The Influence of Apical and Basal Defoliation on the Canopy Structure and Biochemical Composition of Vitis vinifera cv. Shiraz Grapes and Wine
- School of Agriculture and Food, University of Melbourne, Parkville, VIC, Australia
Defoliation is a commonly used viticultural technique to balance the ratio between grapevine vegetation and fruit. Defoliation is conducted around the fruit zone to reduce the leaf photosynthetic area, and to increase sunlight exposure of grape bunches. Apical leaf removal is not commonly practiced, and therefore its influence on canopy structure and resultant wine aroma is not well-studied. This study quantified the influences of apical and basal defoliation on canopy structure parameters using canopy cover photography and computer vision algorithms. The influence of canopy structure changes on the chemical compositions of grapes and wines was investigated over two vintages (2010–2011 and 2015–2016) in Yarra Valley, Australia. The Shiraz grapevines were subjected to five different treatments: no leaf removal (Ctrl); basal (TB) and apical (TD) leaf removal at veraison and intermediate ripeness, respectively. Basal leaf removal significantly reduced the leaf area index and foliage cover and increased canopy porosity, while apical leaf removal had limited influences on canopy parameters. However, the latter tended to result in lower alcohol level in the finished wine. Statistically significant increases in pH and decreases in TA was observed in shaded grapes, while no significant changes in the color profile and volatile compounds of the resultant wine were found. These results suggest that apical leaf removal is an effective method to reduce wine alcohol concentration with minimal influences on wine composition.
Introduction
Climate change has already had many impacts on the global wine industry, some of them that could be considered beneficial or detrimental depending on the growing region (Jones and Webb, 2010; Mira De Orduna, 2010; Mozell and Thach, 2014). The phenological development of grapevines is altered mainly due to the changes in global temperature, carbon dioxide concentration [CO2], and solar radiation, leading to compression of phenological stages, accelerated grape maturation and earlier harvest dates (Mira De Orduna, 2010). One of the major consequences to grape chemistry is the elevated berry sugar level, which is mainly caused by excessive evaporative loss of water, rather than increased photosynthesis (Keller, 2010; Mozell and Thach, 2014). Elevated wine alcohol levels are reported in major existing wine regions of the world (Jones, 2007; Van Leeuwen and Darriet, 2016). Increasing grape sugar and consequently wine alcohol levels is deemed undesirable for the wine industry, which can be related to a range of problems, such as: stuck alcoholic fermentation, increased levels of acetic acid, higher energy requirements for cooling, generation of unwanted by-products, alteration of wine sensory profile, potential influences on human health and reducing competitiveness due to alcohol associated taxation (Erasmus et al., 2003; Coulter et al., 2008; Mira De Orduna, 2010; Ashenfelter and Storchmann, 2016). Thus, techniques controlling sugar concentration in grapes and wine alcohol levels with minimal influences on wine biochemical profile are favored by the wine industry in general.
Four major approaches may be used to reduce alcohol levels in wine: (i) microbiological interventions; (ii) genetic engineering of fermenting yeast; (iii) physical/enzymatic treatment of grapes and wine and (iv) agronomical management in the vineyard. The microbiological approach selects yeast strains with lower ethanol yield during the alcoholic fermentation. Milanovic et al. (2012) observed reduced ethanol production with the co-fermentation of immobilized Strarmerella bombicola and Saccharomyces cerevisiae. Genetic engineering techniques could control the genes expression of S. cerevisiae, and therefore convert sugar into other by-product and create new yeast strains (Ozturk and Anli, 2014).
Physical or enzymatic removal of ethanol can also effectively reduce alcohol level in wine. For example, membrane-based systems are now commonly used in new world wine countries, however, may have some adverse impacts on the aroma of wine (Diban et al., 2008). On the other hand, adding the enzyme glucose oxidase (GOX) during fermentation from the fungus Aspergillus niger catalyzes the conversion of glucose into gluconic acid and hydrogen peroxide, thus leading to lower alcohol in the resultant wine (Biyela et al., 2009; Ozturk and Anli, 2014), but this intervention is controlled in many winegrowing regions.
