Microbial Biocontrol Strategies for Ambrosia Beetles and Their Associated Phytopathogenic Fungi
- 1Red de Estudios Moleculares Avanzados, Centro Regional del Bajío, Instituto de Ecología A.C., Pátzcuaro, Mexico
- 2Unidad de Tecnología Ambiental, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. (CIATEJ), Guadalajara, Mexico
- 3Department of Plant Pathology, UC Davis, Davis, CA, United States
- 4Red de Estudios Moleculares Avanzados, Instituto de Ecología, A.C., Xalapa, Mexico
- 5Cátedra CONACYT - Unidad de Biotecnología Vegetal, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. (CIATEJ), Zapopan, Mexico
- 6Centro de Investigación en Alimentación y Desarrollo A.C. (CIAD), Unidad Cuauhtémoc, Cd. Cuauhtémoc, Chihuahua, Mexico
- 7Cátedra CONACYT - Unidad de Tecnología Ambiental, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. (CIATEJ), Guadalajara, Mexico
Ambrosia beetles and their symbiotic fungi are causing severe damage in natural and agro-ecosystems worldwide, threatening the productivity of several important tree crops such as avocado. Strategies aiming at mitigating their impact include the application of broad-spectrum agrochemicals and the incineration of diseased trees, but the increasing demand for environment-friendly strategies call for exploring biological control for the management of ambrosia beetles and their phytopathogenic fungal symbionts. The aim of this review is to examine the existing knowledge on biocontrol approaches using beneficial microorganisms and microbial natural products with entomopathogenic and antifungal activity against ambrosia beetles and fungi. We show that biocontrol has been mainly focused on the insect, using entomopathogenic fungi (EPF) such as Beauveria spp. or Metarhizium spp. However, recent studies have been integrating EPF with mycoparasitic fungi such as Trichoderma spp. to simultaneously challenge the vector and its fungal symbionts. Novel approaches also include the use of microbial natural products as insect lures or antifungal agents. Contrastingly, the potential of bacteria, including actinobacteria (actinomycetes), as biocontrol agents of ambrosia fungi has been little investigated. We thus suggest that future research should further examine the antifungal activity of bacterial strains, with an emphasis on harsh environments. We also suggest pursuing the isolation of more effective microbial strains with dual biocontrol effect, i.e., exhibiting fungicidal/insecticidal activities. Moreover, additional efforts should aim at determining the best application methods of biocontrol agents in the field to ensure that the positive effects detected in vitro are sustained. Finally, we propose the integration of microbiome studies in pest and disease management strategies as they could provide us with tools to steer the beneficial host plant microbiome and to manipulate the beetle microbiome in order to reduce insect fitness.
Introduction
Ambrosia beetles (Coleoptera: Platypodinae and Scolytinae) are a group of wood-boring insects that characteristically colonize stressed or recently dead trees, burrowing galleries into their hosts where they inoculate and culture their fungal symbionts as a food source for their larvae and maturing adults (Farrell et al., 2001; Biedermann and Taborsky, 2011; Huang et al., 2019). In turn, ambrosia fungi depend on their beetle vectors for dispersal (Ranger et al., 2021), as their spores are transported by the beetles in specialized sac-like structures named mycangia, thus facilitating fungal propagation among host trees (Batra, 1963). Fungi in the ambrosia symbiosis have been identified in at least seven families (Ophiostomataceae, Ceratocystidaceae, Nectriaceae, Bionectriaceae, Saccharomycetaceae, Peniophoraceae, and Meruliaceae) and dominantly belong to the genera Ambrosiella, Fusarium, and Raffaelea (Harrington et al., 2010; Huang et al., 2019; Ranger et al., 2021).
Exotic ambrosia beetle species have been recently acknowledged as potential pests, as some of their fungal symbionts may act as plant pathogens (Hulcr and Dunn, 2011; Cruz et al., 2021). By shifting from colonizing dead or declining trees to attacking living ones, these exotic beetles can become highly invasive (Hulcr and Dunn, 2011). Invasive ambrosia beetles that have inflicted serious damage to tree crops and forest ecosystems include those of the Euwallacea fornicatus species complex, native of Southeast Asia, which form an association with fungi from the ambrosia Fusarium clade (Kasson et al., 2013). Among them, Fusarium euwallaceae and F. kuroshium are responsible for Fusarium dieback (Lynch et al., 2016; Na et al., 2018), a disease that has been reported in the U.S.A. (California and Florida), Israel, and South Africa, infecting more than 58 plant families (Eskalen et al., 2013; Freeman et al., 2013; Carrillo et al., 2016; Paap et al., 2018). By invading the plant vascular tissues, fungi responsible of Fusarium dieback block the transport of nutrients and induce wilting, branch dieback, potentially leading to tree mortality (Eskalen et al., 2013). Another invasive ambrosia beetle with a large economic impact is Xyleborus glabratus, which symbiotic fungus Raffaelea lauricola has been causing extensive mortality of Lauraceae species in the Southeastern part of the U.S.A., including avocado (Persea americana Mill.), by triggering laurel wilt (Lira-Noriega et al., 2018). The development of this disease involves xylem dysfunction and disturbed water transport (Inch and Ploetz, 2012). Other species of Raffaelea are also found in the ambrosia beetle Platypus cylindrus, causing severe harm to Quercus (Fagaceae) trees in the Mediterranean basin (Freeman et al., 2019).
Both Fusarium dieback and laurel wilt, vectored by ambrosia beetles, have hampered avocado production in several regions of the word (Carrillo et al., 2015; Freeman et al., 2019). Moreover, one of the vectors of Fusarium dieback, the ambrosia beetle Euwallacea kuroshio, also known as Kuroshio Shot Hole Borer (KSHB), was recently detected in Tijuana, Mexico, representing a serious threat for the world largest producer and exporter of avocados (García-Ávila et al., 2016). Other crops which production has been hindered by ambrosia beetles and their fungal symbionts include orange (Citrus sinensis (L.) Osbeck), grapevine (Vitis vinifera L.), cacao (Theobroma cacao L.), coffee (Coffea spp.), macadamia (Macadamia integrifolia Maiden & Betche), peach (Prunus persica L. Stokes), and tea (Camellia sinensis (L.) Kuntze) (Gadd and Loos, 1947; Eskalen et al., 2013; Kagezi et al., 2015; Paap et al., 2018; Rugman-Jones et al., 2020; Asman et al., 2021). It is therefore critical to find efficient control strategies for these economically important pests and diseases (Báez-Vallejo et al., 2020).
The application of broad-spectrum agrochemicals is currently the main management practice for the mitigation of the impact caused by ambrosia beetle/fungi complexes. The cryptic habit of the beetles warrants the repeated application of contact insecticides to avoid infestation and of systemic insecticides with long residual efficacy to kill those beetles already inside the trees (Paine et al., 2011; Castrillo et al., 2016; Eatough Jones and Paine, 2018). Early detection and sanitation measures are also implemented to dispose of infested wood and limit the spread of wood borers (Eatough Jones and Paine, 2015; Avery et al., 2018). A wide array of systemic fungicides has been tested to reduce the spread of ambrosia fungi, propiconazole being one of the most promising active ingredients so far (Mayfield et al., 2008; Freeman et al., 2012). The current agrochemical restrictions for the export of crops such as avocado and the increasing demand for environment-friendly strategies call for exploring biological control for the management of ambrosia beetles and their fungal symbionts (Dunlap et al., 2017; Reverchon et al., 2019; Castrejón-Antonio et al., 2020). Here, we define biological control as the use of microorganisms and their natural products as natural antagonists for controlling the populations of pests and plant pathogens. The objective of this review is to examine the current state of biological control approaches aiming at mitigating the damages caused by ambrosia beetles and their associated phytopathogenic fungi, focusing on beneficial microorganisms and microbial natural products with entomopathogenic and antifungal activity. Furthermore, we aim at proposing a new, integrated approach to steer the plant microbiome and enhance the potential benefits of the native microbiota for plant health and protection against ambrosia beetles and their fungal symbionts (Figure 1).

Figure 1. Overview of the different microbe-based approaches for the biocontrol of ambrosia beetles and their pathogenic fungi. The traditional approach has been focused on studying the antagonism displayed by single strains of entomopathogenic fungi (EPF) or Trichoderma spp. against ambrosia beetles or their associated fungi. Recent works have been integrating both strategies to tackle ambrosia fungi and their vectors at the same time, exploring other sources of biocontrol agents. Novel, cutting-edge strategies aim at manipulating the beetle microbiome in order to reduce its fitness, or the host microbiome in order to steer beneficial microorganisms.
Entomopathogenic Microorganisms for the Control of Ambrosia Beetles
Entomopathogenic fungi (EPF) represent a promising sustainable alternative to chemical pesticides and have been used against a broad range of agricultural and forest insect pests (Ocampo-Hernández et al., 2011; Verma et al., 2021). Their use is particularly interesting for the control of ambrosia beetles, as the cryptic nature of these insects effectively protect them from conventional insecticides during most of their life cycle (Castrillo et al., 2016). Several species of EPF have been used against ambrosia beetles with encouraging results, the most commonly tested ones being Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales; Table 1).
