Shelf-Life Extension of Large Yellow Croaker (Larimichthys crocea) Using Active Coatings Containing Lemon Verbena (Lippa citriodora Kunth.) Essential Oil
- 1College of Food Science and Technology, Shanghai Ocean University, Shanghai, China
- 2National Experimental Teaching Demonstration Center for Food Science and Engineering Shanghai Ocean University, Shanghai, China
- 3Shanghai Engineering Research Center of Aquatic Product Processing and Preservation, Shanghai, China
- 4Shanghai Professional Technology Service Platform on Cold Chain Equipment Performance and Energy Saving Evaluation, Shanghai, China
- 5School of Health and Social Care, Shanghai Urban Construction Vocational College, Shanghai, China
- 6Shanghai Guo Qi Testing Services Technology Co., Ltd., Shanghai, China
Active coating could improve the fish quality and extend the shelf life. This study investigates the effect of locust bean gum (LBG) and sodium alginate (SA) active coatings containing lemon verbena (Lippa citriodora Kunth.) essential oil (LVEO) emulsions on microbiological, physicochemical and organoleptic evaluation of large yellow croaker (Larimichthys crocea) samples during refrigerated storage at 4°C. Results showed that LBG-SA coatings incorporated with 0.30 or 0.60% LVEO emulsions significantly inhibited the growth of mesophile bacteria, Pseudomonas spp., H2S-producing bacteria, lactic acid bacteria (LAB) and psychrophilic bacteria, and reduce the productions of trimethylamine (TMA), total volatile basic nitrogen (TVB-N) and ATP-related compounds. Further, the LVEO treatments also retarded the water migration and maintained the organoleptic evaluation results of large yellow croaker during storage at 4°C. In conclusion, the LBG-SA active coatings incorporated with LVEO emulsions maintained the quality and extended the shelf life of large yellow croaker during refrigerated storage.
Introduction
Large yellow croaker (Larimichthys crocea) is an important commercial marine fish in China and cultured extensively due to its flavor and commercial value (1, 2). However, fresh large yellow croaker is highly perishable and results in great economic losses (3, 4) due to lipid oxidation, protein degradation, and the production of undesirable compounds in the presence of microorganisms and related enzymes (5). Freshness is the most important issue relating to its quality and value (6). The fish spoilage could produce trimethylamines (TMA), organic acids, biogenic amines, alcohols, sulfides, ketones and aldehydes with unacceptable off-flavors. Fish spoilage is mainly related to the presence of Gram-negative proteolytic psychrotrophic bacteria, mainly Pseudomonas spp., Shewanella spp., and Enterobacteriaceae (7).
Some preservation techniques have applied to improve the quality and extend the shelf life (8). Using active coatings to delay the microbial growth on the fish surface could improve the fish quality and extend its shelf-life (9). Active coating mainly from food-grade natural materials including polysaccharides (e.g., alginates, gum, chitosan) has been developed as the coating for fish and fish products (10). Natural plant preservative has been paid more and more attention as it have little impact on human health or the environment to control spoilage organisms (11). Sodium alginate (SA) is an anionic polysaccharide and has good film forming properties (12). However, pure SA film still has relatively poor mechanical strength and antimicrobial activity, limiting its application to food packaging if modified properly (13). Locust bean gum (LBG) is a natural high molecular weight (300–1,200 kDa) branched polysaccharide (14). Being non-ionic, its aqueous solubility is not affected by pH or ionic strength of the liquid medium (15). SA could be used as an ion source (anionic) to promote the mucoadhesive property of non-ionic LBG (15, 16).
The incorporation of natural bioactive compounds in the active coating could improve the quality and performance, and essential oils (EOs) are the commonly used categories. Lemon verbena (Lippa citriodora Kunth.) is an aromatic plant native to South America and widely used for medicinal purposes, including antimicrobial, neuroprotective, anticonvulsant, cardioprotective, antigenotoxic, and anti-inflammatory activities (17, 18). Previous research has shown that high percentage of neral in LVEO exhibits antimicrobial and antioxidant activities (19). Nevertheless, the antimicrobial activity of neral against several spoilage organisms has been well-documented in in-vitro trials and applied in food storage (20, 21). However, some chemically active compounds of EOs are rarely present in food matrices, which have negative impact on the chemical food integrity, physical stableness, and the loss of bioactive activity of bioactive compounds (22). LVEO encapsulated by emulsion can conquer these problems by improving the oxidative stability of compounds, limiting the reaction of these compounds with food, protecting their constancy during process of food and maintenance, and providing controlled and targeted release conditions (22–24). Biopolymeric emulsions with high food compatibility could inhibit the microbial growth and lipid oxidation in fish during cold storage.
The research was to explore the effect of LBG and SA based coatings incorporated with LVEO on the quality of refrigerated large yellow croaker. The changes in microbial survival, total volatile basic nitrogen (TVB-N), trimethylamine (TMA), K-value, lipid oxidation, free fatty acids, hardness, and organoleptic evaluation of refrigerated large yellow croaker during storage for 18 days were tested to determine the preservative mechanism of each treatment.
Materials and Methods
Essential Oil From Lemon Verbena
The leaves of lemon verbena were washed with deionized water and then hydro-distillated by a Clevenger-type apparatus for 3 h. LVEO was dried with sodium sulfate anhydrous and then kept at 4°C in sealed brown vials till being used. The LVEO components were analyzed by GC-MS with the method of Homayonpour et al. (10). The conditions were set as follow: Sample volume: 1 μL; Injection port temperature: 280°C; Ion source temperature: 230°C; Initial temperature: 60°C for 1 min; Program rate: 10°C/min; Final temperature: 290°C for 5 min; Septum purge with flow rate 2 mL/min. The LVEO components were recognized with confirmed with those of mass spectra and authentic samples with reference compounds in the NIST 2011. The relative content of each component of LVEO (%) was measured with area under peak.
Preparation of Active Films Incorporated With LVEO Emulsions
The LVEO/lecithin emulsions were prepared according to Liu et al. (16). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of LVEO for Pseudomonas spp. and Shewanella spp. are 0.30 and 0.60%, respectively. Therefore, different concentrations of LVEO (0.15, 0.30, and 0.60%, v/v considering the 1/2 MIC and MIC, as well the MBC concentrations, respectively) and 0.15% (w/v) lecithin were stirred mechanically in a beaker. Then the LVEO/lecithin emulsions were homogenized with a rotor-stator homogenizer (HR-6, Huxi Industrial co., LTD, Shanghai, China) at 15,000 rpm for 5 min. LBG-SA solution was prepared with SA (1.5% w/v, M/G = 2:1, Mw 2.1 × 106 g/mol, viscosity of 200 ± 20 mPa•s, specifications received from supplier) and LBG (0.5% w/v, from Ceratonia siliqua seeds, >75% galactomannan content, M/G = 4:1, Mw 320 kDa, specifications received from supplier). To obtain complete dispersion of LBG-SA, the solution was stirred at 60°C for 4 h. Glycerol (30% w/w based on LBG-SA) as a plasticizer was added to LBG-SA solution and stirred for 1 h. The pH value of 1.5% w/v sodium alginate and 0.5% w/v locust bean gum solutions were about 6.7 and 6.2, respectively. The resultant LBG/-SA coating solution was filtrated through a double-layer degreased gauze to remove any undissolved particles. The prepared LVEO/lecithin emulsions were separately added to the LBG-SA solution and stirred continuously for 2 h. Then, the LBG-SA active coatings solutions incorporated with LVEO emulsions were prepared with ultrasonic assisted treatments at 700 W using a ultrasonic assisted processor and degassed under vacuum.
Preparation of Large Yellow Croaker Samples
Fresh large yellow croaker (700 ± 25 g) were supplied by a local market and randomly divided into four batches. Each batch of samples was immersed in the corresponding freshly prepared active coating solutions for 20 min with a ratio of 1:3 (w/v). Then the large yellow croaker samples were taken out and air-dried at 4°C for 60 min to form the active coating. After that, each large yellow croaker sample was packaged in sterile polyethylene bag and stored at 4°C for the subsequent assessments at 3-day interval. The abbreviation was followed: (1) CK (large yellow croaker samples were treated with LBG-SA active coating without LVEO emulsion); (2) LYC-0.15%LVEO (large yellow croaker samples were treated with LBG-SA active coating incorporated with 0.15% LVEO emulsion); (3) LYC-0.30%LVEO (large yellow croaker samples were treated with LBG-SA active coating incorporated with 0.30% LVEO emulsion); (4) LYC-0.60%LVEO (large yellow croaker samples were treated with LBG/-SA active coating incorporated with 0.60% LVEO emulsion).
Microbiological Analysis
Twenty-five grams fish flesh were fully blended with 225 mL normal saline and then subjected to gradient dilutions. The following microbiological analyses were carried out (25): (i) mesophile bacteria: plate count agar mediums were cultivated at 30°C for 48 h; (ii) Pseudomonas spp.: cetrimide agar mediums were cultivated at 30°C for 72 h; (iii) H2S-producing bacteria: iron agar mediums were cultivated at 30°C for 72 h; lactic acid bacteria: MRS agars were cultivated at 30°C for 72 h; (v) psychrophilic bacteria: plate count agar mediums were cultivated at 30°C for 7 days. Each sample was measured in triplicates.
