Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook
- 1Laboratory of Environmental Nanotechnology, Division of Biochemistry, National Dairy Research Institute, Karnal, India
- 2Centre for Healthy Brain Ageing, School of Psychiatry, University of New South Wales, Sydney, NSW, Australia
- 3Cell Biology and Proteomics Lab, Animal Biotechnology Centre, National Dairy Research Institute, Karnal, India
In the current scenario, it is an urgent requirement to satisfy the nutritional demands of the rapidly growing global population. Using conventional farming, nearly one third of crops get damaged, mainly due to pest infestation, microbial attacks, natural disasters, poor soil quality, and lesser nutrient availability. More innovative technologies are immediately required to overcome these issues. In this regard, nanotechnology has contributed to the agrotechnological revolution that has imminent potential to reform the resilient agricultural system while promising food security. Therefore, nanoparticles are becoming a new-age material to transform modern agricultural practices. The variety of nanoparticle-based formulations, including nano-sized pesticides, herbicides, fungicides, fertilizers, and sensors, have been widely investigated for plant health management and soil improvement. In-depth understanding of plant and nanomaterial interactions opens new avenues toward improving crop practices through increased properties such as disease resistance, crop yield, and nutrient utilization. In this review, we highlight the critical points to address current nanotechnology-based agricultural research that could benefit productivity and food security in future.
Introduction
Agriculture acts as the primary pillar of the developing economy and provides food for a better life. In the current scenario, the field of agriculture has been facing a wide range of challenges, including unpredictable climate change, contamination of soil with various harmful environmental pollutants such as fertilizers and pesticides, and majorly elevating food demands with a growing global population (Pouratashi and Iravani, 2012). A recent report by the United Nations projected that the global population would become 8.5 billion by 2030 and approximately 9 billion by 2050 (https://population.un.org/wpp/Publications/Files/WPP2019). Therefore, to meet the demands of the relentlessly growing population, there is an urgent need to increase food production more than 50%. On the contrary, industrialization leads to depletion of natural resources on which the population's livelihood depends, such as oceans, forests, and ecological biodiversity, at an alarming rate (Kang et al., 2009). Thus, it advocates the need for increased crop production and better food security. Ecological biodiversity plays an essential role in maintaining the delicate balance of the environment and food production as it strengthens agricultural resilience toward the environmental stress that potentially leads to crop failure. More specifically, the biodiversity for food and agriculture comprises livestock, crops, forestry, and aquaculture systems by which human beings are sustained. Oceans and forests are home to numerous animal and plant species that majorly withstand world population for food, fodder, fuel, and fiber. Thus, any issues that could cause ecological disequilibrium significantly affect food security (http://www.fao.org/3/CA3129EN/CA3129EN.pdf; https://www.un.org/sustainabledevelopment/blog/2019/05/nature-decline-unprecedented-report/; Rice and Garcia, 2011). In order to deal with these pertinent issues, newer techniques and strategies are continuously evolving. Given this, one such step taken is the introduction of nanomaterial (NM) based products for revolutionizing modern agriculture practices. These materials bear high reactivity due to their large surface area–to-volume ratio and novel physicochemical properties that provide the clear advantage of required modification according to elevating demand.
Previously, nanotechnology has been utilized in multiple fields, including chemistry, pharmaceutical science, diagnosis, and therapeutics, to name a few (Leso et al., 2019; Stefan and Monchaud, 2019; Li W. Q. et al., 2020). Nanoparticles (NPs), at the forefront of nanotechnology, are incorporated into a variety of consumer products owing to their tremendous potential as compared to their bulk counterparts (Jeevanandam et al., 2018). Therefore, the unique and emerging properties of these new-age materials have drawn unequivocal attention in the food and agriculture sector.
Modern agriculture is transforming into precision agriculture with the help of these new-age materials that are helping to gain maximum output from the available resources. Various types of other NMs, such as nanoclays, nanotubes, and nanowires, possess unique surface chemistry, electrical, and optical properties (Yaqoob et al., 2020) that provide them with better sensitivity, improved detection limits, and rapid response times (Kumbhakar et al., 2014). Fertilizers are necessary to enhance crop production, but at the other end, they decrease soil fertility by disturbing the soil mineral balance (Solanki et al., 2015). Generally, pesticides, fertilizers, and antibiotics are sprayed, which can be run off efficiently. Furthermore, the cost of production of these pesticides and fertilizers is very high, which needs to be controlled. The application of NMs in agriculture aims to reduce nutrient losses to increase yields, reduce the amounts of products for plant protection (Usman et al., 2020), and minimize the cost of production to maximize output. In the current review, we suggest and provide the facts that NMs deliver a better solution to improve agriculture and food systems. However, we also highlight the fact that it is vital to test the extent of toxicity of NM-based products before implementing them in the market. Nanotechnology-based products, such as smart agrochemical delivery systems using NMs as a carrier of active ingredients, are being continuously developed. The waste products of agriculture, such as soy hulls and wheat straw, can be converted into advanced bionanocomposites having improved mechanical and physical properties to be utilized for industrial purposes. Some progress is still needed to gain the practical advantages of nanotechnology, such as better design of processes, risk assessment of nanopesticides and nanofertilizers, and regulations for commercialization of nanoagroproducts (Chen and Yada, 2011; de Oliveira et al., 2014; Amenta et al., 2015). Another challenging issue yet to be resolved includes farming systems to deal better with unpredictable climate variability, including new varieties of crops that can sustain drought, heat, and other environmental stress to convey the whole spectrum of modern farming practices worldwide (Elias et al., 2019). We strongly believe that a broad perspective of nanotechnology is essential to fulfilling the goal of helping foster global agriculture.
NP Interaction With Plants
The agricultural sector is benefiting from advancements in science and technology, which give us new ideas and solutions to combat severe problems. With the advent of nanotechnology, more efficient and fewer contaminant nanoformulations are continuously produced for sustainable agriculture (Fraceto et al., 2016). These new-age materials have the potential to alter the physiology of plants right after entering the complex plant–soil system that can be easily exploited to understand the aftereffects (Figure 1). Moreover, for controlled delivery of active compounds, critical knowledge of the interaction of these NMs with plants, either positive or negative, is required. Thus, they could provide novel avenues toward developing better nanomaterial-based products. It is also believed that the concentration of NMs in the natural environment is much lower than levels considered harmful. However, still, some gaps are left to be filled with comprehensive safety assessments (Batley et al., 2013).
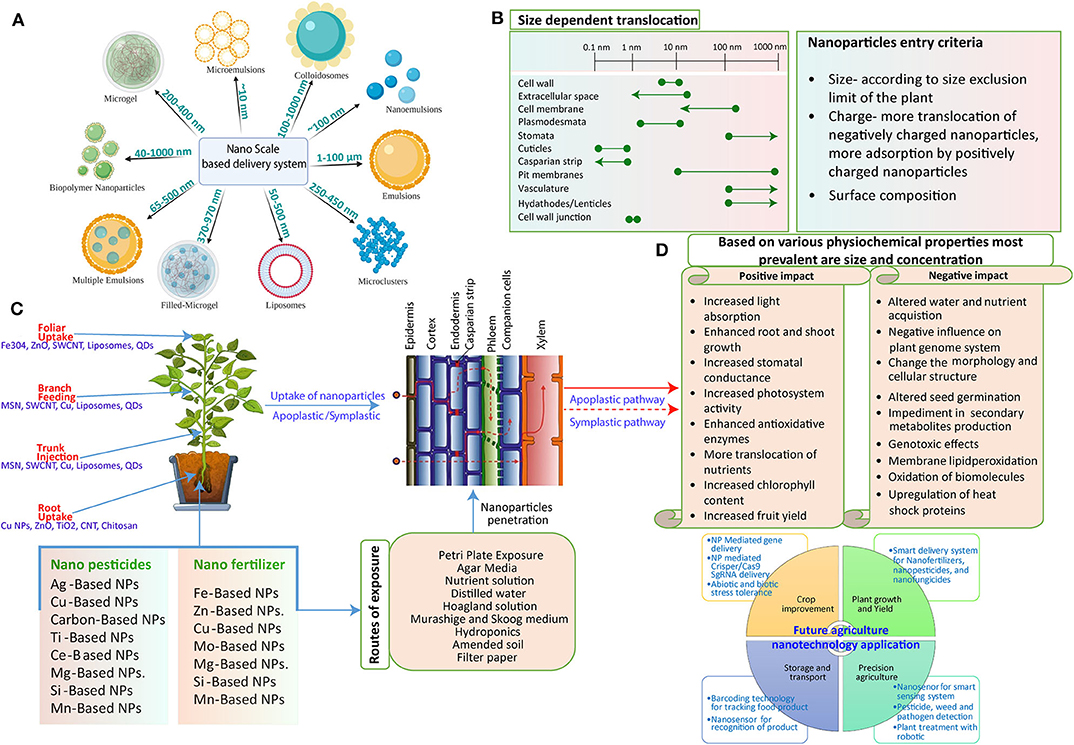
Figure 1. Schematic representation of the potential interaction of NPs with the plant system. (A) Various NPs are used as nano-carriers for the delivery of exogenous cargo. They may vary in their size from 10 nm to more than 100 nm. (B) Interaction mechanism: The mechanism of NP interaction with and subsequent translocation to the plants. After successful penetration through the external layers, NPs move through apoplastic and symplastic pathways to reach different plant cells and tissues. (C) Plant structures impose different exclusion limits to the movement of NPs as size exclusion limit (SEL) of the cell wall is 5–20 nm, Casparian strip with SEL of <1 nm, and SEL of plasmodesmata is 3–50 nm in diameter. Some NPs can form larger pores in cell walls, plasmodesmata, and cuticles, thus facilitating the entry of larger size NPs (line shown with dotted corners). NPs with different surface charges vary in the uptake and translocation through the plant system owing to electrochemical interaction between them. Also, the surface modification of the NPs alters the movement. (D) The various responses of plants to the exposure of NPs. NPs can impose positive as well as negative impacts on plants that are mostly determined by their physiochemical properties; size and concentration are the most prevalent as more concentration leads to negative impact and less concentration imposes a positive impact on the plant system. On the other hand, size plays an essential role as small-size NPs can be easily taken up by the plant that could cause more interaction and thus a more powerful direct impact.
Systemic Approach for Selectivity of NMs: Physicochemical Property-Based Uptake and Interaction
Several factors are responsible for the uptake and transformation of NPs: prominently, plant physiology, physiochemical properties of the NP itself, size, surface charge, shape, and potential interaction with plants (Pérez-de-Luque, 2017).
Size-Dependent Uptake of NPs
Size of the NPs must be considered as a significant parameter to study the uptake in plants as various barriers confined within the plants are in the range of micrometer (mm) to nanometer (nm) (Figure 1A). For instance, the epidermis foliar is made up of the cells that form a cuticle membrane. The epidermis consists of stomata containing two guard cells forming a pore of about 3–12 μm wide and 10–30 μm long during the opening for gaseous exchange. Therefore, NPs can make their way through the plants by virtue of these stomatal openings. Additionally, the permeation properties substantially differ for the cuticle layer on the epidermis and trichrome of the stomata. The cuticle layer, on the other hand, presents in a more significant amount on the leaf epidermis with a size exclusion limit in the nm range (Wang P. et al., 2016; Wang X. et al., 2016; Figures 1B,C). It was described that NPs with a size range of 4–100 nm could cross the cuticle by disrupting the waxy layer (Larue et al., 2014), and fluorescently tagged NPs >50 nm can accumulate in the epidermis underneath the cuticle where stomata are absent (Nadiminti et al., 2013). The polymeric NPs of 43 nm diameter are able to penetrate the leaves of Vicia faba solely through the stomata, whereas particles of 1 μm did not cross at all (Eichert et al., 2008). NPs usually get deposited on the cell wall of the substomatal cavity when entering through the stomata. The likely penetration of small-size NPs, for instance, 20 nm Fe3O4 NPs, were observed through TEM studies in the Nicotiana benthamiana plant (Cai et al., 2020; Figures 1B,C).
The principal size exclusion limit was mainly imposed (Palocci et al., 2017), notably, by these plant cell walls that are usually in the size range of 3.5–20 nm and more often around 5 nm (Carpita et al., 1979; Tepfer and Taylor, 1981). Thus, NPs smaller than 5 nm can traverse the cell wall of intact cells efficiently. In one study, SWCNT was able to form an endocytosis-like structure in the intact cell wall of the Arabidopsis thaliana plant (Shen et al., 2010). It is suggested that NPs within a size range limit according to the cell wall can traverse through it. For example, quantum dots (QDs) fall under the size range of plant cell wall pores (sub-10 nm) that can be easily exploited to make their entry through the plants (Wu et al., 2017a). Li et al. observed the presence of AgNPs of size 24.8–38.6 nm within lettuce leaves when applied through the foliar route. They also demonstrated the in planta transformation of AgNO3 applied with AgNPs that also explains the biotransformation phenomenon (Li W. Q. et al., 2020).
The interaction of NPs with the cell wall creates large-size pores that further facilitate the entry of NPs (Carlson et al., 2008). Further, there are results on the accumulation of larger sized NPs in the cell wall and subsequently in the cytoplasm, but a discrepancy between the claimed pore size of the cell wall (depends on many factors) and the accumulation of larger sized NPs is still maintained. Several studies carried on plant protoplast utilized as a model system to study NP uptake through the plant cell membrane. Moreover, this circumvents the technical difficulties arise in studying uptake as well as quantification of NPs inside the plants mostly due to the internal fluorescence of the plant cell wall and impermeability imposed by plants to certain staining dyes (Torney et al., 2007).
After NP entry into the plants, it is imperative to recognize the path that they follow to transport as it gives an idea of where these NPs might accumulate. NPs follow two important routes for moving up and down in a plant: the apoplast and the symplast. The symplastic pathway allows movement through the cytoplasm of adjacent cells (Roberts and Oparka, 2003), whereas the apoplastic pathway allows flow through the extracellular spaces involving cell walls of neighboring cells and xylem vessels (Sattelmacher, 2001). The cell-to-cell movement takes place through the passage plasmodesmata, serving as a cytoplasmic bridge to facilitate particle movement between neighboring cells. They are about 20–50 nm in diameter, surrounded by 3-nm particles (Dietz and Herth, 2011).
Surface Charge-Dependent Uptake of NPs
NPs have a great degree of freedom that essentially relies on their uptake, absorption, and trafficking inside plants. The physicochemical interaction of NMs with plants under their energy and surface charge results in modifying the surface receptors, transporters, and specific membrane proteins (Juárez-Maldonado et al., 2019). NPs with different surface charge possess the differential capacity for aggregation and surface properties in comparison to their pristine counterparts (Hotze et al., 2010; Figure 1C).
Inside the leaves, a biological membrane, i.e., the cell wall, is present with both hydrophobic and hydrophilic components and unequal distribution of fixed negative charges (surface potential of cellulose fibers and lignin are −15 and −45 mV, respectively) (Santiago et al., 2013; Zeng et al., 2017). Thus, a possible explanation lies in that the negatively charged cell wall favors the uptake of positively charged NPs in the tissues. The negatively charged plant cell walls act as the surface for the ion exchange that potentially promotes the penetration of cationic rather than anionic NPs (Meychik et al., 2005). In contrast to this, transport efficiency is greatly enhanced for negatively charged NPs. The surface charge–dependent uptake and transportation of AuNPs is explained by Zhu et al., who show much higher adsorption of positively charged NPs at root surfaces. However, negatively charged NPs were observed to have a higher internalization rate and translocation (Zhu et al., 2012). In this context, CeO NPs with a positive charge strongly adsorbed onto the root surfaces (negatively charged), whereas negatively charged CeO2 NPs showed low root accumulation but higher shoot internalization, mainly by overcoming electrostatic repulsion (Liu et al., 2019; Figure 1D).
Similarly, Milewska-Hendel et al. comprehensively demonstrate the surface charge–dependent uptake of AuNPs in roots and its transportation from roots to shoots (Milewska-Hendel et al., 2019). It is thought that negatively charged NPs assist both symplastic as well as apoplastic transport into the vascular system through roots to shoots. Thus, the transport performance of the NPs across plants is significantly varied through surface charge modification (Figures 1B,C). Based on the physicochemical properties and concentration of NPs in conjunction with agrochemicals exert positive as well as negative impact on plant health (Figure 1D). The review contains comprehensive information on various impacts, and the future prospect of NPs in agriculture is discussed.
Anatomical Difference and Mode of Application-Related NP Uptake
The anatomical differences in a plant and the surface charge of the NPs are very closely related. A better association of positively charged cerium oxide NPs is found with dicot plants, such as tomato and lettuce, with a larger root surface area. However, better root-to-shoot translocation is observed with neutral and negatively charged NPs (Spielman-Sun et al., 2019). This suggests that anatomical and physiological differences in plants result in differential uptake patterns of NMs, which is also evidenced by observations that diverse crops belonging to different species show a varied pattern of accumulation and absorption of distinct classes (e.g., TiO2, carbon-coated, Au) of NMs (Cifuentes et al., 2010; Larue et al., 2012c; Figures 1B, 2A).
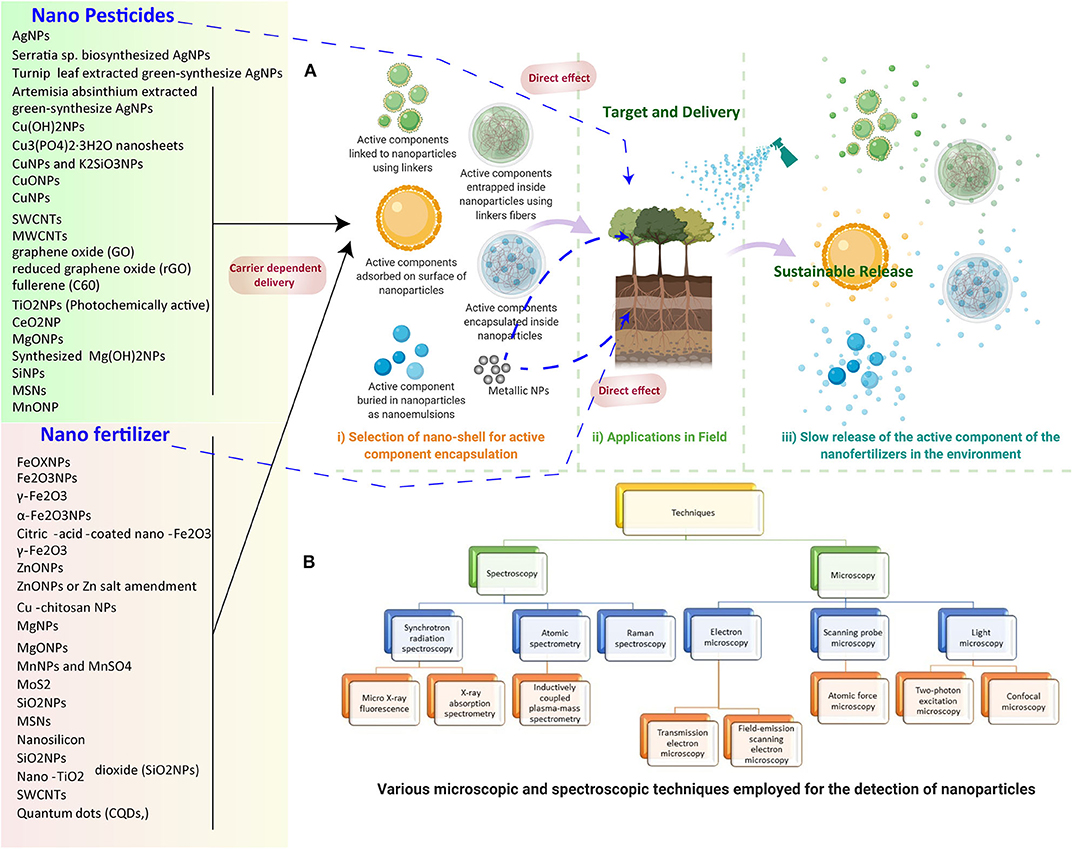
Figure 2. (A) NP-based nano pesticide and fertilizer delivery to the plants and release in the environment. (1) Selection of nano-shells for active component encapsulation. (2) Application in the field includes direct delivery and carrier-mediated delivery. (3) The steady release of the active component of the nano fertilizers in the environment for sustainable release. (B) Various microscopic and spectroscopic techniques employed for the detection of NPs in plants.
The dicots, in comparison to monocot plants, show higher uptake of NPs due to more permeability in the cuticles. This is proven in the case of lipid-based liquid crystalline nanostructures, which are utilized as a delivery vehicle (Nadiminti et al., 2013). This difference is attributed to the different architecture of the cell wall in monocots and dicots. In monocots, the pectin polymer is less tightly bound to the cellulose (Carpita and Gibeaut, 1993). Another significant anatomical difference is in the leaf architecture, such as the presence of dumbbell-shaped stomata in monocot and kidney-shaped stomata in dicot plants, resulting in variable degrees of uptake and translocation efficiencies of NPs (Figures 1B, 2A).
Additionally, the leaves of monocots have parallel veins that are most resistant to tearing and, therefore, have high strength (Avellan et al., 2017; Hu et al., 2020). The ability of plants to take up these NMs also depends upon the mode of their application as roots absorb nutrients from the soil, leaves are meant for the exchange of gas, and cuticles hamper the penetration of any substance freely (Schwab et al., 2016). However, drought-resistant plants develop tighter cell walls, cuticles, Casparian strips, and other barriers that are helpful to minimize water loss during scarce water conditions (Riederer and Schreiber, 2001). Some of the essential factors of the soil, such as organic matter and humic acid, make NMs more stable, which, conversely, enhances their bioavailability (Navarro et al., 2012). The presence of bacteria and mycorrhizal fungi living in association with plants also affects the plant uptake of NPs (Feng et al., 2013). The transport of CuO NPs from root to shoot and its reverse transport from shoots back to roots takes place via xylem and phloem, respectively (Wang Z. et al., 2012).
NPs can also utilize a clathrin-independent endocytic pathway for gaining entry into the plant. Poly (lactic-co-glycolic) acid (PLGA) NPs follow this pathway as treatment of inhibitors of the clathrin-dependent pathway do not hamper its uptake into the grapevine (V. vinifera) protoplast. The size and surface characteristics of the NPs play essential roles in determining the uptake inside the plants as evidenced by a study conducted on foliar uptake of AuNPs on wheat that explains efficient translocation of smaller size NPs with a surface coating of PVP-AuNPs rather than citrate-AuNPs (Avellan et al., 2019). Another study shows no uptake of SWCNT by cucumber seedlings, but in the form of nanosheets, they were able to interact with the external surface of the roots (Cañas et al., 2008). It shows that the uptake of NMs is highly dependent on the type of delivery method used. In this context, Su et al. investigated the fate and mobility of surface-modified AgNP delivery to trees by employing various methods, including direct tree injection and petiole feeding in Mexican lime citrus trees (Su et al., 2020). Further explanation of uptake and transport in conjunction with the fate and transformation of NMs is comprehensively reviewed by different groups (Lv et al., 2019; Su et al., 2019).
Methods to Study Uptake, Quantification, and Translocation of NPs
Potential techniques must be developed to track the interaction taking place between plants and NMs; moreover, more vital information is needed to quantify the uptake and translocation of NPs inside plants and as released in the environment (Figure 2A). In this context, the assimilation and concentration of iron (Fe3O4) NPs in pumpkin (Cucurbita maxima) plants were quantified by employing a vibrating sample magnetometer to confirm the presence in roots and leaves (Zhu et al., 2008). Similarly, Fe3O4 NPs can also be measured and tracked in plant organs by exploiting its magnetic properties, such as temperature and magnetic field dependence of magnetization (Govea-Alcaide et al., 2016). The major problem with tracking and translocation of Fe3O4 NPs is associated with the inability to distinguish between intact Fe3O4 NPs and leached ions, which further can be solved by utilizing magnetic particle spectrometry in conjunction with conventional atomic absorption spectroscopy (Ju et al., 2019; Figure 2B).
