated and reached up to 83 and 12% of the initial nitrogen concentration in paraquat (Dhaouadi and Adhoum, 2009).
Microbial Degradation of Paraquat
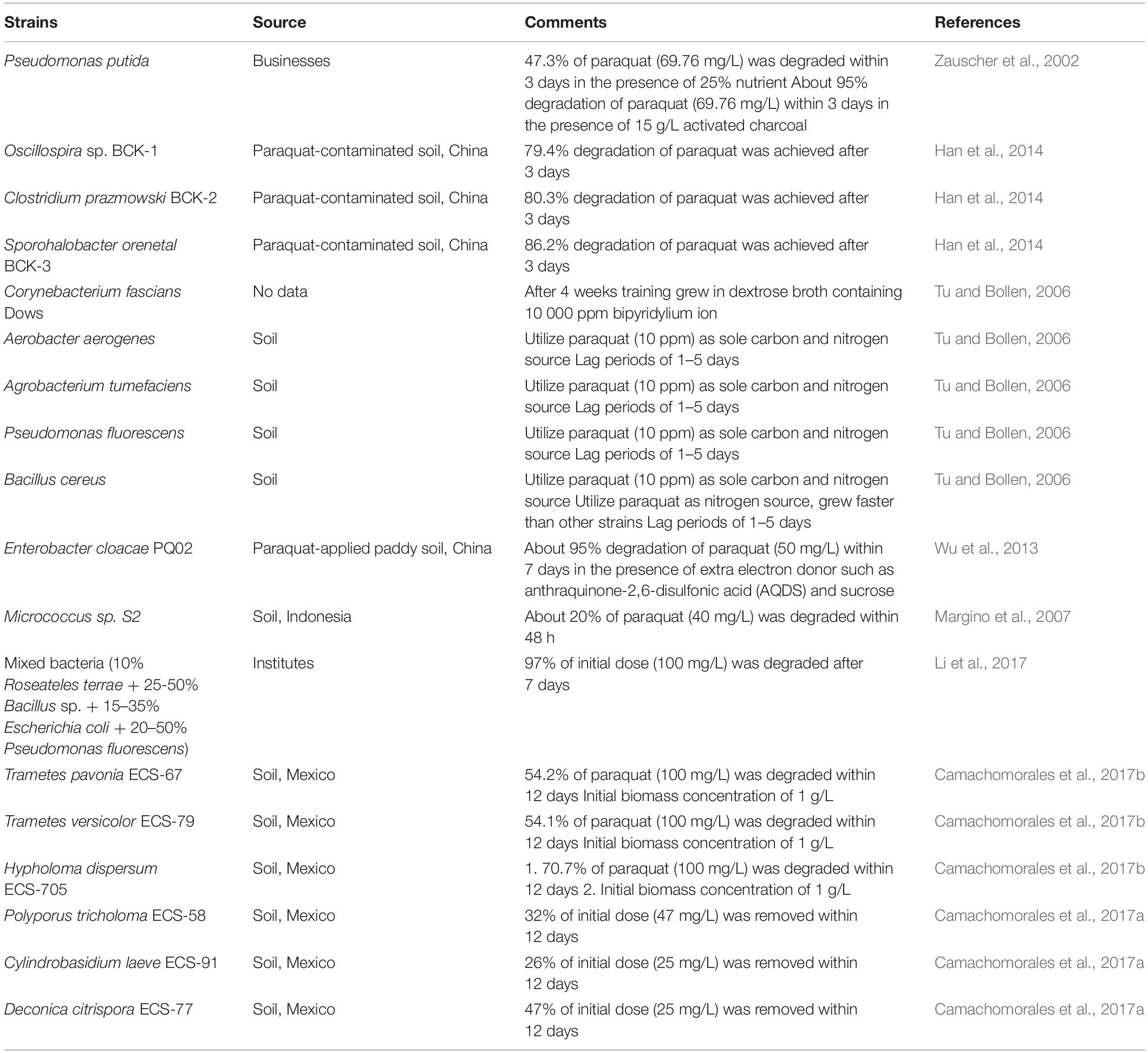
Bacterial and fungal species belonging to different genera have been isolated from paraquat-contaminated soils by enrichment culture techniques and characterized based on biochemical and molecular tools. Studies have confirmed that some bacterial and fungal (Table 1) species can degrade paraquat in soils and slurry. As illustrated in the table, several degrading microorganisms have been isolated from contaminated soils and deposited to respective microbial culture banks. Four bacterial strains including Aerobacter aerogenes, Agrobacterium tumefaciens, Pseudomonas fluorescens, and Bacillus cereus have been characterized for the mineralization of paraquat and can utilize paraquat as a sole growth source of carbon or nitrogen (Tu and Bollen, 2006). Bacteria such as Oscillospira sp. BCK-1, Clostridium prazmowski BCK-2, and Sporohalobacter orenetal BCK-3 efficiently degraded paraquat up to 79.35, 80.26, and 86.22%, respectively, after 3 days of treatment (Han et al., 2014).
