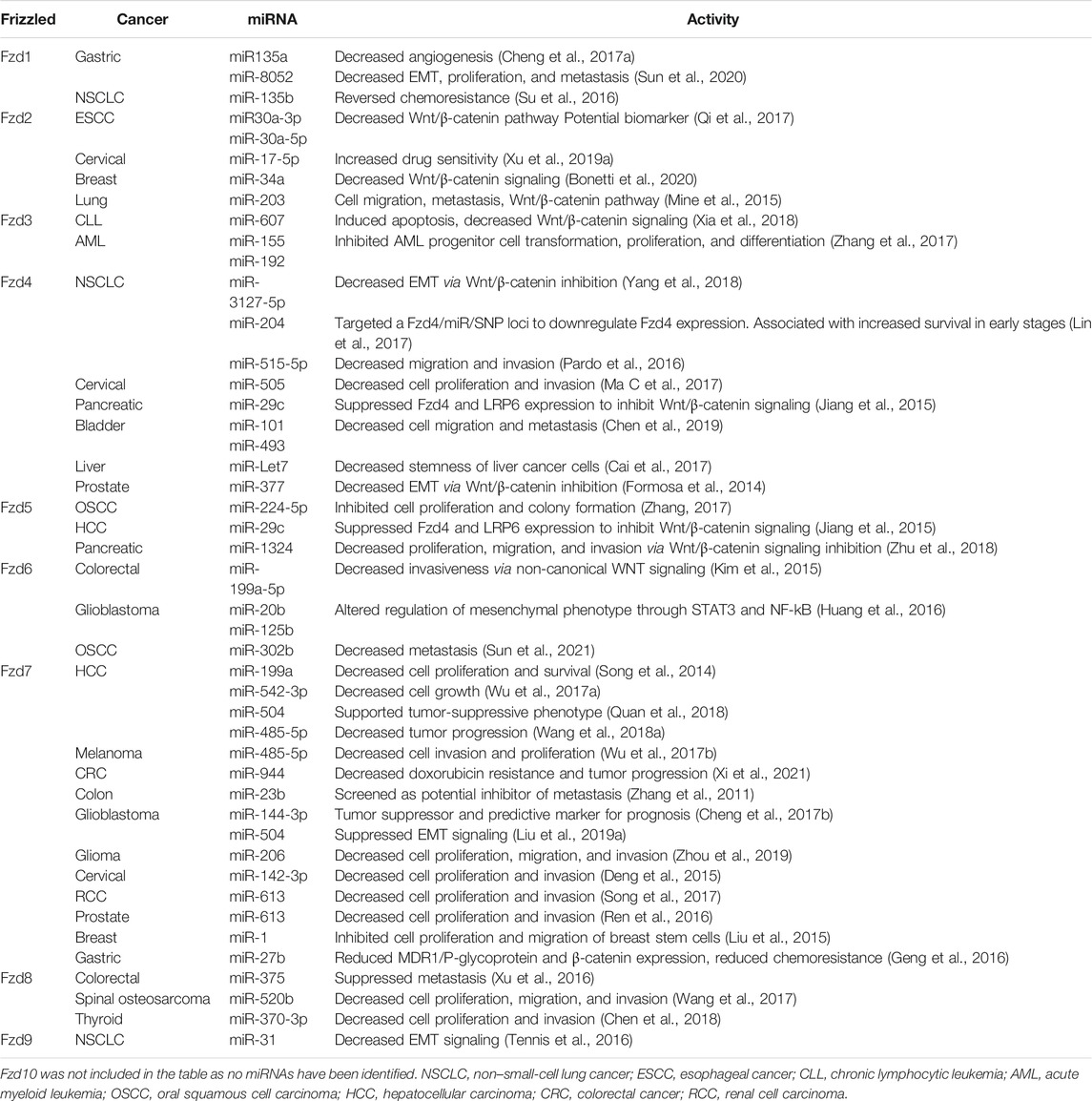
Non-Coding RNA and Frizzled Receptors in Cancer
- Division of Pulmonary Sciences and Critical Care Medicine, School of Medicine, University of Colorado, Aurora, CO, United States
Frizzled receptors have been long recognized for their role in Wnt/β-catenin signaling, a pathway known for its tumorigenic effects. More recent studies of frizzled receptors include efforts to understand non-coding RNA (ncRNA) regulation of these receptors in cancer. It has become increasingly clear that ncRNA molecules are important for regulating the expression of both oncogenic and tumor-suppressive proteins. The three most commonly described ncRNA molecules are microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). Here, we review ncRNA molecules that directly or indirectly affect frizzled protein expression and downstream signaling. Exploring these interactions highlights the potential of incorporating ncRNA molecules into cancer prevention and therapy strategies that target frizzled receptors. Previous investigations of frizzled receptors and ncRNA have established strong promise for a role in cancer progression, but additional studies are needed to provide the substantial pre-clinical evidence required to translate findings to clinical applications.
Introduction
Non-coding RNA molecules are known for their role in regulating tumorigenic pathways. Three different non-coding RNA molecules have been identified, including microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA), all of which lack protein coding capacity. MicroRNAs contain complementary nucleotide sequences to specific 5′ or 3′UTR regions of mRNAs, allowing them to bind to the mRNA and inhibit translation (Fan et al., 2019). lncRNAs regulate both at the transcriptional and translational levels by various mechanisms. Research on lncRNA in cancer has focused on the ability of lncRNA to indirectly control signaling pathways by inhibiting transcription of interfering miRNA molecules, or by binding to mature miRNA as a competing endogenous RNA (ceRNA) (Ponting et al., 2009; Li et al., 2020a). CircRNA is an ssRNA molecule formed from the joining of the 5′ end and 3′-poly-A tail during exon splicing events (Memczak et al., 2013). CircRNA manipulates numerous pathways by complementary binding of specific miRNAs, thus inhibiting their activity, similar to lncRNA (Memczak et al., 2013). The ability of circRNAs to significantly enhance or inhibit tumorigenic pathways, along with their increased stability compared to lncRNA, highlights their potential as therapeutic targets for future research (Memczak et al., 2013). There are 10 frizzled (Fzd) protein receptors, categorized as G-protein–coupled receptors (GPCRs) with a seven-span transmembrane domain that activates multiple tumorigenic signaling pathways following wingless type (WNT) ligand binding. Downstream Fzd/Wnt pathways include both the canonical Wnt/β-catenin pathway and the two non-canonical Wnt/PCP and Wnt/Ca2+ pathways. The canonical Wnt/β-catenin pathway is a common signaling pathway in cancer where Wnt/Fzd binding inhibits the tertiary GSK3β complex from forming. This inhibition prevents the phosphorylation and inactivation of β-catenin in the cytoplasm. This enables β-catenin to translocate to the nucleus where it associates with the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to induce the transcription of tumorigenic target genes such as TGF-β (Hendrickx and Leyns, 2008). Meanwhile, the Wnt/PCP and Wnt/Ca2+ pathways induce cytoskeletal rearrangements, as well as cell polarity and movement by upregulating intracellular phosphorylation cascades.5 Although the role of Fzds in cancer varies by specific Fzd receptor and cancer type, their signaling pathways have emerged as potentially important drug targets due to their critical role in Wnt/β-catenin signaling and cell mobility (Schulte and Kozielewicz, 2020). For instance, compounds that bind to the transmembrane domain of Fzd7 and interfere with Wnt binding could reduce oncogenic signaling in many cancer types (Kalhor et al., 2020). The epilepsy drug carbamazepine binds Fzd8 to suppress β-catenin signaling (Zhao et al., 2020), and Fzd9 may be a critical receptor for a lung cancer chemoprevention drug (Tennis et al., 2010). Identifying non-coding RNAs that enhance or inhibit downstream Fzd signaling will support new approaches to cancer prevention and treatment. Here, we provide a brief review of non-coding RNA associated with Fzd receptors in cancer.