Also, the electron microscopy technique was used to track the translocation of C70 fullerene and MWCNT from roots to aerial parts (leaves) of rice (Oryza sativa); however, the authors did not quantify the amount of NPs taken up by the plants (Lin et al., 2009). In this regard, the uptake of MWCNTs in wheat and rapeseed plants was quantified through transmission electron microscopy (TEM) and the Raman spectroscopy technique (Larue et al., 2012b).
Critical imaging techniques, such as X-ray and computed tomography (CT), are beneficial to detect and localize various NMs in plants. Recently, to localize gold NPs in the roots of A. thaliana, a combination of techniques such as X-ray computed nanotomography (nano-CT), enhanced dark field, and hyperspectral (DF-HSI) imaging was used. The combined use of 2-D (DF-HSI) and 3-D (nano-CT) techniques provides better means to characterize and evaluate the NP–plant interaction at the cellular level (Avellan et al., 2017). Another non-invasive, highly sensitive method to visualize NPs through the combination of autoradiography, positron emission tomography (PET)/CT, TEM, and SEM was demonstrated in lettuce (Lactuca sativa) (Davis et al., 2017). In a pioneering study, the effect and interaction of TiO2 NPs with garlic (Allium sativum) was observed through spectroscopic techniques using time-resolved, laser-induced fluorescence and ultraviolet-visible spectra. The resulting increased chlorophyll content and enhanced photosynthetic activity in leaves of garlic plants were observed as compared to the control. The authors reported a decrease in the intensity ratio of red and far red chlorophyll fluorescence bands, suggesting a rise in photosynthetic activity and chlorophyll content (Bharti et al., 2018). Two-photon excitation microscopy as a novel strategy was employed for in vivo detection of MWCNTs, TiO2, and Cerium oxide NPs in wheat tissues (Wild and Jones, 2009). Other essential techniques, such as microscopy and spectroscopy, are highlighted in Figure 2B.
Although microscopy techniques provide a clear advantage in analyzing NPs in different samples, they have some main limitations, including sample preparation, a partial area of analysis, and limited 3-D imaging, which further requires alternative solutions. In view of this, inductively coupled plasma-mass spectroscopy (ICP-MS), in particular, single-particle ICP-MS (SP-ICP-MS) analysis, is a promising method for detection, characterization, and quantification of NMs (Mozhayeva and Engelhard, 2020). Similarly, SP-ICP-MS is utilized to quantify the uptake of CuO NPs in edible plants, lettuce, kale, and collard greens (Keller et al., 2018).
Mass spectrometry–based analytical techniques help distinguish between different chemical forms of NMs as they can readily be transformed once they enter the plant. One such approach utilized SP-ICP-MS and ESI tandem MS to confirm the fate of ZnO NPs inside the edible plant lettuce (Wojcieszek et al., 2019). The Au NPs were quantified by using ICP-MS in order to observe the uptake mode and accumulation in watermelon plants (Raliya et al., 2016). The significant advantage of these potential techniques lies in either their cumulative power or utilizing them in combination. The simultaneous uptake, retention, and distribution along with the plant were studied by Nath et al. for Ag, Cu, and ZnO NPs in A. thaliana using SP-ICP-MS, SEM, and EDS (Nath et al., 2018). Similarly, the combination of three orthogonal techniques, such as electron microscopy, SP-ICP-MS, and ICP-OES, is used to study the uptake and size distribution of TiO2 NPs in rice plant (Oryza sativa L.) tissues (Deng et al., 2017).
The Physiochemical Response of Plants Toward NPs
NPs for Plant Growth and Seed Germination
The uptake of NMs inside plants largely depends on the size, chemical composition, and functional groups present on their surface and the type of coating. The interaction and uptake of NMs lead to changes at the molecular level and affect the overall physiology of plants (Jin et al., 2017). The promising effect of multiwalled carbon nanotubes (MWCNTs) on the growth (55–64% increase) of tobacco cells involves a unique molecular mechanism. The majority of the activated genes involved are related to cell division (CycB), cell wall extension (NtLRX1), and tobacco aquaporin (NtPIP1) (Khodakovskaya et al., 2012). The ability of NMs to penetrate the hard coating of seeds and allow water importation is the deciding factor for increased growth and vigor. In addition to this, the seed priming technique with the help of nanotechnology is a promising strategy; before sowing, it further corroborates the potential of high-yield value crops (Acharya et al., 2020; Anand et al., 2020).
The application of MWCNTs affects the growth of some important crops, such as barley, soybeans, and corn. MWCNTs aggregate inside the endosperm of exposed seeds, which was confirmed by Raman spectroscopy and TEM imaging (Lahiani et al., 2013). NPs were transported to different parts of the plant and interacted with cellular machinery, thus promoting plant growth. NPs made of mesoporous silica (MSNs) can improve photosynthesis by interacting with chloroplasts, resulting in enhanced seed germination, increased total protein, and chlorophyll content. The highest concentration (2,000 mg/l) of MSNs did not induce stress conditions in any of the plants, which is indicative of its safer application as a smart delivery system (Sun et al., 2016). Silica plays an essential role in providing nutrition as its deficiency consequently makes plants weaker and highly susceptible to biotic and abiotic stress (Rafi et al., 1997). The application of silica also helps to ameliorate the effect of drought stress and increase biomass in stress-affected plants (Ahmad et al., 2007). Maize plants show better response toward the application of silica NPs (SiO2 NPs) prepared from rice husk and thoroughly characterized as compared to its bulk counterpart and control. Silica uptake was enhanced, and roots were elongated in the maize plants; this helps to withstand drought conditions. Root sectioning also reveals the direct uptake and accumulation of SiO2 NPs in the epidermis cells of maize (Suriyaprabha et al., 2012). The detailed impact of SiO2 NPs on agriculture has been extensively reviewed by Rastogi et al. (2019).
Another potential NP such as TiO2 also promotes the growth of plants as evidenced in canola (Brassica napus), which resulted in promoted seed germination as well as seed vigor. The highest effect was seen at a concentration range of 1,200–1,500 mg/l with increased plumule growth and large radicle as compared to the control (Mahmoodzadeh et al., 2013). The effect of any NP can be positive and negative, depending on its additive concentration and size. The smallest TiO2 NPs accumulate more in plants as compared to larger particles (Larue et al., 2012a). Moreover, these NPs can also affect the miRNA levels to initiate the growth-promoting pathways in plants (Boykov et al., 2019).
The positive effect of Fe NPs on the Capsicum annum plant at lower concentrations was found with increased plant growth by increasing chloroplast number and grana stacking. In contrast, when these NPs were applied at higher levels, they caused harm to the plant (Yuan et al., 2018). Other metal oxide NPs, such as ZnO and Cu, are observed to affect seed germination in Vigna mungo, i.e., black gram (Raja et al., 2019). The seed germination and growth-enhancing properties of less studied polyvinylpyrrolidone (PVP) protected platinum NPs in Pisum sativum (Rahman et al., 2020). Another critical and biocompatible NP, chitosan, exerted its effect on seed germination and growth by elevating the levels of indole-3-acetic acid (IAA) concentration in wheat or Triticum aestivum L. even at a shallow concentration (Li et al., 2019).
Recent Advances in NPs for Plant Protection
The application of NPs (described in the previous sections) influence various important factors of the plant's growth and development that include photosynthesis, seedling vigor, and growth of roots and shoots. Plants are found everywhere; therefore, they have to bear extreme environmental conditions, such as drought, salt stress, high temperature, UV radiation, and salt stress. A number of different stress responses is shown by plants, such as alterations in molecular mechanisms, stress-responsive gene expression, and production of antioxidative enzymes that help to play a central role in scavenging the plants in harsh environmental conditions (Rejeb et al., 2014). Plants protect themselves from osmotic stress by producing various organic osmolytes, such as polyols and trehalose, and also different amino acids, such as proline and glycine. NPs provide an efficient way or, in other words, provide support to plants in alleviating this protection mechanism.
NPs Mitigate Abiotic Stress Response
In addition to helping plants grow, NPs also protect them from abiotic stress. Toxic metal binds to the surface of the NP owing to its large surface area and small size, thus reducing its availability. Abiotic stress includes drought, salinity, alkalinity, temperature fluctuations, and mineral and metal toxicity. NPs can mimic the activity of antioxidant enzymes in the form of nano-enzymes that can scavenge from oxidative stress (Sharifi et al., 2020). Photosynthesis is the vital metabolic process in plants and one of the highly susceptible methods; thus, by mitigating oxidative and osmotic stress, its normal functioning can be maintained. In the photosynthetic machinery, photosystem II (PS II), Rubisco, and ATP are the primary targets under stress conditions (e.g., nutrient deficiency, salinity, water, drought, and heat; Figure 3).
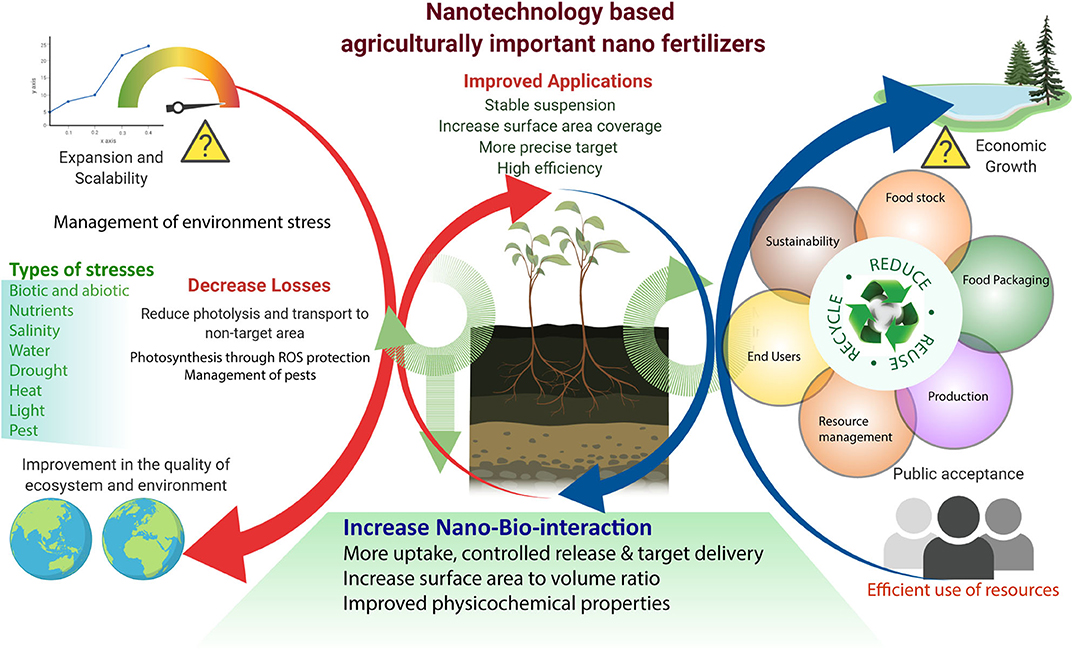
Figure 3. Nanotechnology-based agriculturally important nano fertilizers increase agronomic productivity, efficiency, and reduce environmental stress. Efficient utilization of nanotechnology in agriculture for future sustainability.
Th defense response in plants against abiotic stress was studied; for example, SiO2 NPs improved plant transpiration rate, water use efficiency, total chlorophyll, and carbonic anhydrase activity in the Cucurbita pepo plant in response to a defense mechanism against salt stress (Siddiqui et al., 2014). Similarly, it was found that TiO2 (anatase) changes the photoreduction activity and inhibits linolenic acid in the electron transport chain. It also decreased the rate of oxygen evolution of chloroplasts (Mingyu et al., 2008). In stress conditions, ROS are produced by the cell organelles, and this is the signature of abiotic stress. Plants are also equipped with the enzymatic machinery to deal with the oxidative stress imposed by the environment on them (Figure 3). However, plants face the consequences of such a situation when stress overcomes the defense system. NMs help in mitigating such stress by activating specific genes, accumulating osmolytes, and providing free nutrients and amino acids (Table 1).
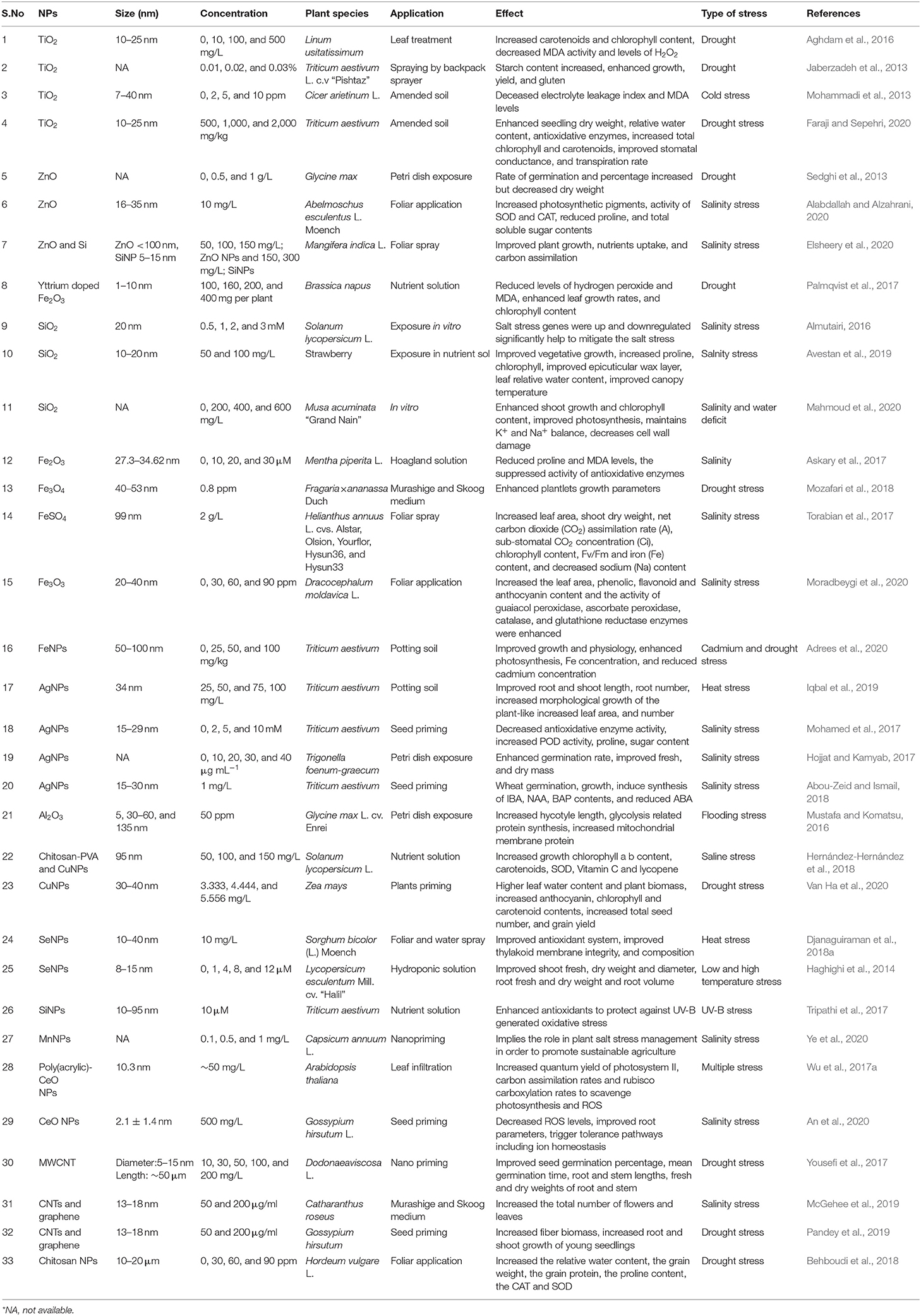
Table 1. Types of NPs, their relevant sizes, and concentration with the application focus and effect found on alleviating abiotic stress response in plants.
Cerium NPs (CeO NPs)
Nanoceria has broad implications on plant health, both positive and negative, depending on the exposure concentration, coating, surface charge, plant species, and growth conditions (Milenković et al., 2019). Nanoceria is a family of CeO NPs with wide application as an antioxidant in the biomedical industry (Liu and Shi, 2019). Although NPs can impose some severe effects on plants, as we look into the positive aspects of these NPs, it outweighs the negative impacts and can be exploited to provide health benefits to plants (Table 1). As we discuss earlier, the potential interaction at the nano–bio interface, these NPs could augment the tolerance against various abiotic stress of plants by regulating essential pathways (Saxena et al., 2016). For instance, abiotic stress leads to excessive ROS production that consequently decreases the photosynthetic performance of the plant and could cause biomolecule oxidation (Wakeel et al., 2020). Thus nano-Ce is well-suitable to mitigate this effect owing to the unique redox potential based on the facile transition between Ce3+ and Ce4+ oxidation states (Collin et al., 2014) and, therefore, acts as an ROS scavenger.
Moreover, a CeO NP with its high Ce3+/Ce4+ ratio mimics superoxide dismutase and produces hydrogen peroxide, but it also mimics catalase activity at a low Ce3+/Ce4+ ratio and shows the scavenging effect (Wang Q. et al., 2012; Wang Z. et al., 2012; Ma et al., 2015; Pulido-Reyes et al., 2015). In addition, the oxidative scavenging effect also is extended to other stresses, which include excess light, heat, and dark chilling. Furthermore, it leads to decrease photosystem II abundance, photochemical efficiency, chlorophyll content, and changed plant morphology (Nievola et al., 2017; Chen et al., 2020; Gao et al., 2020). Wu et al. explained the ameliorative effect of the anionic CeO NPs in the A. thaliana plant against abiotic stress, including excess heat, light, dark, and chilling (Wu et al., 2017b). The salinity stress also imposes a threat to plant physiology by a similar mechanism of oxidative stress. It was found that CeO NPs could alleviate the oxidative stress in Brassica napus. The effect was imposed by NaCl (100 mM) by modifying physiobiochemical properties, such as increased plant biomass, chlorophyll content (which, in turn, increases the Mg2+ uptake), and efficient photosystem at a concentration of 200 and 1,000 mg/kg (Rossi et al., 2016).
The maintenance of the cytosolic Na+/K+ ratio is considered one of the hallmarks of salinity stress (Hauser and Horie, 2010), and NPs can affect this transport efficiently. Wu et al. demonstrated that nanoceria could augment plant tolerance to salinity stress. By directly acting on hydroxyl radical (·OH) generation and affecting potassium fluxes (reducing K+ efflux and improving K+ retention) across the plasma membrane of mesophyll thus improves plant photosynthetic performance and biomass (Wu et al., 2018). The catalytic scavenging activity of CeO NPs has also been utilized to mitigate drought stress, which, in particular, has severe implications for plant growth and yield worldwide. The highly efficient, drought-resistance activity of CeO NPs was observed in foliar-sprayed sorghum plants at a shallow concentration of 10 mg/L.
Additionally, it alleviated the ROS percentage and lipid peroxidation, resulting in improved carbon assimilation rates as well as pollen germination associated with it (Djanaguiraman et al., 2018b). Seedling stage effects of salinity stress could be mitigated by CeO NPs. Seeds primed with poly (acrylic acid)-coated CeO NPs (500 mg/L in water for 24 h) when germinated in salinity stress (200 mM NaCl) showed drastic effects on the seedling roots, including increased length (56%), weight (41%), and root vitality (114%) as compared to the control. The subsequent perturbation of pathways related to the antioxidative enzyme system, ion binding and Ca2+ signaling, and terpene synthesis result in mitigation of oxidative stress and enhanced tolerance against salinity stress (An et al., 2020).
Silicon NPs (SiNPs)
Silicon (Si) is known to be the second most abundant material on Earth after oxygen, and it has gained significant importance in agriculture (Table 1). Si is considered to be midway between an essential and non-essential element for plants because it is not merely responsible for plant survival, but if present, plants can sufficiently benefit (Luyckx et al., 2017). These NPs can directly or indirectly interact with plants, thus conferring morphological and physiological changes in order to provide tolerance against stress. It confers beneficial effects on plant growth; increases their biomass, anatomy, and physiology; modifies tissue differentiation; activates defense systems; and helps in acclimatizing to stress conditions (Babajani et al., 2019).
SiNPs showed anti-stress effects at various concentrations toward drought stress in Hawthorns (Crataegus sp.); the responses varied in the seedlings depending on the concentration applied at different stages of drought stress, i.e., moderate to severe. These effects include enhanced photosynthetic ability; relative water content; membrane electrolyte leakage; and increased levels of chlorophylls, carotenoids, and proline (Ashkavand et al., 2015). The increased tolerance under a saline environment conferred by the application of Nano-SiO2 by accumulating proline maintained the ionic balance, enhanced the antioxidant system, and increased levels of various phytopropanoids, thus leading to osmotic adjustments (Soleymanzadeh et al., 2020). Additionally, under salt stress, SiO2 NPs are shown to increase water use efficiency, stomatal conductance, transpiration rate, and reduced chlorophyll degradation, leading to tolerance from external insult (Haghighi and Pessarakli, 2013). Salt stress is the primary culprit causing significant changes in the epicuticular wax layer.
Interestingly, nano-Si application to strawberry plants resulted in better epicuticular wax structure and thickness as compared to the salt-stressed plant (Avestan et al., 2019). Another work performed on sweet pepper plants (Capsicum annum L.) to study the impact of nano-Si to mitigate salt stress observed significant changes in comparison to their bulk counterpart (Tantawy et al., 2015). Nano-Si was supplied to the plants through an irrigation system at low concentrations (1.0 and 2.0 cm3/L) for weeks, resulting in enhanced leaves and fresh weight. The concentration-dependent effect of SiO2 NPs was also observed on potato plants exposed to different salt stress (NaCl; 50 and 100 mM). The NPs were able to show better stress amelioration at a lower concentration (50 mg/L) and a higher concentration (100 mg/L) (Gowayed et al., 2017). However, lower concentration was found to be more effective. These studies notably suggest a better viewpoint about the positive and negative impact of NPs, contradicting the assumptions of only toxic aspects of NMs. Therefore, it is required to initially study the characteristics of NPs before employing it in any field.
Plant hormones play a vital role during external stress conditions and confer better adaptation to different changing environmental scenarios (Verma et al., 2016; Raza et al., 2019). A plant growth hormone such as Gibberellin has an important role in plant responses against various abiotic stresses including drought, shading, flooding, and low temperature mainly by reducing the plant growth to divert the focus onto withstanding stress response (Colebrook et al., 2014). Plant hormones can also be supplied to the plant by utilizing NMs; for instance, Mesoporous silica NPs (MSN) entrapping abscisic acid were tested on the A. thaliana plant. It significantly showed the prolonged release of hormones and further improved the drought resistance ability of the seedlings (Sun et al., 2018).
Titanium Dioxide NPs (TiO2 NPs)
We have described the antioxidative effects of specific NPs that scavenge ROS produced in response to threat or stress in plants, but there are other NPs that exert their effects through other mechanisms involving gene regulation (Table 1). For example, TiO2 NP exposure (0.01%) increased the chlorophyll content and biomass by triggering a mechanism of antioxidant enzyme activities, resulting in decreased malondialdehyde and hydrogen peroxide and production of proline and soluble sugars, thus maintaining the osmotic balance (Abdel Latef et al., 2018). Similarly, nano-TiO2 was able to trigger the expression of particular essential non-coding RNA considered to play a vital role in the abiotic stress-resistance mechanism. Frazier et al. observed the expression of 11 conserved miRNA in response to TiO2 NPs (0.1, 1, 2.5, and 5%) in tobacco seedlings that are responsible for rescuing plants from heavy metal stress (Frazier et al., 2014).