The biodegradation rate in controlled conditions is influenced by multiple factors including temperature, pH, nutrients, initial concentration, inoculum size and properties of the bacterial or fungal strain (Chen et al., 2015; Cycoń and Piotrowska-Seget, 2016; Zhan et al., 2018b). Zauscher et al. (2002) demonstrated that Pseudomonas putida degraded paraquat up to 95% (69.76 mg/L) in the presence of 15 g/L activated charcoal after 3 days of treatment, whereas only 47.3% of paraquat was degraded after substituting activated carbon with nutrients. It has been reported that Corynebacterium fascians Dows tolerated extremely high concentrations of bipyridylium ion (10000 mg/L) in dextrose broth up to 4 weeks (Tu and Bollen, 2006). Wu et al. (2013) reported that Enterobacter cloacae PQ02 degraded approximately 95% of the initial paraquat dose (50 mg/L) in the presence of extra electron donor anthraquinone-2,6-disulfonic acid (AQDS) and sucrose within 7 days. These carbon sources can easily be utilized by bacteria and accelerate their growth during lag phase. Some studies have revealed that the use of mixed bacterial culture (consortium) resulted in enhanced degradation of pollutants as mixed bacterial culture follows co-metabolism for pollutant degradation (Nawong et al., 2018; Tian et al., 2018). Li et al. (2017) used four microorganisms including Roseateles terrae, Bacillus sp., Escherichia coli, and P. fluorescens, in a mixed culture for paraquat degradation, and achieved 97% degradation of initial paraquat dose (100 mg/L) over 7 days. Bacterial strains exhibited significant degradation ability and provided a potential tool for bioremediation of paraquat-contaminated environments.
Besides bacteria, fungal systems can also effectively degrade paraquat. Lipomyces starkeyi Lod and Rij completely removed paraquat (27 mg/L) from the medium within 3 days. However, when the paraquat concentration was increased twofold (54 mg/L), biomass and paraquat degradation notably decreased to less than 10% (Alexander, 1999). It was noticed that L. starkeyi could degrade paraquat under aerobic conditions. Biodegradation studies revealed that paraquat-degrading microorganisms do not exhibit similar efficiency in degrading different concentrations of paraquat. Camachomorales et al. (2017b) isolated 54 macromycetes from southeastern Mexico, and only three (Trametes pavonia ECS-67, Trametes versicolor ECS-79, and H. dispersum ECS-705) presented 54.2, 54.1, and 70.7% of paraquat (100 mg/L) degradation within 12 days. In another study Camachomorales et al. (2017a) revealed that three other macromycetes including Polyporus tricholoma ECS-58 (32%, 75 mg/L), Cylindrobasidium laeve ECS-91 (26%, 25 mg/L), and Deconica citrispora ECS-77 (47%, 25 mg/L), showed lower paraquat degradation after 12 days of incubation.
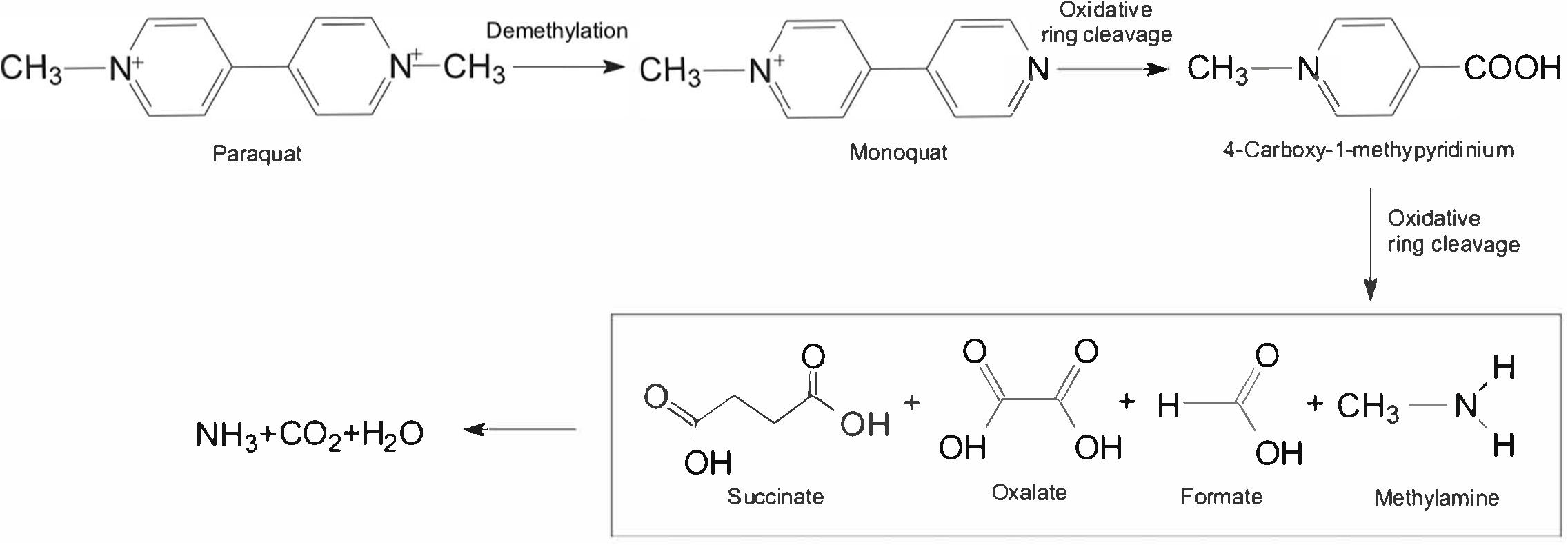
To date, degradation pathways of paraquat in microorganisms have never been reported. As shown in Figure 3, the 1st step in paraquat conversion is demethylation to form monoquat through microbial activity. In the next step, further oxidative ring cleavage of monoquat forms 4-carboxy-1-methylpyridinium ion (Dinis-Oliveira et al., 2008). Pyridinium ring carbons are released as CO2 by 14C-labeling, and 4-carboxy-1-methylpyridinium ion readily degrades in soils into methylamine and CO2 by microbial activity (Singh and Singh, 2016). Methylamine can be used as a source of nitrogen and carbon for microbial growth. During the paraquat biodegradation, ring fragmentation products identified as three carboxylic acids were oxalate, formate and succinate whereas methylamine and carbon dioxide were detected as the ultimate metabolites (Dinis-Oliveira et al., 2008). However, the enzymology of paraquat degradation is rarely reported and other intermediates have not been identified. Paraquat-degrading microbes might utilize the product from upstream pathways of cell energy via glycolytic and tricarboxylic acid pathways. Further studies about paraquat biodegradation are required to detect common metabolites and enzymes responsible for converting different intermediates.