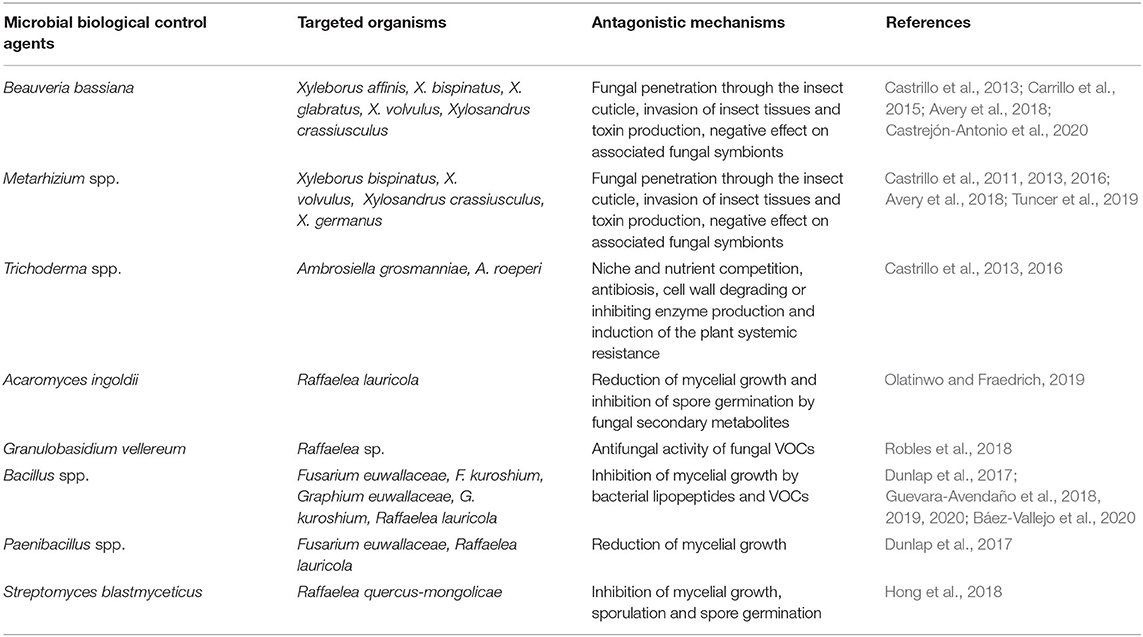
Table 1. Existing microbe-based strategies for the biocontrol of ambrosia beetles and their phytopathogenic fungal symbionts.
Beauveria bassiana is a widely distributed fungus with a broad host range and has been used as the basis for the development of many commercial bioformulations aiming at pest control (Zimmermann, 2007a). The mode of infection of B. bassiana is similar to that of other EPF, and initiates with the attachment of hydrophobic conidia to the insect cuticle, where germination starts. The formation of germ-tubes allows fungal penetration through the cuticle, further favored by the evasion from the insect host immune response. The subsequent fungal proliferation and invasion of insect tissues, coupled with the production of toxins, is followed by outgrowth from the dead host and production of new infective fungal spores (Zimmermann, 2007a; Wang and Wang, 2017; Xu et al., 2019). This EPF has already been described for its potential use in the biological control of several bark beetle species, such as Dendroctonus valens (Zhang et al., 2011; Xu et al., 2019), D. rufipennis (Davis et al., 2018), Ips typographus (Kreutz et al., 2004), or Scolytus amygdali (Batta, 2007). Regarding ambrosia beetles, the entomopathogenic effect of B. bassiana has been successfully tested against the redbay ambrosia beetle X. glabratus with mortality rates ranging from 55 and 71%, where the fungus prevented the reproduction of adult beetles and inhibited the establishment of their pathogenic fungal symbiont, although not affecting their initial boring activity (Carrillo et al., 2015). Similar findings were found in studies where B. bassiana was used to mitigate the impact of Xylosandrus crassiusculus, Xyleborus volvulus, Xyleborus bispinatus, and Xyleborus affinis (Castrillo et al., 2013; Avery et al., 2018; Castrejón-Antonio et al., 2020).
In the reviewed studies reporting the potential of B. bassiana for mitigating the impact of ambrosia beetles, several recommendations were made for an adequate selection of EPF and their optimum application under field conditions. Castrejón-Antonio et al. (2020) highlighted that EPF selection is key to the successful development of commercial biocontrol formulations and recommended that EPF strains be selected for their virulence, enzymatic profile, and adaptation to harsh environmental conditions, the latter to ensure a proper viability and persistence of fungal propagules in the field. This is particularly important since microbial biocontrol agents such as EPF are easily inactivated by natural environmental factors, especially ultraviolet radiation (ultraviolet A; UVA, 320 to 400 nm) and high temperatures (≥35°C), which consequently limit their effectiveness and persistence in open field conditions. Castrejón-Antonio et al. (2020) also emphasized the relevance of considering initial fungal growth parameters, such as mycelial growth, sporulation, and germination rates, since these are key aspects for subsequent massive production. Moreover, it is essential to consider the time elapsed between the application of EPF spores and the substantial mortality and mycosis of the treated beetles under laboratory conditions (especially the time until sporulation on cadavers and spores produced per specimen). This time parameter is key since EPF strains which are capable of producing abundant spores could cause horizontal transmission from an infected beetle (secondary inoculum source) to another within the galleries. Avery et al. (2018) recommended the development of oil-based emulsions as they seem to enhance spore adhesion to the insect cuticle, protect them against UV radiation, and thus favor germination and penetration. Likewise, the microencapsulation of entomopathogenic fungi through the use of different techniques (such as complex coacervation, liquid phase coating, and spray drying) could improve their entomopathogenic action; microencapsulation is commonly applied using different coating materials to effectively improve the stability of fungal spores and to regulate their release (Qiu et al., 2019). Biopolymers like starches, maltodextrins, and gums can be used as covering materials for biopesticides based on EPF because they favor the spore persistence, increasing the resistance to wash-off by rain and dew, and conferring thermo- and photo-tolerance to the active agent (Camacho et al., 2015). Despite the potential of microencapsulation, its use to improve EPF-based formulations is still scarce and, to the best of our knowledge, has not yet been explored for the control of ambrosia beetles. Finally, Castrillo et al. (2013) and Carrillo et al. (2015) emphasized the importance of optimizing the delivery system of EPF in the field to increase beetle mortality rates, suggesting that, for X. glabratus, trunk sprays or treated bait stations could be implemented for fungal delivery. Given the complexity of controlling ambrosia beetles, due to the insect cryptic habits and the cost of protecting large scale plantations, it is also important to conduct studies aimed at finding strains of endophytic EPF that are capable of successfully colonizing tissues of the entire plant (roots, stems, and leaves), where they could exert their bioinsecticidal action (Behie et al., 2015). Brownbridge et al. (2012), for example, proposed using seed coating and root dip as methods to successfully establish B. bassiana as an endophyte in Pinus radiata seedlings, to counteract the negative impact of bark beetles in pine plantations.
Fungi of the genus Metarhizium have also been evaluated as EPF against bark and ambrosial beetles (Castrillo et al., 2011; Tuncer et al., 2019; Mann and Davis, 2021). Metarhizium spp. exhibit several characteristics that are distinctive of successful EPF, such as easy isolation and culture, a large spore production, a broad host range and an efficient infection mechanism through the direct penetration of the cuticle (Zimmermann, 2007b; Mann and Davis, 2021). The entomopathogenic activity of commercial strains of M. brunneum has been reported against X. crassiusculus and Xylosandrus germanus in laboratory and field studies (Castrillo et al., 2011, 2013, 2016), where the authors reported an effect not only on adult females (with mortality rates higher than 50%) but also on their offspring. As with B. bassiana, negative interactions also seemed to occur between M. brunneum and the beetle fungal symbionts, thereby reinforcing the biocontrol activity of EPF. Metarhizium brunneum was also evaluated in laboratory assays against X. crassiusculus, X. volvulus, and X. bispinatus, significantly reducing beetle mean survival time when compared with that of untreated beetles (controls), more markedly so in X. bispinatus (Avery et al., 2018). Finally, Metarhizium anisopliae was tested against X. germanus with promising results, as 100% mortality rates were registered in laboratory conditions (Tuncer et al., 2019). Most studies emphasized the need to carry out field experiments to confirm the efficiency of Metarhizium-based formulations under unfavorable environmental conditions, and to determine the best spore concentration and delivery system for these biopesticides (Gugliuzzo et al., 2021).
The results presented in these studies point to the need to combine EPF with other control strategies for optimal integrated management and control of ambrosia beetle populations. It is thus necessary to examine the compatibility of EPF-based bioformulations with conventional agrochemicals used by the growers to ensure that applied fungicides minimally affect EPF and other potentially beneficial fungi (Avery et al., 2018; Zhou et al., 2018). Prospecting for new native, potentially successful EPF is an approach that still needs to be pursued, as recently recommended by Gugliuzzo et al. (2021), to identify new virulent fungal strains that could be more adapted to their natural conditions than commercial ones. The combination of EPF with mycoparasitic fungi such as Trichoderma spp. also seems like a promising approach, which would tackle not only the insect vector but also its fungal symbionts. Work carried out by Carrillo et al. (2016) showed an indirect negative impact of the commercial strain Trichoderma harzianum T-22 on brood production, probably through the limitation of their fungal food source. The antifungal effect of Trichoderma spp. against ambrosia fungi will be addressed in more detail in the next section of this review. Finally, further exploration of the beetle microbiome may provide new tools to enhance the beneficial effect of EPF, as recently demonstrated by Xu et al. (2019) for the bark beetle D. valens. These authors showed that the beetle gut microbiota interacted with B. bassiana to accelerate the beetle death and that bacteria from the genus Erwinia may be predominantly involved in this interaction. Examining the gut microbiome of ambrosia beetles thus seems like a promising new area of research, as determining shifts in the beetle microbial assemblages following inoculation with EPF could help identify positive feedbacks favoring EPF action (Figure 1).