MicroRNA
MicroRNAs interact with frizzled mRNA transcripts, influencing frizzed protein expression and cancer signaling in various tissues (Figure 1). Fzd1 is moderately expressed in most tissues; however, it is highly expressed in lung, placenta, smooth intestine, and bone tissues. Fzd1 has also been observed to play a role in tumorigenesis in breast cancer, gastric cancer, and non-small cell lung cancer (NSCLC). In an effort to decrease Fzd1 expression and its downstream signaling, several miRNAs have been identified that inhibit Fzd1 mRNA transcription (Su et al., 2016; Cheng et al., 2017a; Sun et al., 2020). For example, miR-135a and miR-8052 act as tumor suppressors in gastric cancer by inhibiting Fzd1 expression, EMT, and cell migration (Cheng et al., 2017a; Sun et al., 2020). It has also been observed that increasing miR-135b expression in chemoresistant NSCLC cell lines decreases Fzd1 expression and increases drug sensitivity (Su et al., 2016). Fzd2, commonly expressed in fetal lung, brain, and kidney, as well as adult cardiac tissue, has been identified as an upregulated tumorigenic receptor in multiple cancers from primary tumors to metastatic tissues (Sagara et al., 1998; Zhang et al., 2015; Fu et al., 2020). MicroRNAs that inhibit Fzd2 expression and signaling lead to inhibition of tumorigenesis. While the mechanism is still unknown, upregulation of CD82 not only decreases c-Met signaling but also upregulates miR-203 expression. Upregulation of miR-203 suppresses both Fzd2 mRNA levels and protein expression in NSCLC cells in vitro (Mine et al., 2015). miRNAs miR-17-5p, miR-30a-5p, miR-30a-3p, and miR-34a also decrease Fzd2 expression and inhibit tumorigenesis in cervical carcinoma, esophageal squamous cell carcinoma, and breast cancer, respectively (Qi et al., 2017; Xu et al., 2019a; Bonetti et al., 2020). miR-17-5p also increases drug sensitivity to cervical carcinoma cells treated with Cas-II-gly by inhibiting both Fzd2 and the lncRNA MALAT1 (Xu et al., 2019a). Fzd3 expression has been observed in the adult skeletal muscle, kidney, pancreas, cerebellum, and cerebral cortex tissues, and it is associated with esophageal carcinoma, lung squamous cell carcinoma, leukemia, myeloma, lymphoma, and Ewing sarcoma (Wong et al., 2013). However, microRNAs associated with Fzd3 have only been identified in leukemias thus far. miR-607 downregulates Fzd3 expression, leading to inhibited Wnt/β-catenin signaling, which decreases chronic lymphocytic leukemia (CLL) progression and induces apoptosis (Kirikoshi et al., 2000; Xia et al., 2018). miR-155 and miR-192 inhibit Fzd3/Wnt/β-catenin signaling in acute myeloid leukemia (AML) and decrease transformation, proliferation, and differentiation of AML progenitor cells (Zhang et al., 2017).
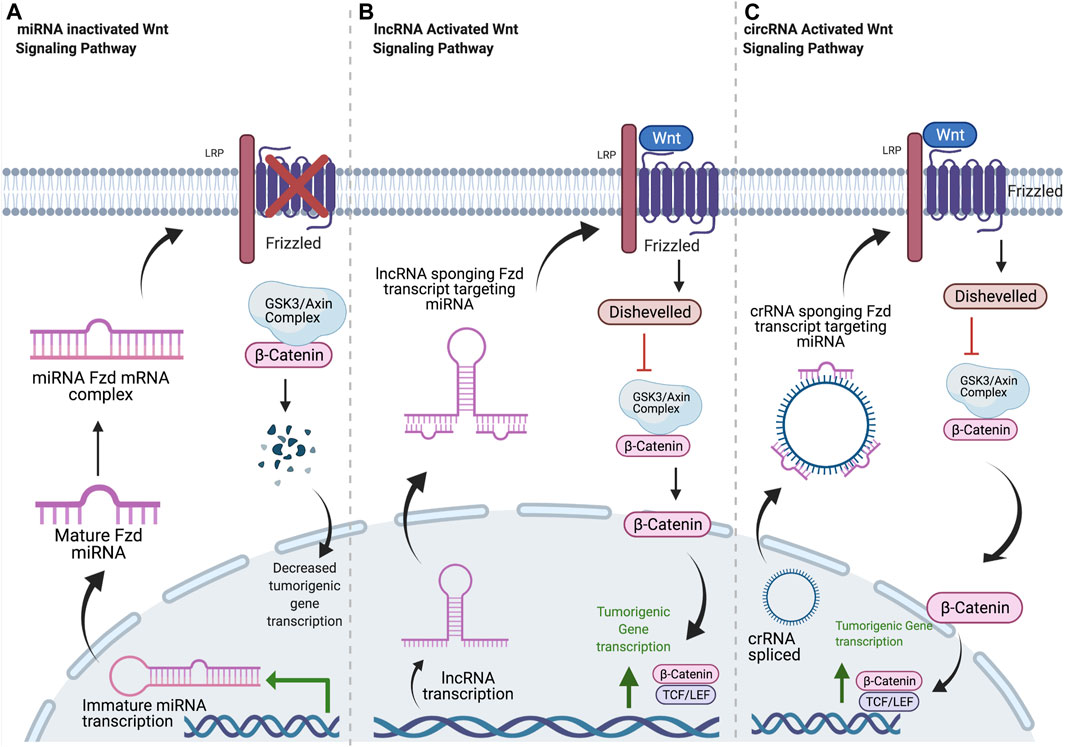
FIGURE 1. Common mechanisms of ncRNA regulation of Frizzled receptors. (A) Mature miRNA oligonucleotides target Fzd transcripts, blocking translation. Decreased Fzd at the plasma membrane results in decreased β-catenin localization to the nucleus and decreased transcription of tumorigenic signaling components. (B) Once in the cytosol, lncRNA transcripts sequester miRNA oligos with complementary sequences. This allows for normal Fzd protein expression, leading to increased β-Catenin signaling and tumorigenicity. (C) circRNA is spliced and released into cytosol. circRNA sponges miRNA oligos with complementary sequences, resulting in increased Fzd expression and increased tumorigenic signaling through β-catenin. Created with BioRender.com.
Fzd4 expression has a moderate baseline expression in most tissues, with high expression observed in digestive and female reproductive tissues (Uhlen et al., 2015; Protein Atlas (2021). Hum, 2021). Fzd4 has exhibited tumorigenic properties in studies of bladder, prostate, glioma, lung, liver, pancreatic, and cervical carcinoma by supporting proliferation, tumor progression, and metastasis through upregulation of canonical Wnt signaling (Zeng et al., 2018). Multiple miRNAs, including miR-515-5p in NSCLC, miR-101, and miR-493 in bladder cancer, and miR-505 in cervical carcinoma, decrease Fzd4 expression accompanied by tumor-suppressive effects (Ueno et al., 2012; Pardo et al., 2016; Ma C et al., 2017; Chen et al., 2019). A biomarker and potential drug target for NSCLC was identified as an miR-SNP (rs713065) within the 3′UTR binding site of the Fzd4 gene that interacts with miR-204, downregulates Fzd4 expression, and is associated with less aggressive NSCLC tumors (Lin et al., 2017). miRNA-Let7 reduces CD24 + 133+, a cellular indication of stemness, by decreasing Fzd4 expression and Wnt/β-catenin signaling in liver cancer (Cai et al., 2017). Meanwhile, miR-377 and miR-3127-5p inhibit EMT via Fzd4 downregulation in prostate cancer and NSCLC, respectively (Formosa et al., 2014; Yang et al., 2018). miR-29c is associated with both Fzd4 and Fzd5 in pancreatic cancer. miR-29c decreases expression of Fzd4, Fzd5, and frizzled co-receptor LRP6, inhibiting pancreatic tumor cell migration and stemness (Jiang et al., 2015).
