- Department of Plant Sciences, School of Life Sciences, University of Hyderabad, Hyderabad, India
Abscisic acid (ABA) is a stress hormone that accumulates under different abiotic and biotic stresses. A typical effect of ABA on leaves is to reduce transpirational water loss by closing stomata and parallelly defend against microbes by restricting their entry through stomatal pores. ABA can also promote the accumulation of polyamines, sphingolipids, and even proline. Stomatal closure by compounds other than ABA also helps plant defense against both abiotic and biotic stress factors. Further, ABA can interact with other hormones, such as methyl jasmonate (MJ) and salicylic acid (SA). Such cross-talk can be an additional factor in plant adaptations against environmental stresses and microbial pathogens. The present review highlights the recent progress in understanding ABA’s multifaceted role under stress conditions, particularly stomatal closure. We point out the importance of reactive oxygen species (ROS), reactive carbonyl species (RCS), nitric oxide (NO), and Ca2+ in guard cells as key signaling components during the ABA-mediated short-term plant defense reactions. The rise in ROS, RCS, NO, and intracellular Ca2+ triggered by ABA can promote additional events involved in long-term adaptive measures, including gene expression, accumulation of compatible solutes to protect the cell, hypersensitive response (HR), and programmed cell death (PCD). Several pathogens can counteract and try to reopen stomata. Similarly, pathogens attempt to trigger PCD of host tissue to their benefit. Yet, ABA-induced effects independent of stomatal closure can delay the pathogen spread and infection within leaves. Stomatal closure and other ABA influences can be among the early steps of defense and a crucial component of plants’ innate immunity response. Stomatal guard cells are quite sensitive to environmental stress and are considered good model systems for signal transduction studies. Further research on the ABA-induced stomatal closure mechanism can help us design strategies for plant/crop adaptations to stress.
Introduction: ABA and Plant Defense
Plants need to respond quickly to diverse stress conditions, as they cannot move away. Stress can be due to abiotic (e.g., drought, salinity, chilling, and high-temperature) or biotic factors (e.g., pathogens, insects, herbivores) (Zhu, 2016; Lamers et al., 2020). Plants developed various adaptation strategies to cope up with these situations. A typical example is the stomatal closure, limiting the water loss and restricting pathogen entry into the leaves (Melotto et al., 2008; Gudesblat et al., 2009a; Sussmilch and McAdam, 2017; Agurla et al., 2018a). Plants accumulate hormones [e.g., abscisic acid (ABA) or salicylic acid (SA) or methyl jasmonate (MJ)] under abiotic stress conditions and elicitors [e.g., flagellin 22 (flg22)] under pathogen attack. Among the hormones, ABA is involved in several abiotic and biotic stress conditions and is therefore considered an essential and versatile compound. In contrast, SA, MJ, and ethylene (ET) help in resistance against biotic stress. Under drought, salinity, or cold stress, ABA accumulation causes stomatal closure to conserve water while up-regulating genes to promote osmotic adjustment in leaves (Lim et al., 2015; Zhao et al., 2017; Niu et al., 2018). The enhanced ABA levels in plants mediate the cross-adaptation against drought and pathogens besides insect herbivores (Lee and Luan, 2012; Nguyen et al., 2016).
Several compounds other than ABA also accumulated in plants in response to different stresses (Table 1). These compounds can close stomata and, in many instances, improve plants’ resistance to pathogens. The plant hormones and elicitors can further regulate transcription factors and induce pathogenesis-related (PR) genes (Bielach et al., 2017; Breen et al., 2017). There can also be a cross-talk between the factors involved in abiotic and biotic stress signaling (Nejat and Mantri, 2017; Saijo and Loo, 2020). However, these compounds either require ABA for their action or interact with ABA to activate defense responses.
Table 1. A spectrum of compounds that accumulate in plant cells along with ABA during biotic/abiotic stress and can promote stomatal closure.
Since its discovery, studies on ABA (a sesquiterpene) and its role in plant processes were studied extensively. Plant processes such as seed dormancy, seed development, promotion of desiccation tolerance, abscission, and, most importantly, stomatal closure were all regulated by ABA (Lim et al., 2015). Further, ABA can be crucial in also non-stress conditions (Yoshida et al., 2019). The action of ABA was complemented by hormones, such as SA (Robert-Seilaniantz et al., 2011; Wang H.Q. et al., 2020). Similarly, some of the secondary messengers triggered by ABA can also participate in plants’ adaptation to abiotic and biotic stress. Examples are reactive oxygen species (ROS), nitric oxide (NO), and cytosolic free Ca2+ (León et al., 2014; Huang et al., 2019). Several compounds like polyamines (PAs), hydrogen sulfide (H2S), and brassinosteroids (BRs) promote drought tolerance by regulating ABA synthesis and vice versa (Jin et al., 2013; Ha et al., 2014; Adamipour et al., 2020).
Readers interested in ABA and its role in plants may refer to some recent reviews (Kumar et al., 2019; Chen K. et al., 2020; Gietler et al., 2020; McAdam and Sussmilch, 2020). Our review emphasizes the role of ABA’s stomatal closure as an adaptive measure against both abiotic and biotic stresses. We have also discussed other compounds that can improve plant adaptations, still involving ABA. The stomatal closure by ABA follows a typical scheme of signal transduction. The interactions of these signaling components with others to synergize the plant’s adaptation against pathogen attacks are described. To limit the length of our article, reviews were cited when available. In some instances, original articles were referred, due to their classic importance.
Increased ABA Levels Under Different Stress Conditions
When plants were exposed to water stress (drought), an increase in ABA was typical due to either synthesis or degradation of ABA or both (Ma et al., 2018; Chen K. et al., 2020; Gietler et al., 2020). The soil-water deficit could be perceived as a signal by roots to trigger ABA’s de novo synthesis (Jiang and Hartung, 2008; Fang and Xiong, 2015; Qi et al., 2018). The increase in ABA of roots in response to drought was correlated with an increase in foliar-ABA concentrations, suggesting drought-induced ABA played a significant role in controlling leaf water potential (Zegada-Lizarazu and Monti, 2019). ABA accumulated in roots was transported to trigger stomatal closure in leaves and limit transpirational water-loss (Haworth et al., 2018). An increase in ABA could also occur in response to temperature-stress (high or low) (Tao et al., 2016; Karimi, 2019) or a newly discovered small peptide, CLE25 (Takahashi et al., 2018). Sato et al. (2018) found that NCED3 could be the trigger to enhance ABA biosynthesis in Arabidopsis under drought stress. Under these conditions, increased ABA and stomatal closure could limit the water-loss and restrict pathogen entry (Wu et al., 2007; Alazem and Lin, 2015). This phenomenon was complemented with additional steps of ABA transport from roots to shoots, conversion of bound ABA into free form to mobilize ABA within leaf (Hewage et al., 2020; Xylogiannis et al., 2020).
An increase in endogenous levels of ABA was also observed when plants were infected with pathogens, for e.g., Phaseolus by Colletotrichum (Dunn et al., 1990), flax by Fusarium (Boba et al., 2020), and nced5 mutant of Arabidopsis by Alternaria (Fan et al., 2009). Similarly, the clonal variation of chestnut susceptibility or resistance to Fusarium was related to ABA levels under infection (Camisón et al., 2019). The exact relationship between endogenous ABA levels and disease susceptibility of plants appeared to be complex, as the relationship depended on the duration of infection, other stresses, and the type of pathogen (Asselbergh et al., 2008). During the early stages of pathogen infection, the increased ABA levels helped in resistance, while at later stages, high levels of ABA made the plants susceptible to pathogens (Maksimov, 2009). The differential effects of ABA on the modulation of pathogen sensitivity need to be examined further, particularly in relation to the predisposition of plant tissue. Readers interested in ABA accumulation mode may refer to relevant articles for further details (Maksimov, 2009; Finkelstein, 2013; Ali et al., 2020; Chen K. et al., 2020).
Stomatal Closure: A First Line of Defense Against Diverse Stress Conditions
Stomatal closure is one of the initial responses of plants to stress conditions to retain water status and provide innate immunity against pathogens (McLachlan et al., 2014; Arnaud and Hwang, 2015; Agurla et al., 2018a). The physical barriers on the plant’s outer surface, such as bark, cuticle, and cell wall, could protect against physical and biological factors. However, the microscopic pores on leaf surfaces called stomata are the accessible entrances to several microbes. Stomata form the gateways for transpiration, photosynthetic gas exchange as well as microbial entry into leaves. Stomatal guard cells are quite dynamic in sensing and responding to external microbial pathogens. Stomatal closure can be an essential strategy to defend against abiotic and biotic factors such as drought or pathogens (Lim et al., 2015; Melotto et al., 2017; Nejat and Mantri, 2017). Several instances of stomatal closure induced by plant pathogens are listed in Table 2. Stomatal closure was triggered by either elicitors or other compounds produced in the leaf in response to pathogens, such as SA, MJ, or PAs. Stomata can sense and respond to microbe-associated molecular patterns, including chitosan, flagellin, and harpin (Zhang L. et al., 2017; Klessig et al., 2018). The sensing of ABA or other compounds and the final response of stomatal closure follows a common signaling pathway involving receptors, protein kinases, secondary messengers, ion channels, ion efflux, and turgor loss in guard cells. Among kinases, OST1 is a primary activating factor NADPH oxidase and raises the ROS levels of guard cells.
Table 2. Several compounds that induce stomatal closure can also promote pathogen resistance of plants.
During ABA-induced stomatal closure, an increase in OST1 kinase was followed by the activation of RBOH D/F, and increases in ROS/NO/Ca2+ levels. In turn, Ca2+ dependent CDPKs activated slow anion channel 1 (SLAC1), S-type anion channel 3 (SLAH3) and K+out channels to promote ion efflux from guard cells and forced stomata to close. However, in presence of flg22 or yeast elicitor, the activity of OST1 did not increase (Montillet et al., 2013; Ye et al., 2015). Albeit in a resting stage, OST1 participated in stomatal closure by variety of signals including PAMPs (e.g., flg 22, yeast elicitor, chitosan) or environmental components, such as high CO2 or high humidity (Melotto et al., 2006; Ye et al., 2015, 2020b; Ye and Murata, 2016; Pantin and Blatt, 2018). Besides its action through ROS/NO/Ca2+, OST1 could directly modulate ion channels to cause stomatal closure (Figure 1). In a recent study, the events involving OST1/SnRK2s were studied in real-time using FRET sensors (Zhang et al., 2020). These experiments provided a visual evidence of the interaction of OST1 with signaling components of ABA and elevated CO2. It is obvious that OST1 is an important point of convergence of signals from abiotic and biotic factors. It would be interesting to assess the mechanism by which OST1 keeps up such dual mode of activation and modulating downstream components, all converging to mediate stomatal closure.