Grapevine canopy management has been commonly used to control sugar accumulation during grape maturation to achieve lower alcohol wines. The rate of sugar accumulation in berries is largely dependent on the ratio of leaf area to fruit weight (LA/FW), and lower sugar accumulation in grape berries can be achieved by reducing leaf area (Ozturk and Anli, 2014). The influences of leaf trimming on grape biochemical composition has been researched on many grapevine cultivars, while limited research has been conducted on V. vinifera cv. Shiraz, which is one of the major cultivars planted in Australia (Kozina et al., 2008; Lanari et al., 2013; Baiano et al., 2015; Feng et al., 2015; Osrečak et al., 2016). In addition, defoliation studies tend to focus on bunch zone leaf removal, which not only modifies the LA/FW ratio, but also increases the direct solar exposure and alter bunch zone microclimate (Spayd et al., 2002). The influence of apical leaf trimming on the canopy structure and biochemical composition of resultant grapes and wines has not been well-studied.
The objective of this research was to quantify the impacts of apical and basal leaf removal at different grapevine phenological stages on the canopy structure of grapevines. Furthermore, this research investigates the consequences of leaf removal on the biochemical composition of grape berries and wine in V. vinifera cv. Shiraz. The findings of this project provide vineyard managers an alternative canopy management method to manipulate grape sugar accumulation and to reduce wine alcohol levels with minimal influences on the wine volatile profile.
Materials and Methods
Vineyard Site
This study was conducted on a commercial Shiraz (Vitis vinifera cv.) vineyard located at Coldstream, Victoria, Australia (latitude −37.72, longitude 145.41, elevation 83 m) in two contrasting growing seasons (2010–2011 and 2015–2016) The weather data was obtained from the closest Bureau of Meteorology weather station (~1.5 km; BOM weather station No. 086383). The mean January temperature (MJT) and total rainfall from budburst (October) to harvest for the two studied growing seasons were 20.4°C; 706.8 mm and 20.7°C; 204 mm, respectively. Grapevines were trained using a vertical shooting positioning trellis system. All vine rows were orientated from north to south. A total of 25 panels of vines were established in each row with 4 vines in each panel. Vine spacing was 2.8 m between rows and 1.8 m between vines. Canopy management practices were restricted to the treatments from this trial. Other agronomical management practices were applied with normal commercial standards. No specific pest and disease pressure was observed during both experimental seasons.
Experimental Design and Berry Measurements
Vitis vinifera L. cv. Shiraz grapevines included in this study were subjected to five treatments: (i) no defoliation treatment (Ctrl); (ii) 5–7 basal leaves surrounding bunches removed from each shoot at Veraison (0–50%) (TB-v) or at mid ripeness (TB-m); (iii) seven fully expanded apical leaves removed from each shoot at Veraison (0–50%) (TD-v) or mid ripeness (TD-m) in the 2015–2016 growing season, and only Ctrl and TA-v treatment in the 2011–2012 growing season. Five replicates were conducted for each treatment with one panel of grapevines as one individual replicate in the 2015–2016 growing season, while 12 replicates were conducted in 2011–2012, following a completely random block design.