Fzd5 is normally highly expressed in fetal liver and adult pancreas tissues and moderately expressed in fetal kidney and adult liver tissues (Saitoh et al., 2001a). Fzd5 is associated with breast, pancreatic, gastric, and liver cancer (Zeng et al., 2018). Only two miRNA molecules have been found so far to manipulate Fzd5 expression in cancer. miR-224-5p is associated with FTH1P3 and Fzd5 in oral squamous cell carcinoma (OSCC). FTH1P3 normally acts as a molecular sponge, inhibiting miR-224-5p activity and upregulating Fzd5 expression. However, upregulation of miR-224-5p interferes with this to decrease Fzd5 protein expression, inhibiting colony formation and proliferation (Zhang, 2017). miR-1324 is involved in the circ_0067934/Fzd5 axis in hepatocellular carcinoma (HCC). Normally, circ_0067934 indirectly upregulates Fzd5 by decreasing miR-1324 expression. However, when miR-1324 levels are increased, decreased proliferation, migration, and invasion occur through inhibition of Fzd5/Wnt/β-catenin signaling (Zhu et al., 2018). Fzd6 is moderately expressed in most adult tissues; however, it is highly expressed in endocrine tissues such as the thyroid and adrenal glands (Uhlen et al., 2015; Protein Atlas (2021). Hum, 2021). Fzd6 is connected with many cancer types, including breast, liver, prostate, colorectal, and lung cancers and leukemia (Corda and Sala, 2017). MicroRNAs targeting Fzd6 have mostly been described as tumor-suppressive; however, Fzd6 has been shown to be both upregulated and downregulated in various cancers. In gastric cancer, miR-21 was shown to directly target and inhibit the expression of Fzd6. Interestingly, the downregulation of Fzd6 was shown to increase cell proliferation and migration (Yan et al., 2016). Conversely, in colorectal cancer, Fzd6 expression is upregulated but can be downregulated by miR-199a-5p (Kim et al., 2015). In glioblastoma, two miRNAs, miR-125b and miR-20b, are differentially regulated in proneural and mesenchymal glioblastomas and are inversely expressed compared to Fzd6 expression (Huang et al., 2016). Fzd6 and miR-302b are inversely expressed in OSCC, where inhibition of Fzd6 by miR-302b suppresses metastasis (Sun et al., 2021).
Fzd7 is moderately expressed in most adult tissues also but exhibits high expression levels in neural and reproductive tissues. Fzd7 is essential for the induction of the neural crest and maintaining intestinal homeostasis throughout adulthood (Phesse et al., 2016). zd7 is associated with hepatocellular carcinoma, breast cancer, melanoma, and gastric cancer (Zeng et al., 2018). Regulation of Fzd7 by miRNA occurs in various cancers. miR-199a, miR-542-3p, miR-504, and miR-27b all inhibit tumorigenesis by directly downregulating Fzd7 expression in HCC (Chen et al., 2013; Song et al., 2014; Wu et al., 2017a; Quan et al., 2018). In glioblastoma, miR-144-3p binds to the 3′UTR of Fzd7 and inhibits proliferation, invasion, and migration (Cheng et al., 2017b). Also, in glioblastoma, Fzd7 is expressed inversely to miR-504 (Liu et al., 2019a). In gastric cancer, Fzd7 induces a tumorigenic phenotype in response to H. pylori that is suppressed by miR-27b (Geng et al., 2016). Increased expression of miR-944 attenuated doxorubicin resistance in colon cancer by targeting Fzd7 (Xi et al., 2021). A genome-wide screen for miR-23b revealed that it may be a direct modulator of CRC metastasis by regulating Fzd7 (Zhang et al., 2011). Inhibition of Fzd7 by miR-485-5p in melanoma cells was observed with 3′UTR luciferase assays and qPCR analysis (Wu et al., 2017b). In cervical cancer cell lines, miR-142-3p expression is decreased, while Fzd7 was decreased when cells were transfected with miR-142-3p mimics (Deng et al., 2015). miR-613 is predicted to bind the Fzd7 3′UTR and decreases Fzd7 expression when transfected into renal cell carcinoma cell lines (Song et al., 2017). In glioma, patient sample studies were shown to overexpress Fzd7, and both in vitro and in vivo interrogation revealed miR-206 targets Fzd7 while also decreasing cell proliferation, migration, and invasion (Zhou et al., 2019). miR-613 has an inverse expressional profile compared to Fzd7 and suppresses cell proliferation and invasion, while overexpression of Fzd7 mitigates this effect (Ren et al., 2016). Last, miR-1 upregulation in breast cancer stem cells directly targets Fzd7 to decrease expression, along with decreasing cell proliferation and invasion (Liu et al., 2015).
Fzd8 is highly expressed in fetal renal and neural tissue, as well as mature renal, pancreatic, cardiac, and skeletal muscle tissue, but has also exhibited tumorigenic influence by supporting cell proliferation, invasion, and metastasis in various cancers (Saitoh et al., 2001b; Li et al., 2017; Yin et al., 2013; Chen et al., 2020). miR-520b and Fzd8 expression is inversely correlated in both osteosarcoma patient samples and cell lines (Wang et al., 2017). In thyroid cancer, miR-370-3p is sponged by other ncRNAs, leading to increased Fzd8 expression (Chen et al., 2018). miR-375 downregulated Fzd8 expression, suppressing metastasis in colorectal cancer (Xu et al., 2016). Finally, miR-99b-5p was shown to directly target Fzd8 in non–small-cell lung cancer, resulting in decreased cell proliferation, invasion, and migration during in vitro analysis (Liu et al., 2019b). Fzd9 expression has been identified in mature lung, brain, skeletal muscle, kidney, and male reproductive tissues (Tomizawa et al., 2009). Similar to Fzd6, Fzd9 miRNA regulation contrasts with the other Fzd proteins. Studies of Fzd9 function show it to be upregulated in triple-negative breast cancer, but it is also tumor-suppressive in NSCLC (de Bastos et al., 2021), (Tennis et al., 2010). However, knowledge of miRNA regulation of Fzd9 is limited, with only one published study showing miR-31 indirectly inhibiting Fzd9 expression and supporting cancer-promoting signaling in NSCLC in vitro and in vivo (Tennis et al., 2016). Despite studies supporting an oncogenic role for Fzd10, miRNAs that regulate it have yet to be identified. Fzd10 is only normally expressed in fetal tissues and not in adult tissues; however, upregulated Fzd10 protein expression is oncogenic, specifically driving EMT, and is associated with a variety of cancers, including colon, melanoma, and gastric (Wang et al., 2018a; Scavo et al., 2018). Fzd10 is likely targeted by miRNA that will be identified in future studies and offer potential antitumorigenic targets. The extensive current knowledge of miRNA that targets Fzd receptors is summarized in Table 1, with miRNA activity, cancer type, and the frizzled transcript being targeted.
Long Non-Coding RNA
The therapeutic potential of targeting lncRNAs was introduced in 2009; however, research regarding their influence on tumorigenic potential related to Fzds has been limited until recently (Ponting et al., 2009). The influence of lncRNA on Fzd protein expression and related downstream signaling and tumor progression is mostly a result of their ability to sequester miRNA transcripts as a competing endogenous RNA (ceRNA) molecule (Figure 1). LEF1-AS1 acts as a ceRNA against miR-328 by upregulating CD44. This resulted in an increase in Fzd2/Wnt/β-catenin signaling by recruiting C-myc to upregulate Fzd2 transcription in prostate cancer (Li et al., 2020b). By in silico and in vitro analysis, MALAT1 influenced Fzd2 expression by competing for miR-17-5p (Xu et al., 2019a). SNHG10 upregulated Fzd3 expression in osteosarcoma, both in vitro and in vivo, by binding miR-182-5p and increasing Wnt/β-catenin signaling and tumorigenesis (Zhu et al., 2020). DLX6-AS1 binds miR-497-5p in vitro and increases Fzd4 expression in pancreatic cancer, leading to increased expression of EMT markers (Yang et al., 2019). Upregulation of HOXD-AS1 expression in ovarian cancer (OC) competitively binds miR-608, an inhibitory miRNA of Fzd4, in vitro and in vivo, leading to high Fzd4 expression that correlates with ovarian cancer cell migration, invasion, and proliferation (Wang et al., 2018b). Fzd5 expression in oral squamous cell carcinoma (OSCC) is increased by FTH1-3, which acts as a ceRNA against miR-224-5p to remove repression of Fzd5 expression and induce proliferation and colony formation in OSCC cells (Zhang, 2017). Both DLX6-AS1 and CASC9 sponge miRNA miR-497-5p, which increases Fzd6 expression, along with tumor growth and metastasis in pancreatic cancer and bladder cancer, respectively (Yang et al., 2019; Zhan et al., 2020). In hepatocellular carcinoma, DSCR8 increases Fzd7 expression by sequestering miR-485-5p (Wang et al., 2018a). In bladder cancer, ROR1-AS1 acts as a ceRNA for miR-504 to increase Fzd7 expression (Chen and Fu, 2020). Increased expression of Fzd7 leads to increased tumorigenesis in both cancer types. In triple negative breast cancer, AWPPH is expression is positively correlated with Fzd7, and it may act as a ceRNA for an unidentified miRNA (Wang et al., 2018c).