Water deficit is a severe issue for plant cultivation as it leads to a loss in vigor and causes severe damage to crops. Nano-TiO2 can improve the hydration status of the plant by increasing the activity of the nitrate reductase (NR) enzyme, which, in turn, enhances the accumulation of osmolytes. The increased NR enzyme activity results in nitric oxide (NO) production that ultimately induces proline and glycine betaine synthesis (Khan et al., 2020). TiO2 NPs tend to show both enzymatic as well as a non-enzymatic defense system against stress in plants. Interestingly, TiO2 NPs are acknowledged for the regulation of other enzymes, such as glutamate hydrogenase, glutamine synthase, and so on, thereby, accumulating more nutrients and also essential oil production (Ahmad et al., 2018). In this regard, Gohari et al. carried out a greenhouse experiment to evaluate the effect of nano-TiO2 (0, 50, 100, and 200 mg L−1) on Moldavian balm (Dracocephalum moldavica L.) plants grown under exacerbated salinity stress (0, 50, and 100 mM NaCl). The TiO2 treated plants (100 mg/L) showed a profound effect on the production of essential oil content (1.19%): geranial, z-citral, geranyl acetate, and geraniol under normal conditions. This implicated protection of aromatic plants against the stress condition by directly modifying their essential oil production profile and composition (Gohari et al., 2020). Karamian et al. carried out another study on medicinal plants showing that drought stress was mitigated by supplying methyl jasmonate (200 μM), salicylic acid (100 μM), and TiO2 NPs (20 ppm). The results suggest that it increased water stress tolerance through activation of enzymatic and non-enzymatic antioxidant defense systems (Karamian et al., 2020).
Nano Pesticides
As more than half of the worldwide population rely on plants and their products for survival, it is a significant concern to enhance plant productivity as well as maintenance of their health. As several biotic and abiotic factors include pathogens, nutrient deficiency, or soil/air pollution, respectively, all equally can affect the health of the plant via inducing damages that, consequently, lead to decreased crop and fruit yield. Frequently, plant disease is caused by a broad range of disease-causing organisms (or pests). Thus, it is an immediate requirement to keep them from causing more plant wilting. Insects are crucial matters in agriculture as they demolish crops and overrun stored food, consequently causing the worsening quality of food and transmission of plant diseases. As per the definition of the U.S. Environmental Protection Agency, pesticides are substances that deliberately prevent, repel, and demolish any pest (Figure 3).
Pesticides are used to enhance and improve the crop yield and efficiency by protecting plants from damaging factors, including plant diseases and insects (Jampílek and Králová, 2017). However, it has been found that the use of pesticides is lethal and toxic for the environment; most of them are hazardous and lethal to human and animal health. Therefore, many pesticides are banned by state or international authorities. This involves several significant and vital issues, including indiscriminate use of pesticides at a high concentration that harms the ecosystem, increasing bioaccumulation, making the soil infertile, and disrupting its microbiota (Meena et al., 2020). Some issues must be taken into consideration to increase agronomic productivity and efficiency and reduce the environmental effect (Figure 3).
The synthesis of effective and safe pesticides is tricky and expensive, but nanotechnology provides a novel and improved solution (Sasson et al., 2007). Nanotechnology already has a significant impact on pharmacy (drug delivery). With the recent developments in the agriculture and food sector, it is promising. Nanotechnology is not restricted to applications for plant protection against pests, but expands to minimize waste, monitor of plant growth, guarantee enhanced food quality, and secure rising of global food production (Jampílek and Králová, 2017). The most frequent trials in nano pesticides are nano herbicides, nano insecticides, nano nematocides, and nano fungicides (Table 2).
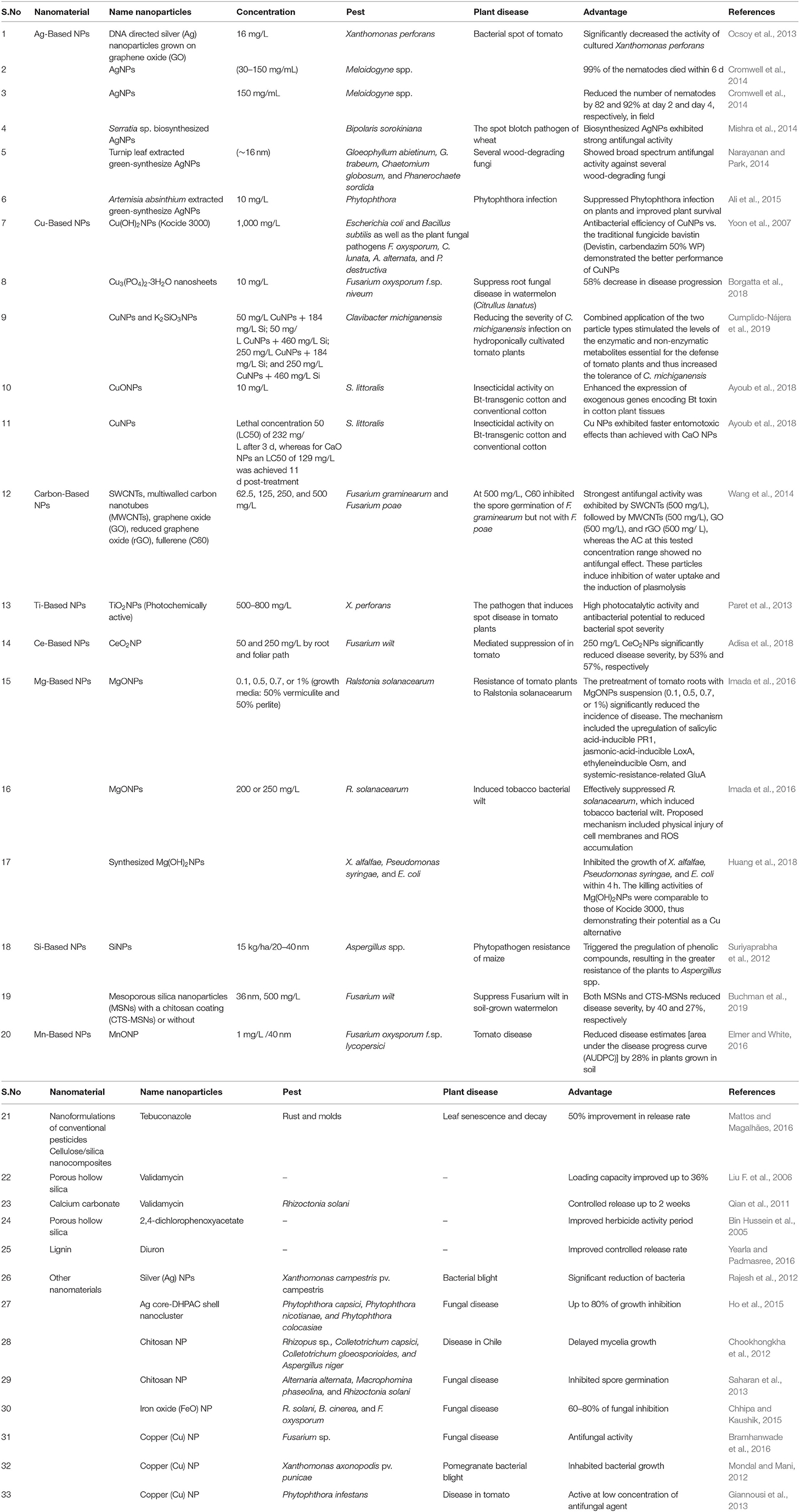
Table 2. Different types of NPs employed for the generation of nano pesticides, their composition, benefits, target pests, and examples of active components.
The best example of a nano insecticide is nanostructured alumina (NSA), which is nanoengineered material that works as an insecticide. It is synthesized through the oxidation of metal, which exhibits a fixed electric charge. Advance nanotechnology designed NSA in such a way so it can combat insects by taking advantage of its system. Insects show electric charges, which are created through triboelectrification. Tribo-charge is a type of contact electricity or electrification generated by two non-conductive bodies or material when they come in contact through friction. Furthermore, an exciting mode of action of NSA takes advantage of insect tribo-charging and is completed in two sequential mechanistic steps. Primarily, negatively charged NSA particles initiate interaction with a positive charge of the insect through strong electrical bonding. This interaction causes dehydration of the insect through the potent sorptive property of the NSA particle, which detaches insects' cuticle insect layer, a fibrous composite of chitin and results in the death of the insect due to dehydration. As hypothesized, the insecticidal property of NSA powder or particle is based on the attachment of the particle to the cuticle of the insect, which further disturbs the water balance and leads to ambient increased humidity. The strong insecticidal efficacy of NSA relies on its small size, intrinsic electric charge, sorbative prosperity, and large surface area (Stadler et al., 2018; Figure 4).
Figure 4. Advantages of nano-based agrochemicals: Nano fertilizers provide nutrients to plants or enhance the effect of fertilizers even when applied in smaller amounts. Uptake of nutrients can be increased by encapsulating the fertilizers in nanoform. Ultimately, it reduces nutrient loss, improves crop quality and yield, and minimizes the risk of environmental degradation.
Moreover, engineered nano pesticides are assisting the conventional cotton and insect-resistant transgenic (Bt cotton) crop, which was designed to combat the bollworm. Insecticidal activity of Cu NPs at a low dose (10 mg/L) has the potential to upregulate the expression of exogenous microbial protein from Bacillus thuringiensis coded through the Bt toxin in plant tissue to improve resistance against the bollworm (Zhao et al., 2020; Table 2). During spot disease in tomato plants, TiO2 and ZnO NPs, photochemically active, act as antimicrobial agents. When TiO2 and ZnO NPs (photochemically active) are exposed to light, they generate excited electrons, which, in the presence of oxygen, synthesize superoxide radicals through direct electron transfer. Using photochemically active TiO2, NPs are engineered to have antimicrobial properties and have a great impact on agriculture as nano pesticides. It has extensive photocatalytic activity and higher antibacterial potential against X. perforans, which causes spot disease in tomato plants (Paret et al., 2013).
Furthermore, the packaging of conventional pesticides with NPs or polymers is highly demanding in the area of the pesticide industry as they are anticipated to address these issues by enhancing pesticide efficiency, increasing production, reducing excess runoff by slow release of active ingredients over a prolonged period (Nuruzzaman et al., 2016), and ultimately protecting the environment. This new field of research comprises the study of the interaction of NPs with insects to improve existing pest control management and provide advanced nanoformulations that remain stable, quickly penetrate the organism, are delivered at the targeted site, are cost-effective, and are more active during field applications. Nanoformulation or nano carriers act as the vehicle to transport and control delivery or release of the active compound, which presents in its core, and is used to conserve an adequate amount of compound during the whole period of insect growth (from the transition from larva to the maturity stage). For instance, sustainable nano carrier construction of chitosan liposome, which coats the inner core, consists of etofenprox or alpha-cypermethrin. Similarly, different types of NMs and nanoformulations of conventional pesticides have been developed that show a significant impact on pest control (Figure 4).
Nanoemulsions
Nanoemulsions refers to the oil-in-water emulsion in which pesticide is dispersed as nanosized droplets in water. The main advantage of producing nanoemulsions is to increase the solubility of pesticidal active ingredients. The nanoemulsions of neem oil show that smaller droplets are efficiently taken up as LC50 is found to be decreased with droplet size (Anjali et al., 2012). However, the higher efficacy of the permethrin nanoemulsion than that of permethrin macroparticles against yellow fever mosquito (Aedes aegypti) is indicative of its enhanced uptake (Kumar et al., 2013). In one study, it is reported that Emamectin-benzoate nanoemulsion is more effective against the Asian rice borer (Chilo suppressalis) compared to coarse emulsions (Fan et al., 2010). In some reports, no significant results were obtained with pesticide colloids, for example, no significant difference in LC50 values was found for nanoemulsion of Chlorpyrifos and a commercially available coarse emulsion against cotton bollworm (Helicoverpa armigera) (Qi-liang et al., 2006). Another important aspect is their controlled or slow release from the colloidal formulations that result in more sustained and prolonged exposure (Figure 4).
Polymer Nano Pesticides
These are polymer-based nanocarriers for encapsulating pesticides that act as a protective reservoir containing polysaccharides and polyesters. In addition to this, they tend to increase dispersion in aqueous media, facilitating the slow and controlled release of pesticides. The polymer nano pesticides have gained lots of attention as they are flexible to design, biodegradable (corn oil, beeswax, and cashew gum), and biocompatible. Polymer-based nanoformulations such as nanogels, nanospheres, or nanofibers could be a better substitute (Nuruzzaman et al., 2016).
Solid NPs as Nano Pesticides
Some NPs that are considered to be antibacterial/pesticidal agents instead act only as nanocarriers, for example, inert dust of alumina, silica, and clays that damage the inner wax coating of the insect cuticle, which, in turn, loses water and dies due to desiccation. This mode of action is considered safer as it is unlikely that insects will develop resistance (Shah and Khan, 2014). SiNPs cause damage to the protective epidermis layer by being physio-sorbed on the lipids, thus causing the death of the insects (Barik et al., 2008). Nanostructured alumina was discovered by Stadler et al. (2010) as an insecticide against two grain pests, R. dominica and S. oryzae. The thin sheets of silicate materials are nanoclays that are produced from volcanic ash. They have been efficiently used as a carrier for controlled release of a potent herbicide 2,4-dichlorophenoxyacetate bin (Bin Hussein et al., 2005).
Nanoherbicides
Weeds are considered to be the biggest threat for damaging crops in a greater quantity by utilizing nutrients that would otherwise be available to plants. Conventional methods, including removing weeds by hand, for eradicating weeds is time-consuming and requires a lot of effort. Many herbicides are currently available in the market that have the potential to kill weeds in a field but also cause harm to the crops and decline the soil fertility. Nanoherbicides can be a better, eco-friendly alternative for effective weed control without leaving any toxic residues in the soil (Pérez-de-Luque, 2017; Table 2). Constant use of the same herbicide for a more extended period could lead to resistance in the weeds. Poly (epsilon-caprolactone) (PCL) nanocapsules have been used as a carrier system for the conventional herbicide atrazine. The atrazine-containing nanocapsules showed more potent post-emergence herbicidal activity on mustard plants than a commercial atrazine formulation (Oliveira et al., 2015). Chitosan NPs were prepared by cross-linking through disulfide bonds of diuron for its controlled delivery based on the concentration of glutathione (Yu et al., 2015).
Nanofungicides
Fungal diseases are the most common and account for more than 70% of damage to major crops such as rice, wheat, barley, groundnut, and cotton (Godfray et al., 2016). These diseases affect society by causing severe loss to crop yield and the economy. Conventional fungicides work to curb these losses but, on the other hand, target non-specific living organisms and cause imbalance to the biodiversity. Therefore, we need to look for alternate approaches to achieve better precision in fungal disease management (Patel et al., 2014). One of the best approaches is the development of NPs as an effective strategy against fungal pathogens. AgNPs, owing to their antimicrobial properties, are widely used for disinfection purposes (Baker et al., 2005). An experiment was conducted to check the effectiveness of AgNPs and Ag ions on plant-pathogenic fungi Bipolaris sorokiniana and Magnaporthe grisea. The ionic and nanoparticulate forms were effective against fungi and significantly reduced disease severity when applied for 3 h (Jo et al., 2009). CuNPs and AgNPs can both suppress the growth of two fungal pathogens Alternaria alternata and Botrytis cinerea (Ouda, 2014). ZnO NPs and MgO NPs show antifungal activity against Alternaria alternate, Rhizopus stolonifer, Fusarium oxysporum, and Mucor plumbeus (Wani and Shah, 2012). An MWCNT-g-PCA hybrid material encapsulated two conventional pesticides, zineb and mancozeb, which was confirmed to be a more potent fungicide against A. alternate (Sarlak et al., 2014).
Nano Fertilizers
Currently, agriculture all across the globe faces a broad spectrum of challenges, such as nutrient deficiency, stagnation in crop yields, diminishing soil organic matter, low water availability, nutrient deficiency in the soil, decreased land area due to urbanization and land degradation, and labor shortages (Godfray et al., 2010). The application of nanoscience and nanotechnology is increasing tremendously, and novel methods are continuously being proposed to produce novel and desirable materials for crop production and management (Table 3). It is one of the most influential ideas of the evolving science of precision agriculture in which farmers efficiently make use of fertilizers and other inputs. The uncontrolled population has prompted the enormous production of conventional fertilizers to increase food production and crop protection, but this ultimately decreases the soil fertility and quality of food. These chemical fertilizers not only add to the tribulations of the subtle ecosystem, but also affect human health as they remain unused. The global demand for fertilizers is expected to rise in the coming years, according to a report submitted by the Food and Agricultural Organization of the United Nations. Therefore, it is imperative to involve intelligent strategies for sustainable agriculture, such as nanotechnology. Nano fertilizers are environmentally friendly fertilizers or smart fertilizers with the potential to increase the application rates of fertilizers and reduce the loss of nutrients from it, mainly phosphorous and nitrogen (Dimkpa and Bindraban, 2017). It offers the gradual and controlled release of nutrients to the targeted site, which helps to prevent the contamination of water bodies and the environment (Dwivedi et al., 2016; Figures 2, 3).
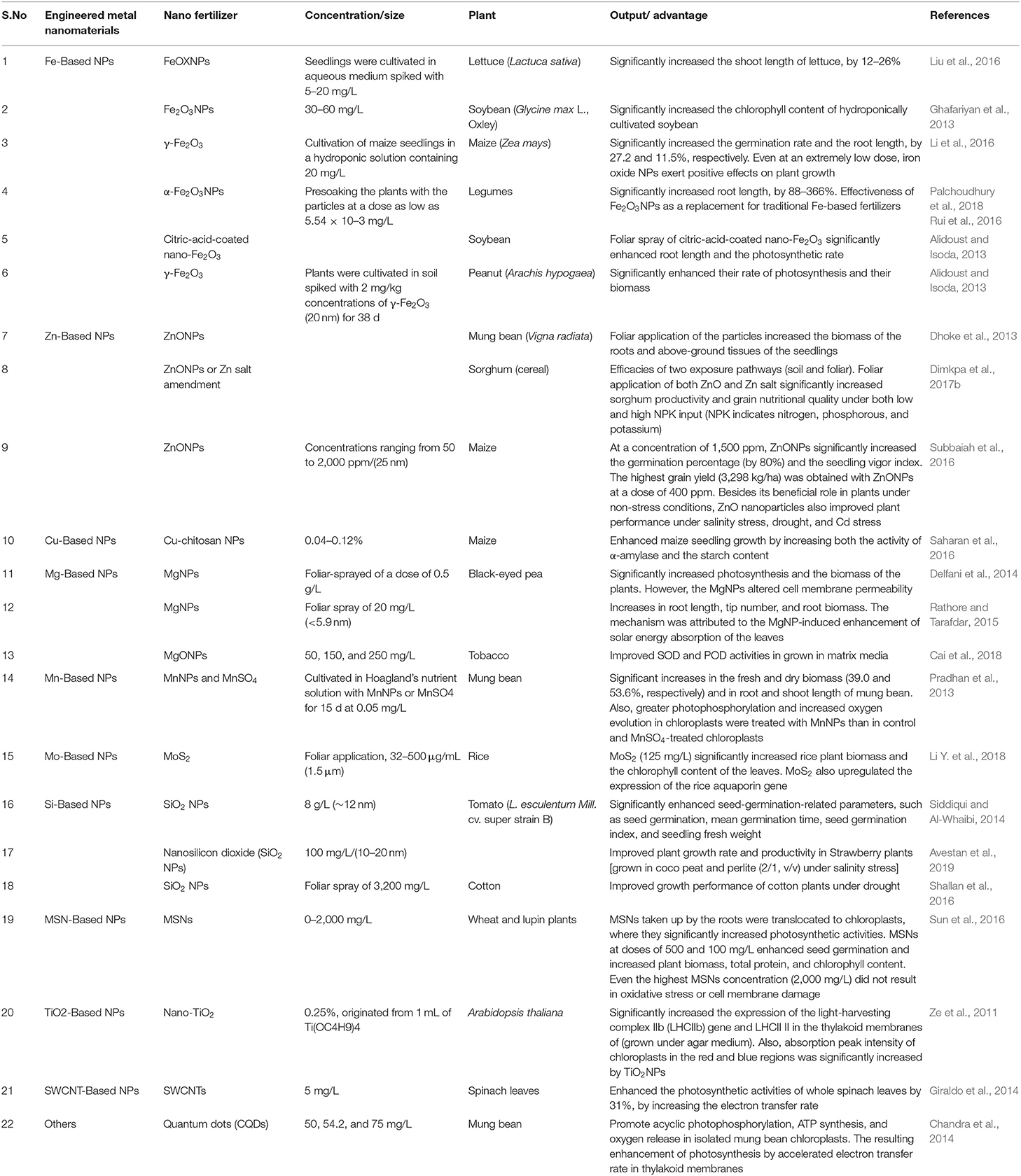
Table 3. Different types of NPs employed for the generation of nano fertilizers, their composition, benefits, and examples of active components.
Nano fertilizers are those that either provide nutrients to plants or enhance the effect of fertilizers even when applied in smaller amounts (Rameshaiah et al., 2015). Uptake of nutrients can be increased by encapsulating the fertilizers in nano form that ultimately reduces nutrient loss, improves crop quality and yield, and also minimizes the risk of environmental degradation (Figure 2). The foliar application of these nano fertilizers is also demonstrated to mitigate the stress in plants (Tarafdar et al., 2012). Nano fertilizers can be divided into three categories based on the nutritional requirements of the plants: (1) macronutrient nano fertilizers, (2) micronutrient nano fertilizers, and (3) nanoparticulate nano fertilizers (Chhipa, 2017). Macronutrient nano fertilizers are composed of a combination of elements, such as potassium (K), magnesium (Mg), nitrogen (N), calcium (Ca), and phosphorus (P). It is estimated that the total consumption of macronutrient fertilizers is projected to increase to 263 million tons (Mt) in 2050, thus showing a critical need for these fertilizers in the agricultural sector. Delfani and his group tested the efficiency of Mg and Fe NPs on the growth of black-eyed peas (Vigna unguiculata) by foliar application and observed enhanced seed weight and photosynthetic ability that, in turn, increased the yield (Delfani et al., 2014). The Ca NPs, together with humic acids, improved seedling growth in peanuts (about 30% increase) with dry biomass reaching 5.78 g per plant (Liu et al., 2005). The synthesized hydroxyapatite [Ca5 (PO4)3 OH] NPs of size 16 nm significantly enhanced the growth rate and yield of soybeans (Glycine max) in comparison to control. These studies suggest that NPs can be applied as potent fertilizers for field crops (Liu and Lal, 2015). For more detail, please refer to Table 3.
Plant micronutrients consist of molybdenum (Mo), copper (Cu), iron (Fe), nickel (Ni), manganese (Mn), and Zinc (Zn), and compared to macronutrients, they are required in much smaller amounts for the healthy growth of crops. Micronutrients applied as composite formulations are more effective in providing enough nutrients and cause less environmental degradation. Micronutrients packed in NPs enhance the nutrient bioavailability and fertilizer use efficiency relative to other conventional ions and salts (Dimkpa and Bindraban, 2016). The composite of three micronutrients NPs (ZnO, CuO, and B2O3) has been successfully established for agronomic fortification to mitigate drought stress in soybean plants (Dimkpa et al., 2017a). Nanoparticulate fertilizers contain other NPs, such as CNTs, TiO2, and SiO2, that enhance the growth of plants. The mixture of TiO2 and SiO2 increased nitrogen fixation, growth enhancement, and improved seed germination in soybeans (Lu et al., 2002; Table 3).