Bioremediation Potential of Paraquat-Degrading Microorganisms
Microbial remediation is the process of transforming highly toxic compounds into low-toxic or non-toxic products after a series of domestication, enrichment, screening and culturing of the strains having degradation characteristics (Chen et al., 2015; Liu et al., 2015; Cycoń and Piotrowska-Seget, 2016). Bioremediation is supposed to be more promising for the removal of chemical pollutants in water and soil environments (Chen et al., 2014; Yang et al., 2018). Physicochemical methods of controlling or mitigating environmental pollution were less effective and more expensive than biological methods of remediation (Arora et al., 2018). The use of microorganisms for bioremediation of contaminated sites may be a viable alternative to conventional clean-up methods because a variety of microorganisms are known to utilize chemical pollutants as a sole carbon or energy source (Gonzalez-Marquez et al., 2019). In agriculture, paraquat residues in the topsoil layer and plant surface are degraded by photolysis, while most of the remaining are absorbed by clay lattice and loose weeding activity. According to Alexander (1999), paraquat is completely degraded by soil microorganisms within 6 years into ammonia, carbon dioxide and water (Figure 3). Hence, microbial bioremediation is considered as an efficient, safe and cost-effective strategy to remove paraquat from contaminated environments. Most of the microorganisms in the environment (90∼99%) cannot be cultured, and paraquat half-life in the soil can be as long as 6.6 years (Alexander, 1999). Therefore, it is necessary to isolate and identify the high-efficiency paraquat-degrading microorganisms and determine their potential for the bioremediation of paraquat-contaminated environments. To solve isolation difficulties, metagenomics-based approaches can be followed for paraquat biodegradation study.
A few paraquat-degrading strains have been isolated, but their degradation efficiency in fields is unstable. Response surface method can optimize the conditions for microbial degradation of pollutants (Chen et al., 2013; Xiao et al., 2015). Hitherto, studies involved in paraquat-degrading microbes are few, and mainly include bacteria. The paraquat degradation rate can nearly reach 100% after adding exogenous electron donor or activated carbon. For example, P. putida and E. cloacae PQ02 showed a 95% paraquat degradation rate after the addition of activated carbon and glucose. It is worth mentioning that in a patent filed by Li et al. (2017), a bacterial consortium achieved 97% paraquat degradation (100 mg/L). There is less information about the fungal degradation of paraquat, and the overall degradation effect of fungi is not as significant as that of bacteria. A recent study on fungi by Camachomorales et al. (2017b) indicated that 70.7% paraquat was removed by Hypholoma dispersum ECS-705 in 12 days. Bacteria metabolize paraquat in two ways: (1) bacteria utilize paraquat as the sole nitrogen or carbon source, and (2) bacteria transform paraquat into low-toxic or non-toxic products through co-metabolism. In both cases, microbial biodegradation of paraquat is quite promising.
Conclusion and Future Perspectives
Large-scale paraquat applications in the agriculture sector are urgently demanded to mitigate the effects of this compound. Established hazardous impacts of paraquat on humans and environment urge us to develop safe, efficient and economical technologies for the remediation of paraquat-contaminated environments. During the last two decades, physicochemical degradation based on AOPs has been developed as an effective measure to remove paraquat residues from sewage. However, considering the high cost and uncontrollable reaction conditions, it is not widely applicable.