Total volatile Basic Nitrogen Determination
TVB-N determination was carried out with the method of Zhuang et al. (26). 5.0 g of minced fish flesh and 45 mL deionized water were homogenized and then centrifuged at 3,040 × g at 4°C for 5 min. 5.0 mL of the supernatant was taken to determine the content of TVB-N using steam distillation method with Kjeldahl equipment (Kjeltec 8400, Foss, Denmark) and TVB-N expressed as mg N/100 g of large yellow croaker muscle. Each sample was measured in triplicates.
Determination of Trimethylamine
TMA value was determined by picric acid colorimetric method according to Li et al. (27). 2.0 g of minced fish flesh and 18 mL trichloroacetic acid (7.5%, w/v) were homogenized and then centrifuged at 11,960 × g at 4°C for 10 min. After that, 5.0 mL of the supernatant were mixed successively with 1 mL of formaldehyde (10%, v/v), 10 mL of anhydrous toluene and 3 mL of saturated potassium carbonate solutions. Subsequently, 5 mL solution extracted from toluene layer was fully blended with 5 mL of picric acid solution (0.02%, w/v). The absorbance of the mixture was measured at 410 nm. TMA content was calculated according to TMA standard curve and expressed as mg 100 g−1 sample. Each sample was measured in triplicates.
Determination of K-Value
ATP-related compounds were determined by a RP-HPLC procedure with the method of Yu et al. (28). 2.0 g of minced fish flesh and 7.5 mL precooled perchloric acid solution (6%, v/v) were homogenized and then centrifuged at 11,960 × g at 4°C for 5 min. The precipitate was extracted again with the same condition. The supernatants were collected and neutralized with KOH solutions to the final pH ranges of 6.5–6.8. After that, the neutralized solution was centrifuged at 3,040 × g at 4°C for 5 min and the supernatant was made up to 25 mL with deionized water. Subsequently, the prepared solution was filtered through a 0.22-μm filter membrane and analysized using HPLC (Waters 2695, Milford, USA) furnished with a Shim-pack VP-ODS C18 column (150 × 46 mm). Each sample was measured in triplicates. K-value was calculated according to Equation (1).
where HxR, Hx, ATP, ADP, AMP, IMP, are hypoxanthine riboside, and hypoxanthine, adenosine triphosphate, adenosine diphosphate, adenosine monophosphate, inosine monophosphate, respectively.
Determination of Peroxide Value
POV was determined by the method described by Quan et al. (29). 1.0 g of minced fish flesh and 11 mL of chloroform/methanol (2:1, v/v) were homogenized and then centrifuged at 11,960 × g at 4°C for 2 min. 7.0 mL of the supernatant and 2 mL of 0.5% sodium chloride solution were homogeneously mixed and then centrifuged at 3,040 × g at 4°C for 5 min to separate the solution into two phases. Three milliliter of lower phase, 2 mL of chloroform/methanol (2:1, v/v), 25 μL of ammonium thiocyanate and 25 μL of iron (II) chloride were mixed uniformly. The reaction mixture stood for 20 min at room temperature for 20 min and the absorbance was measured at 500 nm. Each sample was measured in triplicates. Results were expressed in mmol mequiv. peroxide/100 g sample:
V is the thiosulphate for titration (mL); N is the normality of thiosulphate; W is the weight of lipid (g).
Evaluation of Thiobarbituric Acid Reactive Substances
TBARS was monitored with the method of Vale et al. (30) and expressed as mg of malonaldehyde (MDA)/kg of large yellow croaker sample. Five grams flesh and 20 mL of 20% TBA solution were homogeneously mixed and stood for 1 h. Then the mixture was centrifugated at 11,960 × g at 4°C for 10 min and collected the supernatants. Five milliliter collected supernatant was mixed with 5 mL TBA (0.02 M) and boiled for 40 min. Then the mixture was immediately transferred to ice bath and the absorbance was measured at 532 nm. Each sample was measured in triplicates.
Determination of Free Fatty Acids
The FFAs concentration of the large yellow croaker muscle lipid extract was measured by colorimetric reaction with cupric acetate-pyridine and the absorbance was determined at 715 nm according to Trigo et al. (31). The results were expressed as mmol·kg−1 muscle. Each sample was measured in triplicates.
Water Distribution and Migration
Low field nuclear magnetic resonance (LF-NMR) analysis was carried out according to Li et al. (32). Portions of 2 × 2 × 1.5 cm dorsal muscle was cut off and packaged with polyethylene film. Transverse relaxation (T2) was determined on the LF-NMR analyzer (MesoMR23-060H.I, Newmai co., Ltd., China) with the proton resonance frequency of 20 MHz. The Carr-Purcell-Meiboom-Gill (CPMG) was used to obtain T2 relaxation information to collect decay signals. The primary parameters were as following: SW (the receiver bandwidth frequency) = 100 kHz, RFD (the parameter to control the first data point that acquired) = 0.08, NS (the number of the scans) = 4, P1 (RF 90° pulse width) = 18 μs, P2 (RF 180° pulse width) = 36 μs, RG1 (analog gain) = 20 db, DRG1 (digital gain) = 6 db, PRG (preamplifier gain) = 0, delay DL1 = 0.2 ms and TW (the duration between successive scans) = 2,000 ms. Longitudinal relaxation (T1) was measured by using the inversion-recovery (IR) sequence to confirm the MRI parameters followed the below parameters: P1 = 18 μs, P2 = 36 μs, SW = 200 KHz, RFD = 0.020 ms, RG1 = 20 db, DRG1 = 1, NS = 4, TW = 5,000 ms, PRG = 0, NTI = 20, and DL1 = 0.2 ms. Each measurement was performed in triplicate. Post-processing of NMR T2 data distributed exponential fitting of CPMG decay curves were performed by Multi-Exp Inv Analysis software (Newmai Co., Ltd., China). From the multi-exponential fitting analysis, time constants for each process were calculated from the peak position, and the area under each peak (corresponding to the proportion of water molecules exhibiting that relaxation time) was determined by cumulative integration.
After the measurement of LF-NMR, 1H MRI images of large yellow croaker samples were also determined on MesoMR23-060H.I NMR Analyzer (33). The MRI images, including T1, T2, and proton density weighted images, were acquired by using the spin-echo (SE) sequence. The MRI measurement was performed with time repetition (TR), slice width, and time echo (TE) being 500 ms, 3.0 mm, and 20 ms, respectively. Each sample was measured in triplicates. The MRI images were processed with two software: unified mapping and pseudocolor processing. After unified mapping and pseudocolor processing, the gray level images were converted to the color images.
Determination of Hardness
The hardness of large yellow croaker samples was determined using a TA.XT texture analyzer equipped with P/5 probe (34). Portions of 3 × 2 × 1.5 cm dorsal muscle (about 5.0 g) was cut off and the hardness was measured with the test speed of 1 m/s and sample deformation of 50%. Each sample was measured at least eight points.
Organoleptic Evaluation
The organoleptic evaluation of large yellow croaker samples was determined with the quality index method (QIM) described by Sun et al. (35). This method involves five important quality parameters, namely color, elasticty, mucus, muscular tissue and smell. Fifteen experienced panelists (trained by professional laboratory staff, eight women and seven men, 20–40 years old) conducted the organoleptic evaluation based on QIM with the score scale ranging from 1 to 10. All panelists were trained by professional laboratory staff and had a history in fish assessment and previously at Shanghai Ocean University, joined in another research displayed by Li et al. (36). They were designated based on their taste detection limit, sensitivity and smell of very low LVEO concentrations. Ten represents the best quality of large yellow croaker and the sample will not be accepted once the score is <4.
Statistical Analysis
Data analysis of the quality of large yellow croaker was performed in triplicate (except hardness determination and organoleptic evaluation). The date was analyzed using SPSS 22.0 through one-way ANOVA procedure followed by Duncan's-test. The results were expressed as means ± standard deviation. p < 0.05 was considered to be statistically significant.
Results and Discussions
Chemical Composition of LVEO
The important components of LVEO used included citral (31.79%), neral (23.75%), geraniol (22.01%), and D-limonene (10.36%) obtained from the MS libraries (Supplementary Table 1). The four most abundant components are responsible of the antimicrobial activity of LVEO (37, 38).
Microbiological Analyses
Figure 1 shows the corresponding growth data of TVC, Pseudomonas spp., H2S-producing bacteria, LAB and psychrophilic bacteria of large yellow croaker samples during refrigerated storage at 4°C for 18 days. The low count (2.3 lg CFU/g) of TVC at the beginning indicated fish fresh (39). LVEO treatments could delay the spoilage microbial growth and the LVEO treated large yellow croaker samples had lower TVC than that of CK. On 15th day, CK exceeded the “shelf-life” limit of 7.0 lg CFU/g (40). Incorporation of LVEO to LBG-SA coatings showed the significant antimicrobial activity of coatings, which led to extend the shelf-life by inhibiting the growth of undesirable microorganisms. EOs have been widely used for the food preservation application as having potential antimicrobial activity in active coatings (41–43). The destructions of cell membrane structure and functional characteristics of the cell membrane are considered to be the most important mechanism of EOs against microorganisms (44, 45).