Molecular Mechanism of Nano Fertilizer Uptake, Translocation, and Action
Use of NM as nano fertilizer is possible due to its smaller size than the pore size of the plant cell wall (up to 20 nm), which contributes to nutrient uptake. Nano fertilizers can directly apply to soil and/or leaves. Therefore, there are two significant modes of application of nano fertilizer. First is foliar entry, and second is root entry. Spray on the trough cuticle, stromata, hydathodes, lenticels, and wounds leads to the foliar entry, and root tips, lateral root, root hairs, and rhizodermis provides the root entry. Foliar applications of nano fertilizer prefer or can be done during unfavorable soil and weather conditions (Figure 1).
Additionally, this mode of application promotes the direct entry of nutrients into the plant leaf system, simultaneously reducing the wastage of fertilizer. Hence, foliar application of nano fertilizer leads to higher NUE and also gives a rapid response to the growth of crops or plants (Mahil et al., 2019). Foliar entry is facilitated by nano-pores, the base of the trichome and stromal opening in leaves, easing NP uptake and their transport inside of leaves. For instance, in faba beans (Vicia faba) and more (in C. arabica below 2.5 nm and P. cerasus below 100 nm), it is demonstrated that nanosized particles (43 nm), deeply buried in the bulk of the leaf interior compartment, which suggests the effectiveness of nano fertilizer to enhance nutrient uptake (Eichert et al., 2008).
As nano fertilizer, it is suggested for higher nutrient use efficiency (NUE) because of its higher ability to pass and deliver the nutrients via nanosized (50–60 nm) pores of plasmodesmata, which usually transports ions between cells (Iqbal, 2019). Furthermore, plant root entry also shows significant passage for nutrient uptake, such as being highly porous to nano fertilizer compared to conventional fertilizer or manure. Demonstration using fluorescently labeled monodispersed mesoporous silica NPs suggest they amazingly describe the mechanism of uptake and translocation of the NP. NPs penetrate the root through symplastic and apoplastic pathways and passes to the xylem tissue to areal regions of the plant, such as the leaves and stem (Sun et al., 2014). For instance, ZnO and CeO2 NPs are illustrated by tracing travel patterns from the entrance into the ryegrass root cells and further up to the vascular tissues in soybeans (Glycine max) (López-Moreno et al., 2010). Once entered into the cell, these particles take the way through apoplastically or symplastically, where they travel through the plasmodesmata of one cell to another and finally reach the cytoplasm. In the cytoplasm, they begin to distribute to different cytoplasmic organelles and participate in various metabolic pathways of cells. Also, a higher uptake of NPs in the hydroponic medium as compared to plants grown in the sand has been noticed (Solanki et al., 2015). Hence, nano fertilizers have great potential to transport and deliver nutrients via a smaller channel of plasmodesmata (50–60 nm) from cell to cell. Therefore, higher NUE and less nutrient loss during entrance to transport by nano fertilizer results in higher productivity, ~6–17%, and increased nutritional quality of field crops and plants (Iqbal, 2019; Figure 1).
Sensing System With NPs
The uncontrolled use of pesticides, fertilizers, and heavy metals in agricultural practices needs to pay attention to minimize the loss to the environment. Regular monitoring of the health status of soil and possible occurrence of any disease must be done with the help of emerging technologies. Accurate sensing systems must be developed that should be handy and portable for real-time monitoring of large field areas. Nanotechnology could enhance the performance of any biosensor to agree with its real application in agriculture. Remarkable progress in the nanofabrication technology and sensitive analytical techniques enables responsive sensor development. Nano sensors could demonstrate their potential in many areas, such as crop cultivation and harvesting, pathogen detection, and soil parameters (pH and nutrients), to name a few. NPs have unique surface chemistry, electrical and thermal characteristics, enhanced sensitivities, and enhanced detection limits that make them suitable for better sensing systems (Yao et al., 2014).
NP-Based Biosensor for Pesticide Residue Detection
Traditional methods often employed for pesticide detection, such as high-performance liquid chromatography and GC/MS, are highly reproducible but suffer from low detection limits and require more efforts (sample collection, extraction, analysis) and well-equipped laboratories (GC/MS pesticide detection). The pesticide, heavy metals, and other pollutants present in water and soil are detected by various NMs, such as metal NPs, graphene, CNTs, and QDs (Zhang and Fang, 2010). The use of NMs adds the further advantages of high sensitivity, low sample requirement, and faster detection. The binding of the target molecule with that of the biosensor can be measured by various direct and indirect means, such as recording changes in color, electrical potential, or fluorescence. Another strategy is array-based in which biomolecules can be fixed to a substrate and allows simultaneous measurement of multiple analytes (Ghormade et al., 2011). NMs such as cadmium telluride (CdTe) QDs were employed for the detection of 2,4-dichlorophenoxyactic acid based on a fluoroimmunoassay-based sensing system (Vinayaka et al., 2009).
Furthermore, the fluorescent property of QDs, owing to their high photostability and size-tunable emission properties (Vinayaka and Thakur, 2010), can also be exploited for the detection of other harmful pesticides, such as atrazine (Cummins et al., 2006), pyrethroid (Kranthi et al., 2009), and methiocarb (Hua et al., 2010). The optical-based immunosensors employ several NMs, including QDs and AuNPs, that give enhanced signal amplification. Chen et al. employ QDs conjugated with streptavidin for detecting the pesticide chlorpyrifos in drinking water (Chen et al., 2010). AuNPs can be used for detecting onsite pesticides found in water coming from agricultural fields and other resources (Lisha and Pradeep, 2009). AuNP function is realized for immunochromatographic test strips for the detection of different pesticides. The authors report a single-step, Au-based, lateral-flow immunoassay strip assay for the simultaneous detection of triazophos and carbofuran. Most of the pesticides are inhibitors of an enzyme acetylcholinesterase (AChE), which is crucial to human health. This enzymatic reaction can be utilized to make biosensors; for example, the concentration of a pesticide was measured using a localized surface plasmon resonance fiber-optic biosensor, which monitors AChE inhibition (Guo et al., 2009). The enzyme was immobilized on the surface of AuNPs and alters the light on inhibition with a pesticide (e.g., paraoxon) that enables its quantification.
NPs for Plant Pathogen Detection
NMs can be applied as a rapid diagnostic tool or a biomarker against various plant pathogens and can be used either directly or indirectly for pathogen detection or used as an indicator for detecting specific diseases. AuNP-based immunosensors were utilized for detecting Karnal bunt (Tilletia indica) disease in wheat using surface plasmon resonance (Singh et al., 2010). Fluorescence SiNPs, combined with Ab, were utilized to detect Xanthomonas axonopodis pv. vesicatoria, known to cause bacterial spot disease in Solanaceae plants (Yao et al., 2009). The area has lots of potential but is still in its infancy and has to be realized in real field applications. Another essential strategy is the use of nano-chips, a type of microarray that consists of fluorescent oligo probes to identify hybridization (López et al., 2009). QD fluorescence resonance energy transfer-based immunosensors were developed to detect Phytoplasma aurantifolia that causes witches broom disease of lime and showed high sensitivity (100%) and specificity with a detection limit of 5 ca. P. aurantifolia per μL (Rad et al., 2012). Whenever plants get an infection with any pathogen, they produce unique volatile signatures (plant volatiles), and the detection of these signature compounds could help to confirm the presence of any pathogen infection in plants. Although the occurrence of p-ethylguaiacol released by plants upon infection with fungi Phytophthora cactorum, TiO2 and SnO2 detected it on screen-printed carbon electrodes through an electrochemical sensing system (Fang et al., 2014). Both the NPs exhibited high sensitivity (174–188 μA cm−2 mM−1) with 35–62 nM as a low limit of detection (Fang et al., 2014).
NP-Based Smart Plant Sensing System
The smart plant sensing system offers precise management of costly agrochemicals (i.e., pesticides, fertilizer, nutrients, and water) and mitigates major plant stress events in a changing climate. Increasing total crop yield requires innovative and high-throughput smart plant sensing approaches for developing plant stress-tolerance varieties and increasing the use of limited resources, such as nutrients and water. The smart nano sensors used for the optimization of crop growth and precisely detecting stress and limited resources can communicate through wireless and optical signals for real-time monitoring of plant health status (Giraldo et al., 2019). Regular monitoring of the health status of plants and possible occurrence of any disease must be done with the help of Raman and infrared spectroscopy that generate low signal-to-noise ratios and involve complicated and costly equipment (Wilson et al., 2015; Altangerel et al., 2017). Recently, remote sensing tools have improved the signal-to-noise ratio for monitoring individual plant health status and can directly communicate with hyperspectral imaging cameras and smartphones with a nanobiotechnology-based smart plant sensing system (Baret et al., 2007; Hatfield et al., 2008; Wolfert et al., 2017; Padilla et al., 2018).
Real-time monitoring of plant resource deficits or stressors is based on crucial signaling molecules (i.e., calcium, ROS, NO, glucose, and sucrose) and plant hormones (i.e., ABA, ethylene, jasmonic acid, and methyl salicylate) by a nanotechnology-based smart sensing system (Kim et al., 2010; Gilroy et al., 2014; Giraldo et al., 2019). NMs, having unique surface chemistry, electrical and thermal characteristics, enhanced sensitivities, and enhanced detection limits, are suitable for better sensing systems for crucial signaling molecules in vivo (Yao et al., 2014). These emerging properties of NMs make them fluorescent in low or transparent-background windows and ultra-low photobleaching of living tissue and allow the detection of analytes with high spatiotemporal resolution down to the single-molecule level and millisecond time scales (Guo et al., 2014; Hong et al., 2015).
QDs are fluorescent NM sensors used for highly glucose selective (500–1,000 μM) emission ranges from the visible to the near-infrared (nIR) for assessing plant productivity and stress (Li J. et al., 2018). Short-lived signaling molecules, such as calcium, ROS, NO, glucose, and nitroaromatics, can be real-time monitored, applying SWCNT sensors in the selected region of the leaf that can reported fluorescence intensity with high spatiotemporal resolution (seconds) (Giraldo et al., 2014, 2015; Wong et al., 2017). Analytes bind with the SWCNT sensor humic phase coating, both altering the nIR fluorescence intensity or shifting wavelength. A variable concentration of an analyte present in cells, subcellular space, and the whole plant, for example, calcium and ROS, results in fast spatiotemporal pattern changes in waves with time and subcellular locations (Zhang et al., 2013; Kruss et al., 2014). Integration of different simulations, mainly diffusion and stochastic kinetic, helps to elucidate the complex data with a productive relationship under different resource deficiency and stress conditions. In the future, system biology integration of extensive biological data (affinities, the rate constant, sensitivity, selectivity, and dynamic range) via computational modeling that offers translation of plant chemical signaling into digital information is important for decision and action of agriculture devices.
In the past decades, wearable sensors have been developed through nanotechnological innovation for human clothing and skin application (Heikenfeld et al., 2018). Therefore, it opens a new window that offers wearable nanoelectronic circuits on plant surfaces for wireless communication of volatile compounds with low concentration in real-time. SWCNT channels and graphitic electrodes transferred onto leaf surfaces of live plants can sense trace levels of a gaseous compound down to 5 ppm concentration (Lee et al., 2014). Binding of SWCNT-based sensors equipped with copper complexes to ethylene gas emitted from plant fruits leads to changes in the resistance proportional to the volatile compound concentration (Esser et al., 2012). CNT-based senors are now available in the market for detection of ethylene, which is a plant hormone and crucial indicator of fruit ripping. Furthermore, plants embedded with functionalized SWCNTs with bombolitin peptide are used for real-time monitoring of explosive nitroaromatics (i.e., picric acid) in a smartphone or laboratory-grade camera for changes in nIR fluorescence intensity (Wong et al., 2017). Similarly, green fluorescent protein-based calcium sensors act as a detector for plant stress.
Nanobiotechnology-based sensors have the capacity for real-time monitoring of chemical signaling by phenotyping or agricultural devices embedded with smartphone, meteorological station, or hyperspectral imaging cameras to alleviate the environmental stress and selection of high yielder plant traits, for example, delivery of ZnO and cerium oxide NPs to protect the plant from heat and salinity (increasing potassium retention in leaf mesophyll) by scavenging reactive oxygen (Graham et al., 2016; Wu et al., 2017b, 2018). Similarly, CuO can protect from fungal root disease in watermelon (Citrullus lanatus), and nanocrystals reduce cold damage (Alhamid et al., 2018; Borgatta et al., 2018).
Smart plant sensors still need testing in agriculture field conditions, such as environmental condition, plant growth, and how development can affect their performance. The communication of plant sensors with electronic devices is still limited to controlled laboratory conditions. If the field trials are fruitful, the sensor can provide real-time information regarding the requirement of nutrients, water, pesticides, and fertilizer for specific needs.
NPs for Managing the Agricultural Post-Harvest Waste
Most of the recent literature on the application of nanotechnology emphasizes nano pesticides, nano fertilizers, nanosensors, and crop protection. The abundant amount of lignocellulose has been obtained from agricultural waste that can be utilized efficiently to prepare functional NMs, such as nanocellulose (Shahabi-Ghahafarrokhi et al., 2015), nanocomposites (Othman, 2014), and biochar. Rice and wheat husks can be used to produce value-added products that alleviate concerns about disposing of agricultural waste (Kaur et al., 2016). By utilizing electrospinning, waste cotton fibers and cellulose can be converted into value-added products (Ramakrishna et al., 2006). Cellulose is the most abundant biopolymer that makes the backbone of tree trunks and branches. It is also considered the main component of the paper industry. It can be efficiently utilized for the production and application of nanocellulose and natural fiber (Dai and Fan, 2013; Figure 3). Nanoscale cellulosic materials are very hydrophilic, called aerogels, that offer a wide range of applications. Their properties are very distinct from their bulk parts. Holocellulose aerogels can be widely utilized as adsorption materials owing to the nano-fibrillar structure with high surface area and high adsorbing properties (Muñoz-García et al., 2015). Silica, an inorganic element, can be extracted from the rice husk, which is known for its excellent source of nano and microsilica. The effective use of nano-silica conjugated with validamycin is for the controlled delivery of water-soluble pesticide (Liu F. et al., 2006). Nano silica conjugated with methyl methoxy silane was suggested as a plant growth regulator and nanofungicide against mildew disease (Huang et al., 2015). Some of the nano pesticides are already hitting the market (Table 4), but their relation to the controlled release of agrochemicals is still ongoing and may take several years. We reviewed the detailed information for NP-based companies' related products and properties (for more detail refer to Table 4).
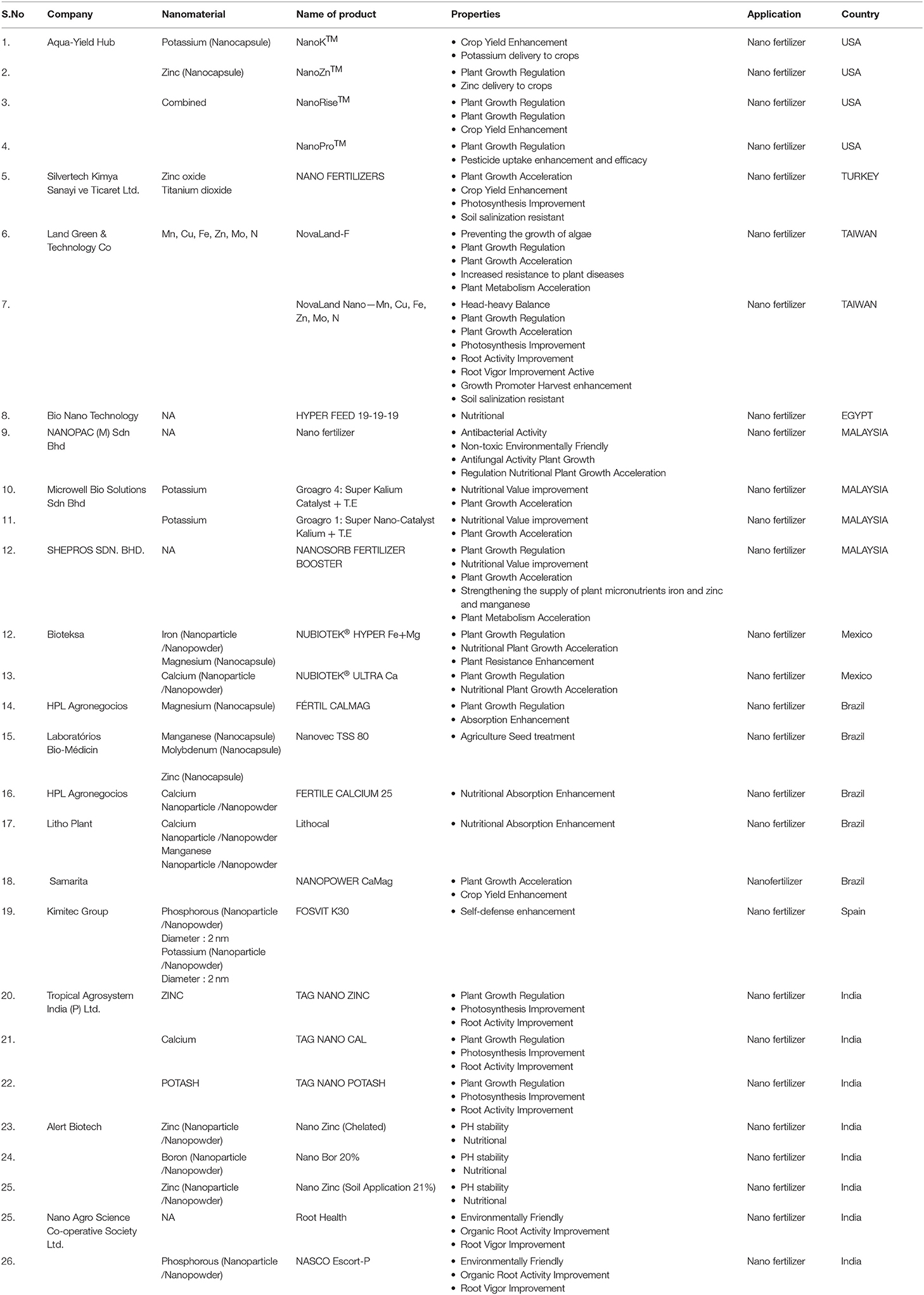
Table 4. List of commercial agricultural nano formulations based product companies, application with respective countries.
Toxicological Impact of NP Risk and Health Hazards in Agriculture Application
Despite huge research, limited progress has been made in understanding the NM risk and health hazards. NP application in agri-food industries majorly lacks the transparent and precise framework for risk governance across the different sectors (Figure 1D). The development of harmonized regulatory frameworks in the field of nanosafety has been focused on safety-by-design synthesis approaches (Kraegeloh et al., 2018; Stone et al., 2018). To share the information among different nanotechnology, fields are required not only to show nanosafety research advancement, But also risk assessment, risk management, policymaking, and to support risk communication among the different stakeholders (for example, society, industries, researchers, policymakers, insurance companies, and the general public). Mostly, nanosafety researchers work with a two-way approach (Bos et al., 2015). The first approach is to understand human health toxicity, which is predominately targeted to organisms, tissues, and cells (for example, mouse skin and immune cells). Second is environmental health risk; communities are primarily concerned with other branches of the tree of life (for example, single-cell organisms, plant tissues, invertebrates, and fish). Subsequently, plant-based nanosafety research has been focused on the intentional or direct exposure of food crops to specific NMs (for example, nano pesticides, nano fertilizer, and nanoherbicides), indirect or unintentional exposure (i.e., spray drifting, leaching, and runoff of NPs; Figure 5).
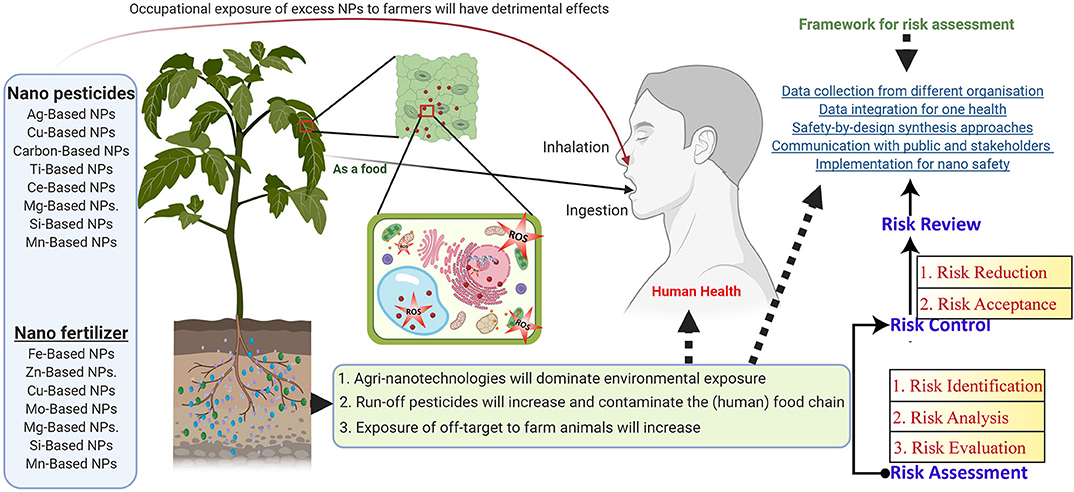
Figure 5. Framework for the risk assessment of nanotechnology in the agri-food sector: The direct delivery of nano pesticides and nano fertilizers to plants can induce ROS, leading to intracellular organelles and function disruptions. Indirect release (i.e., released from agricultural products as well as food processing and packaging) results in the occupational exposure (i.e., from industrial processes, direct contact with nano pesticides and nano fertilizers) to humans. Risk framework for risk assessment, risk management, policymaking, and to support risk communication among the different stakeholders to improve agriculture nanotechnology sustainability.
The primary point that needs to be addressed before exploiting NPs in any field is their physiochemical properties as this could have a direct impact on their potential health and environmental hazards that somehow limit their actual potential as a beneficial entity. Of these characteristics, size and concentration are the foremost properties of the NPs important from the toxicology perspective (Table 5). It can be correlated with the increased surface area with a decreased size that ultimately leads to higher uptake and interaction with the plant system, thus increasing the chances of generating adverse effects (Nel et al., 2006). It has been extensively reviewed by Ma et al. (2010) that NPs of size <5 nm can readily be translocated through cell wall pores and 20 nm through plasmodesmata; thus, it can be perceived that NPs with lower size range can be quickly taken up by the plants and could cause more phytotoxicity even at lower concentrations (Ma et al., 2010; Rico et al., 2011; Wang et al., 2013). As in the case of larger size graphene oxide (GO), the potential translocation from roots to shoots is hindered, thus lowering the bioaccumulation (Chen et al., 2017).