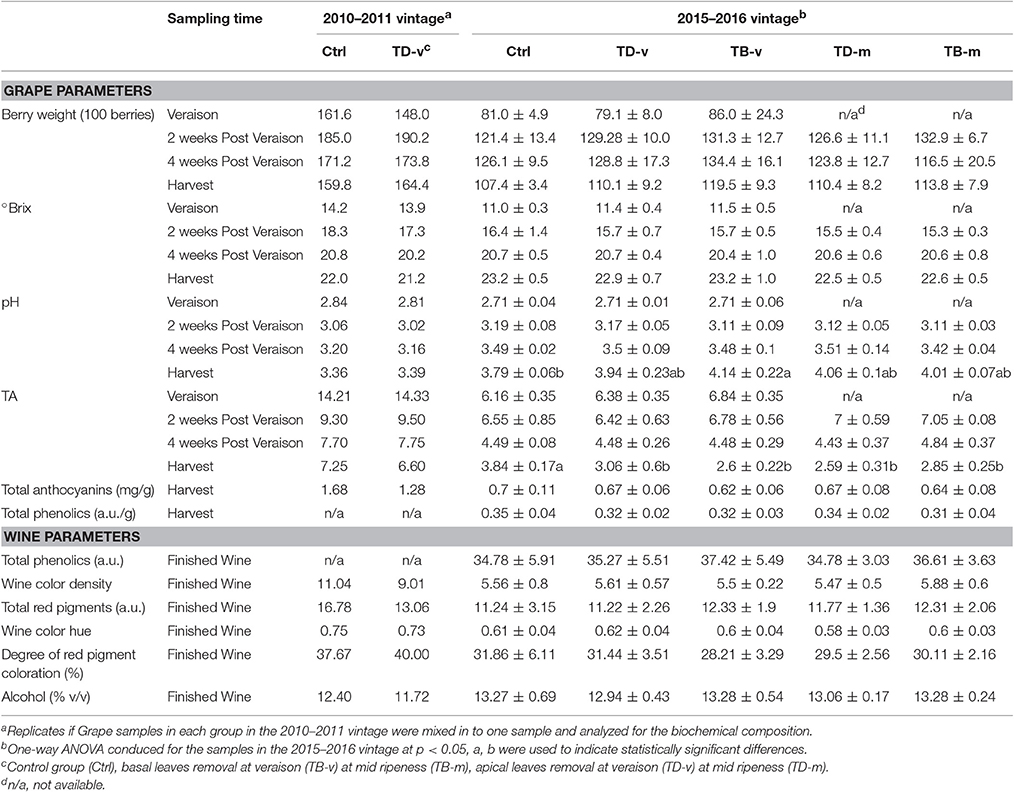
Grape samples (200 g per replicate) were collected in zip-lock plastic bags before treatment at baseline, then fortnightly since treatment until commercial harvest on 5th April 2011 and 2nd March 2016. For each sampling date, fruit was transferred to the laboratory of the winery for immediately analysis in the same day (2011–2012). Samples were stored at −20°C in the winery laboratory, and later on transferred the University of Melbourne in Styrofoam boxes on dry ice, then stored at −20°C until analysis (2015–2016). In the 2011–2012 growing season, grape samples from individual replicates were blended together and subjected to chemical analysis. Total soluble solid (TSS, °Brix), pH, titratable acidity (TA), total anthocyanins, and total phenolics were analyzed using a refractometer, pH meter, alkaline titration, and spectrophotometer, respectively, following the published protocol (Iland, 2004).
Leaf Area Index (LAI) and Canopy Structure Measurements
All the experimental replicates were subjected to canopy structure measurements at mid-ripeness after defoliation treatments in the 2015–2016 growing season following the canopy cover photography method (Fuentes et al., 2008, 2014). For this, an iPhone 5s (Apple lnc. Cupertino, CA, USA) was used to acquire digital images of grapevine canopy using the back camera. Upward-looking images were obtained from under the grapevine canopies at 0° zenith angle and around 20 cm above ground. Three images were obtained from each treated panel around the middle grapevine. Images were labeled and saved in fine JPEG format. The images collected were then analyzed using computer vision algorithms through a customized code written in Matlab. v2015b (Mathworks Inc., Matick, MA, USA; Fuentes et al., 2008, 2014).