Fungi With Antifungal Activity Against Ambrosia Fungi
Fungi are versatile organisms capable of producing a broad range of antimicrobial secondary metabolites and have thus been used as biocontrol agents for a variety of phytopathogenic fungi (Adnan et al., 2019; Segaran and Sathiavelu, 2019). In particular, mycoparasitic fungi, especially those from the genus Trichoderma, have been extensively used as biological control agents of other fungi as they can act as antagonists through combining several inhibition mechanisms. These include niche and nutrient competition, antibiosis, cell wall degrading or inhibiting enzyme production and induction of the plant systemic resistance (Contreras-Cornejo et al., 2016; Adnan et al., 2019). However, until now, the use of fungal antagonists to inhibit the growth of ambrosia fungi has been seldomly explored (Table 1).
Three Trichoderma strains (Trichoderma koningiopsis, T. harzianum, and T. viride), isolated from the rhizosphere of avocado trees, have been recently documented by our research group to reduce the mycelial growth of F. kuroshium by more than 50% (Ruiz-Cisneros et al., unpublished data). The overgrowth of the F. kuroshium colony was observed immediately after both fungi came into contact (Figure 2). Based on these results, we are currently aiming at evaluating the antagonistic potential of these Trichoderma strains against F. kuroshium in situ.
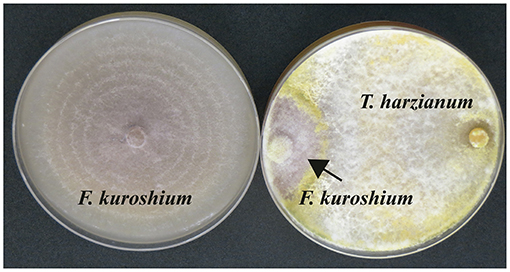
Figure 2. (Left) Culture of F. kuroshium in the absence of the antagonistic fungus (control); (Right) in vitro overgrowth of Trichoderma harzianum on Fusarium kuroshium.
A commercial strain of Trichoderma has been evaluated for its antifungal activity against ambrosia fungi by Castrillo et al. (2013, 2016), with encouraging results. These authors showed that Trichoderma harzianum T-22 could successfully outcompete different strains of Ambrosiella grosmanniae and A. roeperi in vitro, which were isolated from the ambrosia beetles X. germanus and X. crassiusculus, respectively. Moreover, beetle bioassays showed that T. harzianum T-22 could significantly reduce brood production in galleries, most likely through the inhibition of the fungal symbiont growth. The reduction in brood numbers was comparable to that observed when EPF was applied, causing the mortality of female adults before laying eggs (Castrillo et al., 2013, 2016). Future research should also investigate the potential of Trichoderma strains as putative biocontrol agents of ambrosia beetles. As recently reviewed by Poveda (2021), Trichoderma is capable of controlling insect pests through multiple mechanisms such as parasitism, the production of insecticidal secondary metabolites, antifeedant compounds, and repellent metabolites, the activation of systemic plant defense responses and the attraction of insect natural enemies.
More recently, a basidiomycete species, Acaromyces ingoldii, was reported to exhibit antagonism against R. lauricola in dual culture plate assays (Olatinwo and Fraedrich, 2019). Interestingly, these authors evaluated the antifungal activity of A. ingoldii crude extracts, showing that the produced secondary metabolites completely inhibited mycelial growth of R. lauricola and significantly reduced spore germination in vitro. These positive biocontrol effects were however not confirmed in vivo, which calls for further exploration of the adequate extract dosage and mode of application to overcome the observed resilience of R. lauricola. Furthermore, the identity of the antifungal compounds present in these crude extracts should also be determined. The authors suggested that using A. ingoldii as a biological control agent instead of its crude extracts could constitute a better alternative to ensure lasting protection against the phytopathogen, as this fungal isolate is capable of growing endophytically within the host tissue (Olatinwo and Fraedrich, 2019). Screening for other endophytic fungal antagonists from different host bark and xylem tissues could therefore offer more alternatives for the biocontrol of ambrosia fungi.
Another potential alternative for the control of phytopathogenic ambrosia fungi is the use of natural products from cultured or wild superior fungi (mushrooms) that could either affect the phytopathogen mycelial growth or reduce its production of phytotoxic compounds (Alves et al., 2013). For example, it has been reported that organics extracts from culture filtrates and basidiomes of Agaricus subrufescens (pilei and stipe), Lentinula edodes (pilei), and Pleurotus ostreatus significantly reduced the mycelial growth and production of fusaric acid in Fusarium spp. in vitro (Chen and Huang, 2010; Merel et al., 2020). Interestingly, fusaric acid was recently described as one of the potential virulence factors produced by F. kuroshium (Gutiérrez-Sánchez et al., 2021) and is capable of inducing in vitro foliar damage in avocado, in a similar way to the symptomatology observed in Fusarium dieback. Future research should thus explore the biotechnological potential of crude extracts or pure compounds obtained from mushrooms, as a strategy to tackle the negative effects of ambrosia fungi.
In addition to fungal crude extracts, fungal volatile organic compounds (VOCs) have also been investigated for their antagonistic effect against ambrosia fungi. Robles et al. (2018) demonstrated that Granulobasidium vellereum, a wood endophyte of Platanus acerifolia (Aiton) Willd (Platanaceae), could successfully inhibit mycelial growth of Raffaelea sp. associated with Megaplatypus mutatus in vitro, through the emission of fungal VOCs. Chemical analysis of the emitted compounds revealed the presence of esters such as 2-methylpropyl acetate, 2-methylbutyl acetate and 3-methylbutyl acetate, and the sesquiterpene β-caryophyllene, all of which have been previously described for their putative antimicrobial and nematicidal activity (Strobel et al., 2001; Terra et al., 2018; Yalage Don et al., 2020; Hilgers et al., 2021). The use of fungal VOCs as biofumigants seems promising and should be further explored in field experiments in order to determine the best application method to be incorporated into integrated pest and disease management strategies.
Interestingly, the use of fungal VOCs has also been explored as lures for ambrosia beetles (Hulcr et al., 2011; Egonyu and Torto, 2018; Ranger et al., 2021), as beetles showed to be particularly attracted to VOCs emitted by their fungal symbionts. Alcohols such as ethanol, 2-methyl-1-propanol and 3-methyl-1-butanol were detected in the volatile profiles of Ambrosiella sp., R. lauricola, and F. solani associated with Xylosandrus compactus (Kuhns et al., 2014; Egonyu and Torto, 2018). Compound β-caryophyllene, previously described as a potential biofumigant, was also dominantly emitted by the ambrosia fungus F. solani (Egonyu and Torto, 2018). In particular, ambrosia beetles exhibited a strong attraction to ethanol, which is also emitted by susceptible host trees (Ranger et al., 2021) and has been reported to promote the growth of fungal symbionts Ambrosiella spp. while reducing that of other competing fungi (Ranger et al., 2018). Further attention should thus be provided to fungal natural products, as they may constitute a promising source of novel compounds for bioformulations aiming at mitigating the impact of ambrosia beetles and their associated pathogenic fungi.
As previously mentioned, identifying fungal strains able to negatively impact ambrosia beetles and their phytopathogenic fungal symbionts is another promising strategy for the biocontrol of ambrosia complexes. In this context, endophytic EPF hold a large potential for the combined biocontrol of insect pests and fungal phytopathogens (Jaber and Alananbeh, 2018; Shapiro-Ilan et al., 2020; Guigón-López et al., 2021). However, to date, no studies have explored the dual efficacy of EPF for the biocontrol of ambrosia beetles and their symbiotic fungi. Recently, we observed in vitro inhibitions of F. kuroshium mycelial growth, ranging from 38.21 to 42.87%, by three Metarhizium anisopliae strains (Rios-Velasco et al., unpublished data). Based on these very promising results, we suggest the continuous exploration of EPF strains with dual antagonistic effects and that are capable of establishing themselves as endophytes to effectively achieve the biocontrol of both ambrosia beetles and their phytopathogenic symbiotic fungi.
Bacteria With Antifungal Activity Against Ambrosia Fungi
The use of bacteria as biological control agents of fungal diseases vectored by ambrosia beetles has been seldomly explored (Table 1). The first report dates from 2017, when Dunlap et al. investigated the antifungal activity of bacterial strains isolated from different sources against F. euwallaceae and R. lauricola, causal agents of Fusarium dieback and laurel wilt respectively. They identified three Paenibacillus and one Bacillus species as successful inhibitors of both avocado fungal pathogens in vitro. This initial work called for future research to determine the best application methods in the field that would ensure the effective contact between bacterial antagonists and phytopathogenic ambrosia fungi. Another suggestion made by Dunlap et al. (2017) was to include avocado-associated microorganisms in the search for bacterial biocontrol agents of F. euwallaceae and R. lauricola.
Guevara-Avendaño et al. (2018) isolated several rhizobacteria from a Fusarium dieback-infested avocado grove in Escondido, California, U.S.A. with antagonistic activity against F. euwallaceae, Graphium euwallaceae, and G. kuroshium, associated with Euwallacea fornicatus (Polyphagous Shot Hole Borer) and E. kuroshio, respectively. Their dual culture assays evidenced 72 bacterial isolates that were capable of inhibiting the mycelial growth of F. euwallaceae in vitro; from these, five isolates, all identified as Bacillus spp., successfully reduced the growth of both Graphium species. Subsequent investigations determined that crude extracts and fractions obtained from Bacillus sp. 4742, the bacterial strain with the strongest antagonism against G. kuroshium, also displayed antifungal activity against F. kuroshium (Pérez-Molina et al., unpublished results). Chemical profiling of the active extracts and subfractions allowed the identification of cyclic lipopeptidic compounds from the iturin and surfactin families, which have been previously described for their antifungal activities (Malfanova et al., 2012; Torres et al., 2017; Théatre et al., 2021). The inhibition of F. kuroshium by Bacillus natural products was further reported by Guevara-Avendaño et al. (2020) and Báez-Vallejo et al. (2020). In both studies, the analysis of bacterial extracts by ultra-performance liquid chromatography coupled to high-resolution mass spectrometry revealed the presence of cyclic lipopeptides from the iturin, surfactin, and fengycin families (Table 2) as the putative antifungal compounds. Bacillus lipopeptides have been described to alter fungal membranes, produce hyphal damage and vacuolization, and induce the production of resistance structures in pathogenic fungi (Alvarez et al., 2012; Cawoy et al., 2015) and thus constitute a promising source of bioactive compounds. Altogether, these findings call for bacterial natural products to be further investigated for the biological control of phytopathogenic fungi associated with ambrosia beetles.
