A single isolated LGR5+ CBC stem cell can be cultured in vitro into three dimensional intestinal epithelial organoids containing the original phenotype and function of the in vivo epithelium[1],[2]. Unfortunately, the current culture medium Matrigel suffers from major drawbacks including sample variety and pathogenic sources[3],[4]. Therefore, the urge to find an artificial Extracellular Matrix (ECM) is extremely high. Multiple small components of the ECM can be mimicked by the introduction of short bioactive peptide sequences onto a solid scaffold[5]. Supramolecular hydrogels are attractive ECM mimics, since their inherent dynamics allows reciprocal interactions with cells, for example, by the clustering of bioactive cues enhancing the cell attachment[5],[6]. Here we present two diverse supramolecular platforms based on ureidopyrimidinone and benzene-1,3,5-carboxamide (see Figure 1), which were functionalized with laminin derived peptides to provide functional cues for intestinal stem cell attachment.
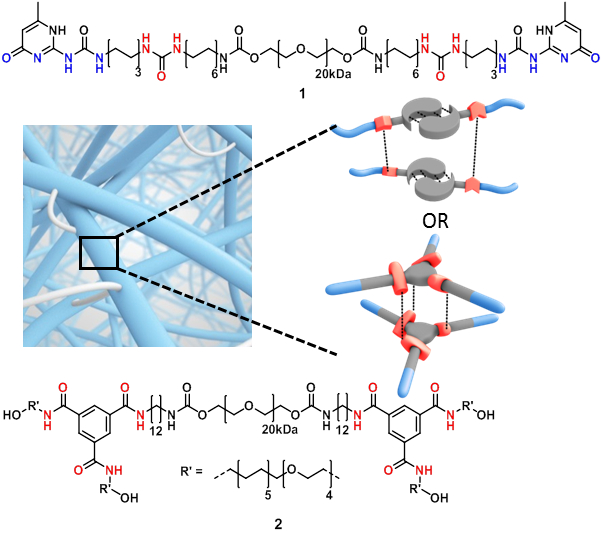
Different supramolecular hydrogels were prepared by varying the concentration of laminin derived supramolecular building blocks with telechelic hydrogelators (1) or (2). The bioactive supramolecular polymers were synthesized using two different approaches; an on-resin approach and a copper catalyzed click reaction strategy. First, 4 laminin derived peptides, developed by Nomizu and Murayama[7],[8], were synthesized using solid phase peptide synthesis using Fmoc chemistry (Figure 2, peptides a-d). For the on-resin approach, an UPy molecule end-functionalized with a carboxylic acid was synthesized and coupled onto the peptide resin resulting in the formation of an amide bond (conjugates 3a-d). In the other strategy, a BTA molecule bearing a monofunctional azide was synthesized, and the peptides were equipped with alkyne functionalities, followed by copper catalyzed cycloaddition resulting in triazole formation (conjugates 4a-d). Subsequently, different compositions of hydrogels were prepared to study the expansion of intestinal stem cells compared to Matrigel. Three concentrations of laminin functionalized supramolecular building blocks (0, 0.5 and 5%) were mixed in 20 wt% of the telechelic hydrogelators (1) and (2) using pH and/or temperature switch resulting in visco-elastic materials. Intestinal stem cells were seeded onto the hydrogels, diluting the hydrogels to 10 wt%, and were imaged after 7 days, counting the expanded cells.
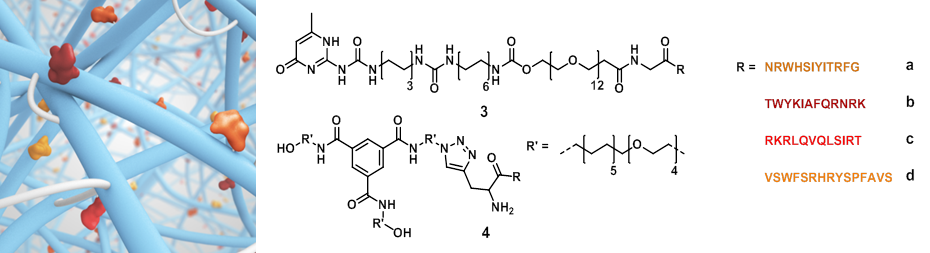
While intestinal stem cells are highly sensitive, they remained viable for over a week, however, no significant expansion was observed. A blocking assay with the individual peptides bearing no supramolecular moieties was performed to study the cell attachment, revealing that only 2 instead of the 4 peptides used were able to sufficiently bind to the cell surface. Presumably, the bioactive guest presentation is not optimal and/or the scaffolds are too dynamic, failing to support sufficient expansion of intestinal stem cells.
Even though much effort has been made to develop a dynamic hydrogel system comprising functional cues, it remains extremely challenging to find the right components and conditions to drive stem cell expansion. The natural ECM consists of many interacting components with diverse functionalities, requiring optimal tuning and functionlization of synthetic systems. The incorporation of growth factor binding sites might bring us a step further.
References:
[1] Sato, T. & Clevers, H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science 340, 1190-1194 (2013).
[2] Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262-265 (2009).
[3] Hughes, C. S., Postovit, L. M. & Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886‐1890 (2010).
[4] Kleinman, H. K. & Martin, G. R. Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology 15 (5), 378-386 (2005).
[5] Boekhoven, J. & Stupp, S. I. 25th Anniversary Article: Supramolecular Materials for Regenerative Medicine. Advanced Materials 26 (11), 1642-1659 (2014).
[6] Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335 (6070), 813-817 (2012).
[7] Nomizu, M. et al. Identification of Cell Binding Sites in the Laminin α1 Chain Carboxyl-terminal Globular Domain by Systematic Screening of Synthetic Peptides. Journal of Biological Chemistry. 270 (35), 20583‐20590 (1995).
[8] Murayama, O., Nishida, H. & Sekiguchi, K. Novel peptide ligands for integrin alpha 6 beta 1 selected from a phage display library. Journal of Biochemistry 120 (2), 445‐451 (1996).