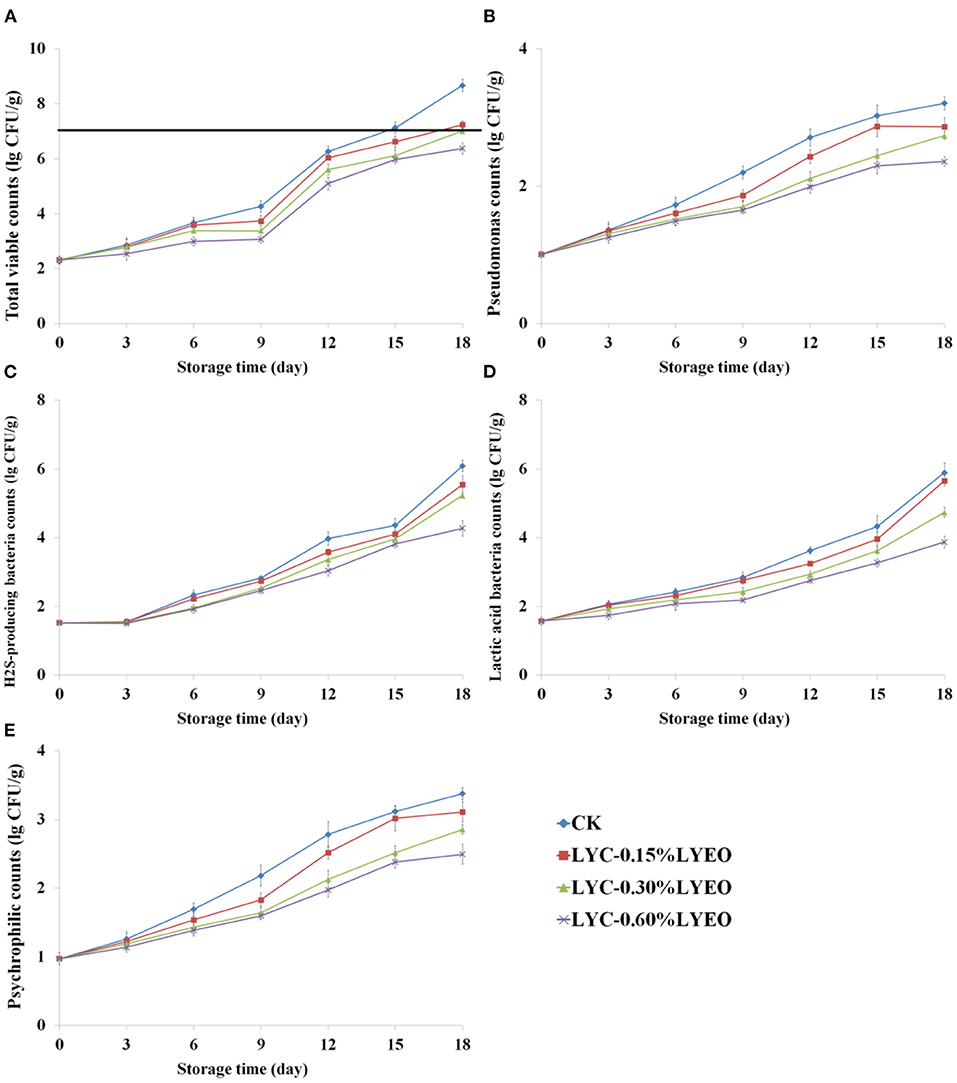
Figure 1. Changes in total viable counts (TVC, A), Pseudomonas spp. counts (B), H2S-producing bacteria counts (C), lactic acid bacteria counts (LAB, D), and psychrophilic counts (E) of large yellow croaker samples during refrigerated storage (CK, large yellow croaker samples were treated with LBG-SA active coating without LVEO emulsion; LYC-0.15%LVEO, large yellow croaker samples were treated with LBG-SA active coating incorporated with 0.15% LVEO emulsion; LYC-0.30%LVEO, large yellow croaker samples were treated with LBG-SA active coating incorporated with 0.30% LVEO emulsion; and LYC-0.60%LVEO, large yellow croaker samples were treated with LBG-SA active coating incorporated with 0.60% LVEO emulsion).
Pseudomonas spp. and H2S-producing bacteria are both known as the specific spoilage organisms (SSOs) in spoiled fish during refrigerated storage (46). The two SSOs also showed similar increase results in this study (Figures 1B,C). Pseudomonas is the predominant aerobic microorganism related to the formation of undesirable odors (47). At the beginning, the number of Pseudomonas spp. was 1.0 lg CFU/g and increased in all large yellow croaker samples during refrigerated storage. There was a significant (p < 0.05) difference in the Pseudomonas spp. count between CK and LVEO treated samples at the end of storage and it was 8.7 lg CFU/g for CK. Therefore, it can be seen that LVEO were effective in delaying the growth of Pseudomonas spp. in large yellow croaker samples, and the inhibitory differences was related to the added concentration of LVEO. Nisar et al. (48) also reported that clove essential oil was also effective against Pseudomonas spp. and the inhibiting effects were increased with the increasing concentration of EO. Myszka et al. (49) reported that green pepper EO could inhibit the growth of Pseudomonas spp. and attenuate the bacterial virulence properties, such as pyocyanin production, elastase and alkaline protease activities.
The number of H2S-producing bacteria (mainly Shewanella spp.) was 1.5 lg CFU/g at the beginning, which increased to 6.1, 5.5, 5.2, and 4.3 lg CFU/g, respectively, in CK, LYC-0.15%LVEO, LYC-0.30%LVEO, LYC-0.60%LVEO, at the end of storage. The number of H2S-producing bacteria in LVEO treated samples were significantly (p < 0.05) lower than that in the CK samples. LYC-0.30%LVEO and LYC-0.60%LVEO samples had the lowest number of Pseudomonas spp. and H2S-producing bacteria in all sampling times, which indicated LBG/SA coating incorporated with 0.30 or 0.60% LVEO could inhibit the growth of the two SSOs in large yellow croaker samples during refrigerated storage. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of LVEO for Pseudomonas spp. and H2S-producing bacteria are 0.30 and 0.60%, respectively. This is the reason that 0.30 and 0.60% of LVEO were used in the experiment. Zhang et al. (50) reported that cinnamon essential oil could also inhibit the growth of Pseudomonas and H2S-producing bacteria in in vacuum-packaged common carp during refrigerated storage and the two microorganisms did not exceed the “shelf-life” limit of 7 lg CFU/g at the end of storage. H2S-producing bacteria could produce fish off-odors even at low cell numbers and the spoilage action includes production of hydrogen sulfide, TMA, methyl mercaptan, and other characteristic compounds (51–53). It is reported that H2S-producing bacteria was responsible for putrescine and cadaverine, however, Pseudomonas spp. contributed most to tyramine (54, 55). In our previous study, H2S-producing bacteria grows from 2.1 to 8.9 lg CFU/g in cultured pufferfish after 18 days at 4°C, producing high levels of hexanal, 1-octen-3-ol, octanal, (E)-2-octenal and 2, 3-butanedione, which are volatile compounds causing strong fishy flavor (27).
The number of LAB was 1.6 lg CFU/g at the beginning (Figure 1D) and progressively increased in all samples during refrigerated storage but with slower rates in LYC-LVEO treated samples. The LAB number of CK, LYC-0.15%LVEO, LYC-0.30%LVEO, LYC-0.60%LVEO reached to 5.9, 5.7, 4.7, and 3.9 lg CFU/g, respectively, at the end of storage. This finding indicated that LAB was not primarily responsible for the spoilage in large yellow croaker samples during refrigerated storage, which is in consistence with trout filets packaged with probiotic carboxymethyl cellulose-sodium caseinate films (56). Although LAB was not the dominant microorganism of fish during refrigerated storage, they might cause the spoilage through producing a sour flavor and biogenic amines (47, 57).
Psychrophilic bacteria are the main microorganisms causing the spoilage of fish during refrigerated storage, thus decreasing the shelf life of fish (58). The number of psychrophilic bacteria was 1.0 lg CFU/g on 0 day and gradually increased with storage time (Figure 1E). The addition of LVEO significantly inhibited the growth of psychrophilic bacteria compared to CK (p < 0.05). Similar trend was found by Shokri et al. (59) with pectin based coatings containing clove essential oil.
In the current research, the counts of TVC, Pseudomonas spp., H2S-producing bacteria, LAB and psychrophilic bacteria of large yellow croaker samples packaged with LVEO active coatings were significantly lower than that of CK during refrigerated storage (p < 0.05). Therefore, using LVEO treatments as an active coating could maintain the microbial quality of large yellow croaker samples during refrigerated storage.
Changes in TVB-N
Volatile nitrogen-containing compounds including ammonia, dimethylamine and TMA, known as TVB-N index, has been regarded as an indicator of spoilage in fish and fish products (60). The initial TVB-N value was determined as 8.17 mg/100 g (Figure 2A), indicting the raw fish being fresh. TVB-N contents of large yellow croaker samples progressively increased to 41.77, 27.72, and 25.26 mg N/100 g in the CK, LYC-0.15%LVEO and LYC-0.30%LVEO samples on 15th day exceeding the upper limit of 25 mg N/100 g (61). However, the LYC-0.60%LVEO samples were still under this limit at the end of storage as the 0.60% LVEO addition could inhibit the growth of SSOs or decrease the capacity of SSOs for oxidative deamination of non-protein nitrogen compounds (62). TVB-N results are consistent with TVC results. The effects of active coatings on TVB-N reduction of large yellow croaker have also been investigated. Shokri et al. (63) found that rainbow trout filets treated by chitosan with Ferulago angulata essential oil nanoemulsion could suppress the TVB-N growth and maintained acceptable freshness during a 16-day storage at 4°C. Dong et al. (64) also stated that active films containing Attapulgite loaded with Allium sativum essence oil pronounced lower TVB-N values in the preservation of large yellow croaker at 4°C under vacuum condition and extended shelf-life up to 9 days with 30 mg N/100 g fish as the TVB-N upper limit.