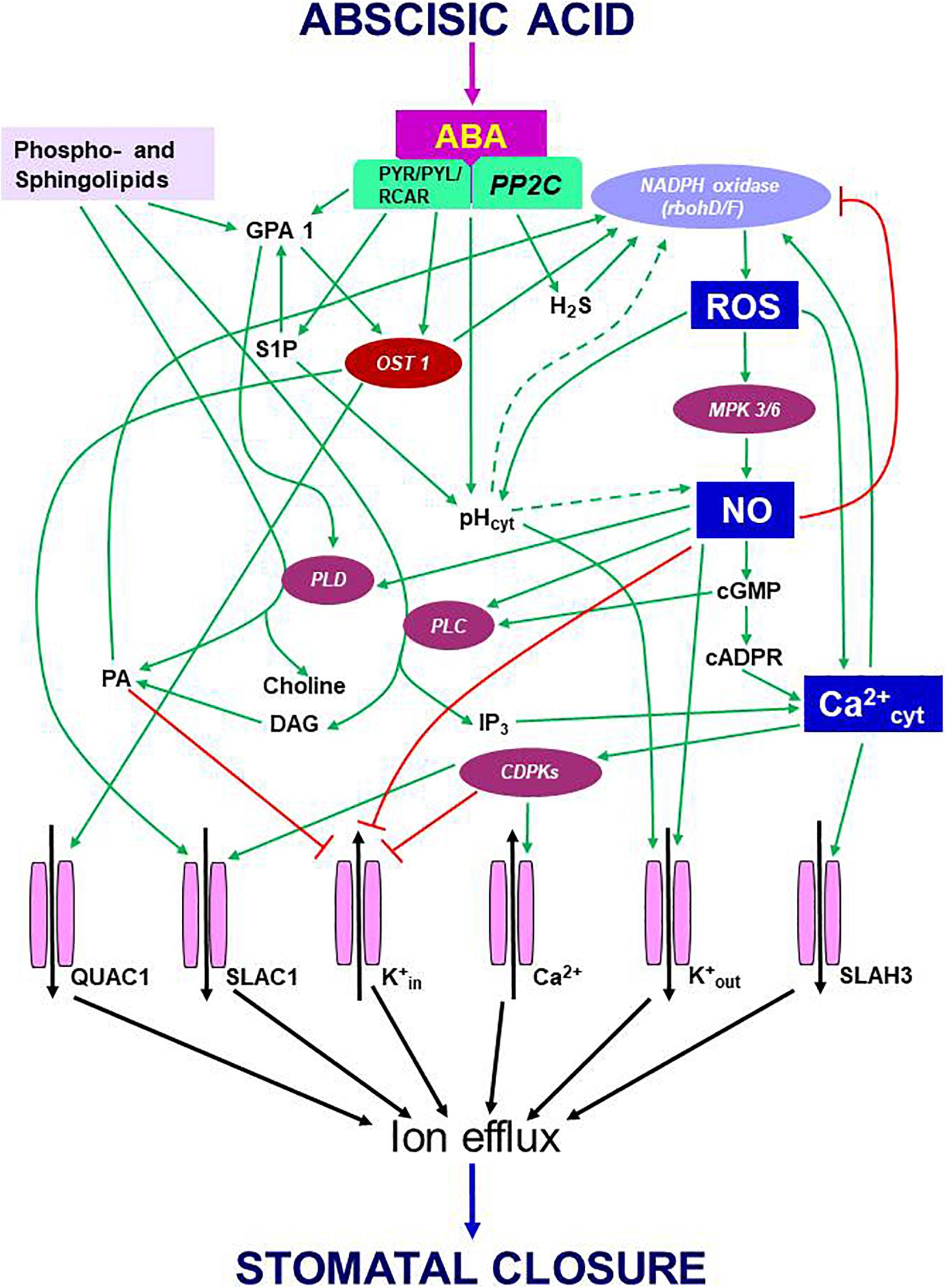
Figure 1. Schematic representation of eventsm during signal transduction pathway induced by ABA leading to stomatal closure. Binding of ABA to its receptor (PYR/PYL/RCAR) blocks the function of PP2C. As a result, OST1, which stays phosphorylated, activates multiple components like NADPH oxidase (to generate ROS) and anion channels [quick anion channel 1 (QUAC1) and slow anion channel 1 (SLAC1)] to trigger anion efflux. The secondary messengers: ROS, NO, and cytosolic Ca2+, exert multiple effects. The rise in cytosolic pH, another secondary messenger, appears to stimulate NADPH oxidase, but neither the origin nor the mode of pH action is understood. The high levels of ROS can promote NO production with the involvement of mitogen-activated protein kinases and elevate pH and Ca2+ in the cytosol. In turn, Ca2+ can activate RBOH-D/F and elevate ROS levels. The rise in NO downregulates K+ inward channels and elevates cytosolic Ca2+ levels through cyclic guanosine monophosphate (cGMP) and cyclic ADP ribose (cADPR). An increase in Ca2+ can activate calcium-dependent protein kinases to facilitate a further influx of Ca2+ from outside. Ca2+-activated calcium-dependent protein kinases stimulate SLAC1 and S-type anion channel 3 while inhibiting K+ influx through K+in channels. When present, NO activates two enzymes, phospholipase C and phospholipase D (PLD), resulting in the synthesis of inositol 1,4,5-triphosphate (IP3) phosphatidic acid. In turn, IP3 releases Ca2+ levels from internal stores of plant cells, while phosphatidic acid can stimulate NADPH oxidase and inhibit the inward K+ channel. ABA can stimulate the formation of sphingosine 1-phosphate (S1P) and phytosphingosine-1P, which activate PLD through G-protein α-subunit 1 (GPA1). NO can promote K+ efflux channels and cytosolic alkalization while inhibiting K+ influx channels via calcium-dependent protein kinases. These three secondary messengers involved in ABA signaling, namely ROS, NO, Ca2+, and their interactions, play a significant role in regulating stomatal closure. Ion channels are terminal points of signal transduction, causing the loss of turgor in guard cells and stomatal closure. Further details are described in the text. Arrows (→) indicate stimulation, and the symbol ⊣ represents inhibition. Abbreviations used here are listed in the Appendix.
There are reports indicating that stomatal closure can be induced by biotic and abiotic stresses in an “OST1-independent manner” (Hsu et al., 2018; Zheng et al., 2018). For e.g., plant elicitor peptides (Peps), a group of damage-associated molecular patterns, can trigger stomatal closure by activating SLAC1 and SLAH3 in an OST1-independent manner (Zheng et al., 2018). Similarly, elevated CO2 can bypass OST1 kinase and activate SLAC1 (Hsu et al., 2018). SLACs may be activated without the involvement of OST1 but other kinases. For e.g., the signaling events in guard cells can utilize MAPK cascade to up-regulate SLAC1/SLAH3 (Jagodzik et al., 2018). MAP kinases 3/6 participated upstream of NO during stomatal closure in darkness (Zhang T.Y. et al., 2017), while MPK 9/12 activated SLAC1 integrating with Ca2+/CDPK system during the cross-talk of ABA and SA (Prodhan et al., 2018), though the exact mechanism is not known. When exposed to a small elicitor peptide, AtPeps, a two-kinase component of BRASSINOSTEROID INSENSITIVE 1-associated receptor kinase 1 (BAK1)/BORTRYTIS-INDUCED KINASE 1 (BIK1) was turned on to activate SLAC1 and SLAH3 in guard cells (Zheng et al., 2018). However, further experiments are necessary to identify the exact components involved in the activation of SLAC1 by MPK 9/12 or BAK1/BIK1.
There can be additional components of a leaf that can provide resistance to microbes as well as environmental stresses, e.g., callose or silicon deposition (Ellinger and Voigt, 2014; Alhousari and Greger, 2018; Islam W. et al., 2020), cuticular waxes (Lewandowska et al., 2020) and trichomes (Fürstenberg-Hägg et al., 2013). ABA was involved in some of these responses. Cuticular wax biosynthesis was ABA-dependent and mediated by MYB94 and MYB96 transcription factors (Lewandowska et al., 2020). A light-induced increase in trichome density and thick leaves was due to high ABA levels in the leaves (Escobar-Bravo et al., 2018). Furthermore, ABA promoted callose synthesis and deposition by negatively regulating callose degrading pathogenesis-related protein 2 (PR2) (Oide et al., 2013). Similarly, ABA up-regulated callose deposition and antiviral RNA silencing mechanism to evade virus attack (Alazem and Lin, 2020).
Stomatal Closure by Compounds Other Than ABA and Their Interactions During Stomata Closure
Besides ABA, several other compounds increase when plants are exposed to stress, which close stomata and help plant defense responses. These compounds can be grouped into three categories: hormones, elicitors, and metabolites (Table 3). The hormones include MJ, SA, ET, and BRs. MJ was the most effective one and induced stomatal closure by elevating pH, ROS, NO, and Ca2+ leading to activation of anion channels, similar to ABA action (Munemasa et al., 2007; Gonugunta et al., 2009; Agurla and Raghavendra, 2016). Further studies in detail are needed to understand the effects of ET as well as BRs on closure.
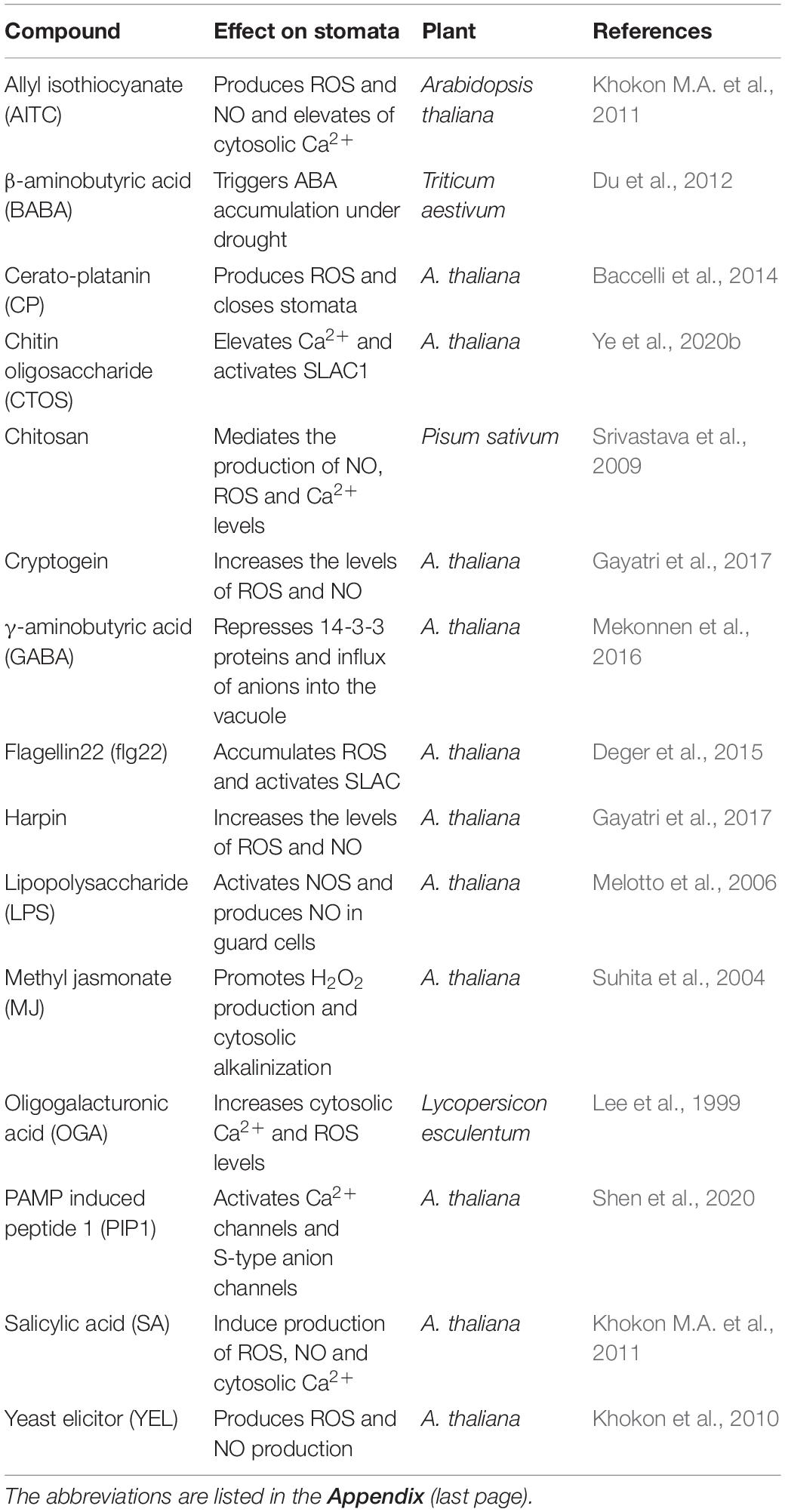
Table 3. A spectrum of hormones (other than ABA)/elicitors/PAMPs and other metabolites capable of inducing stomatal closure and the basis of their action.
Salicylic acid (SA) is considered a plant defense hormone with overlapping functions as an elicitor (Ding and Ding, 2020). SA-induced stomatal closure was mediated by ROS produced primarily through peroxidase (not NADPH oxidase, as in ABA). The other downstream events of NO production and ion channel modulation in guard cells were similar to ABA’s action (Hao et al., 2010; Khokon A.R. et al., 2011; Khokon et al., 2017; Wang et al., 2018). Thus, there is an overlapping of signaling pathways mediated by SA and ABA to cause stomatal closure in Arabidopsis. Several microbial elicitors (chitosan, flg22, harpin, and cryptogein) promoted stomatal closure and prevented pathogens’ entry. These elicitors produce NO and ROS via nitric oxide synthase (NOS) and NADPH oxidase, respectively (Klüsener et al., 2002; Melotto et al., 2006; Agurla and Raghavendra, 2016; Gayatri et al., 2017; Prodhan et al., 2020). The combined action of ROS and NO could be imparting pathogen resistance.
Allyl isothiocyanate (AITC), proline, and PAs are examples of metabolites that accumulate under stress. Despite accumulation in large quantities, proline, a compatible osmolyte, caused only a partial closure (Raghavendra and Reddy, 1987). PAs (including putrescine, spermidine, and spermine) accumulated during water stress and pathogen attack (Alcázar et al., 2010; Hatmi et al., 2018). The oxidation of PAs by polyamine oxidase raised ROS levels, followed by NO to cause stomatal closure similar to ABA (Agurla et al., 2018b). Similarly, AITC promoted stomatal closure and defense responses against biotic components (Khokon M.A. et al., 2011; Ye et al., 2020a).
The hormones, elicitors, and metabolites described above interact markedly with ABA and act in tandem to promote abiotic stress tolerance (Table 4). ABA’s interactions with SA or MJ to work together during stomatal closure and pathogen resistance are well-known (Koo et al., 2020; Wang J. et al., 2020). For e. g., MJ promoted ABA biosynthesis by inducing the AtNCED3 gene expression in Arabidopsis (Hossain et al., 2011). ABA was needed during SA-action on stomata (Wang et al., 2018). Conversely, elevated ABA triggered SA biosynthesis by activating SID2 and promoted stomatal closure (Prodhan et al., 2018). These reports confirm the synergy between MJ, SA, and ABA during stomatal closure.