Bacteria isolated from paraquat-contaminated environments with high degradation capacity and potential for bioremediation are considered as the most promising strategy. However, the published literature generally reveals the use of a single strain for bioremediation testing, which could not produce ideal effects under field conditions. To overcome such problems, consortia consisting of various bacteria could be used for large-scale applications. Members of consortia play various roles in different stages of degradation and can produce better degradation effects than a single strain.
In order to achieve higher degradation efficiency, the relationship between members of such a consortium and their adaptability to adverse environments should be studied. In addition, it is important to screen degrading bacteria that could sustain a wide range of soil environmental factors such as pH, temperature, salinity, heavy metals, and nutrient availability.
The role of functional genes and enzymes in bioremediation of paraquat-contaminated environment is the key to understand degradation mechanism of paraquat. Although many paraquat-degrading microorganisms have been isolated and characterized in previous studies, their functional genes have not yet been reported. Therefore, further studies about related functional genes and enzymes are needed before the field-scale applications of paraquat-degrading microorganisms. Modern high-throughput omics technologies can facilitate to achieve clear information about the metabolic pathway, regulatory genes and enzymes for paraquat biodegradation.
Author Contributions
SC conceived the idea. YH contributed to the writing and prepared the figures and tables. HZ, PB, and SC participated in revising the manuscript. All authors approved the final manuscript for publication.
Funding
This study was partially funded by grants from the National Natural Science Foundation of China (31401763), the National Key Project for Basic Research (2015CB150600), Guangdong Province Science and Technology Innovation Strategy Special Fund (2018B020206001), Guangdong Natural Science Funds for Distinguished Young Scholar (2015A030306038), Guangdong Special Branch Plan for Young Talent with Scientific and Technological Innovation (2017TQ04N026), and the Science and Technology Planning Project of Guangdong Province (2017A010105008).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexander, M. (1999). Biodegradation and Bioremediation, 2nd Edn. New York, NY: Academic Press, 453.
Amondham, W., Parkpian, P., Polprasert, C., Delaune, R., and Jugsujinda, A. (2006). Paraquat adsorption, degradation, and remobilization in tropical soils of Thailand. J. Environ. Sci. Heal. B. 41, 485–507. doi: 10.1080/03601230600701635
Arora, P. K., Srivastava, A., Garg, S. K., and Singh, V. P. (2018). Recent advances in degradation of chloronitrophenols. Bioresour. Technol. 250, 902–909. doi: 10.1016/j.biortech.2017.12.007
Badli, N. A., Ali, R., Bakar, W. A. W. A., and Yuliati, L. (2016). Role of heterojunction ZrTiO4/ZrTi2O6/TiO2 photocatalyst towards the degradation of paraquat dichloride and optimization study by box–behnken design. Arab. J. Chem. 10, 935–943. doi: 10.1016/j.arabjc.2016.02.011
Bai, G. X., Xu, G. S., Li, J., and Gu, J. W. (2014). Optimization of culture conditions for paraquat degradation by lipomyces starkeyi. Ind. Microbiol. 44, 47–50. doi: 10.3969/j.issn.1001-6678.2014.05.009
Bang, Y. J., Kim, J., and Lee, W. J. (2017). Paraquat use among farmers in Korea after the ban. Arch. Environ. Occup. Health. 72, 231–234. doi: 10.1080/19338244.2016.1192982
Bastias-Candia, S., Zolezzi, J. M., and Inestrosa, N. C. (2019). Revisiting the paraquat-induced sporadic Parkinson’s disease-like model. Mol. Neurobiol. 56, 1044–1055. doi: 10.1007/s12035-018-1148-z
Bensaadi, Z., Yeddou-Mezenner, N., Trari, M., and Medjene, F. (2014). Kinetic studies of β-blocker photodegradation on TiO2. J. Environ. Chem. Eng. 2, 1371–1377. doi: 10.1016/j.jece.2014.03.025