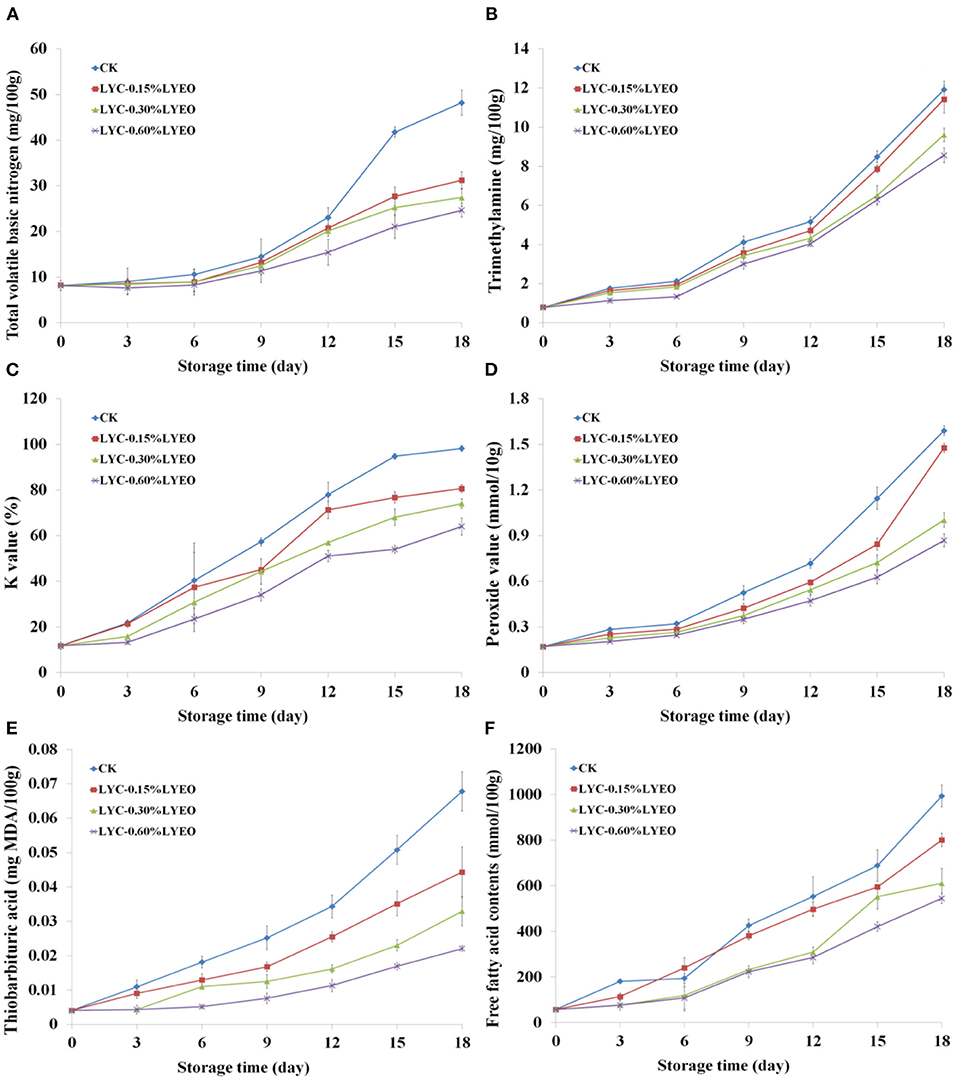
Figure 2. Changes in total volatile basic nitrogen (TVB-N, A), trimethylamine (TMA, B), K-values (C), peroxide value (POV, D), thiobarbituric acid reactive substance (TBARS, E), and free fatty acids contents (FFA, F) of large yellow croaker samples during refrigerated storage.
Changes in TMA
TMA is produced through the breakdown of trimethylamine oxide (TMAO) by bacterial and enzymatic activity; therefore, it can be used as an indicator of freshness for fish and fish products (65). Low initial TMA content (0.79 mg of TMA/100 g fish muscle, Figure 2B) indicates that the large yellow croaker samples were fresh, which was consistent with the relatively low TVC counts. Moreover, H2S-producing bacteria could reduce TMAO to TMA and have low counts (1.5 lg CFU/g) at the beginning. The TMA of all the samples increased significantly (p < 0.05) during refrigerated storage. Jouki et al. suggested 5 mg N/100 g as an upper limit for rainbow trout (66). However, Klnc et al. (67) used 8 mg N/100 g as the limit of acceptability for sea bass. In the current research, the upper limit of TMA, as estimated by the TVB-N and TVC values, was 8 mg TMA/100 g for large yellow croaker samples. On the basis of this limit, CK, LYC-0.15%LVEO, LYC-0.30%LVEO, and LYC-0.60%LVEO samples exceeded the upper limit on 15th, 18th, 18th, and 18th day, respectively.
Changes in K-Values
K-value is widely used to quantify the fish freshness. The adenine nucleotides in degradation products promote spoilage and the formation of off-flavors, causing fish to lose their freshness (68). The K-value of large yellow croaker samples on 0 day was 11.73% (Figure 2C), staying at a very fresh level (K-value <20%), and increased continuously during the whole storage time. The fish samples were considered very fresh till approximately on 6th day for the LYC-0.30%LVEO and LYC-0.60%LVEO samples, comparing with CK on 3th day, indicating that LVEO could inhibit the ATP degradation. The K-value of CK increased with greater speed compared with those of the LVEO treated samples. The CK, LYC-0.15%LVEO, LYC-0.30%LVEO and LYC-0.60%LVEO samples increased to 77.93, 71.33, 68.06, and 64.03% on 12th, 12th, 15th, and 18th day, respectively, exceeding the acceptable limit of 60% (69). The result was similar to Dong et al. (64), who corroborated that Allium sativum essence oil could effectively inhibit the ATP degradation and maintain the high quality of large yellow croaker.
Changes in POV
POV is used to determine the formation of primary lipid oxidation products in fish and fish products during refrigerated storage (70). The POV on 0 day was 0.17 meq peroxide/kg fish (Figure 2D) and increased during refrigerated storage. The POV of all treated large yellow croaker samples increased during refrigerated storage, but at a slower rate in LVEO treated samples comparing with CK. The POV of the CK samples remarkably increased (p < 0.05) to 1.59 meq peroxide/kg fish at the end. This shows the CK sample was oxidized rapidly during refrigerated storage, while lipid oxidation in the LVEO treated large yellow croaker samples occurred more slowly. The delayed lipid oxidation was attributed to the release and diffusion of phenolic compounds presenting in the LBG-SA active coatings to the large yellow croaker samples. The phenolic compounds exhibited antioxidant activities, which are related with their free-radical scavenging ability and metal chelating capacities (71). These findings are consistent with those reported by Shadman et al. (72) who reported that Zataria multiflora Boiss. essential oil was effective in delaying the production of peroxide in rainbow trout (Oncorhynchus mykiss) filets during storage at refrigerated condition.
Changes in TBARS
Fish and other seafood are rich in unsaturated fatty acids, which are easily oxidized by heat, light and enzymes, resulting in undesirable rancid odor and poisoning (73). The increase in TBA may be described by the formation of secondary lipid oxidation products (74). As shown in Figure 2E, the initial amount of MDA in the large yellow croakers was 0.04 mg MDA/kg. TBA value increased in all samples until the end of storage; however, LVEO treated samples reached significantly (p < 0.05) lower TBA values of 0.22–0.44 mg MDA/kg of fish comparing with CK, which attained a higher level of 0.67 mg MDA/kg of fish. A TBA level of 5 mg MDA/kg of fish muscle comprises the threshold for detecting off-odors and off-taste at refrigerated storage (56). In this research, TBA values in all samples were lower than such recommended limits during the entire storage period, which probably LBG-SA active coating can reduce the diffusion of oxygen to the surface of the fish and act as a barrier between the fish and its surroundings, thus inhibiting lipid oxidation (13). It was shown that LVEO treatments had antioxidant activity, due to a high content of neral (75), as evidenced by lower TBA values in the large yellow croaker samples packaged with LVEO coatings. Perumalla and Hettiarachchy (76) reported that the antioxidant activities of the EOs are mainly manifested in the binding of transition metal ion catalysts, the prevention of radical chain initiation, interaction with the free radicals and decomposition of peroxides.
Changes in FFAs
Lipid hydrolysis development was measured by the FFAs formation (31). A progressive FFA formation was observed in all large yellow croaker samples during refrigerated storage (Figure 2F). However, no significant changes in FFAs contents were observed in LYC-0.30%LVEO and LYC-0.60%LVEO samples within the first 3 days. Hydrolysis of glycerol-fatty acid esters is an important change in the lipid content of muscle after fish death resulting in the release of free fatty acids, which is catalyzed by lipases and phospholipases (77). The accumulation of FFAs could be related to the activities of lipase and phospholipase in fish muscle, digestive organs and microorganisms, which were enhanced with storage time. The LVEO presence could produce lower FFAs formation due to the modification of the lipase environment leading to a partial inhibition of its catalytic action (31). Consistent with this research, the employment of LVEO inhibited FFAs formation in the refrigerated large yellow croaker samples.