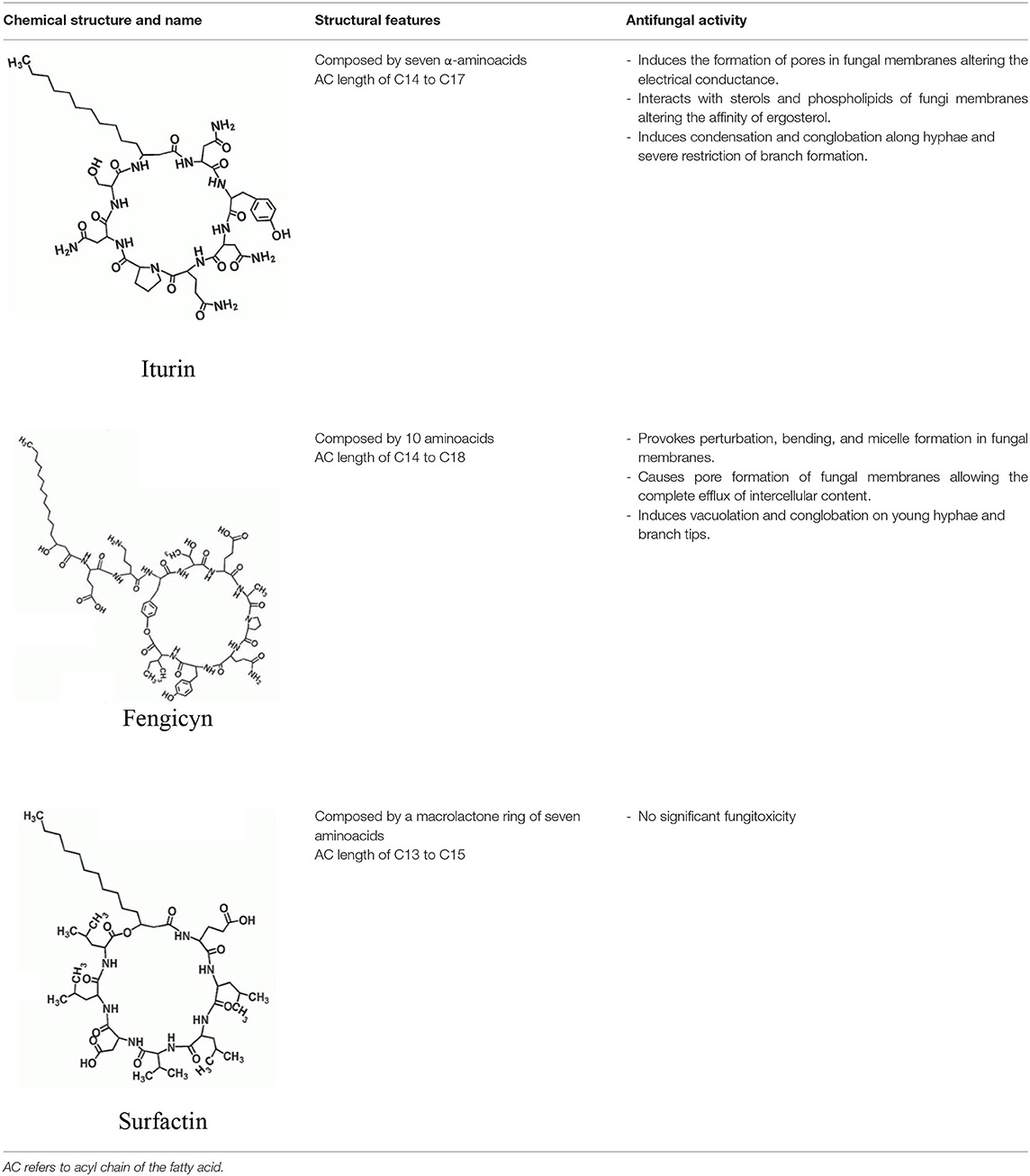
Table 2. Antimicrobial cyclic lipopeptides from Bacillus spp. (adapted from Gong et al., 2015; Caulier et al., 2019).
Cyclic lipopeptides are not the only bacterial compounds to exhibit antifungal activity against phytopathogenic fungi associated with ambrosia beetles. Bacterial VOCs have also been reported to inhibit the growth of F. kuroshium in vitro and induce severe hyphal deformations (Guevara-Avendaño et al., 2019, 2020; Báez-Vallejo et al., 2020; Figure 3). Bacterial VOCs have been shown to play important roles in plant-microbe interactions, through their antimicrobial activity and the induction of the plant systemic resistance (Gutiérrez-Santa Ana et al., 2020a,b; Reverchon and Méndez-Bravo, 2021). Some of the VOCs responsible for the displayed antifungal activity were identified as 2,3,5-trimethylpyrazine, 2-nonanone, 2-decanone, 2-dodecanone, dimethyl disulfide, and dimethyl trisulfide; they were produced by rhizobacteria isolated from avocado and Aiouea effusa (Lauraceae) and belonged to the Bacillus and Pseudomonas genera (Guevara-Avendaño et al., 2019, 2020; Báez-Vallejo et al., 2020). Similarly, Gutiérrez-Santa Ana et al. (2020a) evaluated different Bacillus strains, isolated from different environments (agricultural soils, hydrocarbon-contaminated soils, air, extremophile saline soils), for their antagonistic activity against Fusarium spp. The VOCs emitted by these bacterial strains inhibited the growth of F. solani by up to 24%, whilst inhibition through direct confrontation ranged from 40 to 76%. The main VOCs identified by these authors were alcohols, ketones and alkenes such as 3-methylbutan-1-ol and 2-methylbutan-1-ol, butane-2,3-diol, 3-hydroxybutan-2-one, pentadeca-5,10-diyn-1-ol, undec-1-ene and hexan-1-ol (Gutiérrez-Santa Ana et al., 2020b). In vitro assays showed hyphal deformations and vesicular chlamydospores induced in F. solani after incubation with Bacillus strain for 5 days, and spore reduction with twisted, thinner or broken hyphae in F. kuroshium (Figure 4). Despite these promising results, so far, none of these VOCs has been tested in planta for the control of Fusarium dieback.
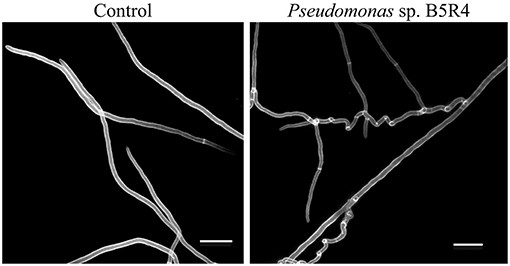
Figure 3. Scanning electron microscopy images of the distortions induced in the hyphal structure of Fusarium kuroshium by VOCs emitted by avocado root endophyte Pseudomonas sp. B5R4.
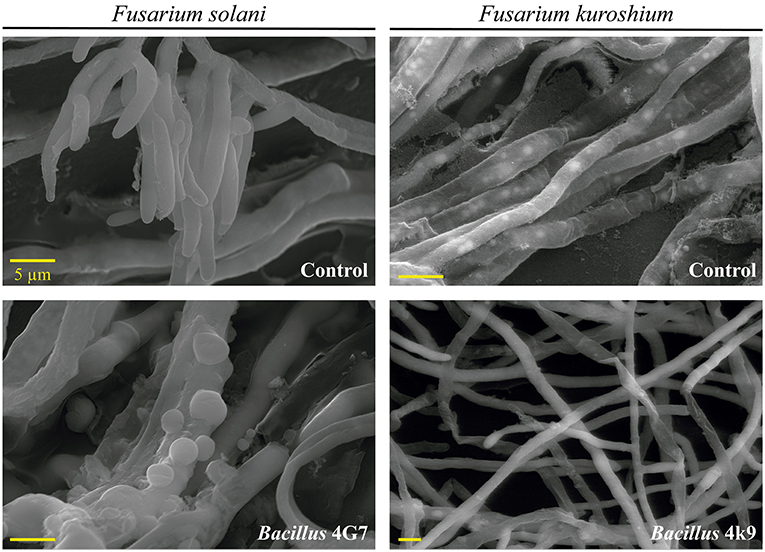
Figure 4. Scanning electron microscopy (SEM) images showing the effect of VOCs emitted by Bacillus 4G7 on F. solani and Bacillus 4k9 on F. kuroshium, after 5 days of incubation.