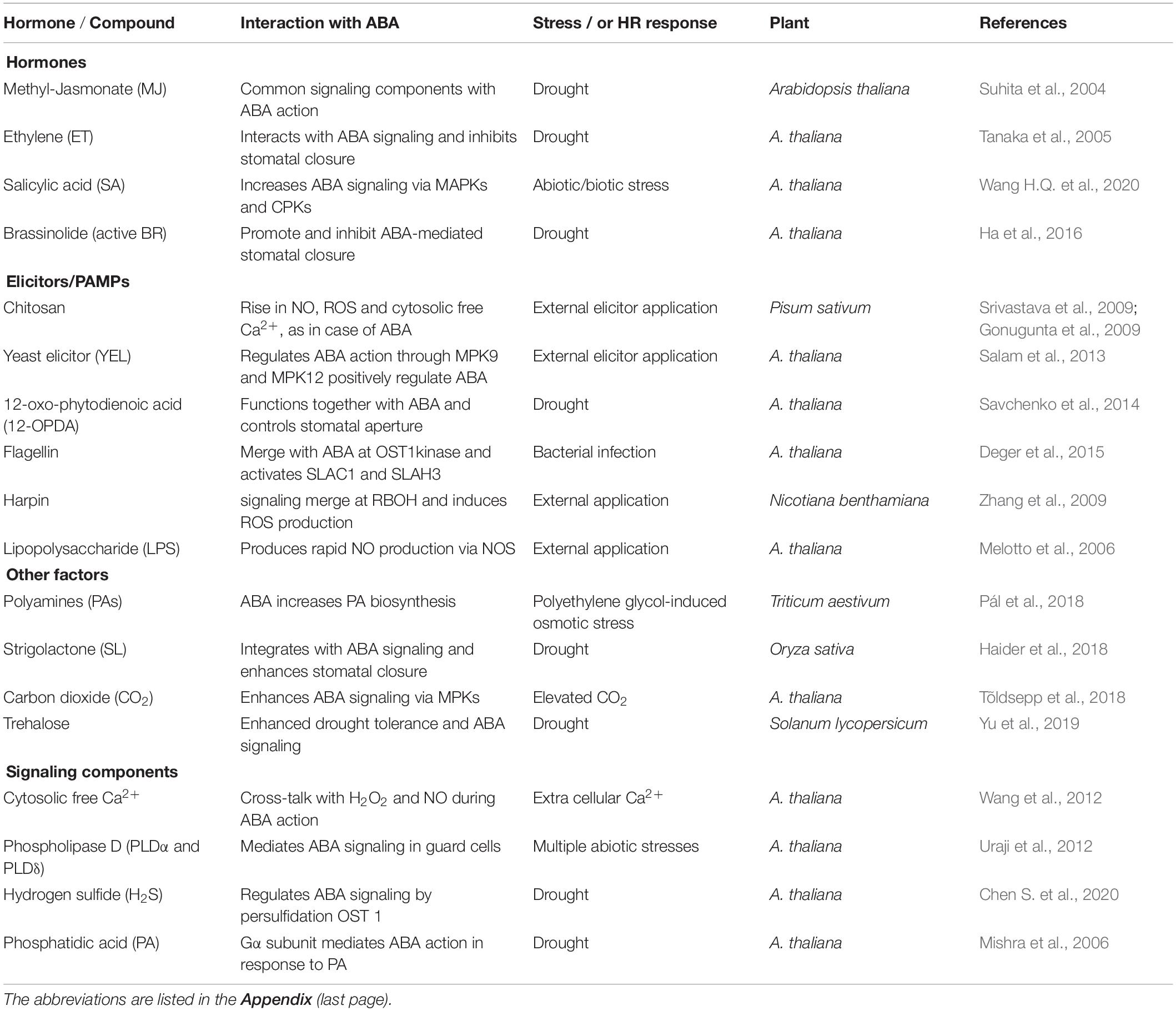
Table 4. Interaction of ABA with other compounds during the stress responses that induce stomatal closure.
An SA-receptor, NPR1, mediated chitosan signaling in guard cells (Prodhan et al., 2020). SA, chitosan, and ABA interacted during stomatal closure by activating MAP kinases (MPK9 and MPK12) (Salam et al., 2012; Khokon et al., 2017). Elevated levels of PAs stimulated biosynthesis of ABA (Yamasaki and Cohen, 2006; Alcázar et al., 2010). In turn, ABA stimulated oxidation of Pas to elevate H2O2 and NO, and stomatal closure (An et al., 2008; Konstantinos et al., 2010). Such interactions could fine-tune ABA’s effects to strengthen the plant defense reactions against both abiotic and biotic stresses. The direct role of PAs and proline in pathogen resistance is not clear.
Countermeasures by Pathogens
The stomatal closure by ABA cannot be a permanent strategy to prevent microbial entry, as pathogens, such as Puccinia, can enter leaves through places other than stomata (Mendgen et al., 1996; Solanki et al., 2019). Therefore, we do not mean to overemphasize the role of ABA-induced stomatal closure as the sole mode of adaptation. Also, stomata need to open subsequently to keep up the gas exchange and normal plant function. At the same time, microbial pathogens initiate counteractive measures to reopen stomata, by either effectors, (such as coronatine or fusicoccin, Schulze-Lefert and Robatzek, 2006; Gudesblat et al., 2009a; Melotto et al., 2017), locking the open-stomata (Prats et al., 2006) or even killing guard cells to prevent their closure (Ye et al., 2020b).
Some of the pathogens secrete a cocktail of cell wall digesting enzymes to facilitate the entry through the epidermis into the leaves (Mendgen et al., 1996). The pathogens can also restrict the biosynthesis/actions of ABA and related hormones (Zeng et al., 2010; Robert-Seilaniantz et al., 2011). Further, pathogens too can trigger programmed cell death (PCD) of host tissue facilitating the spread of infection (Hofius et al., 2017; Huysmans et al., 2017). Despite the counteractive efforts by pathogens, ABA can still contribute to plant defense. It is known that ABA could initiate multifaceted measures involving hypersensitive response (HR) and long-term adaptation on its own or by synergetic interaction with other hormones, such as SA or MJ, to ensure improved resistance (described below).
ABA-Interaction With Gasotransmitters
The role of gasotransmitters in stomatal regulation requires special mention. In addition to NO, two more gaseous signaling molecules (gasotransmitters), hydrogen sulfide (H2S), and carbon monoxide (CO) produced within plant cells are an integral part of ABA-dependent stomatal closure as well as other stress conditions. These three gasotransmitters interacted with ABA-signaling during drought (García-Mata and Lamattina, 2013; Yao et al., 2019; Gahir et al., 2020). Under abiotic stress, ABA could elevate the levels of NO as well as CO or H2S. For e.g., ABA activated heme oxygenase (HO), thereby increased CO levels and caused stomatal closure (Cao et al., 2007; Wang and Liao, 2016). In turn, NO elevated the levels of H2S by regulating H2S producing enzymes (L/D-cysteine desulfhydrases) (Kolupaev et al., 2019; Gahir et al., 2020). Similarly, CO promoted both NO and ROS synthesis, facilitating stomatal closure during abiotic stress (Song et al., 2008; He and He, 2014). Thus, a triangular interaction appears to be operating in guard cells. These interactions and synergistic actions need to be examined further. We, however, feel that among the three gasotransmitters, NO could be the significant signaling molecule. Like in the case of ROS, the production of NO can also be triggered by microbial pathogens to activate defense-related genes (e.g., phenylalanine ammonia-lyase and pathogenesis-related protein-1) that play a significant role in acquired pathogen resistance (Romero-Puertas et al., 2004; Ma and Berkowitz, 2016). NO produced in response to lipopolysaccharide contributed towards resistance against Pst DC3000 (Melotto et al., 2006). The upregulation of H2S production suggested a strong association between H2S and plant defense (Shi et al., 2015; Gahir et al., 2020). Further studies on these protective abilities of gasotransmitters to improve pathogen resistance could help achieve plants’ resilience.
Gasotransmitters exert their actions by mediating post-translational modifications (PTMs) such as S-nitrosylation, nitridation, and persulfidation of target proteins (Scuffi et al., 2016; Kolupaev et al., 2019; Gahir et al., 2020). These PTMs seem to exert different effects. Accumulation of H2S by ABA mediates the persulfidation of SnRK2.6 to promote stomatal closure by ABA (Chen S. et al., 2020). S-nitrosylation, mediated by NO, inhibited OST1/SnRK2.6 kinase activity and limited stomatal closure (Fancy et al., 2017). Detailed experiments on such contrasting effects of NO and H2S would unravel the mechanism of interaction between gasotransmitters and ABA during stomatal closure and plant defense against pathogens.
Signaling Components in Guard Cells Triggered by ABA: Role in Stomatal Closure and Pathogen Resistance
Stomatal closure is the result of turgor loss in guard cells because of increased cation/anion efflux. A well-defined transduction pathway mediates the events during stomatal closure by ABA or other compounds, as illustrated in Figure 1. Binding of ABA to its receptor inactivates protein phosphatase 2C resulting in the activation of OST1 kinase, which stimulates NADPH oxidase (due to phosphorylation) enzyme to generate ROS and then the production of NO. Both ROS and NO can elevate levels of cytosolic Ca2+. The high levels of ROS, NO, and Ca2+ act either directly or together to activate anion/cation efflux channels while inhibiting the influx channels. The final result is the loss of cations/anions from guard cells, resulting in turgor loss and stomatal closure (Agurla et al., 2018a). These three secondary messengers (ROS, NO and Ca2+) can also stimulate the production of other signaling components such as phospholipase C, phospholipase D, phosphatidic acid, and inositol 1,4,5-triphosphate besides raising cytosolic pH, all contributing to stomatal closure. Apart from well-known NO, other gasotransmitters, i.e., CO and H2S, are also involved in ABA-induced stomatal closure.
In recent years, another signaling component, reactive carbonyl species (RCS) was found to play a significant role in stomatal closure. These RCS are products of lipid oxidation, produced and scavenged during various developmental processes, including PCD (Biswas and Mano, 2015). Montillet et al. (2013) suggested that RCS (also called oxylipins) played a dominant role during stomatal closure by biotic factors (e.g., elicitors from pathogens), compared to ROS during the action of ABA (typical of abiotic stress factor). Soon, detailed reports appeared that RCS could function downstream of ROS production during closure by ABA and MJ in Nicotiana tabacum and Arabidopsis thaliana (Islam et al., 2016, 2019; Islam M.M. et al., 2020). Recently, RCS was found to activate CPK6, promote the elevation of Ca2+ and activate SLAC1, leading to stomatal closure (Islam M.M. et al., 2020). These observations imply that RCS and ABA could enable guard cells to respond to both biotic and abiotic stress conditions. Several authors had reviewed the details of the ABA-induced signal transduction pathway (Raghavendra et al., 2010; Munemasa et al., 2015; Sierla et al., 2016; Agurla et al., 2018a; Kolbert et al., 2019; Saito and Uozumi, 2019; Sun et al., 2019).
Several of the signaling components during ABA-induced stomatal closure can protect against pathogens (Table 2). The three major secondary messengers, triggered by ABA (namely ROS, NO, and Ca2+) can initiate defense processes such as stomatal closure and PCD (Sewelam et al., 2016; Suzuki and Katano, 2018). ABA-induced NO can act as a signaling molecule to initiate adaptive responses against abiotic (UV, drought, and salinity) or biotic factors (pathogens or elicitors). The reaction products of ROS and NO (like peroxynitrite) and NO-mediated post-translational modifications can all act together to initiate defense responses (Bellin et al., 2013; León et al., 2014; Arnaud and Hwang, 2015). The rise in cytosolic Ca2+ was often required to induce HR as a plant immunity response (e.g., against microbial pathogens). Other compounds involved in ABA signaling, like phospholipase D and phosphatidic acid, were also associated with plants’ defense against pathogens (Li and Wang, 2019; Moeder et al., 2019). The ability of H2S, a gasotransmitter, to impart resistance against typical plant pathogen (Pseudomonas syringae) suggests a link between stomatal closure and adaptation to plant pathogens (Shi et al., 2015; Gahir et al., 2020). The promotion or inhibition of ROS and NO production by gasotransmitters can be a significant factor during plant defense.
Arabidopsis thaliana had been an excellent model to study and validate the components/mechanisms of plant function. Several mutants of A. thaliana were employed to establish ABA’s signaling components (Table 5). These mutants fall under three groups: those with altered ABA biosynthesis/reception or deficient in signaling compounds or those with altered stomatal response independent of ABA. The mutants who cannot close their stomata also lose their ability to resist pathogens, becoming hypersensitive to pathogens. These observations emphasize the strong association of stomatal closure by ABA or related compounds with altered pathogen resistance.