Blanco-Ayala, T., Andérica-Romero, A. C., and Pedraza-Chaverri, J. (2014). New insights into antioxidant strategies against paraquat toxicity. Free Radic. Res. 48, 628–640. doi: 10.3109/10715762.2014.899694
Camachomorales, R. L., Gerardogerardo, J. L., Karina, G. N., and José, E. S. (2017a). Ligninolytic enzyme production by white rot fungi during paraquat (herbicide) degradation. Rev. Argent. Microbiol. 49, 189–196. doi: 10.1016/j.ram.2016.11.004
Camachomorales, R. L., Karina, G. N., and José, E. S. (2017b). Degradation of the herbicide paraquat by macromycetes isolated from southeastern Mexico. 3 Biotech 7, 324–334. doi: 10.1007/s13205-017-0967-3
Cantavenera, M. J., Catanzaro, I., Loddo, V., Palmisano, L., and Sciandrello, G. (2007). Photocatalytic degradation of paraquat and genotoxicity of its intermediate products. J. Photoch. Photobio. B 185, 277–282. doi: 10.1016/j.jphotochem.2006.06.021
Cha, E. S., Chang, S. S., Gunnell, D., Eddleston, M., Khang, Y. H., and Lee, W. J. (2016). Impact of paraquat regulation on suicide in South Korea. Int. J. Epidemiol. 45, 470–479. doi: 10.1093/ije/dyv304
Chen, S., Chang, C., Deng, Y., An, S., Dong, Y. H., Zhou, J., et al. (2014). Fenpropathrin biodegradation pathway in Bacillus sp. DG-02 and its potential for bioremediation of pyrethroid-contaminated soils. J. Agric. Food Chem. 62, 2147–2157. doi: 10.1021/jf404908j
Chen, S., Deng, Y., Chang, C., Lee, J., Cheng, Y., Cui, Z., et al. (2015). Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci. Rep. 5:8784. doi: 10.1038/srep08784
Chen, S., Dong, Y. H., Chang, C., Deng, Y., Zhang, X. F., Zhong, G., et al. (2013). Characterization of a novel cyfluthrin-degrading bacterial strain Brevibacterium aureum and its biochemical degradation pathway. Bioresour. Technol. 132, 16–23. doi: 10.1016/j.biortech.2013.01.002
Cycoń, M., and Piotrowska-Seget, Z. (2016). Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: a review. Front. Microbiol. 7:1463. doi: 10.3389/fmicb.2016.01463
De Souza, M. L., and Corio, P. (2013). Effect of silver nanoparticles on TiO2-mediated photodegradation of Alizarin Red S. Appl. Catal. B Environ. 13, 325–333. doi: 10.1016/j.apcatb.2013.02.012
Desipio, M. M., Ryan, T., and Dipendu, S. (2018). Photocatalytic decomposition of paraquat under visible light by carbon nitride and hydrogen peroxide. OPTIK. 172, 1047–1056. doi: 10.1016/j.ijleo.2018.07.124
Dhaouadi, A., and Adhoum, N. (2009). Degradation of paraquat herbicide by electrochemical advanced oxidation methods. J. Electroanal. Chem. 637, 33–42. doi: 10.1016/j.jelechem.2009.09.027
Dinis-Oliveira, R. J., Duarte, J. A., Sánchez-Navarro, A., Remião, F., and Carvalho, F. (2008). Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 38, 13–71. doi: 10.1080/10408440701669959
Dos Santos, C. M., and Silva, M. D. (2015). Physiological and biochemical responses of sugarcane to oxidative stress induced by water deficit and paraquat. Acta Physiol. Plant. 37:172. doi: 10.1007/s11738-015-1935-3
Elenga, N., Merlin, C., Le Guern, R., Kom-Tchameni, R., Ducrot, Y. M., Pradier, M., et al. (2018). Clinical features and prognosis of paraquat poisoning in french guiana A review of 62 cases. Medicine 97:e9621. doi: 10.1097/MD.0000000000009621
Fernandes, T., Soares, S. F., Trindade, T., and Daniel-Da-Silva, A. L. (2017). Magnetic hybrid nanosorbents for the uptake of paraquat from water. Nanomaterials 7, E68. doi: 10.3390/nano7030068
Flechel, A., Jolivet, A., Boukhari, R., Misslin-Tritsch, C., Manca, M. F., Wiel, E., et al. (2018). Paraquat poisoning in western french guyana: a public health problem persisting ten years after its withdrawal from the French market. Eur. Rev. Med. Pharmaco. 22, 7034–7038. doi: 10.26355/eurrev-201810-16175
Florêncio, M. H., Pires, E., Castro, A. L., Nunes, M. R., Borges, C., and Costa, F. M. (2004). Photodegradation of diquat and paraquat in aqueous solutions by titanium dioxide: evolution of degradation reactions and characterisation of intermediates. Chemosphere 55, 345–355. doi: 10.1016/j.chemosphere.2003.11.013