The few available reports investigating the potential of bacteria for the control of ambrosia fungi highlight several future research directions. First, most of these reports have been focused on rhizospheric bacteria, which calls for further exploration of other habitats to identify putative bacterial biocontrol agents. Environments subjected to extreme conditions may be an interesting source of biocontrol agents, as they promote the development of stress resistance mechanisms in microorganisms (Rojas-Solis et al., 2020; Tuesta-Popolizio et al., 2021). In particular, the phyllosphere has been characterized as a harsh and highly fluctuating environment, where microorganisms are exposed to oligotrophic conditions, solar radiation, and limited humidity (Vorholt, 2012). Further studies should thus focus on testing bark and leaf bacteria from ambrosia beetle hosts for their antifungal activity against phytopathogenic fungi. Another striking point from these available studies is the focus on Bacillus and Pseudomonas isolates. Although these bacterial genera have been extensively reported for their antifungal activity against a wide range of fungal pathogens (Cazorla et al., 2007; Santoyo et al., 2012; Ossowicki et al., 2017), other bacterial genera may also be explored, in particular those from the Actinobacteria phylum. Actinobacteria are known for secreting a wide array of antimicrobial compounds and it is thus surprising that their potential for the biological control of phytopathogenic ambrosia fungi has seldomly been investigated. An exception is the report by Hong et al. (2018), who showed that Streptomyces blastmyceticus successfully inhibited mycelial growth, sporulation and spore germination of Raffaelea quercus-mongolicae, a close relative of R. lauricola causing oak wilt. They attributed the observed antagonistic activity to the antifungal metabolites produced by Streptomyces. A subsequent study showed that active fractions obtained from S. blastmyceticus induced damage to the plasma membrane of spores and hyphae in several phytopathogenic fungi (Kim et al., 2019). These authors coincidentally mentioned the need for future research to focus on antifungal secondary metabolites and that combined control methods for both pathogenic fungi and insect vectors are necessary for the efficient management of fungal diseases and insect pests in agroecosystems. Preliminary results from our research group also show that actinobacteria of the genus Streptomyces were able to significantly inhibit the growth of F. kuroshium by 15–20% in vitro (Quiñones-Aguilar et al., unpublished). Interestingly, the inoculation of spores from these Streptomyces strains into an artificial medium where Xyleborus ferrugineus females were reared significantly decreased (up to 97%) the number of live females at the end of a 38-day experiment (Rincón-Enríquez et al., 2020).
Microbiome Approaches to Steer Beneficial Microorganisms
An increasing body of literature has been calling for innovative microbe-based approaches for integrated pest management that would consider managing microbiomes to steer beneficial organisms (Mendes et al., 2011; Orozco-Mosqueda et al., 2018; Zhang et al., 2020). These novel strategies would allow to go beyond strain-level antagonistic interactions and induce a general suppressiveness against diseases vectored by ambrosia beetles (Reverchon and Méndez-Bravo, 2021; Figure 1). For example, transplanting new microbiomes into the soil, or steering the existing soil microbiomes to enhance their beneficial effects, could be used to promote plant resistance to ambrosia beetles, following the evidence recently shown by Pineda et al. (2020) for the control of thrips.
Insect and host-plant microbiome studies thus have the potential to shed some light on the complex ecological interactions existing within microbial communities and could consequently help us elucidate how these microbiomes could be manipulated to enhance plant resistance to ambrosia beetles and their associated fungi. Our work on the avocado rhizosphere microbiome has shown that Fusarium dieback decreases the richness and diversity of rhizosphere microbial communities and significantly changed community structure, reducing the abundance of genera such as Sporocytophaga and Cellvibrio, which have been associated with plant growth promotion and chitinase production (Bejarano-Bolívar et al., 2021). However, some potential biocontrol agents such as Myxococcus sp. and Lysobacter sp. were exclusively detected in the rhizosphere of infected trees and should be further tested in antagonism assays (Bejarano-Bolívar et al., 2021). The characterization of the avocado bark microbiome has also highlighted the abundance of bacteria with antifungal activity in the bark environment, such as Sphingomonas or Methylobacterium, which could play a potential role in the biocontrol of Fusarium dieback in avocado trees (Aguirre-von-Wobeser et al., 2021). Collectively, these results could provide a basis for the selection of biocontrol agents that could be used to tackle Fusarium dieback. Finally, the exploration of ambrosia beetle microbiomes could also constitute an initial step for microbiome engineering as a mean to control their associated phytopathogenic fungi, as recently proposed by Ibarra-Juárez et al. (2018). These authors identified bacterial genera Bacillus, Burkholderia, Erwinia, Sphingobacterium, and Stenotrophomonas, among other taxa, as members of the core microbiome of Xyleborus affinis, X. bispinatus, and X. volvulus (Ibarra-Juárez et al., 2018, 2020). These core bacteria are hypothesized to be fundamental drivers of the maintenance of the beetle-fungus farming symbiosis and could thus be targeted in microbiome engineering strategies aiming at reducing pest fitness.
Conclusions
This review discussed the different biocontrol strategies aiming at decreasing the severe ecological and economic impacts of ambrosia beetles and their associated phytopathogenic fungi. Most studies so far have focused on the control of the insect, using EPF such as Beauveria spp. or Metarhizium spp. Recent works have suggested to combine EPF with mycoparasitic fungi such as Trichoderma, to tackle not only the beetle but also its fungal symbionts. Surprisingly, little emphasis has been made on the potential of bacteria, including actinobacteria, as biocontrol agents of ambrosia fungi, which calls for future research to be directed at finding novel microorganisms with antifungal activity, possibly in little explored, extreme environments. Recent studies have also been tackling the potential of microbial natural products as insect lures or antifungal agents. Additional efforts should be made to determine the best delivery method of potential biological control agents (either microbes or their bioactive compounds) in field conditions, as most studies so far have been implemented in vitro. Finally, we propose that microbial engineering methods should be explored in order to steer the plant microbiome and enhance its benefits for plant health and protection against ambrosia beetles and their phytopathogenic fungal symbionts, and to manipulate the beetle microbiomes in order to reduce its fitness.
Author Contributions
FR conceived the topic of the research and wrote a draft of the manuscript. All other authors contributed with additional information, data, tables and figures, and revised the final version of the manuscript.
Funding
This research was funded by the National Fund for Scientific and Technological Development (Fondo Nacional de Desarrollo Científico y Tecnológico or FORDECYT-PRONACES) grant number 292399. We thank Diana Sánchez-Rangel for leading this large project. We are also grateful to Clemente García-Ávila, Abel López Buenfil, and Magnolia Moreno Velázquez at CNRF-SENASICA for providing the facilities to work with F. kuroshium in our experimental research. We thank Alfonso Méndez-Bravo for his assistance with image edition, and Daniel García-Toscano, Olinda Velázquez-López, Mónica Ramírez-Vazquez, and Olga Araceli Patrón-Soberano for providing the microscopy images.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I declare that all sources of funding received for this research have been acknowledged.
References
Adnan, M., Islam, W., Shabbir, A., Khan, K. A., Ghramh, H. A., Huang, Z., et al. (2019). Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 129, 7–18. doi: 10.1016/j.micpath.2019.01.042
Aguirre-von-Wobeser, E., Alonso-Sánchez, A., Méndez-Bravo, A., Espino, L. A. V., and Reverchon, F. (2021). Bark from avocado trees of different geographic locations have consistent microbial communities. Arch. Microbiol. 203, 4593–4607. doi: 10.1007/s00203-021-02449-6