Water Distribution by LF NMR Analysis
LF-NMR is an effective way to evaluate the freshness of fish and MRI is also an assistive method to understand water migration in fish during storage (78). In this study the transverse relaxation time T2 showed a multi-exponential behavior, which suggests that the water is divided into populations in the muscle tissue. T21 ranged from 11.2 to 17.5 ms represent the fraction of strongly bound water. T22 (generally 100–400 ms) relates to the water within the organized protein structures (intra-myofibrillar) and T23 is the water in the space between myofibrils (extra-myofibrillar), which can be more easily mobilized by dripping or cooking and therefore susceptible to fish spoilage (79). The pT21, pT22, and pT23 correspond to the relative amount of bound water, immobilized water and free water, respectively (Table 1). Variations in relaxation times over time were expected in view of the changes in protein structure during deterioration. The pT21 rarely changed for large yellow croaker samples during refrigerated storage as the bound water held within highly organized myofibril structures (36). Some changes in pT2 suggested protein degradation in muscle tissue for large yellow croaker samples during refrigerated storage. The pT22 decreased progressively while pT23 increased for large yellow croaker samples during refrigerated storage. The CK had significant (p < 0.05) lower immobilized water content (from 95.35% at the beginning to 88.27% on 18th day) than that of other samples. However, no significant differences (p > 0.05) were shown in the immobilized water contents between LYC-0.30%LVEO and LYC-0.60%LVEO samples. The pT23 increased during refrigerated storage, however, the LVEO treated large yellow croaker samples had lower free water content than that of CK.
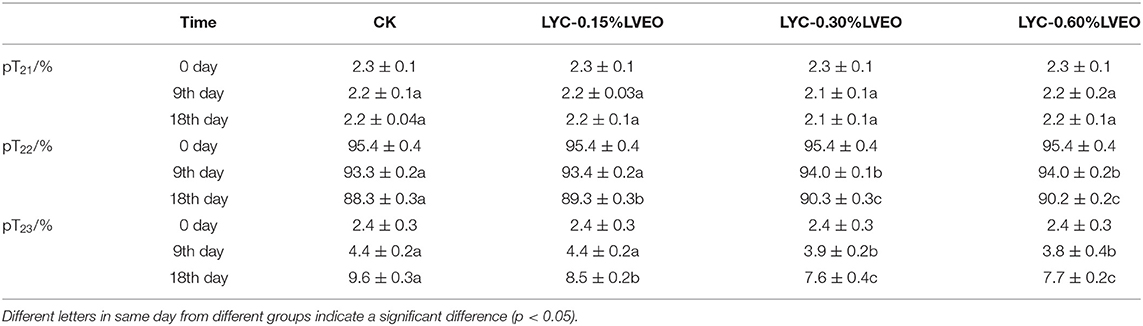
Table 1. Changes in water distribution in different treated large yellow croaker samples on 0 day, 9th day, and 18th day during refrigerated storage.
MRI has attracted more and more attention for providing the visual information of spatial, internal morphological organization and molecular distribution in food matrix (73). Changes of water distribution of refrigerated large yellow croaker samples were investigated by T1 and T2 weighted images with MRI. Corresponding pseudo-color images are shown in Figure 3, in which red is the region with high proton signal density, and blue is the region with low proton signal density. There was no significant difference in MRI brightness of LVEO treated large yellow croaker samples on 9th day (Figure 3). Besides, the brightness of T1 and T2 images varied obscure and the brightness of the samples became darker and bluer during refrigerated storage. The color of CK samples was darker and bluer than other samples on 18th day and the brightness of LVEO treated samples were lighter compared with CK, which indicated the microstructure degradation and destruction of myofibril was more serious in CK sample (80).
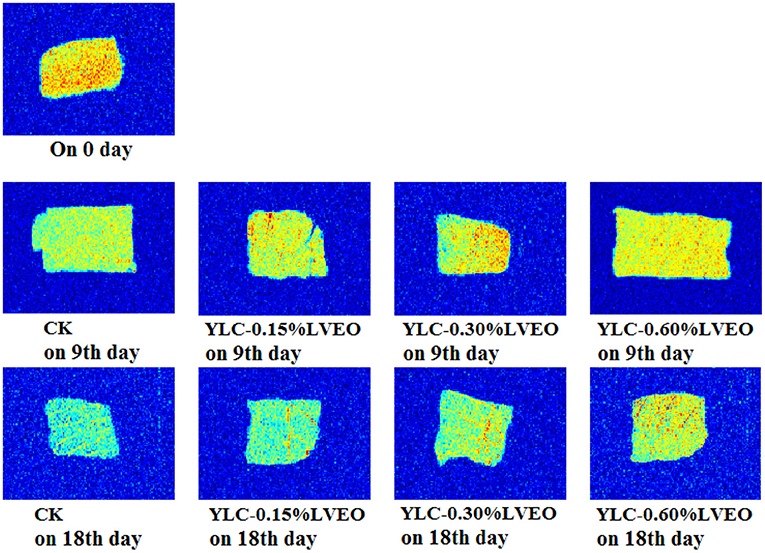
Figure 3. Results of magnetic resonance image (MRI) of large yellow croaker samples under different treatment during refrigerated storage.
Changes in Hardness
The hardness was 5.64 × 103 g on 0 day (Figure 4) and decreased significantly (p < 0.05) in all large yellow croaker samples because the muscle became softer probably due to the autolytic activity of enzymes, the hydrolysis of protein and the destruction of connective tissue (40). The CK samples showed the fastest softening rate, losing about 69.86% of its hardness at the end, and LVEO treated samples had higher values of hardness than CK, which suggested LBG-SA active coatings incorporated with LVEO could decrease the loss of large yellow croaker samples hardness during refrigerated storage. Decreases in hardness of large yellow croaker during refrigerated storage was related to the enzymatic degradation of muscle proteins and thereafter accelerated by microbial activity (53). In the current study, LVEO treated samples reduced the loss of hardness by inhibiting the microbial growth with LVEO.
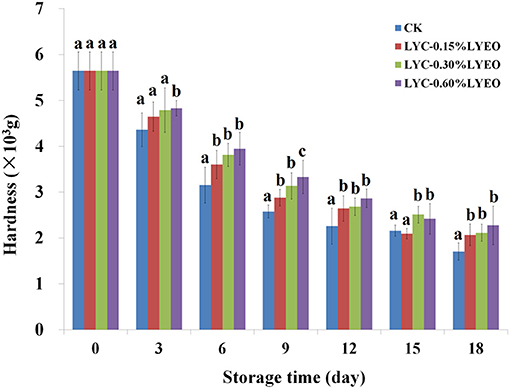
Figure 4. Changes in hardness of large yellow croaker samples during refrigerated storage. Different letters in same day from different groups indicate a significant difference (p < 0.05).
Organoleptic Evaluation Results
The organoleptic evaluation results including color, elasticty, mucus, muscular tissue and smell of large yellow croaker samples during refrigerated storage at 4°C are shown in Figure 5. At the beginning, all samples had high scores proving the good quality and the scores decreased significantly (p < 0.05) with the prolonging of storage time. However, the organoleptic results showed that the scores of the LVEO treated samples were significantly higher than that of CK. Therefore, the method of treating with the LBG-SA coating incorporated with LVEO could effectively delay the quality deterioration and maintain the organoleptic quality of large yellow croaker. At the end of storage, the scores of all the samples were lower than the limit value of 4 and they were considered as unacceptable for large yellow croaker samples in this research. The organoleptic results could directly show whether the large yellow croaker samples have gone spoiled during refrigerated storage. However, it took at least 2 or 3 days to get the chemical and microbiological results. The shelf life of perishable foods can be extended by reducing lipid oxidation and microbial reproduction. The inhibition of lipid oxidation can be attributed to the antioxidant properties of the active coatings, thereby reducing the production of unpleasant odors and flavors. The positive effects of EOs on the organoleptic properties of food have been demonstrated in some research (81). It should be noted that the smell of LVEO was also detected in the organoleptic evaluation; however, the influence on large yellow croaker was limited at this concentration. Besides, some of the observed changed could be due to inhomogeneities since the coating homogeneous and thickness was not measured.