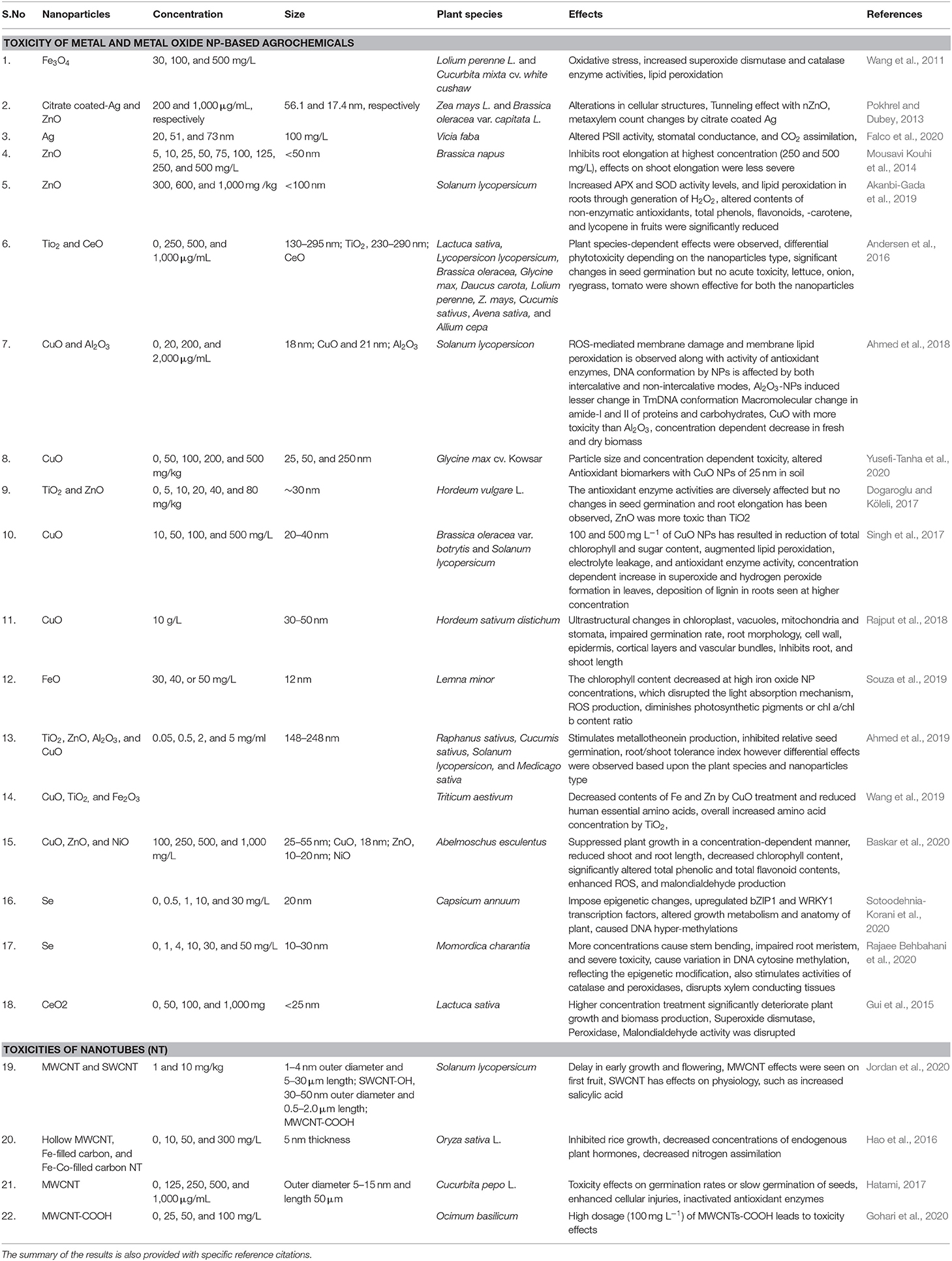
Table 5. Detailed information on the toxicity of NP-based agrochemicals with various concentrations, size, and plant species tested.
It is worth mentioning here that the concentration-dependent effects also mark the critical evidence of NP benefits or toxicity. For instance, ZnO NPs and CeO2 NPs show its toxic effects, such as reduced yield (31.6%), when supplied in higher concentrations (800 mg/kg) and not at lower concentrations (400 mg/kg) in cucumber plants (Zhao et al., 2013b). On the contrary, ZnO NPs promote seed germination and seedling vigor in addition to increased chlorophyll content when supplied in 1,000 ppm concentration to peanut plants (Prasad et al., 2012). The main mechanism of NPs toxicity is more specifically metal NPs relying on the formation of excessive ROS, particularly by shredding ions in the biological environment, thus creating toxic effects (Yan and Chen, 2019). It also interferes with the electron transport chain of mitochondria and chloroplast. Different stress factors also affect the carbon fixation that, in turn, causes photoinhibition and, ultimately, more production of superoxide anion radicals and H2O (Foyer and Noctor, 2005). The resulting overproduced ROS further interacts with the cellular components, causing protein modifications, lipid peroxidation, and damage to DNA (Møller et al., 2007, Huang et al., 2019, Wang et al., 2020). It also has positive implications as it helps in mitigating stress-related issues in plants that show the importance of equilibrium between ROS production and scavenging (Sharma et al., 2012). The increased ROS has substantial implications on the activity of antioxidative enzymes (also shown in Table 5) in plants under NP stress (Faisal et al., 2013; Jiang et al., 2014; da Costa and Sharma, 2016).
Recent reports have also shown that phytohormones play an essential role under external insult response signaling. The NPs cause a decrease in the auxins, cytokinins, and salicylic acid, suggesting hormone imbalance in plants, thus affecting overall metabolism (O'Brien and Benková, 2013; Vankova et al., 2017). A very high concentration of NPs may severely affect photosynthesis, which may result in plant growth suppression or plant death. Several reports have observed significant decrease or inhibition in plant growth as the result of NP exposure (for more detail refer to Table 5).
In light of the reports mentioned above, it can be perceived that several factors come into the picture that have to be considered before realizing the positive utilization of NPs in the agricultural system. Plant–soil interaction is the main driving force for agricultural production; thus, it is critical to study the physicochemical characteristics of the soil system. It is considered to be the paramount sink for the NPs, and hence, their interaction with the soil components could impose a profound impact on the fate of NPs. It has been observed that pH, organic content, and cation exchange capacity results in an increased risk of toxicity (Servin and White, 2016). For instance, loamy clay soil with pH 5.5 reported no toxicity by ZnO NPs even at a concentration of 2,000 mg/kg, whereas, loamy clay soil with pH 7.36 observed noticeable toxicity at a concentration of 45.45 mg/kg (Du et al., 2011). In addition to this, the soil organic matter also plays a significant role in changing the safety issues of the NPs as evident from reduced toxicity of the ZnO NPs when provided with Alginate at a concentration of 400–800 mg/kg. However, ZnO NPs alone cause a significant reduction in plant biomass (Zhao et al., 2013a). NPs also have a direct impact on the soil microbial community structure in a dose-dependent manner as observed by using DNA fingerprinting analysis that showed declining taxa of Rhizobiales, Bradyrhizobiaceae, and Bradyrhizobium (nitrogen fixation process); however, positive effects were also envisaged on Sphingomonadaceae and Streptomycetaceae. It is interesting to note here that NPs are declining the taxa related to nitrogen fixation while increasing the taxa of the community associated with the decomposition process of organic pollutants and biopolymers (Ge et al., 2012). Furthermore, AgNPs showed a dose-dependent impact on the nitrate-reduction activity of Rhizobium and Azotobacter with a 0.2 ppm increase in the activity in Azotobacter (Shahrokh et al., 2014). Environmentally based nanosafety research has primarily targeted accidental environmental releases of NPs during the manufacturing, use, and disposal of nanoformulized agriculture nanoproducts. Mukherjee et al. (2016) studied the comparative toxicity of NPs Al2O2 at ZnO (15 nm), ZnO at KH550 (20 nm), and bare-ZnO (10 nm) to grown green peas (Pisum sativum L.) (Figure 5). Plants treated with Al2O3 at ZnO NP (1,000 mg/kg for 65 days) had higher Zn level in seeds and roots relative to bulk and the other Zn NP exposure. Photosynthetic pigments (Chl-a and carotenoid) significantly increased, but the carbohydrate and protein content remained largely unaffected across all treatments except Al2O3 at ZnO NP. In correspondence, a very high concentration of ZnO NPs (1,000 mg/L) did not significantly alter root elongation and seed germination. In contrast, Cu and Ag-NPs inhibited root growth of zucchini (Cucurbita pepo). The exposure of AgNPs at a concentration of 500 and 100 mg/L resulted in 57% and 41% drastic reduction in plant biomass and transpiration rate, respectively (Stampoulis et al., 2009).
Wang et al. investigated the impact of 200 and 300 (mg/L) ZnO NP exposure, which decreased Arabidopsis growth by ~20 and 80%, respectively, relative to the control. The results show a decrease in the expression of chlorophyll synthesis genes and photosystem structure genes. In contrast, an increase in the expression of carotenoid synthesis genes may contribute to reducing the photosynthesis efficiency linked to overall phytotoxicity in the plants. Asli and Neumann (2009) report that colloidal suspensions of TiO2-NPs (30 nm) accumulated in the root at the cell wall surfaces and decreased cell wall pore size from 6.6 nm to about 3 nm, affecting water transport capacity and, therefore, transpiration of intake plants (Zea mays L.). Genotoxic and phytotoxic impact of CeO2 and TiO2 NP suspensions were investigated on the early growth of barley with concentrations of 0, 500, 1,000, and 2,000 mg /L for 7 days (Mattiello et al., 2015). Genotoxicity [randomly amplified polymorphism DNA (RAPDs) and mitotic index] observed changes in RAPD banding patterns and the chromosomal level with decreasing cell divisions in treated root tip cells compared to control plants. ROS generation and ATP content increased at the concentration of 500 mg/L in both root and shoot (Figure 1D). Authors also observed the presence of CeO2 and TiO2 NPs in exposed root cells through ICP-OES and TEM EDSX microanalysis.
Indeed, theoretically, there may be the direct release of NMs into the environment from agro-nanotechnologies and incorporation into the human exposure pathway through the food chain. The existing challenges facing agricultural nano chemical application are due to the potential migration of NMs through the food chain, so safety assessment is urgently required. There are various modes of exposure of nanoagrochemicals to humans, such as growing demand for food crops (for example, population growth and increasing demand for animal-based products), large food product waste in affluent industries, and other agriculture activities (pre- and post-harvesting of food crops) (Figure 5). In addition, plants present a significant opportunity to facilitate the transfer of NPs to the different tropic levels and ultimately to the food web as they are largely consumed by the lower trophic-level organisms, animals, and people.
It is important to understand the one health concept, especially in the context of agriculture nanotechnology. One cannot minimize the previous research or reports focusing on the need for viewing of modern challenges to health through a one health concept; this cannot be underestimated. Also, many peer-reviewed articles frame their research finding as an example of the demand for a one health approach. We must also appreciate the excellent effort of various international organizations (World Health Organization, the World Organization for Animal Health, and the Food and Agriculture Organization of the United Nations) devoted to promoting the one health concept and spreading the awareness of their importance (Lombi et al., 2019). Here, we propose a transdisciplinary and holistic approach that underpins the one health concept and is crucial for sustainable development and policy implementation of nanotechnology (Figure 5). We are living in an interdependent ecosystem wherein all the organisms on the planet, including humans, animals, plants, and associated microbiota, stay together for their existence by mutual interaction. In particular, the overuse and inappropriate practices of agrochemicals as growth promoters of food crops significantly increase the migration of NMs in the food chain. The nanotoxicity and associated human health hazards require an intelligent testing strategy framework for risk evaluation of agro-nanotechnologies. Along with preliminary assessment (document) of the existing knowledge and the gaps, they categorize and integrate data to develop an intelligent approach to grouping NMs based their properties (for example, particle size, charge, molecular weight, solubility, and composition) and their biological impacts in order to intelligently design next-generation risk-assessment strategies and safety to the risks emerging from this technology.
Future Perspective
Nanotechnology strikes at an indispensable part of numerous domains of the food industry without any doubt. It has promising applications; however, it cannot be overlooked that most of the knowledge we gathered about it is mainly from laboratory experiments. It is almost uncertain about apprehending the practical application while not understanding its impact on environment-related toxicity. Furthermore, not having the guidelines, regulatory bodies, and similar organizations creates obstructions for the selling of novel NP-based agrochemicals. Hence, there is a requirement to execute large trials in forwarding and developing futuristic investigations based on perceived gaps of knowledge. In this setting, we propose six critically important fundamental features:
1. The open government awareness program to educate the common people about food nanotechnology and its applications through formulating an adequate database and proof to assist as logistic support of both the public and food manufacturers.
2. The comprehensive examination of future studies must be featured to determine the risk associated with the usage of NP-based products in the agricultural sector to make a more practical strategy.
3. To identify the minimum concentration dose utilizing the concentration-dependent study in the natural soil system for the NP, which would be non-toxic and can be useful in field applications.
4. To gain a comprehensive understanding of the bioaccumulation capability of NPs during field applications and its effect on nanotoxicity and further knowledge of tropic chain transfer in the ecosystem chain and deciphering the sufficient safety estimate.
5. Broad understanding of soil physico-chemical interaction with NPs for reducing danger toward not distorting the soil microbiota. Further determination of the rotation of soil for modifying the destiny, carrier, and bioavailability of NPs to diminish their following toxicity and their trustworthy and useful utilization in agriculture.
6. Last, we strongly suggest the substitution of synthetic NPs to biosynthesized and performing in-depth research. In this way, utilizing the environmentally friendly protocol may hold comparatively minor or no toxicity, and henceforth, future studies must specifically concentrate on their functional efficiency. In addition, pilot studies must be set in natural conditions (growing plants in soil) to give an explicit depiction of the environmental influence of NPs.
In Addition, Some Safety Measures Should Be Considered
1. It is crucial to harmonize the frameworks of nanosafety regulations focusing on safety-by-design synthesis approaches so that it should not be overused at the toxic level.
2. The physiochemical properties of any new NP-based agrochemical should be analyzed substantially in long-term experiments before exploitation in any field work as it could have a direct impact on health and environmental hazard that will limit the actual potential as a beneficial entity.
3. Environmentally based nanosafety research has to be primarily targeted to study the accidental environmental releases of NPs during the manufacturing, use, and disposal of nanoformulized agriculture nanoproducts.
Conclusion
The present review highlights the different roles of NPs in the agriculture and livestock industry. The implementation of nanotechnology in modern agriculture helps in uplifting the global economy by providing support and advances in different ways. Considering different challenges posed by the increasing population, plant diseases, animal diseases, and climate change, the introduction of NPs significantly contributes to addressing these pressing issues, expanding the use of conventional pesticides and fertilizers to enhance crop production, ultimately causing harm to the natural environment. With the advent of NPs the effectiveness and agronomic efficiency are highly improved compared to traditional resources. The better use of pesticides encapsulated in different nanoformulations provides their better application, and controlled release protects the environment. Several nano pesticides and nano fertilizers are under development, and a few of them are already marketed. The study of NP–plant interaction is an important point to consider to understand the physiological and biochemical responses imposed by plants. Emerging techniques are developed to locate and quantify NPs through the plant system that gives the idea of their transformation and safety aspects in a complex system. Nanotechnology exploits the unique property of NPs and develops nanosensors to detect pesticide residue and various soil parameters, such as pH and moisture.
Consent for Publication
The authors gave their consent for publication of the research results.
Author Contributions
DM, GK, and SA designed and wrote the manuscript. DM, GK, PS, KY, and SA revised and finalized the draft. SA supervised the whole project and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Images were prepared in Adobe Illustrator CC 2017 and BioRender software. We would also like to thank the Director of the Indian Council of Agriculture Research-National Dairy Research Institute (NDRI), India, for providing the necessary facilities to carry out research work. PS was thankful to Department of Biotechnology (DBT) for the SRF fellowship. SA and KY were thankful to the Indian Council of Medical Research (ICMR) for the SRF fellowship.
References
Abdel Latef, A. A. H., Srivastava, A. K., El-sadek, M. S. A., Kordrostami, M., and Tran, L. S. P. (2018). Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land. Degrad. Develop. 29, 1065–1073. doi: 10.1002/ldr.2780
Abou-Zeid, H., and Ismail, G. (2018). The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L to salt stress. Egypt. J. Bot. 58, 73–85. doi: 10.21608/ejbo.2017.1873.1128
Acharya, P., Jayaprakasha, G. K., Crosby, K. M., Jifon, J. L., and Patil, B. S. (2020). Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in texas. Sci. Rep. 10:5037. doi: 10.1038/s41598-020-61696-7
Adisa, I. O., Reddy Pullagurala, V. L., Rawat, S., Hernandez-Viezcas, J. A., Dimkpa, C. O., Elmer, W. H., et al. (2018). Role of cerium compounds in Fusarium wilt suppression and growth enhancement in tomato (Solanum lycopersicum). J. Agricul. Food. Chem. 66, 5959–5970. doi: 10.1021/acs.jafc.8b01345
Adrees, M., Khan, Z. S., Ali, S., Hafeez, M., Khalid, S., Ur Rehman, M. Z., et al. (2020). Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 238:124681. doi: 10.1016/j.chemosphere.2019.124681
Aghdam, M. T., Mohammadi, H., and Ghorbanpour, M. (2016). Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well-watered and drought stress conditions. Braz. J. Bot. 39, 139–146. doi: 10.1007/s40415-015-0227-x
Ahmad, B., Shabbir, A., Jaleel, H., Khan, M. M. A., and Sadiq, Y. (2018). Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant. Biol. 13, 6–15. doi: 10.1016/j.cpb.2018.04.002
Ahmad, F., Aziz, T., Maqsood, M. A., Tahir, M. A., and Kanwal, S. (2007). Effect of silicon application on wheat (Triticum aestivum L.) growth under water deficiency stress. J. Food Agric. 7, 1–7. doi: 10.9755/ejfa.v12i1.5170
Ahmed, B., Khan, M. S., and Musarrat, J. (2018). Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): a study on growth dynamics and plant cell death. Environ. Poll. 240, 802–816. doi: 10.1016/j.envpol.2018.05.015
Ahmed, B., Rizvi, A., Zaidi, A., Khan, M. S., and Musarrat, J. (2019). Understanding the phyto-interaction of heavy metal oxide bulk and nanoparticles: evaluation of seed germination, growth, bioaccumulation, and metallothionein production. RSC Adv. 9, 4210–4225. doi: 10.1039/C8RA09305A
Akanbi-Gada, M. A., Ogunkunle, C. O., Vishwakarma, V., Viswanathan, K., and Fatoba, P. O. (2019). Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ. Technol. Innov. 14:100325. doi: 10.1016/j.eti.2019.100325
Alabdallah, N. M., and Alzahrani, H. S. (2020). The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi. J. Biol. Sci. 27, 3132–3137. doi: 10.1016/j.sjbs.2020.08.005
Alhamid, J. O., Mo, C., Zhang, X., Wang, P., Whiting, M. D., and Zhang, Q. (2018). Cellulose nanocrystals reduce cold damage to reproductive buds in fruit crops. Biosys. Eng. 172, 124–133. doi: 10.1016/j.biosystemseng.2018.06.006
Ali, M., Kim, B., Belfield, K. D., Norman, D., Brennan, M., and Ali, G. S. (2015). Inhibition of Phytophthora parasitica and P. capsici by silver nanoparticles synthesized using aqueous extract of Artemisia absinthium. Phytopathology 105, 1183–1190. doi: 10.1094/PHYTO-01-15-0006-R
Alidoust, D., and Isoda, A. (2013). Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): foliar spray versus soil amendment. Acta Physiologiae. Plantarum. 35, 3365–3375. doi: 10.1007/s11738-013-1369-8
Almutairi, Z. M. (2016). Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (‘Solanum lycopersicum’ L.) seedlings under salt stress. Plant Omics. 9:106.