Alternatives to the ceRNA mechanism have been described for lncRNA, although many studies have yet to identify mechanisms for observed lncRNA effects on targets. VIM antisense RNA 1 (VIM-AS1) expression enhanced tumorigenic pathways by downregulating miR-8052 expression and increasing expression of Fzd1 in human gastric cancer (GC) tissue (Sun et al., 2020). In a microarray of human blood and tissue samples, NR_110882 promoted tumorigenesis in colorectal cancer (CRC) by increasing Fzd2 expression, Wnt2/Fzd2 binding, and Wnt/β-catenin signaling (Tian et al., 2019). GATA6-AS1 induces hypermethylation of the Fzd4 promoter region via EZH2 recruitment in gastric cancer, inhibiting Fzd4 expression and reversing EMT (Li et al., 2020a). AK126698 regulates Fzd8 similarly to miRNA, where it decreases Fzd8 expression by directly binding the mRNA transcript, leading to reduced protein expression, cell proliferation, and migration in NSCLC (Fu et al., 2016). While lncRNA regulation has yet to be described for both Fzd9 and Fzd10, the potential for investigation is clear as they have been shown to be differentially expressed during tumorigenesis in various cancers (Nagayama et al., 2009; Gong et al., 2014; Tennis et al., 2016). LncRNA that affect Fzds may be therapeutic targets by interfering with their ceRNA activity to restore normal Fzd expression or by manipulating other mechanisms of lncRNA activity.
Circular RNA
CircRNA generally leads to upregulated Fzd expression and downstream signaling through various circRNA/miRNA/Fzd pathways. All of the circRNAs associated with Fzds to date have oncogenic effects by sequestering tumor-suppressive miRNAs (Figure 1). In chronic lymphocytic leukemia (CLL) cells, circ-CBFB indirectly upregulates Fzd3 expression and downstream Wnt/β-catenin signaling by inhibiting miR-607 (Xia et al., 2018). Similar mechanisms were observed in CRC spheroid cells where circ_0082096 and circ_006631 upregulate Fzd3 expression by inhibiting miR382, miR-579, miR-224, and miR-548c (Rengganaten et al., 2020). CircRNA_100290 also influences colorectal carcinoma (CRC) by inhibiting miR-516b in CRC cells, indirectly upregulating Fzd4 and the Wnt/β-catenin pathway (Fang et al., 2018). With numerous miRNAs already associated with Fzd4, there is strong potential for further investigation into circRNA that influences Fzd4 signaling in cancer. Meanwhile, circ_0067934 is upregulated in hepatocellular carcinoma (HCC) tissues, and knocking it down significantly reduced cell proliferation, migration, and invasion, while also drastically increasing apoptosis rates in vitro (Zhu et al., 2018). Circ_0067934 inhibits miR-1324, an miRNA that decreases Fzd5 expression and downstream Wnt/β-catenin signaling in pancreatic cancer (Zhu et al., 2018). A similar mechanism was observed in gastric cancer as circ_MTHFD2 sponges miR-124, leading to an increase in Fzd5 protein expression and contributing to multidrug resistance (Xu et al., 2019b). In HCC, increased Fzd7 expression is observed when miR-485-5p is sponged by DSCR8 (Wang et al., 2018a). Fzd7 expression is increased in CRC due to circ_CSPP1 sponging miR-944 and in glioma due to circ_0000177 sequestering miR-638. Both circ_CSPP1 and circ_0000177 expression increased cell proliferation and invasion in these cancers (An et al., 2018; Xi et al., 2021). Fzd8 and NEK6 are overexpressed in thyroid cancer, where NEK6 is a circRNA that targets miR-370-3p, which targets Fzd8 3′UTR (Chen et al., 2018). Fzd1, Fzd2, Fzd6, Fzd9, and Fzd10 currently do not have any circRNA molecules associated with regulation of their expression, despite their obvious role in cancer signaling and tumor progression, highlighting the potential for future research.
Discussion
While studies are still limited, the importance of non-coding RNA in Fzd receptor regulation is clearly emerging. Ongoing research will contribute to improved understanding of the mechanisms of individual Fzd regulation in specific tissue and cellular contexts. Manipulating Fzd expression or activity in cancer cells could offer new approaches to prevention and therapy. Small-molecule drugs could target Fzd-binding pockets, Wnt ligands, co-receptors, or heterotrimeric Gi proteins to control downstream intracellular signaling. Targeting non-coding RNAs present an additional approach to inhibiting oncogenic pathways or enhancing tumor-suppressive pathways. Challenges of targeting Fzds include lack of high-resolution crystal structures for Fzds and context-dependent associations of individual Wnts and Fzds (Schulte and Kozielewicz, 2020; Zhao et al., 2020). Using non-coding RNA to target Fzd activity could circumvent some of these challenges by altering expression, instead of attempting to bind to Fzd receptors or by offering tissue- or disease-specific targeting. The larger volume of studies on miRNA and Fzds points to miRNA as the first potential approach for using non-coding RNA to target Fzds in cancer. This is supported by their demonstrated ability to downregulate oncogenic Fzd expression, inducing antitumorigenic effects both in vitro and in vivo. miRNAs have also been used as biomarkers for tumor prognosis and susceptibility to drug resistance, increasing their appeal as potential mechanistic targets that can be measured as markers of response (Lan et al., 2015; Hanna et al., 2019).
ncRNA molecules have therapeutic potential through regulation of Fzd-initiated oncogenic cell signaling pathways; however, the study of ncRNA in clinical cancer settings has been thus far limited to miRNA. There are clinical trials investigating miRNAs as biomarkers for diagnosis, drug sensitivity, or therapeutic agents in cardiac disease, cancer, neurodegenerative disorders, and viral infections. For example, miR199a-5p and miR-126-3p were found to influence endothelial dysfunction in Fabry disease (FD) and can be used as a diagnostic factor to identify individuals with FD (Cammarata et al., 2018). Similarly, miR-30e was identified as a diagnostic marker in schizophrenic patients following peripheral plasma and peripheral blood mononuclear cell analysis (Sun et al., 2015). Approaches to targeting miRNAs include modified siRNAs, anti-sense oligonucleotides, and small molecules (Wang et al., 2019). miRNA inhibitors and mimics have been administered by intravenous or intratumoral injections in clinical trials. This method of delivery presents obstacles such as off-target effects, poor target reach, low stability, and short circulation time (Hanna et al., 2019; Lee et al., 2019). Nanoparticles represent a potentially improved vector to deliver miRNA drugs that provides increased stability, circulation time, and target accuracy (Lee et al., 2019). This approach was supported by a study that observed a decrease in breast cancer metastasis, following delivery of an miR-708 mimic via nanoparticles (Ramchandani et al., 2019). lncRNA can be targeted by several approaches, including anti-sense oligonucleotides, or CRISPR, although clinical utility of these approaches for cancer is still under study. Advances in ex vivo and in vivo modeling, such as large animals or tissue slice models, are helping to address pre-clinically the safety and efficacy issues that often halt ncRNA drugs early in the development pipeline. A recently investigated alternative, small-molecule inhibitors of lnRNA, may offer a non-sequence targeting option with easier delivery modalities (Chen et al., 2021). With so much still to be learned about lncRNA function and interactions with Fzd, it is likely this approach will not be seen in clinical application for some time. Approaches to modulating circRNAs have been similar to lncRNAs, and they also have potential as therapeutic tools due to their stability and ability to be engineered with multiple binding sites (Rajappa et al., 2020). However, even more so than lncRNA, despite intriguing initial results on the role of circRNA in cancer, considerable additional effort is required to advance circRNA therapy to the clinic. miRNA is currently the most promising ncRNA target in clinical trials, but new therapeutic models will certainly emerge as knowledge and techniques for studying lncRNA and circRNA improve and increase. As research on Fzd receptors and non-coding RNA continues to grow, corresponding investigation of the potential to target Fzd receptor activity through non-coding RNAs will enhance the development of Fzd-based biomarkers, chemoprevention, and chemotherapy.