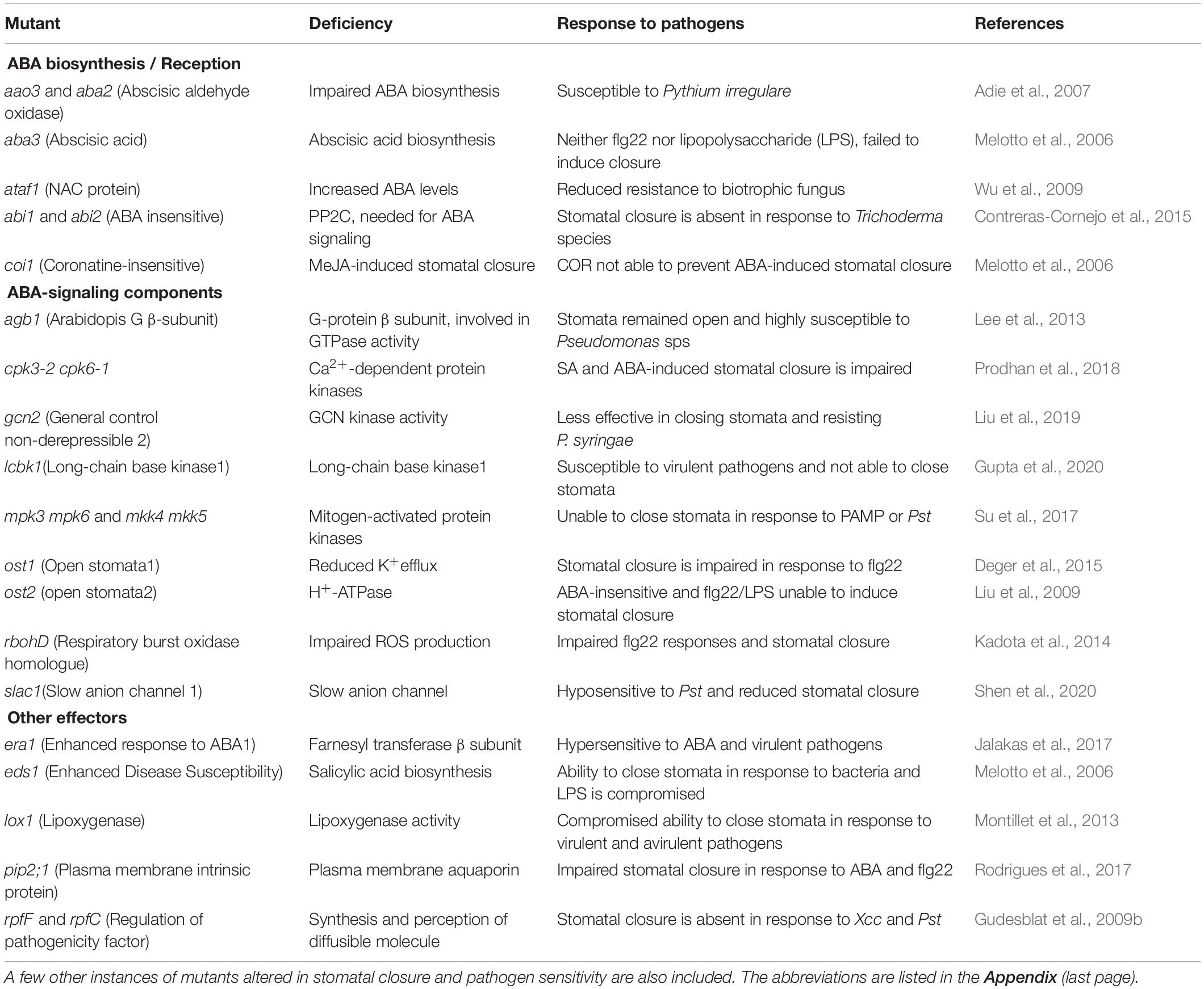
Table 5. List of Arabidopsis mutants deficient in ABA biosynthesis/signaling pathway and their susceptibility to pathogen attack.
Other Points to Be Considered
Evidence is emerging that ABA may not always impart resistance but increase plants’ susceptibility to abiotic or biotic factors by compromising defense responses (Gietler et al., 2020). For e.g., ABA can act differently depending on the pathogen status: pre-entry or post-entry phases. During the early stages, induction of stomatal closure along with stimulation of wax and callose synthesis could reinforce the plant’s defense. However, in the post-invasion stage, ABA can be antagonistic and increase susceptibility to microbes (Alazem and Lin, 2015; Arnaud and Hwang, 2015). Accumulation of ABA at infection site repressed events involving disease resistance against Cercospora in beetroot (Schmidt et al., 2008). Elevated ABA levels promoted sugar transport to fungi and enhanced the infection of wheat by Puccinia striiformis sp. tritici (pst) (Huai et al., 2019). Furthermore, increased ABA levels antagonized plant’s defense responses by suppressing SA or MJ induced defense gene expression, callose deposition, and basal resistance against Fusarium oxysporum or Magnaporthe grisea (Anderson et al., 2004; Jiang et al., 2010; Lim et al., 2015; Ulferts et al., 2015).
We believe that ABA could play a significant role in restricting or at least delaying the pathogen entry and subsequent infections, at least in the case of bacteria (Table 5 and description below). We acknowledge that the role of stomata should not be generalized, as the experiments done on pathogen infection with Arabidopsis (non-host) may not be all applicable with typical host species, such as wheat or barley.
Subsequent Effects of ABA Besides Stomatal Closure Towards Adaptive Responses: Pre-Entry and Post-Entry Phenomena
Pathogen resistance cannot be entirely due to stomatal closure, and the action of ABA needs to continue beyond stomata. Often during infection, an increase in ABA levels led to multiple events that help against abiotic stress and disease resistance (Shafiei et al., 2007). Even if pathogens manage to enter the intercellular spaces, elevated ABA can initiate a spectrum of events that restrict the multiplication and spread of plant pathogens inside leaves. For e.g., ABA can raise the levels of ROS, NO, and Ca2+ (Wendehenne et al., 2004; Moeder et al., 2019; Sadhu et al., 2019). An important consequence of elevated ROS, NO, and Ca2+ is HR response, leading to PCD in several crop species while protecting against pathogens. The HR may also include callose deposition in cell walls, increased cuticular biosynthesis (Luna et al., 2011; Lewandowska et al., 2020), and blocking of plasmodesmata (Huysmans et al., 2017). Further, the modulation by ABA of miRNA can restrict viral replication and the viral movement due to blocked plasmodesmata, thus causing antiviral silencing (Staiger et al., 2013; Alazem and Lin, 2017).
Other ABA-promoted events include the activation of genes involved in either accumulation of compatible solutes for osmotic adaptation or PCD to restrict the spread of pathogens within leaves or enhanced secondary metabolites production (Figure 2). For example, ABRE-binding proteins (AREBs) and ABRE-binding factors (ABFs) up-regulate stress-responsive genes, involved in short-term and long term adaptations to abiotic stresses, including drought, cold, and heat (Verma et al., 2016; Vishwakarma et al., 2017). The accumulation of proline on exposure to water stress or ABA (Stewart, 1980; Planchet et al., 2014) can serve the dual purpose of providing compatible solute for osmotic adjustments in leaves and causing partial stomatal closure in the epidermis (Raghavendra and Reddy, 1987). Elevated proline levels due to ABA can further offer the plant defense against pathogens (Qamar et al., 2015; Christgen and Becker, 2019). There have been claims that proline accumulation is not all ABA-dependent (Savouré et al., 1997). It is not clear if ABA is the master regulator of proline accumulation or a consequence of stomatal closure. ABA and proline’s combined action can be beneficial under hypoxic stress (Cao et al., 2020). Similarly, ABA, proline, and PAs can help together during plant adaptation to osmotic stress (Pál et al., 2018).
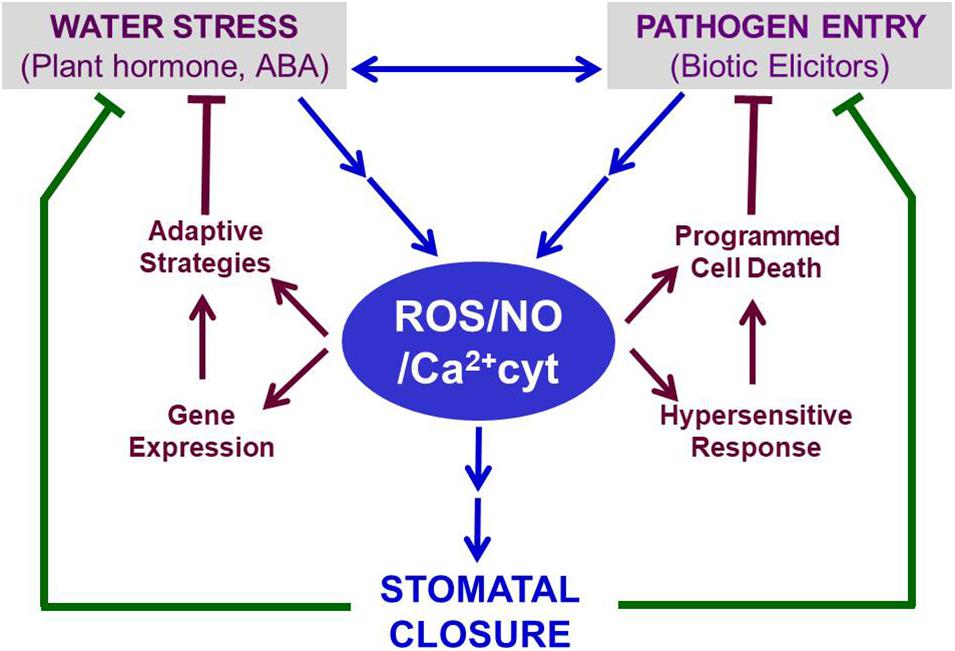
Figure 2. Stomatal closure induced under conditions of abiotic (e.g., drought) or biotic (e.g., pathogens) stress serves as a common defense mechanism. In guard cells, ABA typically raises the levels of ROS, NO, and Ca2+. These three secondary messengers bring out stomatal closure through a series of signaling events (as illustrated in Figure 1). The retention of water within leaves, when stomata are closed, helps to relieve water stress. In parallel, the closed stomata restrict microbial pathogens’ entry into leaves. The trio of ROS, NO, and Ca2+ parallelly induce adaptive events to mitigate water stress and limit pathogen spread by triggering HR and PCD. Thus, ROS, NO, and Ca2+ can be considered vital regulators, participating in ABA-induced defense against abiotic and biotic stress. Further details are described in the text. Abbreviations used here are listed in the Appendix.
Several ABA responses are beneficial during biotic stress, as well. ABA and SA promoted the accumulation of anti-microbial flavan-3-ols, enhancing the plant defense against rust infection in two popular trees, Poplar and Malus (Lu et al., 2017; Ullah et al., 2019). Similarly, the ABA-activated MYC2 transcription factor was essential for defense against Meloidogyne incognita (Xu et al., 2019). PCD is a controlled process and is a consequence of high ROS and NO levels typically up-regulated by ABA (Petrov et al., 2015; You and Chan, 2015). In plants, PCD is often an adaptive response during abiotic/biotic stress (Fagundes et al., 2015; Burke et al., 2020). However, PCD can also aggravate plant disease (Huysmans et al., 2017). ABA was involved in the induction of cell death around the wounded site (Cui et al., 2013). Further experiments are needed to establish if the process of PCD is incidentally associated with ABA or if ABA is the causal factor.
Enhanced production of secondary metabolites is another defense mechanism of plants against stress (Kumar and Sharma, 2018; Khare et al., 2020). Abscisic acid itself is a secondary metabolite produced during stress and can play a significant role in secondary metabolite production when plants encounter abiotic or biotic stress factors (Murcia et al., 2017). ABA-induced increase in flavonoids and other metabolites served as a defensive measure against UV-B radiation (Mazid et al., 2011). Trichoderma harzianum infection increased ABA levels, which helped in osmotic adaptation under drought by restricting water loss and increasing osmolytes, like proline (Mona et al., 2017). Such interactions between ABA and secondary metabolite production are quite exciting and need to be examined in detail.
Conclusion
Based on the extensive literature available, we tried to emphasize the role of ABA-induced stomatal closure as an essential component of plant defense against both limited water and pathogens (Figure 2). ABA’s role is complemented further by the cross-talk and interaction of ABA with other hormones, microbial elicitors, and metabolites. We, therefore, emphasize ABA can play either a direct role or an indirect role as well. The stomatal closure by ABA can be considered a quick short-term response. However, the three vital secondary messengers involved in ABA-signaling, namely ROS, NO, and cytosolic free Ca2+, can promote events, such as osmolyte accumulation, up-regulation of adaptive genes, HR and PCD. These events facilitate the long-term adaptation of plants against abiotic stress as well as pathogens. ABA’s ability to induce stomatal closure may not always be due to changes occurring in guard cells’ secondary messengers. For e.g., chitosan, a microbial elicitor, can cause the death of guard cells (Ye et al., 2020b), likely to make them non-functional. This aspect is thought-provoking and needs to be examined further using ABA or other hormones, such as SA or MJ.
There are emerging areas that are related to the role of ABA and stomatal closure in plant defense. ABA’s ability to induce priming could help plants tolerate heat or drought stress occurring later (Zhang et al., 2019; Wang X. et al., 2020). Similarly, plants could have a transcriptional memory of ABA and MJ that can be useful for long-term adaptations (Avramova, 2019). Because of the well-documented importance, there had been recurring attempts to discover ABA-analogs or ABA-agonists. Stomatal closure and signaling components in guard cells can be excellent model systems to monitor such compounds. A few ABA-analogs were found, which mimic ABA to induce stomatal closure (Puli and Raghavendra, 2012; Vaidya et al., 2019). An extension of such work would open up an exciting possibility of exploiting ABA-analogs to improve plants/crops’ water-use efficiency. There were reports that ABA and or stomatal closure may not be crucial, particularly during fungal pathogen infection of crops, e.g., barley and wheat. A few experiments on mutants of such crop plants deficient in ABA or ability to close stomata may help us clarify the exact situation. Plants are indeed known to employ more than one strategy to overcome stress conditions or to optimize metabolism. ABA-induced stomatal closure is one of the approaches, while plants/pathogens could evoke other strategies, as well.