Alvarez, F., Castro, M., Príncipe, A., Borioli, G., Fischer, S., Mori, G., et al. (2012). The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J. Appl. Microbiol. 112, 159–174. doi: 10.1111/j.1365-2672.2011.05182.x
Alves, M., Ferreira, I. C., Dias, J., Teixeira, V., Martins, A., and Pintado, M. M. (2013). A review on antifungal activity of mushroom (Basidiomycetes) extracts and isolated compounds. Curr. Top. Med. Chem. 13, 2648–2659. doi: 10.2174/15680266113136660191
Asman, A., Rosmana, A., bin Purung, M. H., Amiruddin, A., Amin, N., Sjam, S., et al. (2021). The occurrence of Xylosandrus compactus and its associated fungi on cacao from South Sulawesi, Indonesia: a preliminary study of an emerging threat to the cacao industry. J. Plant Dis. Prot. 128, 303–309. doi: 10.1007/s41348-020-00387-x
Avery, P. B., Bojorque, V., Gámez, C., Duncan, R. E., Carrillo, D., and Cave, R. D. (2018). Spore acquisition and survival of ambrosia beetles associated with the laurel wilt pathogen in avocados after exposure to entomopathogenic fungi. Insects 9:49. doi: 10.3390/insects9020049
Báez-Vallejo, N., Camarena-Pozos, D. A., Monribot-Villanueva, J. L., Ramírez-Vázquez, M., Carrión-Villarnovo, G. L., Guerrero-Analco, J. A., et al. (2020). Forest tree associated bacteria for potential biological control of Fusarium solani and of Fusarium kuroshium, causal agent of Fusarium dieback. Microbiol. Res. 235:126440. doi: 10.1016/j.micres.2020.126440
Batra, L. R. (1963). Ecology of ambrosia fungi and their dissemination by beetles. Trans. Kans. Acad. Sci. 66, 213–236. doi: 10.2307/3626562
Batta, Y. A. (2007). Biocontrol of almond bark beetle (Scolytus amygdali Geurin-Meneville, Coleoptera: Scolytidae) using Beauveria bassiana (Bals.) Vuill. (Deuteromycotina: Hyphomycetes). J. Appl. Microbiol. 103, 1406–1414. doi: 10.1111/j.1365-2672.2007.03369.x
Behie, S. W., Jones, S. J., and Bidochka, M. J. (2015). Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 3, 112–119. doi: 10.1016/j.funeco.2014.08.001
Bejarano-Bolívar, A. A., Lamelas, A., Aguirre von Wobeser, E., Sánchez-Rangel, D., Méndez-Bravo, A, Eskalen, A., et al. (2021). Shifts in the structure of rhizosphere bacterial communities of avocado after Fusarium dieback. Rhizosphere 18:100333. doi: 10.1016/j.rhisph.2021.100333
Biedermann, P. H. W., and Taborsky, M. (2011). Larval helpers and age polyethism in ambrosia beetles. Proc. Natl. Acad. Sci. U.S.A. 108, 17064–17069. doi: 10.1073/pnas.1107758108
Brownbridge, M., Reay, S. D., Nelson, T. L., and Glare, T. R. (2012). Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control 61, 194–200. doi: 10.1016/j.biocontrol.2012.01.002
Camacho, J. E., Behle, R. W., Villamizar, L. F., and Gómez, M. I. (2015). Effect of spray dryer processing parameters on the insecticidal activity of two encapsulated formulations of baculovirus. Biocontrol Sci. Technol. 25, 911–927. doi: 10.1080/09583157.2015.1020761
Carrillo, D., Cruz, L. F., Kendra, P. E., Narvaez, T. I., Montgomery, W. S., Monterroso, A., et al. (2016). Distribution, pest status and fungal associates of Euwallacea nr. fornicatus in Florida avocado groves. Insects 7, 1–11. doi: 10.3390/insects7040055
Carrillo, D., Dunlap, C. A., Avery, P. B., Navarrete, J., Duncan, R. E., Jackson, M. A., et al. (2015). Entomopathogenic fungi as biological control agents for the vector of the laurel wilt disease, the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae). Biol. Control 81, 44–50. doi: 10.1016/j.biocontrol.2014.10.009
Castrejón-Antonio, J. E., Tamez-Guerra, P., Montesinos-Matías, R., Ek-Ramos, M. J., Garza-López, P. M., and Arredondo-Bernal, H. C. (2020). Selection of Beauveria bassiana (Hypocreales: Cordycipitaceae) strains to control Xyleborus affinis (Curculionidae: Scolytinae) females. PeerJ 8:e9472. doi: 10.7717/peerj.9472
Castrillo, L. A., Griggs, M. H., Ranger, C. M., Reding, M. E., and Vandenberg, J. D. (2011). Virulence of commercial strains of Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales) against adult Xylosandrus germanus (Coleoptera: Curculionidae) and impact on brood. Biol. Control 58, 121–126. doi: 10.1016/j.biocontrol.2011.04.010
Castrillo, L. A., Griggs, M. H., and Vandenberg, J. D. (2013). Granulate ambrosia beetle, Xylosandrus crassiusculus (Coleoptera: Curculionidae), survival and brood production following exposure to entomopathogenic and mycoparasitic fungi. Biol. Control 67, 220–226. doi: 10.1016/j.biocontrol.2013.07.015
Castrillo, L. A., Griggs, M. H., and Vandenberg, J. D. (2016). Competition between biological control fungi and fungal symbionts of ambrosia beetles Xylosandrus crassiusculus and X. germanus (Coleoptera: Curculionidae): mycelial interactions and impact on beetle brood production. Biol. Control 103, 138–146. doi: 10.1016/j.biocontrol.2016.09.005
Caulier, S., Nannan, C., Gillis, A., Licciardi, F., Bragard, C., and Mahillon, J. (2019). Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 10:302. doi: 10.3389/fmicb.2019.00302
Cawoy, H., Debois, D., Franzil, L., De Pauw, E., Thonart, P., and Ongena, M. (2015). Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 8, 281–295. doi: 10.1111/1751-7915.12238
Cazorla, F. M., Romero, D., Pérez-García, A., Lugtenberg, B. J. J., Vicente, A. D., and Bloemberg, G. (2007). Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J. Appl. Microbiol. 103, 1950–1959. doi: 10.1111/j.1365-2672.2007.03433.x
Chen, J. T., and Huang, J. W. (2010). Antimicrobial activity of edible mushroom culture filtrates on plant pathogens. Plant Pathol. Bull. 4, 261–270. Available online at: https://www.taiwanphytopath.org/uploads/publication/66591d84701f35ac66ac424cae4d7d32.pdf
Contreras-Cornejo, H. A., Macías-Rodríguez, L., Del-Val, E. K., and Larsen, J. (2016). Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants. FEMS Microbiol. Ecol. 92:fiw036. doi: 10.1093/femsec/fiw036
Cruz, L. F., Cruz, J. C., Carrillo, D., Mtz-Enriquez, A. I., Lamelas, A., Ibarra-Juarez, L. A., et al. (2021). In-vitro evaluation of copper nanoparticles as a potential control agent against the fungal symbionts of the invasive ambrosia beetle Euwallacea fornicatus. Crop Prot. 143:105564. doi: 10.1016/j.cropro.2021.105564
Davis, T. S., Mann, A. J., Malesky, D., Jankowski, E., and Bradley, C. (2018). Laboratory and field evaluation of the entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for population management of spruce beetle, Dendroctonus rufipennis (Coleoptera: Scolytinae), in felled trees and factors limiting pathogen success. Environ. Entomol. 47, 594–602. doi: 10.1093/ee/nvy036
Dunlap, C. A., Lueschow, S., Carrillo, D., and Rooney, A. P. (2017). Screening of bacteria for antagonistic activity against phytopathogens of avocados. Plant Gene 11, 17–22. doi: 10.1016/j.plgene.2016.11.004
Eatough Jones, M., and Paine, T. D. (2015). Effect of chipping and solarization on emergence and boring activity of a recently introduced ambrosia beetle (Euwallacea sp., Coleoptera: Curculionidae: Scolytinae) in southern California. J. Econ. Entomol. 108, 1852–1859. doi: 10.1093/jee/tov169
Eatough Jones, M., and Paine, T. D. (2018). Potential pesticides for control of a recently introduced ambrosia beetle (Euwallacea sp.) in southern California. J. Pest Sci. 91, 237–246. doi: 10.1007/s10340-017-0866-8
Egonyu, J. P., and Torto, B. (2018). Responses of the ambrosia beetle Xylosandrus compactus (Coleoptera: Curculionidea: Scolytinae) to volatile constituents of its symbiotic fungus Fusarium solani (Hypocreales: Nectriaceae). Arthropod Plant Int. 12, 9–20. doi: 10.1007/s11829-017-9552-2
Eskalen, A., Stouthamer, R., Lynch, S. C., Rugman-Jones, P. F., Twizeyimana, M., Gonzalez, A., et al. (2013). Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: scolytinae) vector in southern California. Plant Dis. 97, 938–951. doi: 10.1094/PDIS-11-12-1026-RE
Farrell, B. D., Sequeira, A. S., O'Meara, B. C., Normark, B. B., Chung, J. H., and Jordal, B. H. (2001). The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55, 2011–2027. doi: 10.1554/0014-3820(2001)0552011:TEOAIB2.0.CO;2
Freeman, S., Miller, G., Protasov, A., Maymon, M., Elazar, M., David-Schwartz, R., et al. (2019). Aposymbiotic interactions of three ambrosia beetle fungi with avocado trees. Fungal Ecol. 39, 117–130. doi: 10.1016/j.funeco.2018.11.007
Freeman, S., Sharon, M., Maymon, M., Mendel, Z., Protasov, A., Aoki, T., et al. (2013). Fusarium euwallaceae sp. nov.—a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 105, 1595–1606. doi: 10.3852/13-066
Freeman, S., Sharon, M., Okon-Levy, N., Protasov, A., Eliyahu, M., and Mendel, Z. (2012). Fungicide Screening for Inhibition of the Fungal Symbiont Fusarium sp. nov. in Israel. Available online at: http://www.avocadosource.com/Journals/IABC_2012/S5_04_Freeman.pdf (accessed May 10 2021).