Author Contributions
AS contributed by reading the literature, writing the manuscript, making figures, and editing. KS contributed by reading the literature, writing the manuscript, making figures, and editing. AE contributed by reading the literature and editing. MT contributed by managing, writing the manuscript, reading the literature, and editing.
Funding
All funding for this manuscript was provided by the National Institute of Health. The grant identification number is R01CA214531.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, Y., Chen, X.-m., Yang, Y., Mo, F., Jiang, Y., Sun, D.-l., et al. (2018). LncRNA DLX6-AS1 Promoted Cancer Cell Proliferation and Invasion by Attenuating the Endogenous Function of miR-181b in Pancreatic Cancer. Cancer Cel Int 18, 143. doi:10.1186/s12935-018-0643-7
Bonetti, P., Climent, M., Panebianco, F., Tordonato, C., Santoro, A., Marzi, M. J., et al. (2020). Correction: Dual Role for miR-34a in the Control of Early Progenitor Proliferation and Commitment in the Mammary Gland and in Breast Cancer. Oncogene 39, 2228. doi:10.1038/s41388-019-1094-x
Cai, H., Chen, Y., Yang, X., Ma, S., Wang, Q., Zhang, Y., et al. (2017). Let7b Modulates the Wnt/β-Catenin Pathway in Liver Cancer Cells via Downregulated Frizzled4. Tumour Biol. 39, 101042831771607. doi:10.1177/1010428317716076
Cammarata, G., Scalia, S., Colomba, P., Zizzo, C., Pisani, A., Riccio, E., et al. (2018). A Pilot Study of Circulating microRNAs as Potential Biomarkers of Fabry Disease. Oncotarget 9, 27333–27345. doi:10.18632/oncotarget.25542
Chen, F., Feng, Z., Zhu, J., Liu, P., Yang, C., Huang, R., et al. (2018). Emerging Roles of circRNA_NEK6 Targeting miR-370-3p in the Proliferation and Invasion of Thyroid Cancer via Wnt Signaling Pathway. Cancer Biol. Ther. 19, 1139–1152. doi:10.1080/15384047.2018.1480888
Chen, L., Long, Y., Han, Z., Yuan, Z., Liu, W., Yang, F., et al. (2019). MicroRNA-101 I-nhibits C-ell M-igration and I-nvasion in B-ladder C-ancer via T-argeting FZD4. Exp. Ther. Med. 17, 1476–1485. doi:10.3892/etm.2018.7084
Chen, Q., and Fu, L. (2020). Upregulation of Long Non-coding RNA ROR1-AS1 Promotes Cell Growth and Migration in Bladder Cancer by Regulation of miR-504. PLoS One 15, e0227568. doi:10.1371/journal.pone.0227568
Chen, W., Liu, Z., Mai, W., Xiao, Y., You, X., and Qin, L. (2020). FZD8 Indicates a Poor Prognosis and Promotes Gastric Cancer Invasion and Metastasis via B-Catenin Signaling Pathway. Ann. Clin. Lab. Sci. 50, 13–23.
Chen, Y., Li, Z., Chen, X., and Zhang, S. (2021). Long Non-coding RNAs: From Disease Code to Drug Role. Acta Pharmaceutica Sinica B 11, 340–354. doi:10.1016/j.apsb.2020.10.001
Chen, Z., Ma, T., Huang, C., Zhang, L., Lv, X., Xu, T., et al. (2013). MiR-27a Modulates the MDR1/P-Glycoprotein Expression by Inhibiting FZD7/β-Catenin Pathway in Hepatocellular Carcinoma Cells. Cell Signal. 25, 2693–2701. doi:10.1016/j.cellsig.2013.08.032
Cheng, Z., Liu, F., Zhang, H., Li, X., Li, Y., Li, J., et al. (2017). miR-135a Inhibits Tumor Metastasis and Angiogenesis by Targeting FAK Pathway. Oncotarget 8, 31153–31168. doi:10.18632/oncotarget.16098
Cheng, Z. X., Song, Y. X., Wang, Z. Y., Wang, Y., and Dong, Y. (2017). miR-144-3p Serves as a Tumor Suppressor by Targeting FZD7 and Predicts the Prognosis of Human Glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 21, 4079–4086.
Corda, G., and Sala, A. (2017). Non-canonical WNT/PCP Signalling in Cancer: Fzd6 Takes centre Stage. Oncogenesis 6, e364. doi:10.1038/oncsis.2017.69
de Bastos, D. R., Conceição, M. P. F., Michelli, A. P. P., Leite, J. M. R. S., da Silva, R. A., Cintra, R. C., et al. (2021). An In Silico Analysis Identified FZD9 as a Potential Prognostic Biomarker in Triple-Negative Breast Cancer Patients. Ejbh 17, 42–52. doi:10.4274/ejbh.2020.5804
Deng, B., Zhang, Y., Zhang, S., Wen, F., Miao, Y., and Guo, K. (2015). MicroRNA-142-3p Inhibits Cell Proliferation and Invasion of Cervical Cancer Cells by Targeting FZD7. Tumor Biol. 36, 8065–8073. doi:10.1007/s13277-015-3483-2
Fan, R., Xiao, C., Wan, X., Cha, W., Miao, Y., Zhou, Y., et al. (2019). Small Molecules with Big Roles in microRNA Chemical Biology and microRNA-Targeted Therapeutics. RNA Biol. 16, 707–718. doi:10.1080/15476286.2019.1593094
Fang, G., Ye, B.-L., Hu, B.-R., Ruan, X.-J., and Shi, Y.-X. (2018). CircRNA_100290 Promotes Colorectal Cancer Progression through miR-516b-Induced Downregulation of FZD4 Expression and Wnt/β-Catenin Signaling. Biochem. Biophysical Res. Commun. 504, 184–189. doi:10.1016/j.bbrc.2018.08.152
Formosa, A., Markert, E. K., Lena, A. M., Italiano, D., Finazzi-Agro', E., Levine, A. J., et al. (2014). MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, Mapped to the 14q32.31 Locus, Regulate Proliferation, Apoptosis, Migration and Invasion in Metastatic Prostate Cancer Cells. Oncogene 33, 5173–5182. doi:10.1038/onc.2013.451
Fu, X., Li, H., Liu, C., Hu, B., Li, T., and Wang, Y. (2016). Long Noncoding RNA AK126698 Inhibits Proliferation and Migration of Non-small Cell Lung Cancer Cells by Targeting Frizzled-8 and Suppressing Wnt/β-Catenin Signaling Pathway. Ott 9, 3815–3827. doi:10.2147/OTT.S100633
Fu, Y., Zheng, Q., Mao, Y., Jiang, X., Chen, X., Liu, P., et al. (2020). WNT2-Mediated FZD2 Stabilization Regulates Esophageal Cancer Metastasis via STAT3 Signaling. Front. Oncol. 10, 1168. doi:10.3389/fonc.2020.01168