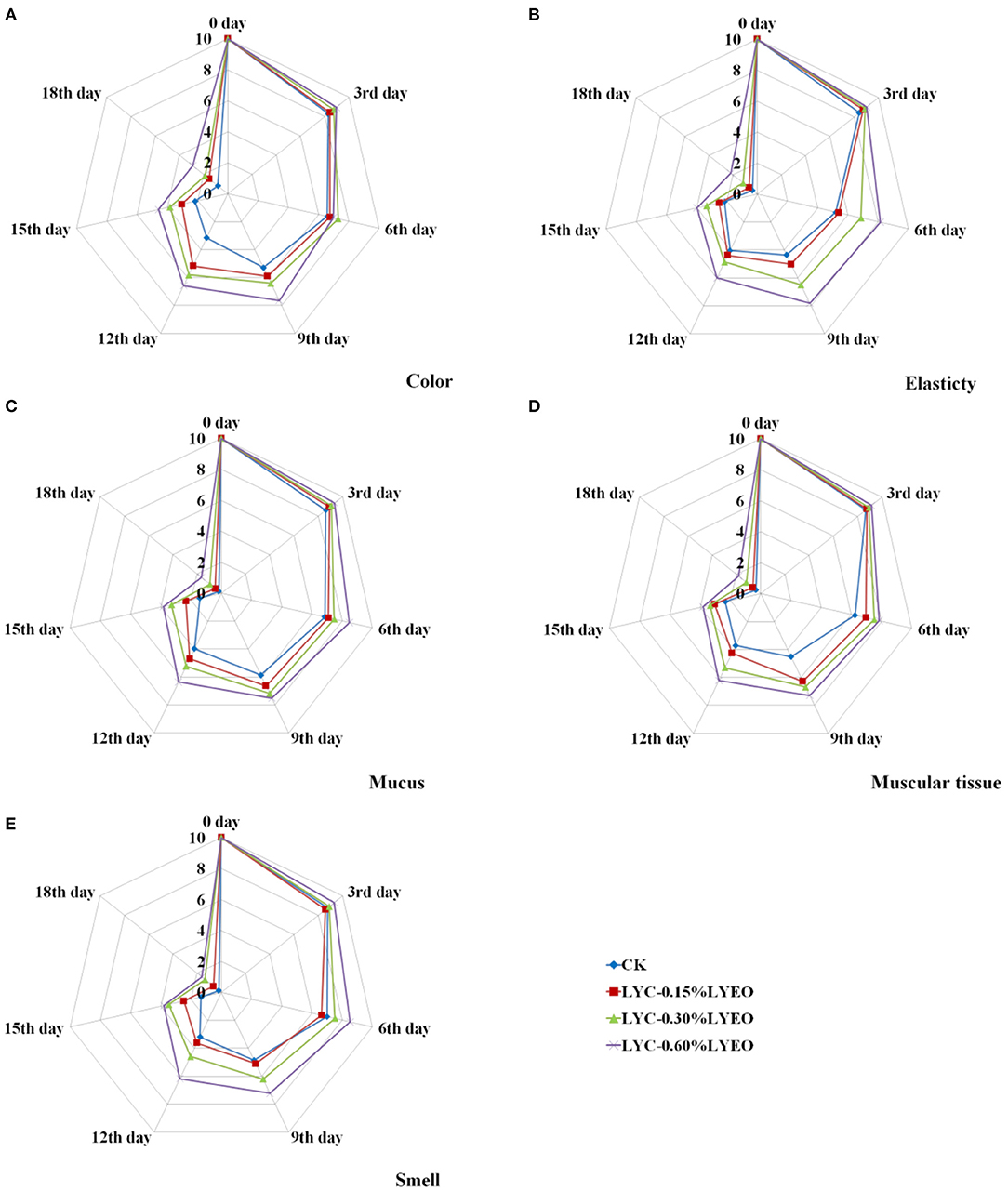
Figure 5. Changes in color (A), elasticty (B), mucus (C), muscular tissue (D), and smell (E) of large yellow croaker samples during refrigerated storage.
Conclusions
The LBG-SA active coatings incorporated with different LVEO concentrations were applied to evaluate the effects on quality improvement of large yellow croaker samples spoilage during refrigerated storage at 4°C for 18 days. This research focused on exploring the effect of LVEO on the quality of large yellow croaker samples during refrigerated storage and the large yellow croaker without LBG-SA active coating was not considered in the experiment. The results of microbiological and physicochemical analyses showed that the LBG-SA films incorporated with 0.30% LVEO and 0.60% LVEO emulsions treated large yellow croaker samples maintained better quality during refrigerated storage, which mainly due to that LVEO could effectively inhibit the growth of SSOs and resist to oxidation to extend the shelf life. LYC-0.30%LVEO and LYC-0.60%LVEO had similar effects in slowing down the spoilage of large yellow croaker; however, 0.60% LVEO addition gave the active coating solution a strong flavor. Therefore, 0.30% LVEO addition combined with refrigerated storage at 4°C could be suitable for maintaining the freshness of large yellow croaker samples and extended the shelf life.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
BL, JM, and JX: conceptualization. BL, XW, XG, LZ, and XM: data curation. BL, XW, and JM: formal analysis. JX: funding acquisition and validation. BL, LZ, and JM: investigation. BL, LZ, JM, and JX: methodology. JM and JX: project administration, writing-review, and editing. BL and JM: software and writing-original draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
LZ was employed by the company Shanghai Guo Qi Testing Services Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded by the China Agriculture Research System (CARS-47), Shanghai Science and Technology Key Project on Agriculture from Shanghai Municipal Agricultural Commission (2019-02-08-00-10-F01143), National Key Research and Development Program (2016YFD0400106), and Shanghai Science and Technology Commission Platform Capacity Construction Project (19DZ2284000).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.678643/full#supplementary-material
References
1. Li X, Liu C, Wang J, Zhou K, Yi S, Zhu W, et al. Effect of hydroxyl radicals on biochemical and functional characteristics of myofibrillar protein from large yellow croaker (Pseudosciaena crocea). J Food Biochem. (2020) 44:e13084. doi: 10.1111/jfbc.13084
2. Li T, Hu W, Li J, Zhang X, Zhu J, Li X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control. (2012) 25:101–6. doi: 10.1016/j.foodcont.2011.10.029
3. Wang Y, Bao X, Wang F, Wang H, Fu L. Dynamic detection of biogenic amines as a quality indicator and their relationship with free amino acids profiles in large yellow croaker (Pseudosciaena crocea). J Food Sci. (2019) 84:254–60. doi: 10.1111/1750-3841.14425
4. Lan WQ, Liu L, Zhang NN, Huang X, Weng ZM, Xie J. Effects of epsilon-polylysine and rosemary extract on the quality of large yellow croaker (Pseudosciaena crocea) stored on ice at 4°C. J Food Biochem. (2020) 44:e13418. doi: 10.1111/jfbc.13418
5. Fu L, Wang C, Ruan X, Li G, Zhao Y, Wang Y. Preservation of large yellow croaker (Pseudosciaena crocea) by Coagulin L1208, a novel bacteriocin produced by Bacillus coagulans L1208. Int J Food Microbiol. (2018) 266:60–8. doi: 10.1016/j.ijfoodmicro.2017.11.012
6. Li T, Li J, Hu W, Chen J, Li H. Protein changes in post mortem large yellow croaker (Pseudosciaena crocea) monitored by SDS-PAGE and proteome analysis. Food Control. (2014) 41:49–55. doi: 10.1016/j.foodcont.2013.12.031
7. Zhu J, Zhao A, Feng L, Gao H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int J Food Microbiol. (2016) 217:146–55. doi: 10.1016/j.ijfoodmicro.2015.10.020
8. Mei J, Xuan M, Xie J. Review on natural preservatives for extending fish shelf life. Foods. (2019) 8:490. doi: 10.3390/foods8100490
9. Wu T, Ge Y, Li Y, Xiang Y, Jiang Y, Hu Y. Quality enhancement of large yellow croaker treated with edible coatings based on chitosan and lysozyme. Int J Biol Macromol. (2018) 120:1072–9. doi: 10.1016/j.ijbiomac.2018.08.188
10. Homayonpour P, Jalali H, Shariatifar N, Amanlou M. Effects of nano-chitosan coatings incorporating with free/nano-encapsulated cumin (Cuminum cyminum L.) essential oil on quality characteristics of sardine fillet. Int J Food Microbiol. (2021) 341:109047. doi: 10.1016/j.ijfoodmicro.2021.109047
11. Baptista RC, Horita CN, Sant'Ana AS. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: a review. Food Res Int. (2020) 127:23. doi: 10.1016/j.foodres.2019.108762
12. Yang M, Li L, Yu S, Liu J, Shi J. High performance of alginate/polyvinyl alcohol composite film based on natural original melanin nanoparticles used as food thermal insulating and UV-vis block. Carbohyd Polym. (2020) 233:115884. doi: 10.1016/j.carbpol.2020.115884
13. Chen J, Wu A, Yang M, Ge Y, Pristijono P, Li J, et al. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control. (2021) 126:108063. doi: 10.1016/j.foodcont.2021.108063
14. Upadhyay M, Adena SKR, Vardhan H, Yadav SK, Mishra B. Locust bean gum and sodium alginate based interpenetrating polymeric network microbeads encapsulating Capecitabine: improved pharmacokinetics, cytotoxicity & in vivo antitumor activity. Mat Sci Eng C-Mater. (2019) 104:109958. doi: 10.1016/j.msec.2019.109958
15. Prajapati VD, Jani GK, Moradiya NG, Randeria NP, Maheriya PM, Nagar BJ. Locust bean gum in the development of sustained release mucoadhesive macromolecules of aceclofenac. Carbohyd Polym. (2014) 113:138–48. doi: 10.1016/j.carbpol.2014.06.061
16. Liu W, Mei J, Xie J. Effect of locust bean gum-sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition. Int J Biol Macromol. (2021) 170:129–39 doi: 10.1016/j.ijbiomac.2020.12.089
17. Solano-Báez AR, Venegas-Portilla A, Rodríguez-Mendoza J, Camacho-Tapia M, Leyva-Mir SG, Márquez-Licona G. First report of neoerysiphe aloysiae causing powdery mildew on lemon verbena (Aloysia citrodora) in Mexico. Plant Sci. (2020) 104:2293. doi: 10.1094/PDIS-02-20-0337-PDN
18. Bahramsoltani R, Rostamiasrabadi P, Shahpiri Z, Marques AM, Rahimi R, Farzaei MH. Aloysia citrodora Paláu (Lemon verbena): a review of phytochemistry and pharmacology. J. Ethnopharmacol. (2018) 222:34–51. doi: 10.1016/j.jep.2018.04.021
19. Vergis J, Gokulakrishnan P, Agarwal RK, Kumar A. Essential oils as natural food antimicrobial agents: a review. Crit Rev Food Sci. (2015) 55:1320–3. doi: 10.1080/10408398.2012.692127
20. Remya S, Mohan CO, Bindu J, Sivaraman GK, Venkateshwarlu G, Ravishankar CN. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J Food Sci Technol. (2016) 53:685–93. doi: 10.1007/s13197-015-2018-6
21. Samba N, Aitfella-Lahlou R, Nelo M, Silva L, Coca R, Rocha P, et al. Chemical composition and antibacterial activity of lippia multiflora moldenke essential oil from different regions of angola. Molecules. (2021) 26:3390. doi: 10.3390/molecules26010155
22. Gibis M, Vogt E, Weiss J. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Funct. (2012) 3:246–54. doi: 10.1039/C1FO10181A
23. Lam SJ, Wong EHH, Boyer C, Qiao GG. Antimicrobial polymeric nanoparticles. Prog Polym Sci. (2018) 76:40–64. doi: 10.1016/j.progpolymsci.2017.07.007