Author Contributions
AR conceived the idea and developed the outline. All authors reviewed the literature and wrote the article. AR edited the final draft of the review. All authors approved the final version.
Funding
Our work on stomatal guard cell was supported by grants (to ASR) of UGC (India-Israel) Joint Research Project [No. 6-4/2017 (IC)] and Council of Scientific and Industrial Research [No. 38 (1404)/15/EMR-II]. SG was supported by a Junior Research Fellowship from University Grant Commission, New Delhi. PB was supported partially by a BBL fellowship (UoH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ABA, Abscisic acid; BRs, Brassinosteroids; CO, Carbon monoxide; ET, Ethylene; Flg22, Flagelling 22; H2S, hydrogen sulfide; HR, Hypersensitive response; MJ, Methyl jasmonate; MPK, Mitogen activated protein kinase; NO, Nitric oxide; OST1, Open stomata 1; PAs, Polyamines; PCD, Programmed cell death; RCS, Reactive carbonyl species; ROS, Reactive oxygen species; SA, Salicylic acid; SLAC1, Slow anion channel 1; SLAH3, S-type anion channel 3.
References
Adamipour, N., Khosh-Khui, M., Salehi, H., Razi, H., Karami, A., and Moghadam, A. (2020). Role of genes and metabolites involved in polyamines synthesis pathways and nitric oxide synthase in stomatal closure on Rosa damascena Mill. under drought stress. Plant Physiol. Biochem. 148, 53–61. doi: 10.1016/j.plaphy.2019.12.033
Adie, B. A., Pérez-Pérez, J., Pérez-Pérez, M. M., Godoy, M., Sánchez-Serrano, J. J., Schmelz, E. A., et al. (2007). ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19, 1665–1681. doi: 10.1105/tpc.106.048041
Agurla, S., Gahir, S., Munemasa, S., Murata, Y., and Raghavendra, A. S. (2018a). Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 1081, 215–232. doi: 10.1007/978-981-13-1244-1_12
Agurla, S., Gayatri, G., and Raghavendra, A. S. (2018b). Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 255, 153–162. doi: 10.1007/s00709-017-1139-3
Agurla, S., and Raghavendra, A. S. (2016). Convergence and divergence of signaling events in guard cells during stomatal closure by plant hormones or microbial elicitors. Front. Plant Sci. 7:1332. doi: 10.3389/fpls.2016.01332
Alazem, M., and Lin, N. S. (2015). Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 16, 529–540. doi: 10.1111/mpp.12204
Alazem, M., and Lin, N. S. (2017). Antiviral roles of abscisic acid in plants. Front. Plant Sci. 8:1760. doi: 10.3389/fpls.2017.01760
Alazem, M., and Lin, N. S. (2020). Interplay between ABA signaling and RNA silencing in plant viral resistance. Curr. Opin. Virol. 42, 1–7. doi: 10.1016/j.coviro.2020.02.002
Alcázar, R., Altabella, T., Marco, F., Bortolotti, C., Reymond, M., Koncz, C., et al. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. doi: 10.1007/s00425-010-1130-0
Alhousari, F., and Greger, M. (2018). Silicon and mechanisms of plant resistance to insect pests. Plants 7, 33. doi: 10.3390/plants7020033
Ali, A., Pardo, J. M., and Yun, D. J. (2020). Desensitization of ABA-signaling: the swing from activation to degradation. Front. Plant Sci. 11:379. doi: 10.3389/fpls.2020.00379
An, Z., Jing, W., Liu, Y., and Zhang, W. (2008). Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 59, 815–825. doi: 10.1093/jxb/erm370
Anderson, J. P., Badruzsaufari, E., Schenk, P. M., Manners, J. M., Desmond, O. J., Ehlert, C., et al. (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16, 3460–3479. doi: 10.1105/tpc.104.025833
Armendariz, A. L., Talano, M. A., Villasuso, A. L., Travaglia, C., Racagni, G. E., Reinoso, H., et al. (2016). Arsenic stress induces changes in lipid signalling and evokes the stomata closure in soybean. Plant Physiol. Biochem. 103, 45–52. doi: 10.1016/j.plaphy.2016.02.041
Arnaud, D., and Hwang, I. (2015). A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol. Plant. 8, 566–581. doi: 10.1016/j.molp.2014.10.012
Asselbergh, B., De Vleesschauwer, D., and Höfte, M. (2008). Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol. Plant Microbe Interact. 21, 709–719. doi: 10.1094/MPMI-21-6-0709
Avramova, Z. (2019). Defence-related priming and responses to recurring drought: two manifestations of plant transcriptional memory mediated by the ABA and JA signalling pathways. Plant Cell Environ. 42, 983–997. doi: 10.1111/pce.13458
Baccelli, I., Lombardi, L., Luti, S., Bernardi, R., Picciarelli, P., Scala, A., et al. (2014). Cerato-platanin induces resistance in Arabidopsis leaves through stomatal perception, overexpression of salicylic acid- and ethylene-signalling genes and camalexin biosynthesis. PLoS One 9:e100959. doi: 10.1371/journal.pone.0100959
Bellin, D., Asai, S., Delledonne, M., and Yoshioka, H. (2013). Nitric oxide as a mediator for defense responses. Mol. Plant Microbe Interact. 26, 271–277. doi: 10.1094/MPMI-09-12-0214-CR
Bielach, A., Hrtyan, M., and Tognetti, V. B. (2017). Plants under stress: involvement of auxin and cytokinin. Int. J. Mol. Sci. 18:1427. doi: 10.3390/ijms18071427
Biswas, M. S., and Mano, J. (2015). Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 168, 885–898. doi: 10.1104/pp.115.256834
Boba, A., Kostyn, K., Kozak, B., Wojtasik, W., Preisner, M., Prescha, A., et al. (2020). Fusarium oxysporum infection activates the plastidial branch of the terpenoid biosynthesis pathway in flax, leading to increased ABA synthesis. Planta 251:50. doi: 10.1007/s00425-020-03339-9
Breen, S., Williams, S. J., Outram, M., Kobe, B., and Solomon, P. S. (2017). Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 22, 871–879. doi: 10.1016/j.tplants.2017.06.013
Burke, R., Schwarze, J., Sherwood, O. L., Jnaid, Y., McCabe, P. F., and Kacprzyk, J. (2020). Stressed to death: The role of transcription factors in plant programmed cell death induced by abiotic and biotic stimuli. Front. Plant Sci. 11:1235. doi: 10.3389/fpls.2020.01235
Camisón, Á, Martín, M. Á, Sánchez-Bel, P., Flors, V., Alcaide, F., Morcuende, D., et al. (2019). Hormone and secondary metabolite profiling in chestnut during susceptible and resistant interactions with Phytophthora cinnamomi. J. Plant Physiol. 241:153030. doi: 10.1016/j.jplph.2019.153030
Cao, X., Wu, L., Wu, M., Zhu, C., Jin, Q., and Zhang, J. (2020). Abscisic acid mediated proline biosynthesis and antioxidant ability in roots of two different rice genotypes under hypoxic stress. BMC Plant Biol. 20:198. doi: 10.1186/s12870-020-02414-3
Cao, Z. Y., Huang, B. K., Wang, Q. Y., Xuan, W., Ling, T. F., and Zhang, B. (2007). Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin. Sci. Bull. 52, 2365–2373. doi: 10.1007/s11434-007-0358-y
Chen, K., Li, G. J., Bressan, R. A., Song, C. P., Zhu, J. K., and Zhao, Y. (2020). Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 62, 25–54. doi: 10.1111/jipb.12899
Chen, S., Jia, H., Wang, X., Shi, C., Wang, X., Ma, P., et al. (2020). Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant. 13, 732–744. doi: 10.1016/j.molp.2020.01.004
Christgen, S. L., and Becker, D. F. (2019). Role of proline in pathogen and host interactions. Antioxid. Redox Signal. 30, 683–709. doi: 10.1089/ars.2017.7335
Contreras-Cornejo, H. A., Macías-Rodríguez, L., Vergara, A. G., and López-Bucio, J. (2015). Trichoderma modulates stomatal aperture and leaf transpiration through an abscisic acid-dependent mechanism in Arabidopsis. J. Plant Growth Regul. 34, 425–432. doi: 10.1007/s00344-014-9471-8
Cui, F., Brosché, M., Sipari, N., Tang, S., and Overmyer, K. (2013). Regulation of ABA dependent wound induced spreading cell death by MYB108. New Phytol. 200, 634–640. doi: 10.1111/nph.12456
Cummins, W. R., Kende, H., and Raschke, K. (1971). Specificity and reversibility of the rapid stomatal response to abscisic acid. Planta 99, 347–351. doi: 10.1007/BF00385826
Deger, A. G., Scherzer, S., Nuhkat, M., Kedzierska, J., Kollist, H., Brosché, M., et al. (2015). Guard cell SLAC1−type anion channels mediate flagellin−induced stomatal closure. New Phytol. 208, 162–173. doi: 10.1111/nph.13435
Desikan, R., Last, K., Harrett-Williams, R., Tagliavia, C., Harter, K., Hooley, R., et al. (2006). Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 47, 907–916.