Altangerel, N., Ariunbold, G. O., Gorman, C., Alkahtani, M. H., Borrego, E. J., Bohlmeyer, D., et al. (2017). In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 114, 3393–3396. doi: 10.1073/pnas.1701328114
Amenta, V., Aschberger, K., Arena, M., Bouwmeester, H., Moniz, F. B., and Brandhoff, P. (2015). Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharma. 73, 463–476. doi: 10.1016/j.yrtph.2015.06.016
An, J., Hu, P., Li, F., Wu, H., Shen, Y., White, J. C., et al. (2020). Emerging investigator series: molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 7, 2214–2228. doi: 10.1039/D0EN00387E
Anand, K. V., Anugraga, A. R., Kannan, M., Singaravelu, G., and Govindaraju, K. (2020). Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigor of green gram (Vigna radiata L.). Mater. Lett. 8:127792. doi: 10.1016/j.matlet.2020.127792
Andersen, C. P., King, G., Plocher, M., Storm, M., Pokhrel, L. R., Johnson, M. G., et al. (2016). Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 35, 2223–2229. doi: 10.1002/etc.3374
Anjali, C. H., Sharma, Y., Mukherjee, A., and Chandrasekaran, N. (2012). Neem oil (Azadirachta indica) nanoemulsion—a potent larvicidal agent against Culex quinquefasciatus. Pest Manag. Sci. 68, 158–163. doi: 10.1002/ps.2233
Ashkavand, P., Tabari, M., Zarafshar, M., Tomášková, I., and Struve, D. (2015). Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Fore. Res. Paper. 76, 350–359. doi: 10.1515/frp-2015-0034
Askary, M., Talebi, S. M., Amini, F., and Bangan, A. D. (2017). Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 63, 65–75. doi: 10.6001/biologija.v63i1.3476
Asli, S., and Neumann, P. M. (2009). Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant. Cell. Environ. 32, 577–584. doi: 10.1111/j.1365-3040.2009.01952.x
Avellan, A., Schwab, F., Masion, A., Chaurand, P., Borschneck, D., Vidal, V., et al. (2017). Nanoparticle uptake in plants: gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ. Sci. Technol. 51, 8682–8691. doi: 10.1021/acs.est.7b01133
Avellan, A., Yun, J., Zhang, Y., Spielman-Sun, E., Unrine, J. M., Thieme, J., et al. (2019). Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 13, 5291–5305. doi: 10.1021/acsnano.8b09781
Avestan, S., Ghasemnezhad, M., Esfahani, M., and Byrt, C. S. (2019). Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 9:246. doi: 10.3390/agronomy9050246
Ayoub, H. A., Khairy, M., Elsaid, S., Rashwan, F. A., and Abdel-Hafez, H. F. (2018). Pesticidal activity of nanostructured metal oxides for generation of alternative pesticide formulations. J. Agricul. Food. Chem. 66, 5491–5498. doi: 10.1021/acs.jafc.8b01600
Babajani, A., Iranbakhsh, A., Ardebili, Z. O., and Eslami, B. (2019). Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Poll. Res. 26, 24430–24444. doi: 10.1007/s11356-019-05676-z
Baker, C., Pradhan, A., Pakstis, L., Pochan, D. J., and Shah, S. I. (2005). Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 5, 244–249. doi: 10.1166/jnn.2005.034
Baret, F., Houlès, V., and Guerif, M. (2007). Quantification of plant stress using remote sensing observations and crop models: the case of nitrogen management. J. Experi. Bot. 58, 869–880. doi: 10.1093/jxb/erl231
Barik, T. K., Sahu, B., and Swain, V. (2008). Nanosilica—from medicine to pest control. Parasitol. Res. 103, 253–258. doi: 10.1007/s00436-008-0975-7
Baskar, V., Safia, N., Sree Preethy, K., Dhivya, S., Thiruvengadam, M., and Sathishkumar, R. (2020). A comparative study of phytotoxic effects of metal oxide (CuO, ZnO and NiO) nanoparticles on in-vitro grown Abelmoschus esculentus. Plant. Biosys Int. J. Deal. Aspec. Plant Biol. 1–10. doi: 10.1080/11263504.2020.1753843
Batley, G. E., Kirby, J. K., and McLaughlin, M. J. (2013). Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc. Chem. Res. 46, 854–862. doi: 10.1021/ar2003368
Behboudi, F., Tahmasebi Sarvestani, Z., Kassaee, M. Z., Modares Sanavi, S. A. M., Sorooshzadeh, A., and Ahmadi, S. B. (2018). Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water. Environ. Nanotechnol. 3, 22–39. doi: 10.22090/JWENT.2018.01.003
Bharti, A. S., Sharma, S., Shukla, N., and Uttam, K. N. (2018). Steady state and time resolved laser-induced fluorescence of garlic plants treated with titanium dioxide nanoparticles. Spectrosco. Lett. 51, 45–54. doi: 10.1080/00387010.2017.1417871
Bin Hussein, M. Z., Yahaya, A. H., Zainal, Z., and Kian, L. H. (2005). Nanocomposite-based controlled release formulation of an herbicide, 2, 4-dichlorophenoxyacetate incapsulated in zinc–aluminium-layered double hydroxide. Sci. Technol. Adv. Mater. 6, 956–962. doi: 10.1016/j.stam.2005.09.004
Borgatta, J., Ma, C., Hudson-Smith, N., Elmer, W., Plaza Pérez, C. D., De La Torre-Roche, R., et al. (2018). Copper based nanomaterials suppress root fungal disease in watermelon (Citrullus lanatus): role of particle morphology, composition and dissolution behavior. ACS. Sustain. Chem. Eng. 61, 4847–14856. doi: 10.1021/acssuschemeng.8b03379
Bos, P. M., Gottardo, S., Scott-Fordsmand, J. J., Van Tongeren, M., Semenzin, E., Fernandes, T. F., et al. (2015). The marina risk assessment strategy: a flexible strategy for efficient information collection and risk assessment of nanomaterials. Int. J. Environ. Res. Public. Health 12, 15007–15021. doi: 10.3390/ijerph121214961
Boykov, I. N., Shuford, E., and Zhang, B. (2019). Nanoparticle titanium dioxide affects the growth and microRNA expression of switchgrass (Panicum virgatum). Genomics 111, 450–456. doi: 10.1016/j.ygeno.2018.03.002
Bramhanwade, K., Shende, S., Bonde, S., Gade, A., and Rai, M. (2016). Fungicidal activity of Cu nanoparticles against fusarium causing crop diseases. Environ. Chem. Lett. 14, 229–235. doi: 10.1007/s10311-015-0543-1
Buchman, J. T., Elmer, W. H., Ma, C., Landy, K. M., White, J. C., and Haynes, C. L. (2019). Chitosan-coated mesoporous silica nanoparticle treatment of citrullus lanatus (watermelon): enhanced fungal disease suppression and modulated expression of stress-related genes. ACS Sustain. Chem. Eng. 7, 19649–19659. doi: 10.1021/acssuschemeng.9b04800
Cai, L., Cai, L., Jia, H., Liu, C., Wang, D., and Sun, X. (2020). Foliar exposure of Fe3O4 nanoparticles on Nicotiana benthamiana: evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hazard. Mater. 393:122415. doi: 10.1016/j.jhazmat.2020.122415
Cai, L., Liu, M., Liu, Z., Yang, H., Sun, X., Chen, J., et al. (2018). MgONPs can boost plant growth: evidence from increased seedling growth, morpho-physiological activities, and Mg uptake in tobacco (Nicotiana tabacum L.). Molecules 23:3375. doi: 10.3390/molecules23123375
Cañas, J. E., Long, M., Nations, S., Vadan, R., Dai, L., Luo, M., et al. (2008). Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 27, 1922–1931. doi: 10.1897/08-117.1
Carlson, C., Hussain, S. M., Schrand, A. M. K, Braydich-Stolle, L., Hess, K. L., Jones, R. L., et al. (2008). Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J. Phys. Chem. B 112, 13608–13619. doi: 10.1021/jp712087m
Carpita, N., Sabularse, D., Montezinos, D., and Delmer, D. P. (1979). Determination of the pore size of cell walls of living plant cells. Science 205, 1144–1147. doi: 10.1126/science.205.4411.1144
Carpita, N. C., and Gibeaut, D. M. (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant. J. 3, 1–30. doi: 10.1111/j.1365-313X.1993.tb00007.x
Chandra, S., Pradhan, S., Mitra, S., Patra, P., Bhattacharya, A., Pramanik, P., et al. (2014). High throughput electron transfer from carbon dots to chloroplast: a rationale of enhanced photosynthesis. Nanoscale 6, 3647–3655. doi: 10.1039/C3NR06079A
Chen, H., and Yada, R. (2011). Nanotechnologies in agriculture: new tools for sustainable development. Trends Food Sci. Tech. 22, 585–594. doi: 10.1016/j.tifs.2011.09.004
Chen, L., Wang, C., Li, H., Qu, X., Yang, S. T., and Chang, X. L. (2017). Bioaccumulation and toxicity of 13C-skeleton labeled graphene oxide in wheat. Environ. Sci. Technol. 51, 10146–10153. doi: 10.1021/acs.est.7b00822
Chen, Y., Ren, H. L., Liu, N., Sai, N., Liu, X., Liu, Z., et al. (2010). A fluoroimmunoassay based on quantum dot–streptavidin conjugate for the detection of chlorpyrifos. J. Agric. Food Chem. 58, 8895–8903. doi: 10.1021/jf101778t
Chen, Y. E., Mao, H. T., Wu, N., Mohi Ud Din, A., Khan, A., Zhang, H. Y., et al. (2020). Salicylic acid protects photosystem II by Alleviating photoinhibition in Arabidopsis thaliana under high light. Int. J. Mol. Sci. 21:1229. doi: 10.3390/ijms21041229
Chhipa, H. (2017). Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15, 15–22. doi: 10.1007/s10311-016-0600-4
Chhipa, H., and Kaushik, N. (2015). “Development of nano-bio-pesticide using Iron and Eucalyptus plant extract and their application in pest management,” in Conference Proceeding of Symposium on Recent Advances in Biotechnology for Food and Fuel (New Delhi: TERI), 19–20.
Chookhongkha, N., Sopondilok, T., and Photchanachai, S. (2012). “Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality,” in International Conference on Postharvest Pest and Disease Management in Exporting Horticultural Crops-PPDM2012 973 (Bankok), 231–237. doi: 10.17660/ActaHortic.2013.973.32
Cifuentes, Z., Custardoy, L., de la Fuente, J. M., Marquina, C., Ibarra, M. R., Rubiales, D., et al. (2010). Absorption and translocation to the aerial part of magnetic carbon-coated nanoparticles through the root of different crop plants. J. Nanobiotechnol. 8:26. doi: 10.1186/1477-3155-8-26
Colebrook, E. H., Thomas, S. G., Phillips, A. L., and Hedden, P. (2014). The role of gibberellin signalling in plant responses to abiotic stress. J. Experiment. Boil. 217, 67–75. doi: 10.1242/jeb.089938
Collin, B., Auffan, M., Johnson, A. C., Kaur, I., Keller, A. A., Lazareva, A., et al. (2014). Environmental release, fate and ecotoxicological effects of manufactured ceria nanomaterials. Environ. Sci. Nano 1, 533–548. doi: 10.1039/C4EN00149D
Cromwell, W. A., Yang, J., Starr, J. L., and Jo, Y. K. (2014). Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J. Nematol. 46, 261–266.
Cummins, C. M., Koivunen, M. E., Stephanian, A., Gee, S. J., Hammock, B. D., and Kennedy, I. M. (2006). Application of europium (III) chelate-dyed nanoparticle labels in a competitive atrazine fluoroimmunoassay on an ITO waveguide. Biosens. Bioelectron. 21, 1077–1085. doi: 10.1016/j.bios.2005.04.003
Cumplido-Nájera, C. F., González-Morales, S., Ortega-Ortíz, H., Cadenas-Pliego, G., Benavides-Mendoza, A., and Juárez-Maldonado, A. (2019). The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Horticult. 245, 82–89. doi: 10.1016/j.scienta.2018.10.007
da Costa, M. V. J., and Sharma, P. K. (2016). Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54, 110–119. doi: 10.1007/s11099-015-0167-5
Dai, D., and Fan, M. (2013). Green modification of natural fibres with nanocellulose. RSC Adv. 3, 4659–4665. doi: 10.1039/c3ra22196b
Davis, R. A., Rippner, D. A., Hausner, S. H., Parikh, S. J., McElrone, A. J., and Sutcliffe, J. L. (2017). In vivo tracking of copper-64 radiolabeled nanoparticles in Lactuca sativa. Environ. Sci. Technol. 51, 12537–12546. doi: 10.1021/acs.est.7b03333
de Oliveira, J. L., Campos, E. V., Bakshi, M., Abhilash, P. C., and Fraceto, L. F. (2014). Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotech. Adv. 32, 1550–1561. doi: 10.1016/j.biotechadv.2014.10.010
Delfani, M., Baradarn Firouzabadi, M., Farrokhi, N., and Makarian, H. (2014). Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 45, 530–540. doi: 10.1080/00103624.2013.863911
Deng, Y., Petersen, E. J., Challis, K. E., Rabb, S. A., Holbrook, R. D., and Ranville, J. F. (2017). Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environ. Sci. Technol. 51, 10615–10623. doi: 10.1021/acs.est.7b01364
Dhoke, S. K., Mahajan, P., Kamble, R., and Khanna, A. (2013). Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol. Dev. 3:e1. doi: 10.4081/nd.2013.e1
Dietz, K. J., and Herth, S. (2011). Plant nanotoxicology. Trends. Plant. Sci. 16, 582–589. doi: 10.1016/j.tplants.2011.08.003
Dimkpa, C. O., and Bindraban, P. S. (2016). Fortification of micronutrients for efficient agronomic production: a review. Agro. Sustain Dev. 36:7. doi: 10.1007/s13593-015-0346-6
Dimkpa, C. O., and Bindraban, P. S. (2017). Nanofertilizers: new products for the industry? J. Agric. Food Chem. 66, 6462–6473. doi: 10.1021/acs.jafc.7b02150
Dimkpa, C. O., Bindraban, P. S., Fugice, J., Agyin-Birikorang, S., Singh, U., and Hellums, D. (2017a). Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agro. Sustain. Dev. 37:5. doi: 10.1007/s13593-016-0412-8
Dimkpa, C. O., White, J. C., Elmer, W. H., and Gardea-Torresdey, J. (2017b). Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agricul. Food. Chem. 65, 8552–8559. doi: 10.1021/acs.jafc.7b02961
Djanaguiraman, M., Belliraj, N., Bossmann, S. H., and Prasad, P. V. (2018a). High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 3, 2479–2491. doi: 10.1021/acsomega.7b01934
Djanaguiraman, M., Nair, R., Giraldo, J. P., and Prasad, P. V. V. (2018b). Cerium oxide nanoparticles decrease drought-induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega 3, 14406–14416. doi: 10.1021/acsomega.8b01894
Dogaroglu, Z. G., and Köleli, N. (2017). TiO2 and ZnO nanoparticles toxicity in barley (Hordeum vulgare L.). Clean Soil Air Water 45:1700096. doi: 10.1002/clen.201700096
Du, W., Sun, Y., Ji, R., Zhu, J., Wu, J., and Guo, H. (2011). TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 13, 822–828. doi: 10.1039/c0em00611d
Dwivedi, S., Saquib, Q., Al-Khedhairy, A. A., and Musarrat, J. (2016). “Understanding the role of nanomaterials in agriculture,” in Microbial Inoculants in Sustainable Agricultural Productivity (New Delhi: Springer), 271–288. doi: 10.1007/978-81-322-2644-4_17
Eichert, T., Kurtz, A., Steiner, U., and Goldbach, H. E. (2008). Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 134, 151–160. doi: 10.1111/j.1399-3054.2008.01135.x
Elias, E. H., Flynn, R., Idowu, O. J., Reyes, J., Sanogo, S., and Schutte, B. J. (2019). Crop vulnerability to weather and climate risk: analysis of interacting systems and adaptation efficacy for sustainable crop production. Sustainability 11:6619. doi: 10.3390/su11236619
Elmer, W. H., and White, J. C. (2016). The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 3, 1072–1079. doi: 10.1039/C6EN00146G
Elsheery, N. I., Helaly, M. N., El-Hoseiny, H. M., and Alam-Eldein, S. M. (2020). Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 10:558. doi: 10.3390/agronomy10040558
Esser, B., Schnorr, J. M., and Swager, T. M. (2012). Selective detection of ethylene gas using carbon nanotube-based devices: utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 51, 5752–5756. doi: 10.1002/anie.201201042
Faisal, M., Saquib, Q., Alatar, A. A., Al-Khedhairy, A. A., Hegazy, A. K., and Musarrat, J. (2013). Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J. Hazard. Mat. 250, 318–332. doi: 10.1016/j.jhazmat.2013.01.063
Falco, W. F., Scherer, M. D., Oliveira, S. L., Wender, H., Colbeck, I., Lawson, T., et al. (2020). Phytotoxicity of silver nanoparticles on Vicia faba: evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci. Total. Environ. 701:134816. doi: 10.1016/j.scitotenv.2019.134816
Fan, P., Gu, Z., Xu, D., Xu, X., and Xu, G. (2010). Action analysis of drops of emamection-benzoate microemulsion on rice leaf. Chine. J. Rice Sci. 24, 503–508. doi: 10.3969/j.issn.1001-7216.2010.05.010
Fang, Y., Umasankar, Y., and Ramasamy, R. P. (2014). Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analyst 139, 3804–3810. doi: 10.1039/C4AN00384E
Faraji, J., and Sepehri, A. (2020). Exogenous nitric oxide improves the protective effects of tio 2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil. Sci. Plant Nutr. 20, 703–714. doi: 10.1007/s42729-019-00158-0
Feng, Y., Cui, X., He, S., Dong, G., Chen, M., Wang, J., et al. (2013). The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ. Sci. Technol. 47, 9496–9504. doi: 10.1021/es402109n
Foyer, C. H., and Noctor, G. (2005). Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant. Cell. 17, 1866–1875. doi: 10.1105/tpc.105.033589
Fraceto, L. F., Grillo, R., de Medeiros, G. A., Scognamiglio, V., Rea, G., and Bartolucci, C. (2016). Nanotechnology in agriculture: which innovation potential does it have? Front. Environ. Sci. 4:20. doi: 10.3389/fenvs.2016.00020
Frazier, T. P., Burklew, C. E., and Zhang, B. (2014). Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Funct. Integ. Genom. 14, 75–83. doi: 10.1007/s10142-013-0341-4
Gao, G., Tester, M. A., and Julkowska, M. M. (2020). The use of high-throughput phenotyping for assessment of heat stress-induced changes in arabidopsis. Plant Phenom. 2020:14. doi: 10.34133/2020/3723916
Ge, Y., Schimel, J. P., and Holden, P. A. (2012). Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl. Environ. Microbial. 78, 6749–6758. doi: 10.1128/AEM.00941-12
Ghafariyan, M. H., Malakouti, M. J., Dadpour, M. R., Stroeve, P., and Mahmoudi, M. (2013). Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 47, 10645–10652. doi: 10.1021/es402249b
Ghormade, V., Deshpande, M. V., and Paknikar, K. M. (2011). Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 29, 792–803. doi: 10.1016/j.biotechadv.2011.06.007
Giannousi, K., Avramidis, I., and Dendrinou-Samara, C. (2013). Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv. 3, 21743–21752. doi: 10.1039/c3ra42118j
Gilroy, S., Suzuki, N., Miller, G., Choi, W. G., Toyota, M., Devireddy, A. R., et al. (2014). A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends. Plant. Sci. 19, 623–630. doi: 10.1016/j.tplants.2014.06.013
Giraldo, J. P., Landry, M. P., Faltermeier, S. M., McNicholas, T. P., Iverson, N. M., Boghossian, A. A., et al. (2014). Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 13, 400–408. doi: 10.1038/nmat3890
Giraldo, J. P., Landry, M. P., Kwak, S. Y., Jain, R. M., Wong, M. H., Iverson, N. M., et al. (2015). A ratiometric sensor using single chirality near-infrared fluorescent carbon nanotubes: Application to in vivo monitoring. Small 11, 3973–3984. doi: 10.1002/smll.201403276
Giraldo, J. P., Wu, H., Newkirk, G. M., and Kruss, S. (2019). Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotech. 14, 541–553. doi: 10.1038/s41565-019-0470-6
Godfray, H. C., Beddington, J. R., Crute, I. R., Haddad, L., Lawrence, D., and Muir, J. F. (2010). Food security: the challenge of feeding 9 billion people. Science 327, 812–818. doi: 10.1126/science.1185383
Godfray, H. C., Mason-D'Croz, D., and Robinson, S. (2016). Food system consequences of a fungal disease epidemic in a major crop. Philos. Trans. R. Soc Lond. B. Biol. Sci. 371:20150467. doi: 10.1098/rstb.2015.0467
Gohari, G., Mohammadi, A., Akbari, A., Panahirad, S., Dadpour, M. R., Fotopoulos, V., et al. (2020). Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 10:912. doi: 10.1038/s41598-020-57794-1
Govea-Alcaide, E., Masunaga, S. H., De Souza, A., Fajardo-Rosabal, L., Effenberger, F. B., Rossi, L. M., et al. (2016). Tracking iron oxide nanoparticles in plant organs using magnetic measurements. J. Nano. Res. 18:305. doi: 10.1007/s11051-016-3610-z
Gowayed, M. H., Al-Zahrani, H. S., and Metwali, E. M. (2017). Improving the salinity tolerance in potato (Solanum tuberosum) by exogenous application of silicon dioxide nanoparticles. Int. J. Agric. Biol. 19, 183–194. doi: 10.17957/IJAB/15.0262
Graham, J. H., Johnson, E. G., Myers, M. E., Young, M., Rajasekaran, P., Das, S., et al. (2016). Potential of nano-formulated zinc oxide for control of citrus canker on grapefruit trees. Plant. Dis. 100, 2442–2447. doi: 10.1094/PDIS-05-16-0598-RE
Gui, X., Zhang, Z., Liu, S., Ma, Y., Zhang, P., He, X., et al. (2015). Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PLoS ONE 10:e0134261. doi: 10.1371/journal.pone.0134261
Guo, Y. R., Liu, S. Y., Gui, W. J., and Zhu, G. N. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal. Biochem. 389, 32–39. doi: 10.1016/j.ab.2009.03.020
Guo, Z., Park, S., Yoon, J., and Shin, I. (2014). Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 43, 16–29. doi: 10.1039/C3CS60271K
Haghighi, M., Abolghasemi, R., and da Silva, J. A. T. (2014). Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Horticult. 178, 231–240. doi: 10.1016/j.scienta.2014.09.006
Haghighi, M., and Pessarakli, M. (2013). Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Horticult. 161, 111–117. doi: 10.1016/j.scienta.2013.06.034
Hao, Y., Yu, F., Lv, R., Ma, C., Zhang, Z., Rui, Y., et al. (2016). Carbon nanotubes filled with different ferromagnetic alloys affect the growth and development of rice seedlings by changing the C: N ratio and plant hormones concentrations. PLoS ONE 11:e0157264. doi: 10.1371/journal.pone.0157264
Hatami, M. (2017). Toxicity assessment of multi-walled carbon nanotubes on Cucurbita pepo L. under well-watered and water-stressed conditions. Ecotoxicol. Environ. Safety 142, 274–283. doi: 10.1016/j.ecoenv.2017.04.018
Hatfield, J. L., Gitelson, A. A., Schepers, J. S., and Walthall, C. L. (2008). Application of spectral remote sensing for agronomic decisions. Agrono. J. 100, 117–131. doi: 10.2134/agronj2006.0370c
Hauser, F., and Horie, T. (2010). A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell. Environ. 33, 552–565. doi: 10.1111/j.1365-3040.2009.02056.x
Heikenfeld, J., Jajack, A., Rogers, J., Gutruf, P., Tian, L., Pan, T., et al. (2018). Wearable sensors: modalities, challenges, and prospects. Lab. Chip. 18, 217–248. doi: 10.1039/C7LC00914C
Hernández-Hernández, H., González-Morales, S., Benavides-Mendoza, A., Ortega-Ortiz, H., Cadenas-Pliego, G., and Juárez-Maldonado, A. (2018). Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 23:178. doi: 10.3390/molecules23010178
Ho, V. A., Le, P. T., Nguyen, T. P., Nguyen, C. K., Nguyen, V. T., and Tran, N. Q. (2015). Silver core-shell nanoclusters exhibiting strong growth inhibition of plant-pathogenic fungi. J. Nanomater. 2015:241614. doi: 10.1155/2015/241614
Hojjat, S. S., and Kamyab, M. (2017). The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russian. Agricult. Sci. 43, 61–65. doi: 10.3103/S1068367417010189
Hong, G., Diao, S., Antaris, A. L., and Dai, H. (2015). Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 115, 10816–10906. doi: 10.1021/acs.chemrev.5b00008
Hotze, E. M., Phenrat, T., and Lowry, G. V. (2010). Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 39, 1909–1924. doi: 10.2134/jeq2009.0462
Hu, P., An, J., Faulkner, M. M., Wu, H., Li, Z., Tian, X., et al. (2020). nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS. Nano 14, 7970–7986. doi: 10.1021/acsnano.9b09178
Hua, X., Qian, G., Yang, J., Hu, B., Fan, J., Qin, N., et al. (2010). Development of an immunochromatographic assay for the rapid detection of chlorpyrifos-methyl in water samples. Biosens. Bioelectron. 26, 189–194. doi: 10.1016/j.bios.2010.06.005
Huang, H., Ullah, F., Zhou, D. X., Yi, M., and Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant. Sci. 10:800. doi: 10.3389/fpls.2019.00800
Huang, S., Wang, L., Liu, L., Hou, Y., and Li, L. (2015). Nanotechnology in agriculture, livestock, and aquaculture in China. A review. Agro. Sustain. Dev. 35, 369–400. doi: 10.1007/s13593-014-0274-x
Huang, Z., Rajasekaran, P., Ozcan, A., and Santra, S. (2018). Antimicrobial magnesium hydroxide nanoparticles as an alternative to Cu biocide for crop protection. J. Agricul. Food. Chem. 66, 8679–8686. doi: 10.1021/acs.jafc.8b01727
Imada, K., Sakai, S., Kajihara, H., Tanaka, S., and Ito, S. (2016). Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant. Pathol. 65, 551–560. doi: 10.1111/ppa.12443
Iqbal, M., Raja, N. I., Hussain, M., Ejaz, M., and Yasmeen, F. (2019). Effect of silver nanoparticles on growth of wheat under heat stress. Ira. J. Sci. Technol. Transac. A Sci. 43, 387–395. doi: 10.1007/s40995-017-0417-4
Iqbal, M. A. (2019). “Nano-fertilizers for sustainable crop production under changing climate: a global perspective,” in Sustainable Crop Production (London, UK: IntechOpen).