24. Niza E, BoŽik M, Bravo I, Clemente-Casares P, Lara-Sanchez A, Juan A, et al. PEI-coated PLA nanoparticles to enhance the antimicrobial activity of carvacrol. Food Chem. (2020) 328:127131 doi: 10.1016/j.foodchem.2020.127131
25. Zhou Q, Li P, Fang S, Liu W, Mei J, Xie J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9°C) storage. Coatings. (2019) 9:489. doi: 10.3390/coatings9080489
26. Zhuang S, Li Y, Hong H, Liu Y, Shu R, Luo Y. Effects of ethyl lauroyl arginate hydrochloride on microbiota, quality and biochemical changes of container-cultured largemouth bass (Micropterus salmonides) fillets during storage at 4°C. Food Chem. (2020) 324:126886. doi: 10.1016/j.foodchem.2020.126886
27. Li P, Zhou Q, Chu Y, Lan W, Mei J, Xie J. Effects of chitosan and sodium alginate active coatings containing ε-polysine on qualities of cultured pufferfish (Takifugu obscurus) during cold storage. Int J Biol Macromol. (2020) 160:418–28. doi: 10.1016/j.ijbiomac.2020.05.092
28. Yu D, Jing D, Yang F, Gao P, Xia W. The factors influencing the flavor characteristics of frozen obscure pufferfish (Takifugu obscurus) during storage: ice crystals, endogenous proteolysis and oxidation. Int J Refrig. (2020) 122:147–55. doi: 10.1016/j.ijrefrig.2020.10.028
29. Quan TH, Benjakul S, Hozzein WN. Quality and storage stability of fish tofu as affected by duck albumen hydrolysate-epigalocatechin gallate conjugate. LWT. (2019) 120:108927. doi: 10.1016/j.lwt.2019.108927
30. do Vale DA, Vieira CB, de Oliveria JM, Vidal MF, de Alcântara LO, da Silva AIM, et al. Determining the wetting capacity of the chitosan coatings from Ucides cordatus and evaluating the shelf-life quality of Scomberomorus brasiliensis fillets. Food Control. (2020) 116:107329. doi: 10.1016/j.foodcont.2020.107329
31. Trigo M, Rodríguez A, Dovale G, Pastén A, Vega-Gálvez A, Aubourg SP. The effect of glazing based on saponin-free quinoa (Chenopodium quinoa) extract on the lipid quality of frozen fatty fish. LWT. (2018) 98:231–6. doi: 10.1016/j.lwt.2018.08.031
32. Li N, Shen Y, Liu W, Mei J, Xie J. Low-field NMR and MRI to analyze the effect of edible coating incorporated with map on qualities of half-smooth tongue sole (Cynoglossus semilaevis Günther) fillets during refrigerated storage. Appl Sci. (2018) 8:1391. doi: 10.3390/app8081391
33. Li M, Li B, Zhang W. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. (2018) 242:16–21. doi: 10.1016/j.foodchem.2017.08.086
34. Mei J, Shen Y, Liu W, Lan W, Li N, Xie J. Effectiveness of sodium alginate active coatings containing bacteriocin EFL4 for the quality improvement of ready-to-eat fresh salmon fillets during cold storage. Coatings. (2020) 10:506. doi: 10.3390/coatings10060506
35. Sun X, Guo X, Ji M, Wu J, Zhu W, Wang J, et al. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4°C. Food Chem. (2019) 272:643–52. doi: 10.1016/j.foodchem.2018.08.040
36. Li P, Peng Y, Mei J, Xie J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT. (2020) 118:108831. doi: 10.1016/j.lwt.2019.108831
37. Thielmann J, Theobald M, Wutz A, Krolo T, Buergy A, Niederhofer J, et al. Litsea cubeba fruit essential oil and its major constituent citral as volatile agents in an antimicrobial packaging material. Food Microbiol. (2021) 96:103725. doi: 10.1016/j.fm.2020.103725
38. Su J, Guo Q, Cai Y, Wang T, Mao L, Gao Y, et al. Effect of ultra-high temperature processing on the physicochemical properties and antibacterial activity of d-limonene emulsions stabilized by β-lactoglobulin/Gum arabic bilayer membranes. Food Chem. (2020) 332:127391. doi: 10.1016/j.foodchem.2020.127391
39. Erkan N, Üretener G, Alpas H, Selçuk A, Özden Ö, Buzrul S. The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innov Food Sci Emerg. (2011) 12:104–10. doi: 10.1016/j.ifset.2010.12.004
40. Tao T, Ding C, Han N, Cui Y, Liu X, Zhang C. Evaluation of pulsed light for inactivation of foodborne pathogens on fresh-cut lettuce: effects on quality attributes during storage. Food Packaging Shelf. (2019) 21:100358. doi: 10.1016/j.fpsl.2019.100358
41. Perdana MI, Ruamcharoen J, Panphon S, Leelakriangsak M. Antimicrobial activity and physical properties of starch/chitosan film incorporated with lemongrass essential oil and its application. LWT. (2021) 141:110934. doi: 10.1016/j.lwt.2021.110934
42. Farsanipour A, Khodanazary A, Hosseini SM. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. Int J Biol Macromol. (2020) 155:766–71. doi: 10.1016/j.ijbiomac.2020.03.228
43. Xiong Y, Kamboj M, Ajlouni S, Fang Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control. (2021) 125:107994. doi: 10.1016/j.foodcont.2021.107994
44. da Silva BD, Bernardes PC, Pinheiro PF, Fantuzzi E, Roberto CD. Chemical composition, extraction sources and action mechanisms of essential oils: natural preservative and limitations of use in meat products. Meat Sci. (2021) 176:108463. doi: 10.1016/j.meatsci.2021.108463
45. Alizadeh Behbahani B, Noshad M, Falah F. Cumin essential oil: phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microb Pathogenesis. (2019) 136:103716. doi: 10.1016/j.micpath.2019.103716
46. Umagiliyage AL, Becerra-Mora N, Kohli P, Fisher DJ, Choudhary R. Antimicrobial efficacy of liposomes containing d-limonene and its effect on the storage life of blueberries. Postharvest Biol Tec. (2017) 128:130–7. doi: 10.1016/j.postharvbio.2017.02.007
47. Singh A, Benjakul S. The combined effect of squid pen chitooligosaccharides and high voltage cold atmospheric plasma on the shelf-life extension of Asian sea bass slices stored at 4°C. Innov Food Sci Emerg. (2020) 64:102339. doi: 10.1016/j.ifset.2020.102339
48. Nisar T, Yang X, Alim A, Iqbal M, Wang Z-C, Guo Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int J Biol Macromol. (2019) 124:1156–66. doi: 10.1016/j.ijbiomac.2018.12.005
49. Myszka K, Olejnik A, Majcher M, Sobieszczańska N, Grygier A, Powierska-Czarny J, et al. Green pepper essential oil as a biopreservative agent for fish-based products: antimicrobial and antivirulence activities against Pseudomonas aeruginosa KM01. LWT. (2019) 108:6–13. doi: 10.1016/j.lwt.2019.03.047
50. Zhang Y, Li D, Lv J, Li Q, Kong C, Luo Y. Effect of cinnamon essential oil on bacterial diversity and shelf-life in vacuum-packaged common carp (Cyprinus carpio) during refrigerated storage. Int J Food Microbiol. (2017) 249:1–8. doi: 10.1016/j.ijfoodmicro.2016.10.008
51. Lee BH, Wu SC, Shen TL, Hsu YY, Chen CH, Hsu WH. The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna fish. Food Chem. (2021) 340:128104. doi: 10.1016/j.foodchem.2020.128104
52. Yang ZQ, Tao XY, Zhang H, Rao SQ, Gao L, Pan ZM, et al. Isolation and characterization of virulent phages infecting Shewanella baltica and Shewanella putrefaciens, and their application for biopreservation of chilled channel catfish (Ictalurus punctatus). Int J Food Microbiol. (2019) 292:107–17. doi: 10.1016/j.ijfoodmicro.2018.12.020
53. Yu Y, Yang S, Lin T, Qian Y, Xie J, Hu C. Effect of cold chain logistic interruptions on lipid oxidation and volatile organic compounds of salmon (Salmo salar) and their correlations with water dynamics. Front Nutr. (2020) 7:155. doi: 10.3389/fnut.2020.00155