Ding, P., and Ding, Y. (2020). Stories of salicylic acid: a plant defense hormone. Trends Plant Sci. 25, 549–565. doi: 10.1016/j.tplants.2020.01.004
Du, Y. L., Wang, Z. Y., Fan, J. W., Turner, N. C., Wang, T., and Li, F. M. (2012). β-Aminobutyric acid increases abscisic acid accumulation and desiccation tolerance and decreases water use but fails to improve grain yield in two spring wheat cultivars under soil drying. J. Exp. Bot. 63, 4849–4860. doi: 10.1093/jxb/ers164
Dunn, R. M., Hedden, P., and Bailey, J. A. (1990). A physiologically-induced resistance of Phaseolus vulgaris to a compatible race of Colletotrichum lindemuthianum is associated with increases in ABA content. Physiol. Mol. Plant Pathol. 36, 339–349. doi: 10.1016/0885-5765(90)90063-4
Ellinger, D., and Voigt, C. A. (2014). Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann. Bot. 114, 1349–1358. doi: 10.1093/aob/mcu120
Escobar-Bravo, R., Ruijgrok, J., Kim, H. K., Grosser, K., Van Dam, N. M., Klinkhamer, P., et al. (2018). Light intensity-mediated induction of trichome-associated allelochemicals increases resistance against thrips in tomato. Plant Cell Physiol. 59, 2462–2475. doi: 10.1093/pcp/pcy166
Fagundes, D., Bohn, B., Cabreira, C., Leipelt, F., Dias, N., Bodanese-Zanettini, M. H., et al. (2015). Caspases in plants: metacaspase gene family in plant stress responses. Funct. Integr. Genomics 15, 639–649. doi: 10.1007/s10142-015-0459-7
Fan, J., Hill, L., Crooks, C., Doerner, P., and Lamb, C. (2009). Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 150, 1750–1761. doi: 10.1104/pp.109.137943
Fancy, N. N., Bahlmann, A. K., and Loake, G. J. (2017). Nitric oxide function in plant abiotic stress. Plant Cell Environ. 40, 462–472. doi: 10.1111/pce.12707
Fang, Y., and Xiong, L. (2015). General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 72, 673–689. doi: 10.1007/s00018-014-1767-0
Finkelstein, R. (2013). Abscisic acid synthesis and response. Arabidopsis Book 11:e0166. doi: 10.1199/tab.0166
Förster, S., Schmidt, L. K., Kopic, E., Anschütz, U., Huang, S., Schlücking, K., et al. (2019). Wounding-induced stomatal closure requires jasmonate-mediated activation of GORK K+ channels by a Ca2+ sensor-kinase CBL1-CIPK5 complex. Dev. Cell 48, 87–99. doi: 10.1016/j.devcel.2018.11.014
Fürstenberg-Hägg, J., Zagrobelny, M., and Bak, S. (2013). Plant defense against insect herbivores. Int. J. Mol. Sci. 14, 10242–10297. doi: 10.3390/ijms140510242
Gahir, S., Bharath, P., and Raghavendra, A. S. (2020). The role of gasotransmitters in movement of stomata: mechanisms of action and importance for plant immunity. Biol. Plant. 64, 623–632. doi: 10.32615/bp.2020.071
García-Mata, C., and Lamattina, L. (2013). Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci. 201-202, 66–73. doi: 10.1016/j.plantsci.2012.11.007
Gayatri, G., Agurla, S., Kuchitsu, K., Anil, K., Podile, A. R., and Raghavendra, A. S. (2017). Stomatal closure and rise in ROS/NO of Arabidopsis guard cells by Tobacco microbial elicitors: Cryptogein and Harpin. Front. Plant Sci. 8:1096. doi: 10.3389/fpls.2017.01096
Gietler, M., Fidler, J., Labudda, M., and Nykiel, M. (2020). Abscisic acid-enemy or savior in the response of cereals to abiotic and biotic stresses? Int. J. Mol. Sci. 21:4607. doi: 10.3390/ijms21134607
Gonugunta, V. K., Srivastava, N., and Raghavendra, A. S. (2009). Cytosolic alkalinization is a common and early messenger preceding the production of ROS and NO during stomatal closure by variable signals, including abscisic acid, methyl jasmonate and chitosan. Plant Signal Behav. 4, 561–564. doi: 10.4161/psb.4.6.8847
Gudesblat, G. E., Torres, P. S., and Vojnov, A. A. (2009a). Stomata and pathogens: warfare at the gates. Plant Signal Behav. 4, 1114–1116. doi: 10.4161/psb.4.12.10062
Gudesblat, G. E., Torres, P. S., and Vojnov, A. A. (2009b). Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. 149, 1017–1027. doi: 10.1104/pp.108.126870
Gupta, P., Roy, S., and Nandi, A. K. (2020). MEDEA-interacting protein LONG-CHAIN BASE KINASE 1 promotes pattern-triggered immunity in Arabidopsis thaliana. Plant Mol. Biol. 103, 173–184. doi: 10.1007/s11103-020-00982-4
Ha, C. V., Leyva-González, M. A., Osakabe, Y., Tran, U. T., Nishiyama, R., Watanabe, Y., et al. (2014). Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. U.S.A. 111, 851–856. doi: 10.1073/pnas.1322135111
Ha, Y., Shang, Y., and Nam, K. H. (2016). Brassinosteroids modulate ABA-induced stomatal closure in Arabidopsis. J. Exp. Bot. 67, 6297–6308. doi: 10.1093/jxb/erw385
Haider, I., Andreo-Jimenez, B., Bruno, M., Bimbo, A., Floková, K., Abuauf, H., et al. (2018). The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 69, 2403–2414. doi: 10.1093/jxb/ery089
Hao, F., Zhao, S., Dong, H., Zhang, H., Sun, L., and Miao, C. (2010). Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J. Integr. Plant Biol. 52, 298–307. doi: 10.1111/j.1744-7909.2010.00920.x
Hatmi, S., Villaume, S., Trotel-Aziz, P., Barka, E. A., Clément, C., and Aziz, A. (2018). Osmotic stress and ABA affect immune response and susceptibility of grapevine berries to gray mold by priming polyamine accumulation. Front. Plant Sci. 9:1010. doi: 10.3389/fpls.2018.01010
Haworth, M., Marino, G., Cosentino, S. L., Brunetti, C., De Carlo, A., Avola, G., et al. (2018). Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environ. Exp. Bot. 147, 116–124. doi: 10.1016/j.envexpbot.2017.11.002
He, H., and He, L. (2014). The role of carbon monoxide signaling in the responses of plants to abiotic stresses. Nitric Oxide 42, 40–43. doi: 10.1016/j.niox.2014.08.011
Hewage, K. A. H., Yang, J. F., Wang, D., Hao, G. F., Yang, G. F., and Zhu, J. K. (2020). Chemical manipulation of abscisic acid signaling: a new approach to abiotic and biotic stress management in agriculture. Adv. Sci. 7:2001265. doi: 10.1002/advs.202001265
Hofius, D., Li, L., Hafrén, A., and Coll, N. S. (2017). Autophagy as an emerging arena for plant-pathogen interactions. Curr. Opin. Plant Biol. 38, 117–123. doi: 10.1016/j.pbi.2017.04.017
Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., Mori, I. C., and Murata, Y. (2011). Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol. 156, 430–438. doi: 10.1104/pp.111.172254
Hou, S., Wang, X., Chen, D., Yang, X., Wang, M., Turrà, D., et al. (2014). The secreted peptide PIP1 amplifies immunity through receptor-like kinase 7. PLoS Pathog. 10:e1004331. doi: 10.1371/journal.ppat.1004331
Hsu, P. K., Takahashi, Y., Munemasa, S., Merilo, E., Laanemets, K., Waadt, R., et al. (2018). Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proc. Natl. Acad. Sci. U.S.A. 115, E9971–E9980. doi: 10.1073/pnas.1809204115
Huai, B., Yang, Q., Qian, Y., Qian, W., Kang, Z., and Liu, J. (2019). ABA-induced sugar transporter TaSTP6 promotes wheat susceptibility to stripe rust. Plant Physiol. 181, 1328–1343. doi: 10.1104/pp.19.00632
Huang, H., Ullah, F., Zhou, D. X., Yi, M., and Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10:800. doi: 10.3389/fpls.2019.00800
Huysmans, M., Lema, A. S., Coll, N. S., and Nowack, M. K. (2017). Dying two deaths – programmed cell death regulation in development and disease. Curr. Opin. Plant Biol. 35, 37–44. doi: 10.1016/j.pbi.2016.11.005
Islam, M. M., Ye, W., Akter, F., Rhaman, M. S., Matsushima, D., Munemasa, S., et al. (2020). Reactive carbonyl species mediate methyl jasmonate-induced stomatal closure. Plant Cell Physiol. 61, 1788–1797. doi: 10.1093/pcp/pcaa107
Islam, M. M., Ye, W., Matsushima, D., Munemasa, S., Okuma, E., Nakamura, Y., et al. (2016). Reactive carbonyl species mediate ABA signaling in guard cells. Plant Cell Physiol. 57, 2552–2563. doi: 10.1093/pcp/pcw166
Islam, M. M., Ye, W., Matsushima, D., Rhaman, M. S., Munemasa, S., Okuma, E., et al. (2019). Reactive carbonyl species function as signal mediators downstream of H2O2 production and regulate [Ca2+]cyt elevation in ABA signal pathway in Arabidopsis guard cells. Plant Cell Physiol. 60, 1146–1159. doi: 10.1093/pcpo/pcz031
Islam, W., Tayyab, M., Khalil, F., Hua, Z., Huang, Z., and Chen, H. (2020). Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 168:104641. doi: 10.1016/j.pestbp.2020.104641
Jagodzik, P., Tajdel-Zielinska, M., Ciesla, A., Marczak, M., and Ludwikow, A. (2018). Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 9:1387. doi: 10.3389/fpls.2018.01387
Jalakas, P., Huang, Y. C., Yeh, Y. H., Zimmerli, L., Merilo, E., Kollist, H., et al. (2017). The role of ENHANCED RESPONSES TO ABA1 (ERA1) in Arabidopsis stomatal responses is beyond ABA signaling. Plant Physiol. 174, 665–671. doi: 10.1104/pp.17.00220
Jia, Q., Kong, D., Li, Q., Sun, S., Song, J., Zhu, Y., et al. (2019). The function of inositol phosphatases in plant tolerance to abiotic stress. Int. J. Mol. Sci. 20:3999. doi: 10.3390/ijms20163999
Jiang, C. J., Shimono, M., Sugano, S., Kojima, M., Yazawa, K., Yoshida, R., et al. (2010). Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol. Plant Microbe Interact. 23, 791–798. doi: 10.1094/MPMI-23-6-0791
Jiang, F., and Hartung, W. (2008). Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. J. Exp. Bot. 59, 37–43. doi: 10.1093/jxb/erm127
Jin, Z., Xue, S., Luo, Y., Tian, B., Fang, H., Li, H., et al. (2013). Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 62, 41–46. doi: 10.1016/j.plaphy.2012.10.017
Kadota, Y., Sklenar, J., Derbyshire, P., Stransfeld, L., Asai, S., Ntoukakis, V., et al. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell. 54, 43–55. doi: 10.1016/j.molcel.2014.02.021
Karimi, R. (2019). Spring frost tolerance increase in Sultana grapevine by early season application of calcium sulfate and zinc sulfate. J. Plant Nutr. 42, 2666–2681.
Khare, S., Singh, N. B., Singh, A., Hussain, I., Niharika, K., Yadav, V., et al. (2020). Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 63, 203–216. doi: 10.1007/s12374-020-09245-7
Khokon, A. R., Okuma, E., Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., et al. (2011). Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34, 434–443.
Khokon, M., Salam, M. A., Jammes, F., Ye, W., Hossain, M. A., Okuma, E., et al. (2017). MPK9 and MPK12 function in SA-induced stomatal closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 81, 1394–1400. doi: 10.1080/09168451.2017.1308244
Khokon, M. A., Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., Mori, I. C., et al. (2010). Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol. 51, 1915–1921. doi: 10.1093/pcp/pcq145
Khokon, M. A., Jahan, M. S., Rahman, T., Hossain, M. A., Muroyama, D., Minami, I., et al. (2011). Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 34, 1900–1906.
Klessig, D. F., Choi, H. W., and Dempsey, D. A. (2018). Systemic acquired resistance and salicylic acid: past, present, and future. Mol. Plant Microbe Interact. 31, 871–888. doi: 10.1094/MPMI-03-18-0067-CR
Klüsener, B., Young, J. J., Murata, Y., Allen, G. J., Mori, I. C., Hugouvieux, V., et al. (2002). Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 130, 2152–2163. doi: 10.1104/pp.012187
Kolbert, Z., Feigl, G., Freschi, L., and Poór, P. (2019). Gasotransmitters in action: nitric oxide-ethylene crosstalk during plant growth and abiotic stress responses. Antioxidants 8:167. doi: 10.3390/antiox8060167
Kolupaev, Y. E., Karpets, Y. V., Beschasniy, S. P., and Dmitriev, A. P. (2019). Gasotransmitters and their role in adaptive reactions of plant cells. Cytol. Genet. 53, 392–406. doi: 10.3103/S0095452719050098
Konstantinos, P. A., Imene, T., Panagiotis, M. N., and Roubelakis-Angelakis, K. A. (2010). ABA-dependent amine oxidases-derived H2O2 affects stomata conductance. Plant Signal Behav. 5, 1153–1156. doi: 10.4161/psb.5.9.12679
Koo, Y. M., Heo, A. Y., and Choi, H. W. (2020). Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 36, 1–10. doi: 10.5423/PPJ.RW.12.2019.0295
Kumar, I., and Sharma, R. K. (2018). Production of secondary metabolites in plants under abiotic stress: an overview. Significan. Bioeng. Biosci. 2, 1–5. doi: 10.31031/SBB.2018.02.000545
Kumar, M., Kesawat, M. S., Ali, A., Lee, S. C., Gill, S. S., and Kim, A. (2019). Integration of abscisic acid signaling with other signaling pathways in plant stress responses and development. Plants 8:592. doi: 10.3390/plants8120592
Kurusu, T., Mitsuka, D., Yagi, C., Kitahata, N., Tsutsui, T., Ueda, T., et al. (2018). Involvement of S-type anion channels in disease resistance against an oomycete pathogen in Arabidopsis seedlings. Commun. Integr. Biol. 11, 1–6. doi: 10.1080/19420889.2018.1495007
Lamers, J., van der Meer, T., and Testerink, C. (2020). How plants sense and respond to stressful environments. Plant Physiol. 182, 1624–1635. doi: 10.1104/pp.19.01464
Lee, S., Choi, H., Suh, S., Doo, I. S., Oh, K. Y., Choi, E. J., et al. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121, 147–152. doi: 10.1104/pp.121.1.147
Lee, S., Rojas, C. M., Ishiga, Y., Pandey, S., and Mysore, K. S. (2013). Arabidopsis heterotrimeric G-proteins play a critical role in host and nonhost resistance against Pseudomonas syringae pathogens. PLoS One 8:e82445. doi: 10.1371/journal.pone.0082445
Lee, S. C., and Luan, S. (2012). ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 35, 53–60. doi: 10.1111/j.1365-3040.2011.02426.x
León, J., Castillo, M. C., Coego, A., Lozano-Juste, J., and Mir, R. (2014). Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J. Exp. Bot. 65, 907–921. doi: 10.1093/jxb/ert454
Lewandowska, M., Keyl, A., and Feussner, I. (2020). Wax biosynthesis in response to danger: its regulation upon abiotic and biotic stress. New Phytol. 227, 698–713. doi: 10.1111/nph.16571
Li, J., and Wang, X. (2019). Phospholipase D and phosphatidic acid in plant immunity. Plant Sci. 279, 45–50. doi: 10.1016/j.plantsci.2018.05.021
Lim, C. W., Baek, W., Jung, J., Kim, J. H., and Lee, S. C. (2015). Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 16, 15251–15270. doi: 10.3390/ijms160715251
Liu, J., Elmore, J. M., Fuglsang, A. T., Palmgren, M. G., Staskawicz, B. J., and Coaker, G. (2009). RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 7:e1000139. doi: 10.1371/journal.pbio.1000139
Liu, X., Afrin, T., and Pajerowska-Mukhtar, K. M. (2019). Arabidopsis GCN2 kinase contributes to ABA homeostasis and stomatal immunity. Commun. Biol. 2:302. doi: 10.1038/s42003-019-0544-x
Lu, Y., Chen, Q., Bu, Y., Luo, R., Hao, S., Zhang, J., et al. (2017). Flavonoid accumulation plays an important role in the rust resistance of Malus plant leaves. Front. Plant Sci. 8:1286. doi: 10.3389/fpls.2017.01286
Luna, E., Pastor, V., Robert, J., Flors, V., Mauch-Mani, B., and Ton, J. (2011). Callose deposition: a multifaceted plant defense response. Mol. Plant Microbe Interact. 24, 183–193. doi: 10.1094/MPMI-07-10-0149
Lv, S., Zhang, Y., Li, C., Liu, Z., Yang, N., Pan, L., et al. (2018). Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 217, 290–304. doi: 10.1111/nph.14813
Ma, Y., and Berkowitz, G. A. (2016). NO and Ca2+: critical components of cytosolic signaling systems involved in stomatal immune responses. Adv. Bot. Res. 77, 285–323. doi: 10.1016/bs.abr.2015.11.001
Ma, Y., Cao, J., He, J., Chen, Q., Li, X., and Yang, Y. (2018). Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 19:3643. doi: 10.3390/ijms19113643
Maksimov, I. V. (2009). Abscisic acid in the plants-pathogen interaction. Russ. J. Plant Physiol. 56:742. doi: 10.1134/S102144370906003X
Malcheska, F., Ahmad, A., Batool, S., Müller, H. M., Ludwig-Müller, J., Kreuzwieser, J., et al. (2017). Drought-enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant Physiol. 174, 798–814. doi: 10.1104/pp.16.01784
Manthe, B., Schulz, M., and Schnabl, H. (1992). Effects of salicylic acid on growth and stomatal movements of Vicia faba L.: evidence for salicylic acid metabolization. J. Chem. Ecol. 18, 1525–1539. doi: 10.1007/BF00993226
Mazid, M., Khan, T. A., and Mohammad, F. (2011). Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 3, 232–249.