Frimpong, J. O., Ofori, E. S. K., Yeboah, S., Marri, D., Offei, B. K., Apaatah, F., et al. (2018). Evaluating the impact of synthetic herbicides on soil dwelling macrobes an the physical state of soil in an agro-ecosystem. Ecotox. Environ. Safe 156, 205–215. doi: 10.1016/j.ecoenv.2018.03.034
Gao, Z. C., Lin, Y. L., Xu, B., Pan, Y., Xia, S. J., Gao, N. Y., et al. (2017). Degradation of acrylamide by the UV/chlorine advanced oxidation process. Chemosphere 187, 268–276. doi: 10.1016/j.chemosphere.2017.08.085
Gonzalez-Marquez, A., Loera-Corral, O., Santacruz-Juarez, E., Tlecuitl-Beristain, S., Garcia-Davila, J., Viniegra-Gonzalez, G., et al. (2019). Biodegradation patterns of the endocrine disrupting pollutant di(2-ethylhexyl) phthalate by Fusarium culmorum. Ecotox. Environ. Safe 170, 293–299. doi: 10.1016/j.ecoenv.2018.11.140
Gu, Z., Gao, M. L., Lu, L. F., Liu, Y. N., and Yang, S. F. (2015). Montmorillonite functionalized with zwitterionic surfactant as a highly efficient adsorbent for herbicides. Ind. Eng. Chem. Res. 54, 4947–4955. doi: 10.1021/acs.iecr.5b00438
Guegan, R., Giovanela, M., Warmont, F., and Motelica-Heino, M. (2015). Nonionic organoclay: a ‘Swiss Army knife’ for the adsorption of organic micro-pollutants? J. Colloid Interf. Sci. 437, 71–79. doi: 10.1016/j.jcis.2014.09.043
Hamad, D., Dhib, R., and Mehrvar, M. (2016). Photochemical degradation of aqueous polyvinyl alcohol in a continuous UV/H2O2 process: experimental and statistical analysis. J. Polym. Environ. 24, 72–83. doi: 10.1007/s10924-016-0750-2
Han, X., Yuan, R., Wang, G. Q., and Zhang, C. J. (2014). Isolation of paraquat degrading bacteria and identification of degradation characteristics. Anhui Agri. Sci. Bull. 20, 38–39. doi: 10.16377/j.cnki.issn1007-7731.2014.08.004
Hance, R. J., Byast, T. H., and Smith, P. D. (1980). Apparent decomposition of paraquat in soil. Soil Biol. Biochem. 12, 447–448. doi: 10.1016/0038-0717(80)90025-5
Hoshina, C., Omura, T., Okuda, K., Tanaka, H., Asari, M., Isozaki, S., et al. (2018). Paraquat toxicity is attenuated by 4-phenylbutyrate-induced phosphorylation of ERK2 via PI3K in A549 cells. Biochem. Bioph. Rese. Co. 503, 809–814. doi: 10.1016/j.bbrc.2018.06.080
Javier, B. F., Real, F. J., Acero, J. L., and Casas, F. (2017). Assessment of the UV/Cl2 advanced oxidation process for the degradation of the emerging contaminants amitriptyline hydrochloride, methyl salicylate and 2-phenoxyethanol in water systems. Environ. Technol. 38, 1–9. doi: 10.1080/09593330.2016.1269836
Kanchanatip, E., Grisdanurak, N., Thongruang, R., and Neramittagapong, A. (2011). Degradation of paraquat under visible light over fullerene modified V-TiO2. React. Kinet. Mech. Cat. 103, 227–237. doi: 10.1007/s11144-011-0293-4
Kearney, P. C., Ruth, J. M., Zeng, Q., and Mazzocchi, P. (1985). UV ozonation of paraquat. J. Agric. Food Chem. 33, 953–957. doi: 10.1021/jf00065a044
Keawkumay, C., Rakmae, S., Rongchapo, W., Suppakarn, N., Prayoonpokarach, S., and Wittayakun, J. (2017). Adsorption of paraquat and pirimiphos-methyl by montmorillonite modified with tetradecylammonium chloride and intragallery templating method. Adsorpt. Sci. Technol. 35, 357–371. doi: 10.1177/0263617416677351
Khataee, A., Sajjadi, S., Hasanzadeh, A., Vahid, B., and Joo, S. W. (2017). One-step preparation of nanostructured martite catalyst and graphite electrode by glow discharge plasma for heterogeneous electro-Fenton like process. J. Environ. Manage. 199, 31–45. doi: 10.1016/j.jenvman.2017.04.095
Khongthon, W., Jovanovic, G., Yokochi, A., Sangvanich, P., and Pavarajarn, V. (2016). Degradation of diuron via an electrochemical advanced oxidation process in a microscale-based reactor. Chem. Eng. J. 292, 298–307. doi: 10.1016/j.cej.2016.02.042
Li, Y., Ge, X. Z., Wang, X. Y., and Gao, R. (2017). The Invention Discloses a Compound Bacterial Agent used to Degrade Paraquat and a Preparation Method. China. Patent No CN 106520618 A. Beijing: National Intellectual Property Administration.