Jaberzadeh, A., Moaveni, P., Moghadam, H. R., and Zahedi, H. (2013). Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 41, 201–207. doi: 10.15835/nbha4119093
Jampílek, J., and Králová, K. (2017). “Nanopesticides: preparation, targeting, and controlled release,” in New Pesticides and Soil Sensors, ed A. M. Grumezescu (Academic Press), 81–127. doi: 10.1016/B978-0-12-804299-1.00004-7
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., and Danquah, M. K. (2018). Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotech. 9, 1050–1074. doi: 10.3762/bjnano.9.98
Jiang, H. S., Qiu, X. N., Li, G. B., Li, W., and Yin, L. Y. (2014). Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 33, 1398–1405. doi: 10.1002/etc.2577
Jin, Y., Fan, X., Li, X., Zhang, Z., Sun, L., Fu, Z., et al. (2017). Distinct physiological and molecular responses in Arabidopsis thaliana exposed to aluminum oxide nanoparticles and ionic aluminum. Environ. Pollut. 228, 517–527. doi: 10.1016/j.envpol.2017.04.073
Jo, Y. K., Kim, B. H., and Jung, G. (2009). Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 93, 1037–1043. doi: 10.1094/PDIS-93-10-1037
Jordan, J. T., Oates, R. P., Subbiah, S., Payton, P. R., Singh, K. P., Shah, S. A., et al. (2020). Carbon nanotubes affect early growth, flowering time and phytohormones in tomato. Chemosphere 256:127042. doi: 10.1016/j.chemosphere.2020.127042
Ju, M., Navarreto-Lugo, M., Wickramasinghe, S., Milbrandt, N. B., McWhorter, A., and Samia, A. C. (2019). Exploring the chelation-based plant strategy for iron oxide nanoparticle uptake in garden cress (Lepidium sativum) using magnetic particle spectrometry. Nanoscale 11, 18582–18594. doi: 10.1039/C9NR05477D
Juárez-Maldonado, A., Ortega-Ortíz, H., Morales-Díaz, A. B., González-Morales, S., Morelos-Moreno, Á., Sandoval-Rangel, A., et al. (2019). Nanoparticles and nanomaterials as plant biostimulants. Int. J. Molec. Sci. 20:162. doi: 10.3390/ijms20010162
Kang, Y., Khan, S., and Ma, X. (2009). Climate change impacts on crop yield, crop water productivity and food security–a review. Progress Natural Sci. 19, 1665–1674. doi: 10.1016/j.pnsc.2009.08.001
Karamian, R., Ghasemlou, F., and Amiri, H. (2020). Physiological evaluation of drought stress tolerance and recovery in Verbascum sinuatum plants treated with methyl jasmonate, salicylic acid and titanium dioxide nanoparticles. Plant Biosys. Int. J. Deal. Aspect. Plant. Biol. 154, 277–287. doi: 10.1080/11263504.2019.1591535
Kaur, T., Singh, G. P., Kaur, G., Kaur, S., and Gill, P. K. (2016). Synthesis of biogenic silicon/silica (Si/SiO2) nanocomposites from rice husks and wheat bran through various microorganisms. Mater. Res. Expr. 3:085026. doi: 10.1088/2053-1591/3/8/085026
Keller, A. A., Huang, Y., and Nelson, J. (2018). Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS. J. Nano. Res. 20:101. doi: 10.1007/s11051-018-4192-8
Khan, M. N., AlSolami, M. A., Basahi, R. A., Siddiqui, M. H., Al-Huqail, A. A., Abbas, Z. K., et al. (2020). Nitric oxide is involved in nano-titanium dioxide-induced activation of antioxidant defense system and accumulation of osmolytes under water-deficit stress in Vicia faba L. Ecotoxicol. Environ Saf. 190:110152. doi: 10.1016/j.ecoenv.2019.110152
Khodakovskaya, M. V., De Silva, K., Biris, A. S., Dervishi, E., and Villagarcia, H. (2012). Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6, 2128–2135. doi: 10.1021/nn204643g
Kim, T. H., Böhmer, M., Hu, H., Nishimura, N., and Schroeder, J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Ann. Review. Plant. Boil. 61, 561–591. doi: 10.1146/annurev-arplant-042809-112226
Kraegeloh, A., Suarez-Merino, B., Sluijters, T., and Micheletti, C. (2018). Implementation of safe-by-design for nanomaterial development and safe innovation: why we need a comprehensive approach. Nanomaterials 8:239. doi: 10.3390/nano8040239
Kranthi, K. R., Davis, M., Mayee, C. D., Russell, D. A., Shukla, R. M., Satija, U., et al. (2009). Development of a colloidal-gold based lateral-flow immunoassay kit for ‘quality-control’ assessment of pyrethroid and endosulfan formulations in a novel single strip format. Crop Prot. 28, 428–434. doi: 10.1016/j.cropro.2009.01.003
Kruss, S., Landry, M. P., Vander Ende, E., Lima, B. M., Reuel, N. F., Zhang, J., et al. (2014). Neurotransmitter detection using corona phase molecular recognition on fluorescent single-walled carbon nanotube sensors. J. Amer. Chem. Soc. 136, 713–724. doi: 10.1021/ja410433b
Kumar, R. S., Shiny, P. J., Anjali, C. H., Jerobin, J., Goshen, K. M., and Magdassi, S. (2013). Distinctive effects of nano-sized permethrin in the environment. Environ. Sci. Pollut. Res. Int. 20, 2593–2602. doi: 10.1007/s11356-012-1161-0
Kumbhakar, P., Ray, S. S., and Stepanov, A. L. (2014). Optical properties of nanoparticles and nanocomposites. J. Nanomater. 2014:181365. doi: 10.1155/2014/181365
Lahiani, M. H., Dervishi, E., Chen, J., Nima, Z., Gaume, A., Biris, A. S., et al. (2013). Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces. 5, 7965–7973. doi: 10.1021/am402052x
Larue, C., Castillo-Michel, H., Sobanska, S., Trcera, N., Sorieul, S., Cécillon, L., et al. (2014). Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Hazard Mat. 273, 17–26. doi: 10.1016/j.jhazmat.2014.03.014
Larue, C., Laurette, J., Herlin-Boime, N., Khodja, H., Fayard, B., and Flank, A. M. (2012a). Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci. Total Environ. 431, 197–208. doi: 10.1016/j.scitotenv.2012.04.073
Larue, C., Pinault, M., Czarny, B., Georgin, D., Jaillard, D., Bendiab, N., et al. (2012b). Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J. Hazard. Mater. 227, 155–163. doi: 10.1016/j.jhazmat.2012.05.033
Larue, C., Veronesi, G., Flank, A. M., Surble, S., Herlin-Boime, N., and Carrière, M. (2012c). Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A. 75, 722–734. doi: 10.1080/15287394.2012.689800
Lee, K., Park, J., Lee, M. S., Kim, J., Hyun, B. G., Kang, D. J., et al. (2014). In-situ synthesis of carbon nanotube–graphite electronic devices and their integrations onto surfaces of live plants and insects. Nano. Lett. 14, 2647–2654. doi: 10.1021/nl500513n
Leso, V., Fontana, L., and Iavicoli, I. (2019). Biomedical nanotechnology: occupational views. Nano Today. 24, 10–14. doi: 10.1016/j.nantod.2018.11.002
Li, J., Hu, J., Ma, C., Wang, Y., Wu, C., Huang, J., et al. (2016). Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 159, 326–334. doi: 10.1016/j.chemosphere.2016.05.083
Li, J., Wu, H., Santana, I., Fahlgren, M., and Giraldo, J. P. (2018). Standoff optical glucose sensing in photosynthetic organisms by a quantum dot fluorescent probe. ACS. Appl. Mater. Interf. 10, 28279–28289. doi: 10.1021/acsami.8b07179
Li, R., He, J., Xie, H., Wang, W., Bose, S. K., Sun, Y., et al. (2019). Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Boil. Macromolec. 126, 91–100. doi: 10.1016/j.ijbiomac.2018.12.118
Li, W. Q., Qing, T., Li, C. C., Li, F., Ge, F., Fei, J. J., et al. (2020). Integration of subcellular partitioning and chemical forms to understand silver nanoparticles toxicity to lettuce (Lactuca sativa L.) under different exposure pathways. Chemosphere 258:127349. doi: 10.1016/j.chemosphere.2020.127349
Li, Y., Jin, Q., Yang, D., and Cui, J. (2018). Molybdenum sulfide induce growth enhancement effect of rice (Oryza sativa L.) through regulating the synthesis of chlorophyll and the expression of aquaporin gene. J. Agricul. Food. Chem. 66, 4013–4021. doi: 10.1021/acs.jafc.7b05940
Lin, S., Reppert, J., Hu, Q., Hudson, J. S., Reid, M. L., Ratnikova, T. A., et al. (2009). Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5, 1128–1132. doi: 10.1002/smll.200801556
Lisha, K. P., and Pradeep, T. (2009). Enhanced visual detection of pesticides using gold nanoparticles. J. Environ. Sci. Health Part B 44, 697–705. doi: 10.1080/03601230903163814
Liu, F., Wen, L. X., Li, Z. Z., Yu, W., Sun, H. Y., and Chen, J. F. (2006). Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mater. Res Bull. 41, 2268–2275. doi: 10.1016/j.materresbull.2006.04.014
Liu, M., Feng, S., Ma, Y., Xie, C., He, X., Ding, Y., et al. (2019). Influence of surface charge on the phytotoxicity, transformation, and translocation of CeO2 nanoparticles in cucumber plants. ACS Appl. Mater. Interf. 11, 16905–16913. doi: 10.1021/acsami.9b01627
Liu, R., and Lal, R. (2015). Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 4:5686. doi: 10.1038/srep05686
Liu, R., Zhang, H., and Lal, R. (2016). Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water. Air. Soil. Poll. 227:42. doi: 10.1007/s11270-015-2738-2
Liu, X., Zhang, F., Zhang, S., He, X., Wang, R., Fei, Z., et al. (2005). Responses of peanut to nano-calcium carbonate. Plant Nutri. Ferti. Sci. 11, 385–389.
Liu, X. M., Feng, Z. B., Zhang, F. D., Zhang, S. Q., and He, X. S. (2006). Preparation and testing of cementing and coating nano-subnanocomposites of slow/controlled-release fertilizer. Agricult. Sci. China 5, 700–706. doi: 10.1016/S1671-2927(06)60113-2
Liu, Y., and Shi, J. (2019). Antioxidative nanomaterials and biomedical applications. Nano Today 27, 146–177. doi: 10.1016/j.nantod.2019.05.008
Lombi, E., Donner, E., Dusinska, M., and Wickson, F. (2019). A one health approach to managing the applications and implications of nanotechnologies in agriculture. Nat. Nanotechnol. 14, 523–531. doi: 10.1038/s41565-019-0460-8
López, M. M., Llop, P., Olmos, A., Marco-Noales, E., Cambra, M., and Bertolini, E. (2009). Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr. Issues Mol. Biol. 11, 13–46.
López-Moreno, M. L., de la Rosa, G., Hernández-Viezcas, J. Á., Castillo-Michel, H., Botez, C. E., Peralta-Videa, J. R., et al. (2010). Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 44, 7315–7320. doi: 10.1021/es903891g
Lu, C., Zhang, C., Wen, J., Wu, G., and Tao, M. (2002). Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 21, 168–171.
Luyckx, M., Hausman, J. F., Lutts, S., and Guerriero, G. (2017). Silicon and plants: current knowledge and technological perspectives. Front. Plant. Sci. 8:411. doi: 10.3389/fpls.2017.00411
Lv, J., Christie, P., and Zhang, S. (2019). Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ. Sci. Nano 6, 41–59. doi: 10.1039/C8EN00645H
Ma, C., Rui, Y., Liu, S., Li, X., Xing, B., and Liu, L. (2015). Phytotoxic mechanism of nanoparticles: destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci. Rep. 5:11618. doi: 10.1038/srep11618
Ma, X., Geiser-Lee, J., Deng, Y., and Kolmakov, A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total. Environ. 408, 3053–3061. doi: 10.1016/j.scitotenv.2010.03.031
Mahil, T., Baburai, N., and Aravinda, K. (2019). Foliar application of nanofertilizers in agricultural crops- A review. J. Farm Sci. 32, 239–249.
Mahmoodzadeh, H., Nabavi, M., and Kashefi, H. (2013). Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornamen. Horticult. Plants. 3, 25–32.
Mahmoud, L. M., Dutt, M., Shalan, A. M., El-Kady, M. E., El-Boray, M. S., Shabana, Y. M., et al. (2020). Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. South Afr. J. Bot. 132, 155–163. doi: 10.1016/j.sajb.2020.04.027
Mattiello, A., Filippi, A., Pošćić, F., Musetti, R., Salvatici, M. C., Giordano, C., et al. (2015). Evidence of phytotoxicity and genotoxicity in Hordeum vulgare L. exposed to CeO2 and TiO2 nanoparticles. Frontiers. Plant. Sci. 6:1043. doi: 10.3389/fpls.2015.01043
Mattos, B. D., and Magalhães, W. L. (2016). Biogenic nanosilica blended by nanofibrillated cellulose as support for slow-release of tebuconazole. J. Nano Res. 18:274. doi: 10.1007/s11051-016-3586-8
McGehee, D. L., Alimohammadi, M., and Khodakovskaya, M. V. (2019). Carbon-based nanomaterials as stimulators of production of pharmaceutically active alkaloids in cell culture of Catharanthus roseus. Nanotechnology 30:275102. doi: 10.1088/1361-6528/ab1286
Meena, R. S., Kumar, S., Datta, R., Lal, R., Vijayakumar, V., Brtnicky, M., et al. (2020). Impact of agrochemicals on soil microbiota and management: a review. Land 9:34. doi: 10.3390/land9020034
Meychik, N. R., Nikolaeva, J. I., and Yermakov, I. P. (2005). Ion exchange properties of the root cell walls isolated from the halophyte plants (Suaeda altissima L.) grown under conditions of different salinity. Plant Soil 277:163. doi: 10.1007/s11104-005-6806-z
Milenković, I., Mitrović, A., Algarra, M., Lázaro-Martínez, J. M., Rodríguez-Castellón, E., Maksimović, V., et al. (2019). Interaction of carbohydrate coated cerium-oxide nanoparticles with wheat and pea: stress induction potential and effect on development. Plant 8:478. doi: 10.3390/plants8110478
Milewska-Hendel, A., Zubko, M., Stróz, D., and Kurczyńska, E. U. (2019). Effect of nanoparticles surface charge on the Arabidopsis thaliana (L.) roots development and their movement into the root cells and protoplasts. Int. J. Molec. Sci. 20:1650. doi: 10.3390/ijms20071650
Mingyu, S., Chao, L., Chunxiang, Q., Lei, Z., Liang, C., and Hao, H. (2008). Nano-anatase relieves the inhibition of electron transport caused by linolenic acid in chloroplasts of spinach. Biol. Trace Ele. Res. 122, 73–81. doi: 10.1007/s12011-007-8055-x
Mishra, S., Singh, B. R., Singh, A., Keswani, C., Naqvi, A. H., and Singh, H. B. (2014). Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 9:e97881. doi: 10.1371/journal.pone.0097881
Mohamed, A. K. S., Qayyum, M. F., Abdel-Hadi, A. M., Rehman, R. A., Ali, S., and Rizwan, M. (2017). Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agro. Soil Sci. 63, 1736–1747. doi: 10.1080/03650340.2017.1300256
Mohammadi, R., Maali-Amiri, R., and Abbasi, A. (2013). Effect of TiO2 nanoparticles on chickpea response to cold stress. Biolo. Trace Elem. Res. 152, 403–410. doi: 10.1007/s12011-013-9631-x
Møller, I. M., Jensen, P. E., and Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481. doi: 10.1146/annurev.arplant.58.032806.103946
Mondal, K. K., and Mani, C. (2012). Investigation of the antibacterial properties of nanocopper against Xanthomonas axonopodis pv. punicae, the incitant of pomegranate bacterial blight. Annal. Microbiol. 62, 889–893. doi: 10.1007/s13213-011-0382-7
Moradbeygi, H., Jamei, R., Heidari, R., and Darvishzadeh, R. (2020). Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Horticult. 272:109537. doi: 10.1016/j.scienta.2020.109537
Mousavi Kouhi, S. M., Lahouti, M., Ganjeali, A., and Entezari, M. H. (2014). Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): investigating a wide range of concentrations. Toxicol. Environ. Chem. 96, 861–868. doi: 10.1080/02772248.2014.994517
Mozafari, A., Havas, F., and Ghaderi, N. (2018). Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria× ananassa Duch.) to cope with drought stress. Plant. Cell. Tiss.Organ. Cult. 132, 511–523. doi: 10.1007/s11240-017-1347-8
Mozhayeva, D., and Engelhard, C. (2020). A critical review of single particle inductively coupled plasma mass spectrometry–a step towards an ideal method for nanomaterial characterization. J. Anal. Atom. Spectrom. 43, 5290–5294. doi: 10.1039/C9JA00206E
Mukherjee, A., Sun, Y., Morelius, E., Tamez, C., Bandyopadhyay, S., Niu, G., et al. (2016). Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 6:1242. doi: 10.3389/fpls.2015.01242
Muñoz-García, R. O., Hernández, M. E., Ortiz, G. G., Fernández, V. V., Arellano, M. R., and Sánchez-Díaz, J. C. (2015). A novel polyacrylamide-based hydrogel crosslinked with cellulose acetate and prepared by precipitation polymerization. Química Nova 38, 1031–1036. doi: 10.5935/0100-4042.20150119
Mustafa, G., and Komatsu, S. (2016). Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J. Proteo. Res. 15, 4464–4475. doi: 10.1021/acs.jproteome.6b00572
Nadiminti, P. P., Dong, Y. D., Sayer, C., Hay, P., Rookes, J. E., Boyd, B. J., et al. (2013). Nanostructured liquid crystalline particles as an alternative delivery vehicle for plant agrochemicals. ACS Appl. Mat. Interf. 5, 1818–1826. doi: 10.1021/am303208t
Narayanan, K. B., and Park, H. H. (2014). Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant. Pathol. 140, 185–192. doi: 10.1007/s10658-014-0399-4
Nath, J., Dror, I., Landa, P., Vanek, T., Kaplan-Ashiri, I., and Berkowitz, B. (2018). Synthesis and characterization of isotopically-labeled silver, copper and zinc oxide nanoparticles for tracing studies in plants. Environ. Pollut. 242, 1827–1837. doi: 10.1016/j.envpol.2018.07.084
Navarro, D. A., Bisson, M. A., and Aga, D. S. (2012). Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J. Hazard. Mater. 211, 427–435. doi: 10.1016/j.jhazmat.2011.12.012
Nel, A., Xia, T., Mädler, L., and Li, N. (2006). Toxic potential of materials at the nanolevel. Science 311, 622–627. doi: 10.1126/science.1114397
Nievola, C. C., Carvalho, C. P., Carvalho, V., and Rodrigues, E. (2017). Rapid responses of plants to temperature changes. Temperature. 4, 371–405. doi: 10.1080/23328940.2017.1377812
Nuruzzaman, M. D., Rahman, M. M., Liu, Y., and Naidu, R. (2016). Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J. Agric. Food Chem. 64, 1447–1483. doi: 10.1021/acs.jafc.5b05214
O'Brien, J. A., and Benková, E. (2013). Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 4:451. doi: 10.3389/fpls.2013.00451
Ocsoy, I., Paret, M. L., Ocsoy, M. A., Kunwar, S., Chen, T., You, M., et al. (2013). Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. Acs Nano. 7, 8972–8980. doi: 10.1021/nn4034794
Oliveira, H. C., Stolf-Moreira, R., Martinez, C. B., Grillo, R., de Jesus, M. B., and Fraceto, L. F. (2015). Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS ONE 10:e0132971. doi: 10.1371/journal.pone.0132971
Othman, S. H. (2014). Bio-nanocomposite materials for food packaging applications: types of biopolymer and nano-sized filler. Agric. Agricul. Sci. Proc. 2, 296–303. doi: 10.1016/j.aaspro.2014.11.042
Ouda, S. M. (2014). Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 9, 34–42. doi: 10.3923/jm.2014.34.42
Padilla, F. M., Gallardo, M., Peña-Fleitas, M. T., De Souza, R., and Thompson, R. B. (2018). Proximal optical sensors for nitrogen management of vegetable crops: a review. Sensors 18:2083. doi: 10.3390/s18072083
Palchoudhury, S., Jungjohann, K. L., Weerasena, L., Arabshahi, A., Gharge, U., Albattah, A., et al. (2018). Enhanced legume root growth with pre-soaking in α-Fe2O3 nanoparticle fertilizer. RSC Adv. 8, 24075–24083. doi: 10.1039/C8RA04680H
Palmqvist, N. M., Seisenbaeva, G. A., Svedlindh, P., and Kessler, V. G. (2017). Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res. Lett. 12:631. doi: 10.1186/s11671-017-2404-2
Palocci, C., Valletta, A., Chronopoulou, L., Donati, L., Bramosanti, M., Brasili, E., et al. (2017). Endocytic pathways involved in PLGA nanoparticle uptake by grapevine cells and role of cell wall and membrane in size selection. Plant Cell Rep. 36, 1917–1928. doi: 10.1007/s00299-017-2206-0
Pandey, K., Anas, M., Hicks, V. K., Green, M. J., and Khodakovskaya, M. V. (2019). Improvement of commercially Valuable traits of industrial crops by application of carbon-based nanomaterials. Scien. Rep. 9:19358. doi: 10.1038/s41598-019-55903-3
Paret, M. L., Vallad, G. E., Averett, D. R., Jones, J. B., and Olson, S. M. (2013). Photocatalysis: effect of light-activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology 103, 228–236. doi: 10.1094/PHYTO-08-12-0183-R
Patel, N., Desai, P., Patel, N., Jha, A., and Gautam, H. K. (2014). Agronanotechnology for plant fungal disease management: a review. Int. J. Curr. Microbiol. App. Sci. 3, 71–84.
Pérez-de-Luque, A. (2017). Interaction of nanomaterials with plants: what do we need for real applications in agriculture. Front. Environ. Sci. 5:12. doi: 10.3389/fenvs.2017.00012
Pokhrel, L. R., and Dubey, B. (2013). Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 452, 321–332. doi: 10.1016/j.scitotenv.2013.02.059
Pouratashi, M., and Iravani, H. (2012). Farmers' knowledge of integrated pest management and learning style preferences: implications for information delivery. Int. J. Pest Manage. 58, 347–353. doi: 10.1080/09670874.2012.724468
Pradhan, S., Patra, P., Das, S., Chandra, S., Mitra, S., Dey, K. K., et al. (2013). Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study. Environ. Sci. Technol. 47, 13122–13131. doi: 10.1021/es402659t
Prasad, T. N. V. K.V., Sudhakar, P., Sreenivasulu, Y., Latha, P., Munaswamy, V., Reddy, K. R., et al. (2012). Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant. Nut. 35, 905–927. doi: 10.1080/01904167.2012.663443
Pulido-Reyes, G., Rodea-Palomares, I., Das, S., Sakthivel, T. S., Leganes, F., Rosal, R., et al. (2015). Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states. Sci. Rep. 5:15613. doi: 10.1038/srep15613
Qian, K., Shi, T., Tang, T., Zhang, S., Liu, X., and Cao, Y. (2011). Preparation and characterization of nano-sized calcium carbonate as controlled release pesticide carrier for validamycin against Rhizoctonia solani. Microchim. Acta 173, 51–57. doi: 10.1007/s00604-010-0523-x
Qi-liang, H. U., Wen-ji, Z. H., Feng-min, L. I., Dong-mei, S. H., Chun-hua, Z. H., and Xiao-li, B. U. (2006). Solubilization of chlorpyrifos in the mixed system of surfactants and the bioactivity evaluation. J. Chin. J. Pesticide Sci. 8, 71–76.
Rad, F., Mohsenifar, A., Tabatabaei, M., Safarnejad, M. R., Shahryari, F., Safarpour, H., et al. (2012). Detection of candidatus phytoplasma aurantifolia with a quantum dots FRET-based biosensor. J. Plant Pathol. 1, 525–534. doi: 10.4454/JPP.FA.2012.054
Rafi, M. M., Epstein, E., and Falk, R. H. (1997). Silicon deprivation causes physical abnormalities in wheat (Triticum aestivum L). J. Plant Physiol. 151, 497–501. doi: 10.1016/S0176-1617(97)80017-X
Rahman, M. S., Chakraborty, A., Mazumdar, S., Nandi, N. C., Bhuiyan, M. N., and Alauddin, S. M. (2020). Effects of poly (vinylpyrrolidone) protected platinum nanoparticles on seed germination and growth performance of Pisum sativum. NanoStruc. NanoObj. 21:100408. doi: 10.1016/j.nanoso.2019.100408
Raja, K., Sowmya, R., Sudhagar, R., Moorthy, P. S., Govindaraju, K., and Subramanian, K. S. (2019). Biogenic ZnO and Cu nanoparticles to improve seed germination quality in blackgram (Vigna mungo). Mater. Lett. 235, 164–167. doi: 10.1016/j.matlet.2018.10.038
Rajaee Behbahani, S., Iranbakhsh, A., Ebadi, M., Majd, A., and Ardebili, Z. O. (2020). Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE 15:e0235556. doi: 10.1371/journal.pone.0235556
Rajesh, S., Raja, D. P., Rathi, J. M., and Sahayaraj, K. (2012). Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J. Biopesti. 5:119.