Geng, Y., Lu, X., Wu, X., Xue, L., Wang, X., and Xu, J. (2016). MicroRNA-27b Suppresses Helicobacter Pylori-Induced Gastric Tumorigenesis through Negatively Regulating Frizzled7. Oncol. Rep. 35, 2441–2450. doi:10.3892/or.2016.4572
Gong, C., Qu, S., Lv, X.-B., Liu, B., Tan, W., Nie, Y., et al. (2014). BRMS1L Suppresses Breast Cancer Metastasis by Inducing Epigenetic Silence of FZD10. Nat. Commun. 5, 5406. doi:10.1038/ncomms6406
Hanna, J., Hossain, G. S., and Kocerha, J. (2019). The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 10, 478. doi:10.3389/fgene.2019.00478
Hendrickx, M., and Leyns, L. (2008). Non-conventional Frizzled Ligands and Wnt Receptors. Dev. Growth Differ. 50, 229–243. doi:10.1111/j.1440-169X.2008.01016.x
Huang, T., Alvarez, A. A., Pangeni, R. P., M. Horbinski, C., Lu, S., Kim, S.-H., et al. (2016). A Regulatory Circuit of miR-125b/miR-20b and Wnt Signalling Controls Glioblastoma Phenotypes through FZD6-Modulated Pathways. Nat. Commun. 7, 12885. doi:10.1038/ncomms12885
Jiang, J., Yu, C., Chen, M., Zhang, H., Tian, S., and Sun, C. (2015). Reduction of miR-29c Enhances Pancreatic Cancer Cell Migration and Stem Cell-like Phenotype. Oncotarget 6, 2767–2778. doi:10.18632/oncotarget.3089
Kalhor, H., Rahimi, H., Akbari Eidgahi, M. R., and Teimoori-Toolabi, L. (2020). Novel Small Molecules against Two Binding Sites of Wnt2 Protein as Potential Drug Candidates for Colorectal Cancer: A Structure Based Virtual Screening Approach. Iran J. Pharm. Res. 19, 160–174. doi:10.22037/ijpr.2019.15297.13037
Kim, B.-K., Yoo, H.-I., Kim, I., Park, J., and Yoon, S. K. (2015). FZD6 Expression Is Negatively Regulated by miR-199a-5p in Human Colorectal Cancer. BMB Rep. 48, 360–366. doi:10.5483/bmbrep.2015.48.6.031
Kirikoshi, H., Koike, J., Sagara, N., Saitoh, T., Tokuhara, M., Tanaka, K., et al. (2000). Molecular Cloning and Genomic Structure of Human Frizzled-3 at Chromosome 8p21. Biochem. Biophysical Res. Commun. 271, 8–14. doi:10.1006/bbrc.2000.2578
Lan, H., Lu, H., Wang, X., and Jin, H. (2015). MicroRNAs as Potential Biomarkers in Cancer: Opportunities and Challenges. Biomed. Res. Int. 2015, 1–17. doi:10.1155/2015/125094
Lee, S. W. L., Paoletti, C., Campisi, M., Osaki, T., Adriani, G., Kamm, R. D., et al. (2019). MicroRNA Delivery through Nanoparticles. J. Controlled Release 313, 80–95. doi:10.1016/j.jconrel.2019.10.007
Li, Q., Ye, L., Zhang, X., Wang, M., Lin, C., Huang, S., et al. (2017). FZD8, a Target of P53, Promotes Bone Metastasis in Prostate Cancer by Activating Canonical Wnt/β-Catenin Signaling. Cancer Lett. 402, 166–176. doi:10.1016/j.canlet.2017.05.029
Li, W., Yang, G., Yang, D., Li, D., and Sun, Q. (2020). LncRNA LEF1-AS1 Promotes Metastasis of Prostatic Carcinoma via the Wnt/β-Catenin Pathway. Cancer Cel Int 20, 543. doi:10.1186/s12935-020-01624-x
Li, Z.-T., Zhang, X., Wang, D.-W., Xu, J., Kou, K.-J., Wang, Z.-W., et al. (2020). Overexpressed lncRNA GATA6-AS1 Inhibits LNM and EMT via FZD4 through the Wnt/β-Catenin Signaling Pathway in GC. Mol. Ther. - Nucleic Acids 19, 827–840. doi:10.1016/j.omtn.2019.09.034
Lin, J., Zandi, R., Shao, R., Gu, J., Ye, Y., Wang, J., et al. (2017). A miR-SNP Biomarker Linked to an Increased Lung Cancer Survival by miRNA-Mediated Down-Regulation of FZD4 Expression and Wnt Signaling. Sci. Rep. 7, 9029. doi:10.1038/s41598-017-09604-4
Liu, Q., Guan, Y., Li, Z., Wang, Y., Liu, Y., Cui, R., et al. (2019). miR-504 Suppresses Mesenchymal Phenotype of Glioblastoma by Directly Targeting the FZD7-Mediated Wnt-β-Catenin Pathway. J. Exp. Clin. Cancer Res. 38, 358. doi:10.1186/s13046-019-1370-1
Liu, R., Chen, Y., Shou, T., Hu, J., and Qing, C. (2019). miRNA-99b-5p Targets FZD8 to Inhibit Non-small Cell Lung Cancer Proliferation, Migration and Invasion. Ott Vol. 12, 2615–2621. doi:10.2147/OTT.S199196
Liu, T., Hu, K., Zhao, Z., Chen, G., Ou, X., Zhang, H., et al. (2015). MicroRNA-1 Down-Regulates Proliferation and Migration of Breast Cancer Stem Cells by Inhibiting the Wnt/β-Catenin Pathway. Oncotarget 6, 41638–41649. doi:10.18632/oncotarget.5873
Ma C, X. B., Husaiyin, S., Wang, L., Wusainahong, K., Ma, J., Zhu, K., et al. (2017). MicroRNA-505 Predicts Prognosis and Acts as Tumor Inhibitor in Cervical Carcinoma with Inverse Association with FZD4. Biomed. Pharmacother. 92, 586–594. doi:10.1016/j.biopha.2017.04.028
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 495, 333–338. doi:10.1038/nature11928
Mine, M., Yamaguchi, K., Sugiura, T., Chigita, S., Yoshihama, N., Yoshihama, R., et al. (2015). miR-203 Inhibits Frizzled-2 Expression via CD82/KAI1 Expression in Human Lung Carcinoma Cells. PLoS One 10, e0131350. doi:10.1371/journal.pone.0131350
Nagayama, S., Yamada, E., Kohno, Y., Aoyama, T., Fukukawa, C., Kubo, H., et al. (2009). Inverse Correlation of the Up-Regulation of FZD10 Expression and the Activation of β-catenin in Synchronous Colorectal Tumors. Cancer Sci. 100, 405–412. doi:10.1111/j.1349-7006.2008.01052.x
Pardo, O. E., Castellano, L., Munro, C. E., Hu, Y., Mauri, F., Krell, J., et al. (2016). miR‐515‐5p Controls Cancer Cell Migration through MARK 4 Regulation. EMBO Rep. 17, 570–584. doi:10.15252/embr.201540970
Phesse, T., Flanagan, D., and Vincan, E. (2016). Frizzled7: A Promising Achille's Heel for Targeting the Wnt Receptor Complex to Treat Cancer. Cancers 8, 50. doi:10.3390/cancers8050050
Ponting, C. P., Oliver, P. L., and Reik, W. (2009). Evolution and Functions of Long Noncoding RNAs. Cell 136, 629–641. doi:10.1016/j.cell.2009.02.006
Protein Atlas (2021). Human Protein Atlas. Available at: http://www.proteinatlas.org.