McAdam, S., and Sussmilch, F. C. (2020). The evolving role of abscisic acid in cell function and plant development over geological time. Semin. Cell Dev. Biol. 109, 39–45. doi: 10.1016/j.semcdb.2020.06.006
McLachlan, D. H., Kopischke, M., and Robatzek, S. (2014). Gate control: guard cell regulation by microbial stress. New Phytol. 203, 1049–1063. doi: 10.1111/nph.12916
Mekonnen, D. W., Flügge, U. I., and Ludewig, F. (2016). Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 245, 25–34. doi: 10.1016/j.plantsci.2016.01.005
Melotto, M., Underwood, W., and He, S. Y. (2008). Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 46, 101–122. doi: 10.1146/annurev.phyto.121107.104959
Melotto, M., Underwood, W., Koczan, J., Nomura, K., and He, S. Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. doi: 10.1016/j.cell.2006.06.054
Melotto, M., Zhang, L., Oblessuc, P. R., and He, S. Y. (2017). Stomatal defense a decade later. Plant Physiol. 174, 561–571. doi: 10.1104/pp.16.01853
Mendgen, K., Hahn, M., and Deising, H. (1996). Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Ann. Rev. Phytopathol. 34, 367–386.
Mishra, G., Zhang, W., Deng, F., Zhao, J., and Wang, X. A. (2006). Bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312, 264–266. doi: 10.1126/science
Moeder, W., Phan, V., and Yoshioka, K. (2019). Ca2+ to the rescue – Ca2+ channels and signaling in plant immunity. Plant Sci. 279, 19–26. doi: 10.1016/j.plantsci.2018.04.012
Mona, S. A., Hashem, A., Abd_Allah, E. F., Alqarawi, A. A., Soliman, D. W. K., Wirth, S., et al. (2017). Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 16, 1751–1757. doi: 10.1016/S2095-3119(17)61695-2
Montillet, J. L., Leonhardt, N., Mondy, S., Tranchimand, S., Rumeau, D., Boudsocq, M., et al. (2013). An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 11:e1001513. doi: 10.1371/journal.pbio.1001513
Munemasa, S., Hauser, F., Park, J., Waadt, R., Brandt, B., and Schroeder, J. I. (2015). Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 28, 154–162. doi: 10.1016/j.pbi.2015.10.010
Munemasa, S., Oda, K., Watanabe-Sugimoto, M., Nakamura, Y., Shimoishi, Y., and Murata, Y. (2007). The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 143, 1398–1407. doi: 10.1104/pp.106.091298
Murcia, G., Fontana, A., Pontin, M., Baraldi, R., Bertazza, G., and Piccoli, P. N. (2017). ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 135, 34–52. doi: 10.1016/j.phytochem.2016.12.007
Nakashima, K., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2014). The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 5:170. doi: 10.3389/fpls.2014.00170
Narula, K., Elagamey, E., Abdellatef, M., Sinha, A., Ghosh, S., Chakraborty, N., et al. (2020). Chitosan-triggered immunity to Fusarium in chickpea is associated with changes in the plant extracellular matrix architecture, stomatal closure and remodeling of the plant metabolome and proteome. Plant J. 103, 561–583. doi: 10.1111/tpj.1475
Nejat, N., and Mantri, N. (2017). Plant immune system: crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 23, 1–16. doi: 10.21775/cimb.023.001
Ng, C. K., Carr, K., McAinsh, M. R., Powell, B., and Hetherington, A. M. (2001). Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 410, 596–599. doi: 10.1038/35069092
Nguyen, D., D’Agostino, N., Tytgat, T. O., Sun, P., Lortzing, T., Visser, E. J., et al. (2016). Drought and flooding have distinct effects on herbivore-induced responses and resistance in Solanum dulcamara. Plant Cell Environ. 39, 1485–1499. doi: 10.1111/pce.12708
Niu, M., Xie, J., Chen, C., Cao, H., Sun, J., Kong, Q., et al. (2018). An early ABA-induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 69, 4945–4960. doi: 10.1093/jxb/ery251
Novák, J., Pavlů, J., Novák, O., Nožková-Hlaváčková, V., Špundová, M., Hlavinka, J., et al. (2013). High cytokinin levels induce a hypersensitive-like response in tobacco. Ann. Bot. 112, 41–55. doi: 10.1093/aob/mct092
Oide, S., Bejai, S., Staal, J., Guan, N., Kaliff, M., and Dixelius, C. (2013). A novel role of PR2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 200, 1187–1199. doi: 10.1111/nph.12436
Pál, M., Tajti, J., Szalai, G., Peeva, V., Végh, B., and Janda, T. (2018). Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 8:12839. doi: 10.1038/s41598-018-31297-6
Pantin, F., and Blatt, M. R. (2018). Stomatal response to humidity: blurring the boundary between active and passive movement. Plant Physiol. 176, 485–488. doi: 10.1104/pp.17.01699
Petrov, V., Hille, J., Mueller-Roeber, B., and Gechev, T. S. (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6:69. doi: 10.3389/fpls.2015.00069
Planchet, E., Verdu, I., Delahaie, J., Cukier, C., Girard, C., Morère-Le Paven, M. C., et al. (2014). Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicago truncatula. J. Exp. Bot. 65, 2161–2170. doi: 10.1093/jxb/eru088
Prats, E., Gay, A. P., Mur, L. A., Thomas, B. J., and Carver, T. L. (2006). Stomatal lock-open, a consequence of epidermal cell death, follows transient suppression of stomatal opening in barley attacked by Blumeria graminis. J. Exp. Bot. 57, 2211–2226. doi: 10.1093/jxb/erj186
Prodhan, M. Y., Munemasa, S., Nahar, M. N., Nakamura, Y., and Murata, Y. (2018). Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol. 178, 441–450. doi: 10.1104/pp.18.00321
Prodhan, Y., Issak, M., Munemasa, S., Nakamura, Y., and Murata, Y. (2020). Salicylic acid receptor NPR1 is involved in guard cell chitosan signaling. Biosci. Biotechnol. Biochem. 84, 963–969. doi: 10.1080/09168451.2020.1718485
Puli, M. R., and Raghavendra, A. S. (2012). Pyrabactin, an ABA agonist, induced stomatal closure and changes in signalling components of guard cells in abaxial epidermis of Pisum sativum. J. Exp. Bot. 63, 1349–1356. doi: 10.1093/jxb/err364
Qamar, A., Mysore, K. S., and Senthil-Kumar, M. (2015). Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 6:503. doi: 10.3389/fpls.2015.00503
Qi, J., Song, C. P., Wang, B., Zhou, J., Kangasjärvi, J., Zhu, J. K., et al. (2018). Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60, 805–826. doi: 10.1111/jipb.12654
Raghavendra, A. S., Gonugunta, V. K., Christmann, A., and Grill, E. (2010). ABA perception and signalling. Trends Plant Sci. 15, 395–401. doi: 10.1016/j.tplants.2010.04.006
Raghavendra, A. S., and Reddy, K. B. (1987). Action of proline on stomata differs from that of abscisic acid, G-substances, or methyl jasmonate. Plant Physiol. 83, 732–734. doi: 10.1104/pp.83.4.732
Robert-Seilaniantz, A., Grant, M., and Jones, J. D. (2011). Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Ann. Rev. Phytopathol. 49, 317–343. doi: 10.1146/annurev-phyto-073009-114447
Rodrigues, O., Reshetnyak, G., Grondin, A., Saijo, Y., Leonhardt, N., Maurel, C., et al. (2017). Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. U.S.A. 114, 9200–9205. doi: 10.1073/pnas.1704754114
Romero-Puertas, M. C., Perazzolli, M., Zago, E. D., and Delledonne, M. (2004). Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol. 6, 795–803. doi: 10.1111/j.1462-5822.2004.00428.x
Sadhu, A., Moriyasu, Y., Acharya, K., and Bandyopadhyay, M. (2019). Nitric oxide and ROS mediate autophagy and regulate Alternaria alternata toxin-induced cell death in tobacco BY-2 cells. Sci. Rep. 9:8973. doi: 10.1038/s41598-019-45470-y
Saijo, Y., and Loo, E. P. (2020). Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 225, 87–104. doi: 10.1111/nph.15989
Saito, S., and Uozumi, N. (2019). Guard cell membrane anion transport systems and their regulatory components: An elaborate mechanism controlling stress-induced stomatal closure. Plants 8:9. doi: 10.3390/plants8010009
Salam, M. A., Jammes, F., Hossain, M. A., Ye, W., Nakamura, Y., Mori, I., et al. (2013). Two guard cell-preferential MAPKs, MPK9 and MPK12, regulate YEL signalling in Arabidopsis guard cells. Plant Biol. 15, 436–442.
Salam, M. A., Jammes, F., Hossain, M. A., Ye, W., Nakamura, Y., Mori, I. C., et al. (2012). MAP kinases, MPK9 and MPK12, regulate chitosan-induced stomatal closure. Biosci. Biotechnol. Biochem. 76, 1785–1787. doi: 10.1271/bbb.120228
Sato, H., Takasaki, H., Takahashi, F., Suzuki, T., Iuchi, S., Mitsuda, N., et al. (2018). Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. U.S.A. 115, E11178–E11187. doi: 10.1073/pnas.1811491115
Savchenko, T., Kolla, V. A., Wang, C. Q., Nasafi, Z., Hicks, D. R., Phadungchob, B., et al. (2014). Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol. 164, 1151–1160. doi: 10.1104/pp.113.234310
Savouré, A., Hua, X. J., Bertauche, N., Van Montagu, M., and Verbruggen, N. (1997). Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol. Gen. Genet. 254, 104–109. doi: 10.1007/s004380050397
Schmidt, K., Pflugmacher, M., Klages, S., Mäser, A., Mock, A., and Stahl, D. J. (2008). Accumulation of the hormone abscisic acid (ABA) at the infection site of the fungus Cercospora beticola supports the role of ABA as a repressor of plant defence in sugar beet. Mol. Plant Pathol. 9, 661–673. doi: 10.1111/j.1364-3703.2008.00491.x
Schulze-Lefert, P., and Robatzek, S. (2006). Plant pathogens trick guard cells into opening the gates. Cell 126, 831–834. doi: 10.1016/j.cell.2006.08.020
Scuffi, D., Lamattina, L., and García-Mata, C. (2016). Gasotransmitters and stomatal closure: is there redundancy, concerted action, or both? Front. Plant Sci. 7:277. doi: 10.3389/fpls.2016.00277
Sewelam, N., Kazan, K., and Schenk, P. M. (2016). Global plant stress signaling: reactive oxygen species at the cross-road. Front. Plant Sci. 7:187. doi: 10.3389/fpls.2016.00187
Shafiei, R., Hang, C., Kang, J. G., and Loake, G. J. (2007). Identification of loci controlling non-host disease resistance in Arabidopsis against the leaf rust pathogen Puccinia triticina. Mol. Plant Pathol. 8, 773–784. doi: 10.1111/j.1364-3703.2007.00431.x
Shen, J., Diao, W., Zhang, L., Acharya, B. R., Wang, M., Zhao, X., et al. (2020). Secreted peptide PIP1 induces stomatal closure by activation of guard cell anion channels in Arabidopsis. Front. Plant Sci. 11:1029. doi: 10.3389/fpls.2020.01029
Shi, H., Ye, T., Han, N., Bian, H., Liu, X., and Chan, Z. (2015). Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J. Integr. Plant Biol. 57, 628–640. doi: 10.1111/jipb.12302
Sierla, M., Waszczak, C., Vahisalu, T., and Kangasjärvi, J. (2016). Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171, 1569–1580. doi: 10.1104/pp.16.00328
Solanki, S., Ameen, G., Borowicz, P., and Brueggeman, R. S. (2019). Shedding light on penetration of cereal host stomata by wheat stem rust using improved methodology. Sci. Rep. 9:7939. doi: 10.1038/s41598-019-44280-6
Song, S., Huang, H., Gao, H., Wang, J., Wu, D., Liu, X., et al. (2014). Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26, 263–279. doi: 10.1105/tpc.113.120394
Song, X. G., She, X. P., and Zhang, B. (2008). Carbon monoxide-induced stomatal closure in Vicia faba is dependent on nitric oxide synthesis. Physiol. Plant. 132, 514–525.