Liu, G., Wang, L., Yang, H. G., Cheng, H. M., and Lu, G. Q. (2010). Titania-based photocatalysts-crystal growth, doping and heterostructuring. J. Mater. Chem. 20, 831–843. doi: 10.1039/B909930A
Liu, J., Chen, S., Ding, J., Xiao, Y., Han, H., and Zhong, G. (2015). Sugarcane bagasse as support for immobilization of Bacillus pumilus HZ-2 and its use in bioremediation of mesotrione-contaminated soils. Appl. Microbiol. Biotechnol. 99, 10839–10851. doi: 10.1007/s00253-015-6935-0
Liu, X., Li, Y. Z., Gan, Q., and Feng, C. G. (2014). Preparation of H3PW12O40/MCM-48 and its photocatalytic degradation of pesticides. Spectrosc. Spect. Anal. 34, 2157–2161. doi: 10.3964/j.issn.1000-0593201408-2157-05
Madani, M., Harir, M., Zrineh, A., and El Azzouzi, M. (2015). Photodegradation of imazethapyr herbicide by using slurry and supported TiO2: efficiency comparison. Arab. J. Chem. 8, 181–185. doi: 10.1016/j.arabjc.2011.03.013
Margino, S., Martani, E., and Magdalena, M. (2007). Superoxide dismutase of Micrococcus sp. S2 and its involve in paraquat detoxification. Indones. J. Biotechnol. 12, 973–979. doi: 10.22146/ijbiotech.7768
Mercurio, P., Flores, F., Mueller, J. F., Carter, S., and Negri, A. P. (2014). Glyphosate persistence in seawater. Mar. Pollut. Bull. 85, 385–390. doi: 10.1016/j.marpolbul.2014.01.021
Nawong, C., Umsakul, K., and Sermwittayawong, N. (2018). Rubber gloves biodegradation by a consortium, mixed culture and pure culture isolated from soil samples. Brazi. J. Microbiol. 49, 481–488. doi: 10.1016/j.bjm.2017.07.006
Ong, W. J., Tan, L. L., Chai, S. P., Yong, S. T., and Mohamed, A. R. (2014). Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environment remediation. ChemSusChem 7, 690–719. doi: 10.1002/chin.201423225
Paraquat Information Center (2018). Paraquat Information Center. Available at: https://paraquat.com/en/use/crops (accessed November 16, 2018)Google Scholar
Pateiro-Moure, M., Nóvoa-Muñoz, J. C., Arias-Estévez, M., López-Periago, E., Martínez-Carballo, E., and Simal-Gándara, J. (2009). Quaternary herbicides retention by the amendment of acid soils with a bentonite-based waste from wineries. J. Hazard. Mater. 164, 769–775. doi: 10.1016/j.jhazmat.2008.08.071
Phuinthiang, P., and Kajitvichyanukul, P. (2019). Degradation of paraquat from contaminated water using green TiO2 nanoparticles synthesized from Coffea arabica L. in photocatalytic process. Water Sci. Technol. 79, 905–910. doi: 10.2166/wst.2018.493
Pukcothanung, Y., Siritanon, T., and Rangsriwatananon, K. (2018). The efficiency of zeolite Y and surfactant-modified zeolite Y for removal of 2, 4-dichlorophenoxyacetic acid and 1, 1’-dimethyl-4, 4’-bipyridinium ion. Micropor. Mesopor. Mat. 258, 131–140. doi: 10.1016/j.micromeso.2017.08.035
Rashidipour, M., Maleki, A., Kordi, S., Birjandi, M., Pajouhi, N., Mohammadi, E., et al. (2019). Pectin/chitosan/tripolyphosphate nanoparticles: efficient carriers for reducing soil sorption, cytotoxicity, and mutagenicity of paraquat and enhancing its herbicide activity. J. Agric. Food Chem. 67, 5736–5745. doi: 10.1021/acs.jafc.9b01106
Rashidzadeh, A., Olad, A., and Hejazi, M. J. (2017). Controlled release systems based on intercalated paraquat onto montmorillonite and clinoptilolite clays encapsulated with sodium alginate. Adv. Polym. Tech. 36, 177–185. doi: 10.1002/adv.21597
Reczek, C. R., Birsoy, K., Kong, H., Martínez-Reyes, I., Wang, T., Gao, P., et al. (2017). A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat. Chem. Biol. 13, 1274–1279. doi: 10.1038/nchembio.2499
Roberts, T. R., Dyson, J. S., and Lane, M. C. G. (2002). Deactivation of the biological activity of paraquat in the soil environment: a review of long-term environmental fate. J. Agric. Food Chem. 50, 3623–3631. doi: 10.1021/jf011323x
Rosman, N., Salleh, W. N. W., Mohamed, M. A., Jaafar, J., Ismail, A. F., and Harun, Z. (2018). Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interf. Sci. 532, 236–260. doi: 10.1016/j.jcis.2018.07.118
Setif, P. (2015). Electron-transfer kinetics in cyanobacterial cells: methyl viologen is a poor inhibitor of linear electron flow. BBA-Bioenergetics 1847, 212–222. doi: 10.1016/j.bbabio.2014.10.008
Shadnia, S., Ebadollahi-Natanzi, A., Ahmadzadeh, S., Karami-Mohajeri, S., Pourshojaei, Y., and Rahimi, H. R. (2018). Delayed death following paraquat poisoning: three case reports and a literature review. Toxicol. Res. 7, 745–753. doi: 10.1039/c8tx00120k