Gadd, C. H., and Loos, C. A. (1947). The ambrosia fungus of Xyleborus fornicatus Eich. Trans. Br. Mycol. Soc. 30, 13–18. doi: 10.1016/S0007-1536(47)80003-8
García-Ávila, C. D. J., Trujillo-Arriaga, F. J., López-Buenfil, J. A., González-Gómez, R., Carrillo, D., Cruz, L. F., et al. (2016). First report of Euwallacea nr. fornicatus (Coleoptera: Curculionidae) in Mexico. Fla. Entomol. 99, 555–556. doi: 10.1653/024.099.0335
Gong, A. D., Li, H. P., Yuan, Q. S., Song, X. S., Yao, W., He, W. J., et al. (2015). Antagonistic mechanism of iturin and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 10:e0116871. doi: 10.1371/journal.pone.0116871
Guevara-Avendaño, E., Bejarano-Bolívar, A. A., Kiel-Martínez, A. L., Ramírez-Vázquez, M., Méndez-Bravo, A., Aguirre von Wobeser, E., et al. (2019). Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiol. Res. 219, 74–83. doi: 10.1016/j.micres.2018.11.009
Guevara-Avendaño, E., Bravo-Castillo, K. R., Monribot-Villanueva, J. L., Kiel-Martínez, A. L., Ramírez-Vázquez, M., Guerrero-Analco, J. A., et al. (2020). Diffusible and volatile organic compounds produced by avocado rhizobacteria exhibit antifungal effects against Fusarium kuroshium. Braz. J. Microbiol. 51, 861–873. doi: 10.1007/s42770-020-00249-6
Guevara-Avendaño, E., Carrillo, J. D., Ndinga-Muniania, C., Moreno, K., Méndez-Bravo, A., Guerrero-Analco, J. A., et al. (2018). Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. nr. fornicatus, and Phytophthora cinnamomi. Antonie Van Leeuwenhoek 111, 563–572. doi: 10.1007/s10482-017-0977-5
Gugliuzzo, A., Biedermann, P. H. W., Carrillo, D., Castrillo, L. A., and Egonyu, J. P. (2021). Recent advances toward the sustainable management of invasive Xylosandrus ambrosia beetles. J. Pest Sci. 94, 615–637. doi: 10.1007/s10340-021-01382-3
Guigón-López, C., Holguín-Ibarra, P. D., Torres-Zapien, J. H., García-Cruz, I., Villapando, I., and Salas-Salazar, N. A. (2021). Metarhizium anisopliae reduces conidial germination and mycelium growth of the apple gray mold Botrytis cinerea. Biol. Control 160:104660. doi: 10.1016/j.biocontrol.2021.104660
Gutiérrez-Sánchez, A., Plasencia, J., Monribot-Villanueva, J. L., Rodríguez-Haas, J. B., López-Buenfil, J. A., García-Ávila, J. C., et al. (2021). Characterization of the exo-metabolome of the emergent phytopathogen Fusarium kuroshium sp. nov., a causal agent of Fusarium dieback. Toxins 13:268. doi: 10.3390/toxins13040268
Gutiérrez-Santa Ana, A., Carrillo-Cerda, H. A., Rodríguez-Campos, J., Kirchmayr, M. R., Contreras-Ramos, S. M., and Velázquez-Fernández, J. B. (2020a). Volatile emission compounds from plant growth-promoting bacteria are responsible for the antifungal activity against F. solani. 3 Biotech 10:292. doi: 10.1007/s13205-020-02290-6
Gutiérrez-Santa Ana, A., Carrillo-Cerda, H. A., Rodríguez-Campos, J., Velázquez-Fernández, J. B., Patrón-Soberano, O. A., and Contreras-Ramos, S. M. (2020b). Dynamics of volatilomes emitted during cross-talking of plant-growth-promoting bacteria and the phytopathogen, Fusarium solani. World J. Microbiol. Biotechnol. 36:152. doi: 10.1007/s11274-020-02928-w
Harrington, T. C., Aghayeva, D. N., and Fraedrich, S. W. (2010). New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111, 337–361. doi: 10.5248/111.337
Hilgers, F., Habash, S. S., Loeschcke, A., Ackermann, Y. S., Neumann, S., Heck, A., et al. (2021). Heterologous production of β-caryophyllene and evaluation of its activity against plant pathogenic fungi. Microorganisms 9:168. doi: 10.3390/microorganisms9010168
Hong, A. R., Yun, J. H., Yi, S. H., Lee, J. H., Seo, S. T., and Lee, J. K. (2018). Screening of antifungal microorganisms with strong biological activity against oak wilt fungus, Raffaelea quercus-mongolicae. J. For. Environ. Sci. 34, 395–404. doi: 10.7747/JFES.2018.34.5.395
Huang, Y. T., Skelton, J., and Hulcr, J. (2019). Multiple evolutionary origins lead to diversity in the metabolic profiles of ambrosia fungi. Fungal Ecol. 38, 80–88. doi: 10.1016/j.funeco.2018.03.006
Hulcr, J., and Dunn, R. R. (2011). The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc. R. Soc. B Biol. Sci. 278, 2866–2873. doi: 10.1098/rspb.2011.1130
Hulcr, J., Mann, R., and Stelinski, L. L. (2011). The scent of a partner: ambrosia beetles are attracted to volatiles from their fungal symbionts. J. Chem. Ecol. 37, 1374–1377. doi: 10.1007/s10886-011-0046-x
Ibarra-Juárez, L. A., Burton, M. A. J., Biedermann, P. H. W., Cruz, L., Desgarennes, D., Ibarra-Laclette, E., et al. (2020). Evidence for succession and putative metabolic roles of fungi and bacteria in the farming mutualism of the ambrosia beetle Xyleborus affinis. MSystems 5, e00541–e00520. doi: 10.1128/mSystems.00541-20
Ibarra-Juárez, L. A., Desgarennes, D., Vázquez-Rosas-Landa, M., Villafan, E., Alonso-Sánchez, A., Ferrera-Rodríguez, O., et al. (2018). Impact of rearing conditions on the ambrosia beetle's microbiome. Life 8:63. doi: 10.3390/life8040063
Inch, S. A., and Ploetz, R. C. (2012). Impact of laurel wilt, caused by Raffaelea lauricola, on xylem function in avocado, Persea americana. For. Pathol. 42, 239–245. doi: 10.1111/j.1439-0329.2011.00749.x
Jaber, L. R., and Alananbeh, K. M. (2018). Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol. Control 126, 117–126. doi: 10.1016/j.biocontrol.2018.08.007
Kagezi, G., Kucel, P., Olal, S., Pinard, F., Seruyange, J., Musoli, P., et al. (2015). In vitro inhibitory effect of selected fungicides on mycelial growth of ambrosia fungus associated with the black coffee twig borer, Xylosandrus compactus Eichhoff (Coleoptera: Curculionidae) in Uganda. Afr. J. Agric. Res. 10, 2322–2328. doi: 10.5897/AJAR12.1705
Kasson, M. T., O'Donnell, K., Rooney, A. P., Sink, S., Ploetz, R. C., Ploetz, J. N., et al. (2013). An inordinate fondness for Fusarium: Phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet. Biol. 56, 147–157. doi: 10.1016/j_fgb.2013.04.004.
Kim, Y. J., Kim, J. H., and Rho, J. Y. (2019). Antifungal activities of Streptomyces blastmyceticus strain 12-6 against plant pathogenic fungi. Mycobiology 47, 329–334. doi: 10.1080/12298093.2019.1635425
Kreutz, J., Vaupel, O., and Zimmermann, G. (2004). Efficacy of Beauveria bassiana (Bals.) Vuill. against the spruce bark beetle, Ips typographus L., in the laboratory under various conditions. J. Appl. Entomol. 128, 384–389. doi: 10.1111/j.1439-0418.2004.00813.x
Kuhns, E. H., Tribuiani, Y., Martini, X., Meyer, W. L., Peña, J., Hulcr, J., et al. (2014). Volatiles from the symbiotic fungus Raffaelea lauricola are synrgistic with Manuka lures for increased capture of the Redbay ambrosia beetle Xyleborus glabratus. Agric. For. Entomol. 16, 87–94. doi: 10.1111/afe.12037
Lira-Noriega, A., Soberón, J., and Equihua, J. (2018). Potential invasion of exotic ambrosia beetles Xyleborus glabratus and Euwallacea sp. in Mexico: a major threat for native and cultivated forest ecosystems. Sci. Rep. 8:10179. doi: 10.1038/_s41598-018-28517-4
Lynch, S. C., Twizeyimana, M., Mayorquin, J. S., Wang, D. H., Na, F., Kayim, M., et al. (2016). Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.— two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 108, 313–329. doi: 10.3852/15-063
Malfanova, N., Franzil, L., Lugtenberg, B., Chebotar, V., and Ongena, M. (2012). Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch. Microbiol. 194, 893–899. doi: 10.1007/s00203-012-0823-0
Mann, A. J., and Davis, T. S. (2021). Entomopathogenic fungi to control bark beetles: a review of ecological recommendations. Pest Manag. Sci. 77, 3841–3846. doi: 10.1002/ps.6364
Mayfield, A. E. III., Barnard, E. L., Smith, J. A., Bernick, S. C., Eickwort, J. M., and Dreaden, T. J. (2008). Effect of propiconazole on laurel wilt disease development in redbay trees and on the pathogen in vitro. Arboricult. Urban For. 34, 317–324. Available online at: https://www.avocadosource.com/papers/Research_Articles/MayfieldAlbert2008c.pdf
Mendes, R., Kruijt, M., De Bruijn, I., Dekkers, E., van der Voort, M., Schneider, J. H., et al. (2011). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100. doi: 10.1126/science.1203980
Merel, D., Savoie, J.-M., Mata, G., Salmones, D., Ortega, C., Atanasova, V., et al. (2020). Methanolic extracts from cultivated mushrooms affect the production of fumonisins B and fusaric acid by Fusarium verticillioides. Toxins 12:366. doi: 10.3390/toxins12060366
Na, F., Carrillo, J. D., Mayorquin, J. S., Ndinga-Muniania, C., Stajich, J. E., Stouthamer, R., et al. (2018). Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of Kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause Fusarium dieback on woody host species in California. Plant Dis. 102, 1154–1164. doi: 10.1094/PDIS-07-17-1042-RE
Ocampo-Hernández, J. A., Tamez-Guerra, P., Pineda, S., Tamayo-Mejía, F., Guzmán-Franco, A., Figueroa de la Rosa, J. I., et al. (2011). Susceptibility of the Mexican bean beetle Epilachna varivestis Mulsant (Coleoptera: Coccinellidae) to endemic isolates of Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Clavicipitaceae). J. Pest Sci. 84, 471–477. doi: 10.1007/s10340-011-0369-y
Olatinwo, R., and Fraedrich, S. (2019). An acaromyces species associated with bark beetles from southern pine has inhibitory properties against Raffaelea lauricola, the causal pathogen of laurel wilt disease of Redbay. Plant Heal. Prog. 20, 220–228. doi: 10.1094/PHP-06-19-0039-RS