Srivastava, N., Gonugunta, V. K., Puli, M. R., and Raghavendra, A. S. (2009). Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta 229, 757–765. doi: 10.1007/s00425-008-0855-5
Staiger, D., Korneli, C., Lummer, M., and Navarro, L. (2013). Emerging role for RNA-based regulation in plant immunity. New Phytol. 197, 394–404. doi: 10.1111/nph.12022
Stewart, C. R. (1980). The mechanism of abscisic acid-induced proline accumulation in barley leaves. Plant Physiol. 66, 230–233. doi: 10.1104/pp.66.2.230
Su, J., Zhang, M., Zhang, L., Sun, T., Liu, Y., Lukowitz, W., et al. (2017). Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell 29, 526–542. doi: 10.1105/tpc.16.00577
Suhita, D., Raghavendra, A. S., Kwak, J. M., and Vavasseur, A. (2004). Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 134, 1536–1545. doi: 10.1104/pp.103.032250
Sun, L. R., Yue, C. M., and Hao, F. S. (2019). Update on roles of nitric oxide in regulating stomatal closure. Plant Signal Behav. 14:e1649569. doi: 10.1080/15592324.2019.1649569
Sussmilch, F. C., and McAdam, S. (2017). Surviving a dry future: abscisic acid (ABA)-mediated plant mechanisms for conserving water under low humidity. Plants 6:54. doi: 10.3390/plants6040054
Suzuki, N., and Katano, K. (2018). Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 9:490. doi: 10.3389/fpls.2018.00490
Takahashi, F., Suzuki, T., Osakabe, Y., Betsuyaku, S., Kondo, Y., Dohmae, N., et al. (2018). A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556, 235–238. doi: 10.1038/s41586-018-0009-2
Tanaka, Y., Sano, T., Tamaoki, M., Nakajima, N., Kondo, N., and Hasezawa, S. (2005). Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 138, 2337–2343. doi: 10.1104/pp.105.063503
Tao, Z. Q., Chen, Y. Q., Chao, L. I., Zou, J. X., Peng, Y. A. N., Yuan, S. F., et al. (2016). The causes and impacts for heat stress in spring maize during grain filling in the North China Plain-A review. J. Integr. Agric. 15, 2677–2687. doi: 10.1016/S2095-3119(16)61409-0
Tõldsepp, K., Zhang, J., Takahashi, Y., Sindarovska, Y., Hõrak, H., Ceciliato, P. H. O., et al. (2018). Mitogen-activated protein kinases MPK4 and MPK12 are key components mediating CO2 -induced stomatal movements. Plant J. 96, 1018–1035. doi: 10.1111/tpj.14087
Ulferts, S., Delventhal, R., Splivallo, R., Karlovsky, P., and Schaffrath, U. (2015). Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol. 15:7. doi: 10.1186/s12870-014-0409-x
Ullah, C., Tsai, C. J., Unsicker, S. B., Xue, L., Reichelt, M., Gershenzon, J., et al. (2019). Salicylic acid activates poplar defense against the biotrophic rust fungus Melampsora larici-populina via increased biosynthesis of catechin and proanthocyanidins. New Phytol. 221, 960–975. doi: 10.1111/nph.15396
Uraji, M., Katagiri, T., Okuma, E., Ye, W., Hossain, M. A., Masuda, C., et al. (2012). Cooperative function of PLDδ and PLDα1 in abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 159, 450–460. doi: 10.1104/pp.112.195578
Vaidya, A. S., Helander, J., Peterson, F. C., Elzinga, D., Dejonghe, W., Kaundal, A., et al. (2019). Dynamic control of plant water use using designed ABA receptor agonists. Science 366:eaaw8848. doi: 10.1126/science.aaw8848
Verma, V., Ravindran, P., and Kumar, P. P. (2016). Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16:86. doi: 10.1186/s12870-016-0771-y
Vishwakarma, K., Upadhyay, N., Kumar, N., Yadav, G., Singh, J., Mishra, R. K., et al. (2017). Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front. Plant Sci. 8:161. doi: 10.3389/fpls.2017.00161
Wang, H. Q., Sun, L. P., Wang, L. X., Fang, X. W., Li, Z. Q., Zhang, F. F., et al. (2020). Ethylene mediates salicylic-acid-induced stomatal closure by controlling reactive oxygen species and nitric oxide production in Arabidopsis. Plant Sci. 294:110464. doi: 10.1016/j.plantsci.2020.110464
Wang, J., Song, L., Gong, X., Xu, J., and Li, M. (2020). Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 21:1446. doi: 10.3390/ijms21041446
Wang, M., and Liao, W. (2016). Carbon monoxide as a signaling molecule in plants. Front. Plant Sci. 7:572. doi: 10.3389/fpls.2016.00572
Wang, W., Wang, X., Huang, M., Cai, J., Zhou, Q., Dai, T., et al. (2018). Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front. Plant Sci. 9:1137. doi: 10.3389/fpls.2018.01137
Wang, W. H., Yi, X. Q., Han, A. D., Liu, T. W., Chen, J., and Wu, F. H. (2012). Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. J. Exp. Bot. 63, 177–190. doi: 10.1093/jxb/err259
Wang, X., Kong, L., Zhi, P., and Chang, C. (2020). Update on cuticular wax biosynthesis and its roles in plant disease resistance. Int. J. Mol. Sci. 21:5514. doi: 10.3390/ijms21155514
Wendehenne, D., Durner, J., and Klessig, D. F. (2004). Nitric oxide: a new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 7, 449–455. doi: 10.1016/j.pbi.2004.04.002
Wilkinson, S., and Davies, W. J. (2010). Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 33, 510–525. doi: 10.1111/j.1365-3040.2009.02052.x
Wu, L., Chen, X., Ren, H., Zhang, Z., Zhang, H., Wang, J., et al. (2007). ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta 226, 815–825. doi: 10.1007/s00425-007-0528-9
Wu, L., Wu, H., Chen, L., Zhang, H., and Gao, X. (2017). Induction of systemic disease resistance in Nicotiana benthamiana by the cyclodipeptides cyclo (l-Pro-l-Pro) and cyclo (d-Pro-d-Pro). Mol. Plant Pathol. 18, 67–74. doi: 10.1111/mpp.12381
Wu, Y., Deng, Z., Lai, J., Zhang, Y., Yang, C., Yin, B., et al. (2009). Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Res. 19, 1279–1290. doi: 10.1038/cr.2009.108
Xu, X., Fang, P., Zhang, H., Chi, C., Song, L., Xia, X., et al. (2019). Strigolactones positively regulate defense against root-knot nematodes in tomato. J. Exp. Bot. 70, 1325–1337. doi: 10.1093/jxb/ery439
Xylogiannis, E., Sofo, A., Dichio, B., Montanaro, G., and Mininni, A. N. (2020). Root-to-shoot signaling and leaf water-use efficiency in peach trees under localized irrigation. Agronomy 10:437. doi: 10.3390/agronomy10030437
Yamasaki, H., and Cohen, M. F. (2006). NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 11, 522–524. doi: 10.1016/j.tplants.2006.09.009
Yang, C., Li, W., Cao, J., Meng, F., Yu, Y., Huang, J., et al. (2017). Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 89, 338–353. doi: 10.1111/tpj.13388
Yao, Y., Yang, Y., Li, C., Huang, D., Zhang, J., Wang, C., et al. (2019). Research progress on the functions of gasotransmitters in plant responses to abiotic stresses. Plants (Basel) 8:605. doi: 10.3390/plants8120605
Ye, W., Adachi, Y., Munemasa, S., Nakamura, Y., Mori, I. C., and Murata, Y. (2015). Open stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant Cell Physiol. 56, 1239–1248. doi: 10.1093/pcp/pcv051
Ye, W., Ando, E., Rhaman, M. S., Tahjib-Ul-Arif, M., Okuma, E., Nakamura, Y., et al. (2020a). Inhibition of light-induced stomatal opening by allyl isothiocyanate does not require guard cell cytosolic Ca2+ signaling. J. Exp. Bot. 71, 2922–2932. doi: 10.1093/jxb/eraa073
Ye, W., Munemasa, S., Shinya, T., Wu, W., Ma, T., Lu, J., et al. (2020b). Stomatal immunity against fungal invasion comprises not only chitin-induced stomatal closure but also chitosan-induced guard cell death. Proc. Natl. Acad. Sci. U.S.A. 117, 20932–20942. doi: 10.1073/pnas.1922319117
Ye, W., and Murata, Y. (2016). Microbe associated molecular pattern signaling in guard cells. Front. Plant Sci. 7:583. doi: 10.3389/fpls.2016.00583
Yoshida, T., Christmann, A., Yamaguchi-Shinozaki, K., Grill, E., and Fernie, A. R. (2019). Revisiting the basal role of ABA - roles outside of stress. Trends Plant Sci. 24, 625–635. doi: 10.1016/j.tplants.2019.04.008
You, J., and Chan, Z. (2015). ROS Regulation during abiotic stress responses in crop plants. Front. Plant Sci. 6:1092. doi: 10.3389/fpls.2015.01092
Yu, W., Zhao, R., Wang, L., Zhang, S., Li, R., Sheng, J., et al. (2019). ABA signaling rather than ABA metabolism is involved in trehalose-induced drought tolerance in tomato plants. Planta 250, 643–655. doi: 10.1007/s00425-019-03195-2
Zegada-Lizarazu, W., and Monti, A. (2019). Deep root growth, ABA adjustments and root water uptake response to soil water deficit in giant reed. Ann. Bot. 124, 605–616. doi: 10.1093/aob/mcz001
Zeng, W., Melotto, M., and He, S. Y. (2010). Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 21, 599–603. doi: 10.1016/j.copbio.2010.05.006
Zhang, H., Fang, Q., Zhang, Z., Wang, Y., and Zheng, X. (2009). The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. J. Exp. Bot. 60, 3109–3122. doi: 10.1093/jxb/erp146
Zhang, L., Takahashi, Y., Hsu, P. K., Kollist, H., Merilo, E., Krysan, P. J., et al. (2020). FRET kinase sensor development reveals SnRK2/OST1 activation by ABA but not by MeJA and high CO2 during stomatal closure. eLife 9:e56351. doi: 10.7554/eLife.56351
Zhang, L., Zhang, F., Melotto, M., Yao, J., and He, S. Y. (2017). Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 68, 1371–1385. doi: 10.1093/jxb/erw478
Zhang, T. Y., Li, F. C., Fan, C. M., Li, X., Zhang, F. F., and He, J. M. (2017). Role and interrelationship of MEK1-MPK6 cascade, hydrogen peroxide and nitric oxide in darkness-induced stomatal closure. Plant Sci. 262, 190–199. doi: 10.1016/j.plantsci.2017.06.010
Zhang, X., Wang, X., Zhuang, L., Gao, Y., and Huang, B. (2019). Abscisic acid mediation of drought priming-enhanced heat tolerance in tall fescue (Festuca arundinacea) and Arabidopsis. Physiol. Plant. 167, 488–501. doi: 10.1111/ppl.12975
Zhao, Y., Gao, J., Im Kim, J., Chen, K., Bressan, R. A., and Zhu, J. K. (2017). Control of plant water use by ABA induction of senescence and dormancy: an overlooked lesson from evolution. Plant Cell Physiol. 58, 1319–1327. doi: 10.1093/pcp/pcx086
Zheng, X., Kang, S., Jing, Y., Ren, Z., Li, L., Zhou, J. M., et al. (2018). Danger-associated peptides close stomata by OST1-independent activation of anion channels in guard cells. Plant Cell 30, 1132–1146. doi: 10.1105/tpc.17.00701
Zhu, J. K. (2016). Abiotic stress signaling and responses in plants. Cell 167, 313–324. doi: 10.1016/j.cell.2016.08.029
Appendix
Keywords: pathogen resistance, water use, stress adaptation, guard cells, signaling components
Citation: Bharath P, Gahir S and Raghavendra AS (2021) Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 12:615114. doi: 10.3389/fpls.2021.615114
Received: 08 October 2020; Accepted: 10 February 2021;
Published: 04 March 2021.
Edited by:
Victoria Pastor, Jaume I University, SpainReviewed by:
Carlos García-Mata, National University of Mar del Plata, ArgentinaWenxiu Ye, Shanghai Jiao Tong University, China
Copyright © 2021 Bharath, Gahir and Raghavendra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agepati S. Raghavendra, as_raghavendra@yahoo.com; asrsl@uohyd.ernet.in
†These authors have contributed equally to this work
 Pulimamidi Bharath
Pulimamidi Bharath Shashibhushan Gahir
Shashibhushan Gahir Agepati S. Raghavendra
Agepati S. Raghavendra