Qi, B., Wang, Y., Chen, Z.-J., Li, X.-N., Qi, Y., Yang, Y., et al. (2017). Down-regulation of miR-30a-3p/5p Promotes Esophageal Squamous Cell Carcinoma Cell Proliferation by Activating the Wnt Signaling Pathway. Wjg 23, 7965–7977. doi:10.3748/wjg.v23.i45.7965
Quan, H., Li, B., and Yang, J. (2018). MicroRNA-504 Functions as a Tumor Suppressor in Hepatocellular Carcinoma through Inhibiting Frizzled-7-Mediated-Wnt/β-Catenin Signaling. Biomed. Pharmacother. 107, 754–762. doi:10.1016/j.biopha.2018.07.150
Rajappa, A., Banerjee, S., Sharma, V., and Khandelia, P. (2020). Circular RNAs: Emerging Role in Cancer Diagnostics and Therapeutics. Front. Mol. Biosci. 7, 577938. doi:10.3389/fmolb.2020.577938
Ramchandani, D., Lee, S. K., Yomtoubian, S., Han, M. S., Tung, C.-H., and Mittal, V. (2019). Nanoparticle Delivery of miR-708 Mimetic Impairs Breast Cancer Metastasis. Mol. Cancer Ther. 18, 579–591. doi:10.1158/1535-7163.MCT-18-0702
Ren, W., Li, C., Duan, W., Du, S., Yang, F., Zhou, J., et al. (2016). MicroRNA-613 Represses Prostate Cancer Cell Proliferation and Invasion through Targeting Frizzled7. Biochem. Biophysical Res. Commun. 469, 633–638. doi:10.1016/j.bbrc.2015.12.054
Rengganaten, V., Huang, C.-J., Tsai, P.-H., Wang, M.-L., Yang, Y.-P., Lan, Y.-T., et al. (2020). Mapping a Circular RNA-microRNA-mRNA-Signaling Regulatory Axis that Modulates Stemness Properties of Cancer Stem Cell Populations in Colorectal Cancer Spheroid Cells. Ijms 21, 7864. doi:10.3390/ijms21217864
Sagara, N., Toda, G., Hirai, M., Terada, M., and Katoh, M. (1998). Molecular Cloning, Differential Expression, and Chromosomal Localization of HumanFrizzled-1, Frizzled-2,andFrizzled-7. Biochem. Biophysical Res. Commun. 252, 117–122. doi:10.1006/bbrc.1998.9607
Saitoh, T., Hirai, M., and Katoh, M. (2001). Molecular Cloning and Characterization of Human Frizzled-5 Gene on Chromosome 2q33.3-q34 Region. Int. J. Oncol. 19, 105–110. doi:10.3892/ijo.19.1.105
Saitoh, T., Hirai, M., and Katoh, M. (2001). Molecular Cloning and Characterization of Human Frizzled-8 Gene on Chromosome 10p11.2. Int. J. Oncol. 18, 991–996. doi:10.3892/ijo.18.5.991
Scavo, M. P., Fucci, L., Caldarola, L., Mangia, A., Azzariti, A., Simone, G., et al. (2018). Frizzled-10 and Cancer Progression: Is it a New Prognostic Marker? Oncotarget 9, 824–830. doi:10.18632/oncotarget.23159
Schulte, G., and Kozielewicz, P. (2020). Structural Insight into Class F Receptors - what Have We Learnt Regarding Agonist‐induced Activation? Basic Clin. Pharmacol. Toxicol. 126, 17–24. doi:10.1111/bcpt.13235
Song, H., Nan, Y., Wang, X., Zhang, G., Zong, S., and Kong, X. (2017). MicroRNA-613 Inhibits Proliferation and Invasion of Renal Cell Carcinoma Cells through Targeting FZD7. Mol. Med. Rep. 16, 4279–4286. doi:10.3892/mmr.2017.7076
Song, J., Gao, L., Yang, G., Tang, S., Xie, H., Wang, Y., et al. (2014). MiR-199a Regulates Cell Proliferation and Survival by Targeting FZD7. PLoS One 9, e110074. doi:10.1371/journal.pone.0110074
Su, W., Mo, Y., Wu, F., Guo, K., Li, J., Luo, Y., et al. (2016). miR-135b Reverses Chemoresistance of Non-small Cell Lung Cancer Cells by Downregulation of FZD1. Biomed. Pharmacother. 84, 123–129. doi:10.1016/j.biopha.2016.09.027
Sun, J. G., Li, X. B., Yin, R. H., and Li, X. F. (2020). lncRNA VIM-AS1 P-romotes C-ell P-roliferation, M-etastasis and E-pithelial-mesenchymal T-ransition by A-ctivating the Wnt/β-catenin P-athway in G-astric C-ancer. Mol. Med. Rep. 22, 4567–4578. doi:10.3892/mmr.2020.11577
Sun, S., Wang, J., Liu, J., Yin, F., Xin, C., Zeng, X., et al. (2021). MiR-302b Suppresses Tumor Metastasis by Targeting Frizzled 6 in OSCC. J. Dent Res. 100, 739–745. doi:10.1177/0022034520986551
Sun, X.-y., Zhang, J., Niu, W., Guo, W., Song, H.-t., Li, H.-y., et al. (2015). A Preliminary Analysis of microRNA as Potential Clinical Biomarker for Schizophrenia. Am. J. Med. Genet. 168, 170–178. doi:10.1002/ajmg.b.32292
Tennis, M. A., New, M. L., McArthur, D. G., Merrick, D. T., Dwyer-Nield, L. D., and Keith, R. L. (2016). Prostacyclin Reverses the Cigarette Smoke-Induced Decrease in Pulmonary Frizzled 9 Expression through miR-31. Sci. Rep. 6, 28519. doi:10.1038/srep28519
Tennis, M. A., Van Scoyk, M., Heasley, L. E., Vandervest, K., Weiser-Evans, M., Freeman, S., et al. (2010). Prostacyclin Inhibits Non-small Cell Lung Cancer Growth by a Frizzled 9-dependent Pathway that Is Blocked by Secreted Frizzled-Related Protein 1. Neoplasia 12, 244–IN6. doi:10.1593/neo.91690
Tian, Y., Xu, Y., Wang, H., Shu, R., Sun, L., Zeng, Y., et al. (2019). Comprehensive Analysis of Microarray Expression Profiles of circRNAs and lncRNAs with Associated Co-expression Networks in Human Colorectal Cancer. Funct. Integr. Genomics 19, 311–327. doi:10.1007/s10142-018-0641-9
Tomizawa, T., Tomizawa, M., and Yokosuka, O. (2009). SiRNA of Frizzled-9 Suppresses Proliferation and Motility of Hepatoma Cells. Int. J. Oncol. 35, 861–866. doi:10.3892/ijo_00000400
Ueno, K., Hirata, H., Majid, S., Yamamura, S., Shahryari, V., Tabatabai, Z. L., et al. (2012). Tumor Suppressor microRNA-493 Decreases Cell Motility and Migration Ability in Human Bladder Cancer Cells by Downregulating RhoC and FZD4. Mol. Cancer Ther. 11, 244–253. doi:10.1158/1535-7163.MCT-11-0592
Uhlen, M., Fagerberg, L., Hallstrom, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., et al. (2015). Tissue-based Map of the Human Proteome. Science 347, 1260419. doi:10.1126/science.1260419
Wang, J., Pang, W., Zuo, Z., Zhang, W., and He, W. (2017). MicroRNA-520b Suppresses Proliferation, Migration, and Invasion of Spinal Osteosarcoma Cells via Downregulation of Frizzled-8. Oncol. Res. 25, 1297–1304. doi:10.3727/096504017X14873430389189
Wang, K., Li, X., Song, C., and Li, M. (2018). LncRNA AWPPH Promotes the Growth of Triple-Negative Breast Cancer by Up-Regulating Frizzled Homolog 7 (FZD7). Biosci. Rep. 38, BSR20181223. doi:10.1042/BSR20181223
Wang, W.-T., Han, C., Sun, Y.-M., Chen, T.-Q., and Chen, Y.-Q. (2019). Noncoding RNAs in Cancer Therapy Resistance and Targeted Drug Development. J. Hematol. Oncol. 12, 55. doi:10.1186/s13045-019-0748-z
Wang, Y., Zhang, W., Wang, Y., and Wang, S. (2018). HOXD-AS1 Promotes Cell Proliferation, Migration and Invasion through miR-608/FZD4 axis in Ovarian Cancer. Am. J. Cancer Res. 8, 170–182.