54. Hao R, Liu Y, Sun L, Xia L, Jia H, Li Q, et al. Sodium alginate coating with plant extract affected microbial communities, biogenic amine formation and quality properties of abalone (Haliotis discus hannai Ino) during chill storage. LWT. (2017) 81:1–9. doi: 10.1016/j.lwt.2017.03.031
55. Li M, Tian L, Zhao G, Zhao Q, Gao X, Huang X, et al. Formation of biogenic amines and growth of spoilage-related microorganisms in pork stored under different packaging conditions applying PCA. Meat Sci. (2014) 96:843–8. doi: 10.1016/j.meatsci.2013.09.023
56. Mozaffarzogh M, Misaghi A, Shahbazi Y, Kamkar A. Evaluation of probiotic carboxymethyl cellulose-sodium caseinate films and their application in extending shelf life quality of fresh trout fillets. LWT. (2020) 126:109305. doi: 10.1016/j.lwt.2020.109305
57. Bekhit AEA, Holman BWB, Giteru SG, Hopkins DL. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: a review. Trends Food Sci Tech. (2021) 109:280–302. doi: 10.1016/j.tifs.2021.01.006
58. Vieira BB, Mafra JF, Bispo ASDR, Ferreira MA, Silva FDL, Rodrigues AVN, et al. Combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT. (2019) 116:108546. doi: 10.1016/j.lwt.2019.108546
59. Shokri S, Ehsani A. Efficacy of whey protein coating incorporated with lactoperoxidase and alpha-tocopherol in shelf life extension of Pike-Perch fillets during refrigeration. LWT. (2017) 85:225–31. doi: 10.1016/j.lwt.2017.07.026
60. Prabhakar PK, Vatsa S, Srivastav PP, Pathak SS. A comprehensive review on freshness of fish and assessment: analytical methods and recent innovations. Food Res Int. (2020) 133:109157. doi: 10.1016/j.foodres.2020.109157
61. Volpe MG, Siano F, Paolucci M, Sacco A, Sorrentino A, Malinconico M, et al. Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchusmykiss) fillets. LWT. (2015) 60:615–22. doi: 10.1016/j.lwt.2014.08.048
62. Fan W, Chi Y, Zhang S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. (2008) 108:148–53. doi: 10.1016/j.foodchem.2007.10.057
63. Shokri S, Parastouei K, Taghdir M, Abbaszadeh S. Application an edible active coating based on chitosan—Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4°C. Int J Biol Macromol. (2020) 153:846–54. doi: 10.1016/j.ijbiomac.2020.03.080
64. Dong Z, Luo C, Guo Y, Ahmed I, Pavase TR, Lv L, et al. Characterization of new active packaging based on PP/LDPE composite films containing attapulgite loaded with Allium sativum essence oil and its application for large yellow croaker (Pseudosciaena crocea) fillets. Food Packaging Shelf. (2019) 20:100320. doi: 10.1016/j.fpsl.2019.100320
65. Sarika AP, Lipton AP, Aishwarya MS. Biopreservative efficacy of bacteriocin GP1 of Lactobacillus rhamnosus GP1 on stored fish filets. Front Nutr. (2019) 6:29. doi: 10.3389/fnut.2019.00029
66. Jouki M, Yazdi FT, Mortazavi SA, Koocheki A, Khazaei N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int J Food Microbiol. (2014) 174:88–97. doi: 10.1016/j.ijfoodmicro.2014.01.001
67. Klnc B, Cakl S, Cadun A, Dncer T, Tolasa S. Comparison of effects of slurry ice and flake ice pretreatments on the quality of aquacultured sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) stored at 4°C. Food Chem. (2007) 104:1611–7. doi: 10.1016/j.foodchem.2007.03.002
68. Li Y, Jia S, Hong H, Zhang L, Zhuang S, Sun X, et al. Assessment of bacterial contributions to the biochemical changes of chill-stored blunt snout bream (Megalobrama amblycephala) fillets: protein degradation and volatile organic compounds accumulation. Food Microbiol. (2020) 91:103495. doi: 10.1016/j.fm.2020.103495
69. Zhou Q, Li P, Fang S, Mei J, Xie J. Preservative effects of gelatin active coating containing eugenol and higher CO2 concentration modified atmosphere packaging on Chinese sea bass (Lateolabrax maculatus) during superchilling (−0.9°C) storage. Molecules. (2020) 25:3390. doi: 10.3390/molecules25040871
70. Li T, Li J, Hu W. Changes in microbiological, physicochemical and muscle proteins of post mortem large yellow croaker (Pseudosciaena crocea). Food Control. (2013) 34:514–20. doi: 10.1016/j.foodcont.2013.05.028
71. Cao TL, Song KB. Development of bioactive Bombacaceae gum films containing cinnamon leaf essential oil and their application in packaging of fresh salmon fillets. LWT. (2020) 131:109647. doi: 10.1016/j.lwt.2020.109647
72. Shadman S, Hosseini SE, Langroudi HE, Shabani S. Evaluation of the effect of a sunflower oil-based nanoemulsion with Zataria multiflora Boiss. essential oil on the physicochemical properties of rainbow trout (Oncorhynchus mykiss) fillets during cold storage. LWT. (2017) 79:511–7. doi: 10.1016/j.lwt.2016.01.073
73. Ghasemi-Varnamkhastia M, Apetrei C, Lozano J, Anyog A. Potential use of electronic noses, electronic tongues and biosensors as multisensor systems for spoilage examination in foods. Trends Food Sci Technol. (2018) 80:71–92. doi: 10.1016/j.tifs.2018.07.018
74. Noori SMA, Khanzadi S, Fazlara A, Najafzadehvarzi H, Azizzadeh M. Effect of lactic acid and ajwain (Carum copticum) on the biogenic amines and quality of refrigerated common carp (Cyprinus carpio). LWT. (2018) 97:434–9. doi: 10.1016/j.lwt.2018.07.014
75. Rahmanzadeh Ishkeh S, Asghari M, Shirzad H, Alirezalu A, Ghasemi G. Lemon verbena (Lippia citrodora) essential oil effects on antioxidant capacity and phytochemical content of raspberry (Rubus ulmifolius subsp. sanctus). Sci Hortic. (2019) 248:297–304. doi: 10.1016/j.scienta.2018.12.040
76. Perumalla AVS, Hettiarachchy NS. Green tea and grape seed extracts-Potential applications in food safety and quality. Food Res Int. (2011) 44:827–39. doi: 10.1016/j.foodres.2011.01.022
77. Chaijan M, Benjakul S, Visessanguan W, Faustman C. Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem. (2006) 99:83–91. doi: 10.1016/j.foodchem.2005.07.022
78. Zhao X, Chen L, Wongmaneepratip W, He Y, Zhao L, Yang H. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. (2021) 354:129581. doi: 10.1016/j.foodchem.2021.129581
79. Carneiro CS, Mársico ET, Ribeiro ROR, Conte-Júnior CA, Mano SB, et al. Low-field nuclear magnetic resonance (LF NMR 1H) to assess the mobility of water during storage of salted fish (Sardinella brasiliensis). J Food Eng. (2016) 169:321–5. doi: 10.1016/j.jfoodeng.2015.09.010
80. Wang S, Xiang W, Fan H, Xie J, Qian YF. Study on the mobility of water and its correlation with the spoilage process of salmon (Salmo solar) stored at 0 and 4°C by low-field nuclear magnetic resonance (LF NMR 1H). J Food Sci Technol. (2018) 55:173–82. doi: 10.1007/s13197-017-2880-5
Keywords: active coating, essential oil, large yellow croaker, total volatile basic nitrogen, shelf-life extension
Citation: Li B, Wang X, Gao X, Ma X, Zhang L, Mei J and Xie J (2021) Shelf-Life Extension of Large Yellow Croaker (Larimichthys crocea) Using Active Coatings Containing Lemon Verbena (Lippa citriodora Kunth.) Essential Oil. Front. Nutr. 8:678643. doi: 10.3389/fnut.2021.678643
Received: 10 March 2021; Accepted: 25 June 2021;
Published: 20 July 2021.
Edited by:
Enrique Barrajón-Catalán, Miguel Hernández University of Elche, SpainReviewed by:
Tao Feng, Shanghai Institute of Technology, ChinaPatricio Román Santagapita, University of Buenos Aires, Argentina
Qinghui Ai, Ocean University of China, China
Copyright © 2021 Li, Wang, Gao, Ma, Zhang, Mei and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Mei, jmei@shou.edu.cn; Jing Xie, jxie@shou.edu.cn
 Bo Li1,2,3,4,5
Bo Li1,2,3,4,5  Leilei Zhang
Leilei Zhang Jun Mei
Jun Mei Jing Xie
Jing Xie