Orozco-Mosqueda, M. C., Rocha-Granados, M. C., Glick, B. R., and Santoyo, G. (2018). Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 208, 25–31. doi: 10.1016/j.micres.2018.01.005
Ossowicki, A., Jafra, S., and Garbeva, P. (2017). The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 12:e0174362. doi: 10.1371/journal.pone.0174362
Paap, T., De Beer, Z. W., Migliorini, D., Nel, W. J., and Wingfield, M. J. (2018). The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: a new invasion in South Africa. Australas. Plant Pathol. 47, 231–237. doi: 10.1007/s13313-018-0545-022
Paine, T. D., Hanlon, C. C., and Byrne, F. J. (2011). Potential risks of systemic imidacloprid to parasitoid natural enemies of a cerambycid attacking Eucalyptus. Biol. Control 56, 175–178. doi: 10.1016/j.biocontrol.2010.08.007
Pineda, A., Kaplan, I., Hannula, S. E., Ghanem, W., and Bezemer, T. M. (2020). Conditioning the soil microbiome through plant–soil feedbacks suppresses an aboveground insect pest. New Phytol. 226, 595–608. doi: 10.1111/nph.16385
Poveda, J. (2021). Trichoderma as biocontrol agent against pests: new uses for a mycoparasite. Biol. Control 159:104634. doi: 10.1016/j.biocontrol.2021.104634
Qiu, H. L., Fox, E. G. P., Qin, C. S., Zhao, D. Y., Yang, H., and Xu, J. Z. (2019). Microcapsuled entomopathogenic fungus against fire ants, Solenopsis invicta. Biol. Control 134, 141–149. doi: 10.1016/j.biocontrol.2019.03.018
Ranger, C. M., Biedermann, P. H. W., Phuntumart, V., Beligala, G. U., Ghosh, S., Palmquist, D. E., et al. (2018). Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl. Acad. Sci. U.S.A. 115, 4447–4452. doi: 10.1073/pnas.1716852115
Ranger, C. M., Dzurenko, M., Barnett, J., Geedi, R., Castrillo, L., Ethington, M., et al. (2021). Electrophysiological and behavioral responses of an ambrosia beetle to volatiles of its nutritional fungal symbiont. J. Chem. Ecol. 47, 463–475. doi: 10.1007/s10886-021-01263-0
Reverchon, F., García-Quiroz, W., Guevara-Avendaño, E., Solís-García, I. A., Ferrera-Rodríguez, O., and Lorea-Hernández, F. (2019). Antifungal potential of Lauraceae rhizobacteria from a tropical montane cloud forest against Fusarium spp. Braz. J. Microbiol. 50, 583–592. doi: 10.1007/s42770-019-00094-2
Reverchon, F., and Méndez-Bravo, A. (2021). “Plant-mediated above- belowground interactions: a phytobiome story,” in Plant-Animal Interactions: Source of Biodiversity, eds K. Del-Claro and H. M. Torezan-Silingardi (Cham: Springer), 205–231. doi: 10.1007/978-3-030-66877-8_8
Rincón-Enríquez, G., Ibarra-Juárez, L. A., Trinidad-Cruz, J., and Quiñones-Aguilar, E. E. (2020). Control biológico de escarabajos ambrosiales con actinobacterias. Rev. Mex. Fitopatol. 38 S87. Available online at: https://www.smf.org.mx/rmf/suplemento/suplemento382020/Suplemento_2020_RMF_FINAL.pdf
Robles, C. A., Ceriani-Nakamurakare, E., Slodowicz, M., González-Audino, P., and Carmarán, C. C. (2018). Granulobasidium vellereum (Ellis andCragin) Jülich, a promising biological control agent. Biol. Control 117, 99–108. doi: 10.1016/j.biocontrol.2017.10.012
Rojas-Solis, D., Vences-Guzmán, M. A., Sohlenkamp, C., and Santoyo, G. (2020). Bacillus toyonensis COPE52 modifies lipid and fatty acid composition, exhibits antifungal activity, and stimulates growth of tomato plants under saline conditions. Curr. Microbiol. 77, 2735–2744. doi: 10.1007/s00284-020-02069-1
Rugman-Jones, P. F., Au, M., Ebrahimi, V., Eskalen, A., Gillett, C. P., Honsberger, D., et al. (2020). One becomes two: second species of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex is established on two Hawaiian Islands. PeerJ 8:e9987. doi: 10.7717/peerj.9987
Santoyo, G., Orozco-Mosqueda, M. D. C., and Govindappa, M. (2012). Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci. Technol. 22, 855–872. doi: 10.1080/09583157.2012.694413
Segaran, G., and Sathiavelu, M. (2019). Fungal endophytes: a potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol. 21:101284. doi: 10.1016/j.bcab.2019.101284
Shapiro-Ilan, D. I., Ramakuwela, T., Bock, C. H., Hatting, J., Vega, F. E., Wells, L., et al. (2020). Initial studies on beneficial fungi that can live inside pecan trees and provide protection from insects and disease. Pecan Grower 23, 50–58. Available online at: https://www.sappa.za.org/wp-content/uploads/docs/2020/11/Shapiro-Ilan-et-al-Pecan-Grower-endophyte-article-2020-jh-27-Oct.pdf
Strobel, G. A., Dirkse, E., Sears, J., and Markworth, C. (2001). Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147, 2943–2950. doi: 10.1099/mic.0.032540-0
Terra, W. C., Campos, V. P., Martins, S. J., Costa, L. S. A. S., da Silva, J. C. P., Barros, A. F., et al. (2018). Volatile organic molecules from Fusarium oxysporum strain 21 with nematicidal activity against Meloidogyne incognita. Crop Protec. 106, 125–131. doi: 10.1016/j.cropro.2017.12.022
Théatre, A., Cano-Prieto, C., Bartolini, M., Laurin, Y., Deleu, M., Niehren, J., et al. (2021). The surfactin-like lipopeptides from Bacillus spp.: natural biodiversity and synthetic biology for a broader application range. Front. Bioeng. Biotechnol. 9:118. doi: 10.3389/fbioe.2021.623701
Torres, M. J., Brandan, C. P., Sabat,é, D. C., Petroselli, G., Erra-Balsells, R., and Audisio, M. C. (2017). Biological activity of the lipopeptide- producing Bacillus amyloliquefaciens PGPBacCA1 on common bean Phaseolus vulgaris L. pathogens. Biol. Control 105, 93–99. doi: 10.1016/j.biocontrol.2016.12.001
Tuesta-Popolizio, D. A., Velázquez-Fernández, J. B., Rodriguez-Campos, J., and Contreras-Ramos, S. M. (2021). Isolation and identification of extremophilic bacteria with potential as plant growth promoters (PGPB) of a geothermal site: a case study. Geomicrobiol. J. 38, 436–450. doi: 10.1080/01490451.2021.1879972
Tuncer, C., Kushiyev, R., Erper, I., Ozdemir, I. O., and Saruhan, I. (2019). Efficacy of native isolates of Metarhizium anisopliae and Beauveria bassiana against the invasive ambrosia beetle, Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egypt. J. Biol. Pest Control 29, 1–6. doi: 10.1186/s41938-019-0132-x
Verma, D. K., Ramírez Guzmán, K. N., Mohapatra, B., Talukdar, D., Chávez-González, M. L., Kumar, V., et al. (2021). “Recent trends in plant- and microbe-based biopesticide for sustainable crop production and environmental security,” in Recent Developments in Microbial Technologies, eds R. Prasad, V. Kumar, J. Singh, and C. P. Upadhyaya (Singapore: Springer), 1–37. doi: 10.1007/978-981-15-4439-2_1
Vorholt, J. A. (2012). Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840. doi: 10.1038/nrmicro2910
Wang, C., and Wang, S. (2017). Insect pathogenic fungi: genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol. 62, 73–90. doi: 10.1146/annurev-ento-031616-035509
Xu, L., Deng, J., Zhou, F., Cheng, C., Zhang, L., Zhang, J., et al. (2019). Gut microbiota in an invasive bark beetle infected by a pathogenic fungus accelerates beetle mortality. J. Pest Sci. 92, 343–351. doi: 10.1007/s10340-018-0999-4
Yalage Don, S. M., Schmidtke, L. M., Gambetta, J. M., and Steel, C. C. (2020). Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 10:4498. doi: 10.1038/s41598-020-61471-8
Zhang, J., Wei, L., Yang, J., Ahmed, W., Wang, Y., Fu, L., et al. (2020). Probiotic consortia: reshaping the rhizospheric microbiome and its role in suppressing root-rot disease of Panax notoginseng. Front. Microbiol. 11:701. doi: 10.3389/fmicb.2020.00701
Zhang, L. W., Liu, Y. J., Yao, J., Wang, B., Huang, B., Li, Z. Z., et al. (2011). Evaluation of Beauveria bassiana (Hyphomycetes) isolates as potential agents for control of Dendroctonus valens. Insect Sci. 18, 209–216. doi: 10.1111/j.1744-7917.2010.01361.x
Zhou, Y., Avery, P. B., Carrillo, D., Duncan, R. H., Lukowsky, A., Cave, R. D., et al. (2018). Identification of the Achilles heels of the laurel wilt pathogen and its beetle vector. Appl. Microbiol. Biotechnol. 102, 5673–5684. doi: 10.1007/s00253-018-9037-y
Zimmermann, G. (2007a). Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 17, 553–596. doi: 10.1080/09583150701309006
Keywords: antifungal activity, bioactive microbial products, entomopathogenic fungi, microbiome engineering, Trichoderma
Citation: Reverchon F, Contreras-Ramos SM, Eskalen A, Guerrero-Analco JA, Quiñones-Aguilar EE, Rios-Velasco C and Velázquez-Fernández JB (2021) Microbial Biocontrol Strategies for Ambrosia Beetles and Their Associated Phytopathogenic Fungi. Front. Sustain. Food Syst. 5:737977. doi: 10.3389/fsufs.2021.737977
Received: 08 July 2021; Accepted: 11 August 2021;
Published: 07 September 2021.
Edited by:
Nicolas Desoignies, HEPH Condorcet, BelgiumReviewed by:
Martin Hill, Rhodes University, South AfricaGitanjali Devi, Assam Agricultural University, India
Copyright © 2021 Reverchon, Contreras-Ramos, Eskalen, Guerrero-Analco, Quiñones-Aguilar, Rios-Velasco and Velázquez-Fernández. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frédérique Reverchon, frederique.reverchon@inecol.mx
 Frédérique Reverchon
Frédérique Reverchon Silvia M. Contreras-Ramos
Silvia M. Contreras-Ramos Akif Eskalen3
Akif Eskalen3  José A. Guerrero-Analco
José A. Guerrero-Analco Evangelina E. Quiñones-Aguilar
Evangelina E. Quiñones-Aguilar Claudio Rios-Velasco
Claudio Rios-Velasco