Sieliechi, J. M., and Thue, P. S. (2015). Removal of paraquat from drinking water by activated carbon prepared from waste wood. Desalin. Water Treat. 55, 986–998. doi: 10.1080/19443994.2014.922504
Singh, B., and Singh, K. (2016). Microbial degradation of herbicides. Crit. Rev. Microbiol. 42, 245–261. doi: 10.3109/1040841x.2014.929564
Slade, P. (1965). Photochemical degradation of paraquat. Nature. 207, 515–516. doi: 10.1038/207515a0
Sorolla, M. G., Dalida, M. L., Khemthong, P., and Grisdanurak, N. (2012). Photocatalytic degradation of paraquat using nanosized Cu-TiO2/SBA-15 under UV and visible light. J. Environ. Sci. 24, 1125–1132. doi: 10.1016/s1001-0742(11)60874-7
Tamano, H., Morioka, H., Nishio, R., Takeuchi, A., and Takeda, A. (2019). Blockade of rapid influx of extracellular Zn2+ into nigral dopaminergic neurons overcomes paraquat-induced Parkinson’s disease in rats. Mol. Neurobiol. 56, 4539–4548. doi: 10.1007/s12035-018-1398-9
Tian, X. M., Wang, X. L., Peng, S. T., Wang, Z., Zhou, R., and Tian, H. (2018). Isolation, screening, and crude oil degradation characteristics of hydrocarbons-degrading bacteria for treatment of oily wastewater. Water Sci. Technol. 78, 2626–2638. doi: 10.2166/wst.2019.025
Tsai, W. T. (2013). A review on environmental exposure and health risks of herbicide paraquat. Toxicol. Environ. Chem. 95, 197–206. doi: 10.1080/02772248.2012.761999
Tsai, W. T., Lai, C. W., and Hsien, K. J. (2004). Adsorption kinetics of herbicide paraquat from aqueous solution onto activated bleaching earth. Chemosphere. 55, 829–837. doi: 10.1016/j.chemosphere.2003.11.043
Tu, C. M., and Bollen, W. B. (2006). Interaction between paraquat and microbes in soils. Weed Res. 8, 38–45. doi: 10.1111/j.1365-3180.1968.tb01399.x
Verssimo, G., Bast, A., and Weseler, A. R. (2017). Paraquat disrupts the anti-inflammatory action of cortisol in human macrophages in vitro: therapeutic implications for paraquat intoxications. Toxicol. Res. 6, 232–241. doi: 10.1039/c6tx00406g
Vilhunen, S., and Sillanpaa, M. (2010). Recent developments in photochemical and chemical AOPs in water treatment: a mini-review. Rev. Environ. Sci. Bio. 9, 323–330. doi: 10.1007/s11157-010-9216-5
Wang, S., Seiwert, B., Kästner, M., Miltner, A., Schäffer, A., Reemtsma, T., et al. (2016). (Bio) degradation of glyphosate in water-sediment microcosms–a stable isotope co-labeling approach. Water Res. 99, 91–100. doi: 10.1016/j.watres.2016.04.041
Wu, C. Y., Liu, J. K., Chen, S. S., Deng, X., and Li, Q. F. (2013). Isolation and characterization of paraquat-degrading extracellular humus-reducing bacteria from vegetable field. Adv. Mat. Res. 807-809, 1026–1030. doi: 10.4028/www.scientific.net/amr.807-809.1026
Xiao, Y., Chen, S., Gao, Y., Hu, W., Hu, M., and Zhong, G. (2015). Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Appl. Microbiol. Biotechnol. 99, 2849–2859. doi: 10.1007/s00253-014-6164-y
Yang, J., Feng, Y., Zhan, H., Liu, J., Yang, F., Zhang, K., et al. (2018). Characterization of a pyrethroid-degrading Pseudomonas fulva strain P31 and biochemical degradation pathway of D-phenothrin. Front. Microbiol. 9:1003. doi: 10.3389/fmicb.2018.01003
Ye, P., and Lemley, A. T. (2008). Adsorption effect on the degradation of carbaryl, mecoprop, and paraquat by anodic Fenton treatment in an SWy-2 montmorillonite clay slurry. J. Agric. Food Chem. 56, 10200–10207. doi: 10.1021/jf801922r
Zauscher, F., Chalela, G., and Kopytko, M. (2002). Biodegradation of two commercial herbicides (Gramoxone and Matancha) by the bacteria Pseudomonas putida. Electron. J. Biotechn. 5, 182–195. doi: 10.2225/vol5-issue2-fulltext-1
Zhan, H., Feng, Y., Fan, X., and Chen, S. (2018a). Recent advances in glyphosate biodegradation. Appl. Microbiol. Biot. 102, 5033–5043. doi: 10.1007/s00253-018-9035-0
Keywords: paraquat, bioremediation, microbial degradation, degradation pathways, oxidation
Citation: Huang Y, Zhan H, Bhatt P and Chen S (2019) Paraquat Degradation From Contaminated Environments: Current Achievements and Perspectives. Front. Microbiol. 10:1754. doi: 10.3389/fmicb.2019.01754
Received: 14 March 2019; Accepted: 15 July 2019;
Published: 02 August 2019.
Edited by:
Qiang Wang, Institute of Hydrobiology (CAS), ChinaReviewed by:
Jian He, Nanjing Agricultural University, ChinaHongzhi Tang, Shanghai Jiao Tong University, China
Copyright © 2019 Huang, Zhan, Bhatt and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohua Chen, shchen@scau.edu.cn
 Yaohua Huang
Yaohua Huang Hui Zhan
Hui Zhan Pankaj Bhatt
Pankaj Bhatt Shaohua Chen
Shaohua Chen