Wang, Y., Sun, L., Wang, L., Liu, Z., Li, Q., Yao, B., et al. (2018). Long Non-coding RNA DSCR8 Acts as a Molecular Sponge for miR-485-5p to Activate Wnt/β-Catenin Signal Pathway in Hepatocellular Carcinoma. Cell Death Dis 9, 851. doi:10.1038/s41419-018-0937-7
Wong, S. C. C., He, C. W., Chan, C. M. L., Chan, A. K. C., Wong, H. T., Cheung, M. T., et al. (2013). Clinical Significance of Frizzled Homolog 3 Protein in Colorectal Cancer Patients. PLoS One 8, e79481. doi:10.1371/journal.pone.0079481
Wu, J., Li, J., Ren, J., and Zhang, D. (2017). MicroRNA-485-5p Represses Melanoma Cell Invasion and Proliferation by Suppressing Frizzled7. Biomed. Pharmacother. 90, 303–310. doi:10.1016/j.biopha.2017.03.064
Wu, W., Dang, S., Feng, Q., Liang, J., Wang, Y., and Fan, N. (2017). MicroRNA-542-3p Inhibits the Growth of Hepatocellular Carcinoma Cells by Targeting FZD7/Wnt Signaling Pathway. Biochem. Biophysical Res. Commun. 482, 100–105. doi:10.1016/j.bbrc.2016.10.136
Xi, L., Liu, Q., Zhang, W., Luo, L., Song, J., Liu, R., et al. (2021). Circular RNA circCSPP1 Knockdown Attenuates Doxorubicin Resistance and Suppresses Tumor Progression of Colorectal Cancer via miR-944/FZD7 axis. Cancer Cel Int 21, 153. doi:10.1186/s12935-021-01855-6
Xia, L. W. L., Wu, L., Bao, J., Li, Q., Chen, X., Xia, H., et al. (2018). Circular RNA Circ-CBFB Promotes Proliferation and Inhibits Apoptosis in Chronic Lymphocytic Leukemia through Regulating miR-607/FZD3/Wnt/β-Catenin Pathway. Biochem. Biophysical Res. Commun. 503, 385–390. doi:10.1016/j.bbrc.2018.06.045
Xu, L., Wen, T., Liu, Z., Xu, F., Yang, L., Liu, J., et al. (2016). MicroRNA-375 Suppresses Human Colorectal Cancer Metastasis by Targeting Frizzled 8. Oncotarget 7, 40644–40656. doi:10.18632/oncotarget.9811
Xu, Q. Y., Xie, M. J., Huang, J., and Wang, Z. W. (2019). Effect of Circ MTHFD2 on Resistance to Pemetrexed in Gastric Cancer through Regulating Expression of miR-124. Eur. Rev. Med. Pharmacol. Sci. 23, 10290–10299. doi:10.26355/eurrev_201912_19667
Xu, Y., Zhang, Q., Lin, F., Zhu, L., Huang, F., Zhao, L., et al. (2019). Casiopeina II-gly A-cts on lncRNA MALAT1 by miR-17-5p to I-nhibit FZD2 E-xpression via the Wnt S-ignaling P-athway during the T-reatment of C-ervical C-arcinoma. Oncol. Rep. 42, 1365–1379. doi:10.3892/or.2019.7268
Yan, J., Liu, T., Zhou, X., Dang, Y., Yin, C., and Zhang, G. (2016). FZD6, Targeted by miR-21, Represses Gastric Cancer Cell Proliferation and Migration via Activating Non-canonical Wnt Pathway. Am. J. Transl Res. 8, 2354–2364.
Yang, J., Ye, Z., Mei, D., Gu, H., and Zhang, J. (2019). Long Noncoding RNA DLX6-AS1 Promotes Tumorigenesis by Modulating miR-497-5p/FZD4/ FZD6/Wnt/β-Catenin Pathway in Pancreatic Cancer. Cmar 11, 4209–4221. doi:10.2147/CMAR.S194453
Yang, Y., Sun, Y., Wu, Y., Tang, D., Ding, X., Xu, W., et al. (2018). Downregulation of miR-3127-5p Promotes Epithelial-Mesenchymal Transition via FZD4 Regulation of Wnt/β-Catenin Signaling in Non-small-cell Lung Cancer. Mol. Carcinogenesis 57, 842–853. doi:10.1002/mc.22805
Yin, S., Xu, L., Bonfil, R. D., Banerjee, S., Sarkar, F. H., Sethi, S., et al. (2013). Tumor-initiating Cells and FZD8 Play a Major Role in Drug Resistance in Triple-Negative Breast Cancer. Mol. Cancer Ther. 12, 491–498. doi:10.1158/1535-7163.MCT-12-1090
Zeng, C.-M., Chen, Z., and Fu, L. (2018). Frizzled Receptors as Potential Therapeutic Targets in Human Cancers. Ijms 19, 1543. doi:10.3390/ijms19051543
Zhan, Y., Zhang, L., Yu, S., Wen, J., Liu, Y., and Zhang, X. (2020). Long Non-coding RNA CASC9 Promotes Tumor Growth and Metastasis via Modulating FZD6/Wnt/β-Catenin Signaling Pathway in Bladder Cancer. J. Exp. Clin. Cancer Res. 39, 136. doi:10.1186/s13046-020-01624-9
Zhang, C.-Z. (2017). Long Non-coding RNA FTH1P3 Facilitates Oral Squamous Cell Carcinoma Progression by Acting as a Molecular Sponge of miR-224-5p to Modulate Fizzled 5 Expression. Gene 607, 47–55. doi:10.1016/j.gene.2017.01.009
Zhang, E., Li, Z., Xu, Z., Duan, W., Sun, C., and Lu, L. (2015). Frizzled2 Mediates the Migration and Invasion of Human Oral Squamous Cell Carcinoma Cells through the Regulation of the Signal Transducer and Activator of Transcription-3 Signaling Pathway. Oncol. Rep. 34, 3061–3067. doi:10.3892/or.2015.4285
Zhang, H., Hao, Y., Yang, J., Zhou, Y., Li, J., Yin, S., et al. (2011). Genome-wide Functional Screening of miR-23b as a Pleiotropic Modulator Suppressing Cancer Metastasis. Nat. Commun. 2, 554. doi:10.1038/ncomms1555
Zhang, H., Zhang, C., Feng, R., Zhang, H., Gao, M., and Ye, L. (2017). Investigating the microRNA-mRNA Regulatory Network in Acute Myeloid Leukemia. Oncol. Lett. 14, 3981–3988. doi:10.3892/ol.2017.6686
Zhao, Y., Ren, J., Hillier, J., Lu, W., and Jones, E. Y. (2020). Antiepileptic Drug Carbamazepine Binds to a Novel Pocket on the Wnt Receptor Frizzled-8. J. Med. Chem. 63, 3252–3260. doi:10.1021/acs.jmedchem.9b02020
Zhou, F., Cao, W., Xu, R., Zhang, J., Yu, T., Xu, X., et al. (2019). MicroRNA-206 Attenuates Glioma Cell Proliferation, Migration, and Invasion by Blocking the WNT/β-catenin Pathway via Direct Targeting of Frizzled 7 mRNA. Am. J. Transl Res. 11, 4584–4601.
Zhu, Q., Lu, G., Luo, Z., Gui, F., Wu, J., Zhang, D., et al. (2018). CircRNA Circ_0067934 Promotes Tumor Growth and Metastasis in Hepatocellular Carcinoma through Regulation of miR-1324/FZD5/Wnt/β-Catenin axis. Biochem. Biophysical Res. Commun. 497, 626–632. doi:10.1016/j.bbrc.2018.02.119
Keywords: non-coding RNA, cancer, frizzled, Wnt/B-catenin, miRNA, lncRNA, circRNA, chemopreventation
Citation: Smith AJ, Sompel KM, Elango A and Tennis MA (2021) Non-Coding RNA and Frizzled Receptors in Cancer. Front. Mol. Biosci. 8:712546. doi: 10.3389/fmolb.2021.712546
Received: 20 May 2021; Accepted: 30 August 2021;
Published: 04 October 2021.
Edited by:
Florence Pinet, Facteurs de Risque et Déterminants Moléculaires des Maladies Liées au Vieillissement, FranceReviewed by:
Agnese Po, Sapienza University of Rome, ItalyPaola Infante, Italian Institute of Technology (IIT), Italy
Damjan Glavač, University of Ljubljana, Slovenia
Copyright © 2021 Smith, Sompel, Elango and Tennis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meredith A. Tennis, Meredith.Tennis@cuanschutz.edu
 Alex J. Smith
Alex J. Smith Kayla M. Sompel
Kayla M. Sompel  Meredith A. Tennis
Meredith A. Tennis