Rajput, V., Minkina, T., Fedorenko, A., Sushkova, S., Mandzhieva, S., Lysenko, V., et al. (2018). Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total. Environ. 645, 1103–1113. doi: 10.1016/j.scitotenv.2018.07.211
Raliya, R., Franke, C., Chavalmane, S., Nair, R., Reed, N., and Biswas, P. (2016). Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 7:1288. doi: 10.3389/fpls.2016.01288
Ramakrishna, S., Fujihara, K., Teo, W. E., Yong, T., Ma, Z., and Ramaseshan, R. (2006). Electrospun nanofibers: solving global issues. Mater. Today 9, 40–50. doi: 10.1016/S1369-7021(06)71389-X
Rameshaiah, G. N., Pallavi, J., and Shabnam, S. (2015). Nano fertilizers and nano sensors–an attempt for developing smart agriculture. Int. J. Engineer. Res. Gen. Sci. 3, 314–320.
Rastogi, A., Tripathi, D. K., Yadav, S., Chauhan, D. K., Živčák, M., Ghorbanpour, M., et al. (2019). Application of silicon nanoparticles in agriculture. 3Biotech 9:90. doi: 10.1007/s13205-019-1626-7
Rathore, I., and Tarafdar, J. C. (2015). Perspectives of biosynthesized magnesium nanoparticles in foliar application of wheat plant. J. Bionanosci. 9, 209–214. doi: 10.1166/jbns.2015.1296
Raza, A., Mehmood, S. S., Tabassum, J., and Batool, R. (2019). “Targeting plant hormones to develop abiotic stress resistance in wheat,” in Wheat Production in Changing Environments (Singapore: Springer), 557–577. doi: 10.1007/978-981-13-6883-7_22
Rejeb, I. B., Pastor, V., and Mauch-Mani, B. (2014). Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3, 458–475. doi: 10.3390/plants3040458
Rice, J. C., and Garcia, S. M. (2011). Fisheries, food security, climate change, and biodiversity: characteristics of the sector and perspectives on emerging issues. ICES J. Marine Sci. 68, 1343–1353. doi: 10.1093/icesjms/fsr041
Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2011). Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agricul. Food. Chem. 59, 3485–3498. doi: 10.1021/jf104517j
Riederer, M., and Schreiber, L. (2001). Protecting against water loss: analysis of the barrier properties of plant cuticles. J. Exp. Bot. 52, 2023–2032. doi: 10.1093/jexbot/52.363.2023
Roberts, A., and Oparka, K. J. (2003). Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26, 103–124. doi: 10.1046/j.1365-3040.2003.00950.x
Rossi, L., Zhang, W., Lombardini, L., and Ma, X. (2016). The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Poll. 219, 28–36. doi: 10.1016/j.envpol.2016.09.060
Rui, M., Ma, C., Hao, Y., Guo, J., Rui, Y., Tang, X., et al. (2016). Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant. Sci. 7:815. doi: 10.3389/fpls.2016.00815
Saharan, V., Kumaraswamy, R. V., Choudhary, R. C., Kumari, S., Pal, A., Raliya, R., et al. (2016). Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agricul. Food. Chem. 64, 6148–6155. doi: 10.1021/acs.jafc.6b02239
Saharan, V., Mehrotra, A., Khatik, R., Rawal, P., Sharma, S. S., and Pal, A. (2013). Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Boilog. Macromole 62, 677–683. doi: 10.1016/j.ijbiomac.2013.10.012
Santiago, M., Pagay, V., and Stroock, A. D. (2013). Impact of electroviscosity on the hydraulic conductance of the bordered pit membrane: a theoretical investigation. Plant Physiol. 163, 999–1011. doi: 10.1104/pp.113.219774
Sarlak, N., Taherifar, A., and Salehi, F. (2014). Synthesis of nanopesticides by encapsulating pesticide nanoparticles using functionalized carbon nanotubes and application of new nanocomposite for plant disease treatment. J. Agric. Food Chem. 62, 4833–4838. doi: 10.1021/jf404720d
Sasson, Y., Levy-Ruso, G., Toledano, O., and Ishaaya, I. (2007). “Nanosuspensions: emerging novel agrochemical formulations,” in Insecticides Design Using Advanced Technologies (Berlin; Heidelberg: Springer), 1–39. doi: 10.1007/978-3-540-46907-0_1
Sattelmacher, B. (2001). The apoplast and its significance for plant mineral nutrition. N. Phytol. 149, 167–192. doi: 10.1046/j.1469-8137.2001.00034.x
Saxena, R., Tomar, R. S., and Kumar, M. (2016). Exploring nanobiotechnology to mitigate abiotic stress in crop plants. J. Pharma. Sci. Res. 8:974.
Schwab, F., Zhai, G., Kern, M., Turner, A., Schnoor, J. L., and Wiesner, M. R. (2016). Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–critical review. Nanotoxicology 10, 257–278. doi: 10.3109/17435390.2015.1048326
Sedghi, M., Hadi, M., and Toluie, S. G. (2013). Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Annal. West Univ. Timisoara ser Biol. 16, 73–78.
Servin, A. D., and White, J. C. (2016). Nanotechnology in agriculture: next steps for understanding engineered nanoparticle exposure and risk. NanoImpact 1, 9–12. doi: 10.1016/j.impact.2015.12.002
Shah, M. A., and Khan, A. A. (2014). Use of diatomaceous earth for the management of stored-product pests. Int. J. Pest Manag. 60, 100–113. doi: 10.1080/09670874.2014.918674
Shahabi-Ghahafarrokhi, I., Khodaiyan, F., Mousavi, M., and Yousefi, H. (2015). Preparation and characterization of nanocellulose from beer industrial residues using acid hydrolysis/ultrasound. Fibers Polym. 16, 529–536. doi: 10.1007/s12221-015-0529-4
Shahrokh, S., Hosseinkhani, B., and Emtiazi, G. (2014). The impact of nano-silver on bacterial aerobic nitrate reductase. J. Bioproces. Biotechn. 4:4. doi: 10.4172/2155-9821.1000162
Shallan, M. A., Hassan, H. M., Namich, A. A., and Ibrahim, A. A. (2016). Biochemical and physiological effects of TiO2 and SiO2 nanoparticles on cotton plant under drought stress. Res. J. Pharma. Bio. Chem. Sci. 7, 1540–1551.
Sharifi, M., Faryabi, K., Talaei, A. J., Shekha, M. S., Ale-Ebrahim, M., and Salihi, A. (2020). Antioxidant properties of gold nanozyme: a review. J. Mol. Liq. 297:112004. doi: 10.1016/j.molliq.2019.112004
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:26. doi: 10.1155/2012/217037
Shen, C. X., Zhang, Q. F., Li, J., Bi, F. C., and Yao, N. (2010). Induction of programmed cell death in arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 97, 1602–1609. doi: 10.3732/ajb.1000073
Siddiqui, M. H., and Al-Whaibi, M. H. (2014). Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi. J. Biol. Sci. 21, 13–17. doi: 10.1016/j.sjbs.2013.04.005
Siddiqui, M. H., Al-Whaibi, M. H., Faisal, M., and Al Sahli, A. A. (2014). Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 33, 2429–2437. doi: 10.1002/etc.2697
Singh, A., Singh, N. B., Hussain, I., and Singh, H. (2017). Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and brassica oleracea var. botrytis. J. Biotechnol. 262, 11–27. doi: 10.1016/j.jbiotec.2017.09.016
Singh, S., Singh, M., Agrawal, V. V., and Kumar, A. (2010). An attempt to develop surface plasmon resonance based immunosensor for Karnal bunt (Tilletia indica) diagnosis based on the experience of nano-gold based lateral flow immuno-dipstick test. Thin Solid Films 519, 1156–1159. doi: 10.1016/j.tsf.2010.08.061
Solanki, P., Bhargava, A., Chhipa, H., Jain, N., and Panwar, J. (2015). “Nano-fertilizers and their smart delivery system,” in Nanotechnologies in Food and Agriculture, ed M. Rai (Cham: Springer), 81–101. doi: 10.1007/978-3-319-14024-7_4
Soleymanzadeh, R., Iranbakhsh, A., Habibi, G., and Ardebili, Z. O. (2020). Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta. Biolog. Cracoviensia. Botan. 62, 33–42.
Sotoodehnia-Korani, S., Iranbakhsh, A., Ebadi, M., Majd, A., and Ardebili, Z. O. (2020). Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum; an in vitro study. Environ. Poll. 265:114727. doi: 10.1016/j.envpol.2020.114727
Souza, L. R. R., Bernardes, L. E., Barbetta, M. F. S., and da Veiga, M. A. M. S. (2019). Iron oxide nanoparticle phytotoxicity to the aquatic plant Lemna minor: effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ. Sci. Poll. Res. 26, 24121–24131. doi: 10.1007/s11356-019-05713-x
Spielman-Sun, E., Avellan, A., Bland, G. D., Tappero, R. V., Acerbo, A. S., Unrine, J. M., et al. (2019). Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ. Sci. Nano 6, 2508–2519. doi: 10.1039/C9EN00626E
Stadler, T., Buteler, M., Valdez, S. R., and Gitto, J. G. (2018). Particulate nanoinsecticides: a new concept in insect pest management. Insectic. Agricul. Toxicol. 83:72448. doi: 10.5772/intechopen.72448
Stadler, T., Buteler, M., and Weaver, D. K. (2010). Novel use of nanostructured alumina as an insecticide. Pest Manage. Sci. 66, 577–579. doi: 10.1002/ps.1915
Stampoulis, D., Sinha, S. K., and White, J. C. (2009). Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 43, 9473–9479. doi: 10.1021/es901695c
Stefan, L., and Monchaud, D. (2019). Applications of guanine quartets in nanotechnology and chemical biology. Nat. Rev. Chem. 3, 650–668. doi: 10.1038/s41570-019-0132-0
Stone, V., Führ, M., Feindt, P. H., Bouwmeester, H., Linkov, I., Sabella, S., et al. (2018). The essential elements of a risk governance framework for current and future nanotechnologies. Risk. Anal. 38, 1321–1331. doi: 10.1111/risa.12954
Su, Y., Ashworth, V., Kim, C., Adeleye, A. S., Rolshausen, P., Roper, C., et al. (2019). Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environ. Sci. Nano 6, 2311–2331. doi: 10.1039/C9EN00461K
Su, Y., Ashworth, V. E., Geitner, N. K., Wiesner, M. R., Ginnan, N., Rolshausen, P., et al. (2020). Delivery, fate, and mobility of silver nanoparticles in citrus trees. ACS Nano 14, 2966–2981 doi: 10.1021/acsnano.9b07733
Subbaiah, L. V., Prasad, T. N. V. K. V., Krishna, T. G., Sudhakar, P., Reddy, B. R., and Pradeep, T. (2016). Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agricul. Food. Chem. 64, 3778–3788. doi: 10.1021/acs.jafc.6b00838
Sun, D., Hussain, H., Yi, Z., Siegele, R., Cresswell, T., Kong, L., et al. (2014). Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant. Cell. Rep. 33, 1389–1402. doi: 10.1007/s00299-014-1624-5
Sun, D., Hussain, H. I., Yi, Z., Rookes, J. E., Kong, L., and Cahill, D. M. (2016). Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 152, 81–91. doi: 10.1016/j.chemosphere.2016.02.096
Sun, D., Hussain, H. I., Yi, Z., Rookes, J. E., Kong, L., and Cahill, D. M. (2018). Delivery of abscisic acid to plants using glutathione responsive mesoporous silica nanoparticles. J. Nanosci. Nanotech. 18, 1615–1625. doi: 10.1166/jnn.2018.14262
Suriyaprabha, R., Karunakaran, G., Yuvakkumar, R., Rajendran, V., and Kannan, N. (2012). Silica nanoparticles for increased silica availability in maize (Zea mays. L) seeds under hydroponic conditions. Curr. Nanosci. 8, 902–908. doi: 10.2174/157341312803989033
Tantawy, A. S., Salama, Y. A. M., El-Nemr, M. A., and Abdel-Mawgoud, A. M. R. (2015). Nano silicon application improves salinity tolerance of sweet pepper plants. Int. J. Chem.Tech. Res. 8, 11–17.
Tarafdar, J. C., Xiong, Y., Wang, W. N., Quinl, D., and Biswas, P. (2012). Standardization of size, shape and concentration of nanoparticle for plant application. Appl. Biol. Res. 14, 138–144.
Tepfer, M., and Taylor, I. E. (1981). The permeability of plant cell walls as measured by gel filtration chromatography. Science 213, 761–763. doi: 10.1126/science.213.4509.761
Torabian, S., Zahedi, M., and Khoshgoftar, A. H. (2017). Effects of foliar spray of nano-particles of FeSO4 on the growth and ion content of sunflower under saline condition. J. Plant. Nutr. 40, 615–623. doi: 10.1080/01904167.2016.1240187
Torney, F., Trewyn, B. G., Lin, V. S. Y., and Wang, K. (2007). Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotech. 2, 295–300. doi: 10.1038/nnano.2007.108
Tripathi, D. K., Singh, S., Singh, V. P., Prasad, S. M., Dubey, N. K., and Chauhan, D. K. (2017). Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 110, 70–81. doi: 10.1016/j.plaphy.2016.06.026
Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., ur Rehman, H., et al. (2020). Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total. Environ. 721:137778. doi: 10.1016/j.scitotenv.2020.137778
Van Ha, C., Van Nguyen, D., Nguyen, H. M., Le, N. T., Nguyen, K. H., Le, H. M., et al. (2020). Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. bioRxiv [Preprint]. doi: 10.1101/2020.02.24.963132
Vankova, R., Landa, P., Podlipna, R., Dobrev, P. I., Prerostova, S., Langhansova, L., et al. (2017). ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci.Total. Environ. 593, 535–542. doi: 10.1016/j.scitotenv.2017.03.160
Verma, V., Ravindran, P., and Kumar, P. P. (2016). Plant hormone-mediated regulation of stress responses. BMC Plant. Boil. 16:86. doi: 10.1186/s12870-016-0771-y
Vinayaka, A. C., Basheer, S., and Thakur, M. S. (2009). Bioconjugation of CdTe quantum dot for the detection of 2, 4-dichlorophenoxyacetic acid by competitive fluoroimmunoassay based biosensor. Biosens. Bioelectron. 24, 1615–1620. doi: 10.1016/j.bios.2008.08.042
Vinayaka, A. C., and Thakur, M. S. (2010). Focus on quantum dots as potential fluorescent probes for monitoring food toxicants and foodborne pathogens. Anal. Bioanal. Chem. 397, 1445–1455. doi: 10.1007/s00216-010-3683-y
Wakeel, A., Xu, M., and Gan, Y. (2020). Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Molec. Sci. 21:728. doi: 10.3390/ijms21030728
Wang, H., Kou, X., Pei, Z., Xiao, J. Q., Shan, X., and Xing, B. (2011). Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 5, 30–42. doi: 10.3109/17435390.2010.489206
Wang, J., Koo, Y., Alexander, A., Yang, Y., Westerhof, S., Zhang, Q., et al. (2013). Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 47, 5442–5449. doi: 10.1021/es4004334
Wang, P., Lombi, E., Zhao, F. J., and Kopittke, P. M. (2016). Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 21, 699–712. doi: 10.1016/j.tplants.2016.04.005
Wang, Q., Ma, X., Zhang, W., Pei, H., and Chen, Y. (2012). The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 4, 1105–1112. doi: 10.1039/c2mt20149f
Wang, S., Wu, X. M., Liu, C. H., Shang, J. Y., Gao, F., and Guo, H. S. (2020). Verticillium dahliae chromatin remodeling facilitates the DNA damage repair in response to plant ROS stress. PLoS Pathog. 16:e1008481. doi: 10.1371/journal.ppat.1008481
Wang, X., Liu, X., Chen, J., Han, H., and Yuan, Z. (2014). Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 68, 798–806. doi: 10.1016/j.carbon.2013.11.072
Wang, X., Yang, X., Chen, S., Li, Q., Wang, W., Hou, C., et al. (2016). Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant. Sci. 6:1243. doi: 10.3389/fpls.2015.01243
Wang, Y., Jiang, F., Ma, C., Rui, Y., Tsang, D. C., and Xing, B. (2019). Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J. Environ. Manage. 241, 319–327. doi: 10.1016/j.jenvman.2019.04.041
Wang, Z., Xie, X., Zhao, J., Liu, X., Feng, W., White, J. C., et al. (2012). Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 46, 4434–4441. doi: 10.1021/es204212z
Wani, A. H., and Shah, M. A. (2012). A unique and profound effect of MgO and ZnO nanoparticles on some plant pathogenic fungi. J. Appl. Pharma. Sci. 2:4.
Wild, E., and Jones, K. C. (2009). Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ. Sci. Technol. 43, 5290–5294. doi: 10.1021/es900065h
Wilson, R. H., Nadeau, K. P., Jaworski, F. B., Tromberg, B. J., and Durkin, A. J. (2015). Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J. Biomed. Optics. 20:030901. doi: 10.1117/1.JBO.20.3.030901
Wojcieszek, J., Jiménez-Lamana, J., Bierla, K., Asztemborska, M., Ruzik, L., Jarosz, M., et al. (2019). Elucidation of the fate of zinc in model plants using single particle ICP-MS and ESI tandem MS. J. Anal. Atom. Spectrom. 34, 683–693. doi: 10.1039/C8JA00390D
Wolfert, S., Ge, L., Verdouw, C., and Bogaardt, M. J. (2017). Big data in smart farming–a review. Agricult. Syst. 153, 69–80. doi: 10.1016/j.agsy.2017.01.023
Wong, M. H., Giraldo, J. P., Kwak, S. Y., Koman, V. B., Sinclair, R., Lew, T. T. S., et al. (2017). Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mat. 16, 264–272. doi: 10.1038/nmat4771
Wu, H., Santana, I., Dansie, J., and Giraldo, J. P. (2017a). In vivo delivery of nanoparticles into plant leaves. Curr. Protoc. Chem. Biol. 9, 269–284. doi: 10.1002/cpch.29
Wu, H., Shabala, L., Shabala, S., and Giraldo, J. P. (2018). Hydroxyl radical scavenging by cerium oxide nanoparticles improves arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 5, 1567–1583. doi: 10.1039/C8EN00323H
Wu, H., Tito, N., and Giraldo, J. P. (2017b). Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 11, 11283–11297. doi: 10.1021/acsnano.7b05723
Yan, A., and Chen, Z. (2019). Impacts of silver nanoparticles on plants: a focus on the phytotoxicity and underlying mechanism. Int. J. Mole. Sci. 20:1003. doi: 10.3390/ijms20051003
Yao, J., Yang, M., and Duan, Y. (2014). Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: new insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 114, 6130–6178. doi: 10.1021/cr200359p
Yao, K. S., Li, S. J., Tzeng, K. C., Cheng, T. C., Chang, C. Y., and Chiu, C. Y. (2009). Fluorescence silica nano probe as a biomarker for rapid detection of plant pathogens. Adv. Mater. Res. 79, 513–516. doi: 10.4028/www.scientific.net/AMR.79-82.513
Yaqoob, A. A., Parveen, T., Umar, K., and Mohamad, M. N. (2020). Role of nanomaterials in the treatment of wastewater: a review. Water 12:495. doi: 10.3390/w12020495
Ye, Y., Cota-Ruiz, K., Hernández-Viezcas, J. A., Valdés, C., Medina-Velo, I. A., Turley, R. S., et al. (2020). Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: a sustainable approach for agriculture. ACS. Sustain. Chem. Eng. 8, 1427–1436. doi: 10.1021/acssuschemeng.9b05615
Yearla, S. R., and Padmasree, K. (2016). Exploitation of subabul stem lignin as a matrix in controlled release agrochemical nanoformulations: a case study with herbicide diuron. Environ. Sci. Poll. Res. 23, 18085–18098. doi: 10.1007/s11356-016-6983-8
Yoon, K. Y., Byeon, J. H., Park, J. H., and Hwang, J. (2007). Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total. Environ. 373, 572–575. doi: 10.1016/j.scitotenv.2006.11.007
Yousefi, S., Kartoolinejad, D., and Naghdi, R. (2017). Effects of priming with multi-walled carbon nanotubes on seed physiological characteristics of Hopbush (Dodonaeaviscosa L.) under drought stress. Int. J. Environ. Stud. 74, 528–539. doi: 10.1080/00207233.2017.1325627
Yu, Z., Sun, X., Song, H., Wang, W., Ye, Z., Shi, L., et al. (2015). Glutathione-responsive carboxymethyl chitosan nanoparticles for controlled release of herbicides. Mater. Sci. Appl. 6:591. doi: 10.4236/msa.2015.66062
Yuan, J., Chen, Y., Li, H., Lu, J., Zhao, H., and Liu, M. (2018). New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 8:3228. doi: 10.1038/s41598-017-18055-w
Yusefi-Tanha, E., Fallah, S., Rostamnejadi, A., and Pokhrel, L. R. (2020). Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total. Environ. 715:136994. doi: 10.1016/j.scitotenv.2020.136994
Ze, Y., Liu, C., Wang, L., Hong, M., and Hong, F. (2011). The regulation of TiO 2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol. Trace. Elem. Res. 143, 1131–1141. doi: 10.1007/s12011-010-8901-0
Zeng, Y., Himmel, M. E., and Ding, S. Y. (2017). Visualizing chemical functionality in plant cell walls. Biotechnol. Biofuel. 10, 1–16. doi: 10.1186/s13068-017-0953-3
Zhang, J., Landry, M. P., Barone, P. W., Kim, J. H., Lin, S., Ulissi, Z. W., et al. (2013). Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 8, 959–968. doi: 10.1038/nnano.2013.236
Zhang, L., and Fang, M. (2010). Nanomaterials in pollution trace detection and environmental improvement. Nano Today 5, 128–142. doi: 10.1016/j.nantod.2010.03.002
Zhao, L., Hernandez-Viezcas, J. A., Peralta-Videa, J. R., Bandyopadhyay, S., Peng, B., Munoz, B., et al. (2013a). ZnO nanoparticle fate in soil and zinc bioaccumulation in corn plants (Zea mays) influenced by alginate. Environ. Sci. Proces. Impact. 15, 260–266. doi: 10.1039/C2EM30610G
Zhao, L., Lu, L., Wang, A., Zhang, H., Huang, M., Wu, H., et al. (2020). Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J. Agricul. Food. Chem. 68, 1935–1947. doi: 10.1021/acs.jafc.9b06615
Zhao, L., Sun, Y., Hernandez-Viezcas, J. A., Servin, A. D., Hong, J., Niu, G., et al. (2013b). Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J. Agricul. Food. Chem. 61, 11945–11951. doi: 10.1021/jf404328e
Zhu, H., Han, J., Xiao, J. Q., and Jin, Y. (2008). Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 10, 713–717. doi: 10.1039/b805998e
Keywords: food, agriculture, nanotechnology, nano-pesticides, biosynthesized toxicity, bioavailability, sustainability, soil
Citation: Mittal D, Kaur G, Singh P, Yadav K and Ali SA (2020) Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2:579954. doi: 10.3389/fnano.2020.579954
Received: 03 July 2020; Accepted: 30 September 2020;
Published: 04 December 2020.
Edited by:
Mietek Jaroniec, Kent State University, United StatesReviewed by:
Vasileios Fotopoulos, Cyprus University of Technology, CyprusAnshu Rastogi, Poznan University of Life Sciences, Poland
Copyright © 2020 Mittal, Kaur, Singh, Yadav and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Azmal Ali, alisyedazmal@gmail.com; azmal786@gmail.com; orcid.org/0000-0003-3024-9379
 Deepti Mittal
Deepti Mittal Gurjeet Kaur
Gurjeet Kaur Parul Singh3
Parul Singh3  Syed Azmal Ali
Syed Azmal Ali