Microvinification
Additional 2 kg of grape bunches were collected from each replicate at harvest and cooled down to 4°C on dry ice to be transferred to the laboratory. Small scale fermentation was performed following the protocol established by the Irymple Research Centre of the Department of Economics, Development, Jobs, Transport, and Resources of the Victorian Government (DEDJTR) as described earlier (Kilmister et al., 2014). Briefly, harvested grapes were destemmed, crushed in sanitized 1.5–2 L containers, pH of the musts were adjusted to 3.4–3.5 with tartaric solution (10%), then diammonium phosphate (10%) at 1 ml/L of juice was added plus commercial yeast at 0.2 g/L (EC1118). Primary fermentation in microvinification were done at 18°C in a temperature controlled room for 7 days until Baume readings reached 1–2 for pressing using a ratchet style press. Wines were transferred into clean 500 ml glass bottles, with addition of potassium metabisulfite (10%) at 0.5 ml/L and copper sulfate (0.4%) at 1 ml/L, and placed in at 16°C controlled cool room for 14 days. After, wines were racked into new bottles filled with CO2, and placed in a 1–2°C cool room for stabilization. Wines were then bottled into clean 375 ml bottles and placed in 14°C cool room until analysis.
Preparation of Samples and Headspace Solid-Phase Microextraction and Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS) Analysis of Wine Volatiles
Wine samples were prepared for wine volatile analysis based on the protocol proposed by Siebert et al. (2005) with some modifications. For this, 1 ml of wine samples were diluted with 9 ml of milli Q water into HS-SPME vial (Agilent Technologies, 20 ml) with addition of 2 g of sodium chloride and 200 μL of 4-octanol (Internal standard; 10 mg/L) and ethyl non-anoate (quality control; 10 ml/L). The vial and its contents were shaken at 220 rpm and heated to 35°C. A polydimethylsiloxane (PDMS, Agilent) 100 μm fiber was exposed to the headspace and agitated for 10 min.
An Agilent Technologies 6850 series II (GC; Agilent Technologies, Santa Clara, CA) equipped with an Agilent PAL 120 multipurpose auto-sampler and coupled with an Agilent 5,873 mass selective detector (MSD) was used for volatile assessments. The instruments were controlled using Agilent G1701EA MSC ChemStation software in conjunction with Agilent PAL Sampler Software Control B.01.04 for ChemStation. The GC was fitted with a J&W DB-wax column (30 m × 0.25 mm, 0.25 μm film df) with helium as carrier gas (ultrahigh purity, BOC Australia, North Ryde, NSW, Australia), and the flow rate was 2.0 ml/min in constant flow mode. The GC inlet was fitted with a borosilicate glass SPME inlet liner (Agilent, 6.3 mm o.d., 78.5 mm length) held at 220°C.
The SPME fiber was desorbed in the pulsed splitless mode and the splitter at 50:1, was opened after 30 s. The fiber was allowed to bake in the inlet for 10 min. The oven was started at 40°C, held for 4 min, then increased to 220°C at 5°C, and held for 20 min. The MS source, quadruple and transfer line was held at 230°C, 106°C, and 250°C, respectively. The MS was operated in positive EI mode at 70 eV with scanning over a mass acquisition range of 35–350 m/z. The standards solution of 62 commonly found compounds in wine were prepared at 13 scales dilutions in model wine solution (13% alcohol, titrate buffer, pH 3.2), and analyzed using the GC as wine samples to generate standard calibration curves for volatiles quantification. Wine volatiles were identified by comparing the mass spectra and retention indices with the NIST library in ChemStation and NIST Chemistry Webbook database and standard solutions obtained. All compounds were quantified based on GC peak ratio of individual compounds and internal standard, and the calibration curves generated from the standard solutions. The concentration of ethyl non-anoate (Quality control), blank SPME runs and blank internals standards were checked regularly.
Statistical Analysis
The grapevine canopy structure parameters, grape and wine biochemical test results and wine volatiles of different treatment groups were subjected to one-way analysis of variance (ANOVA) at p < 0.05 significance level (CoStat, version 6.4, CoHort software, Monterey, USA).
Results and Discussion
Leaf Area Index Analysis
The impacts of defoliation on the canopy structure of the grapevine was quantified using leaf area index (LAI), foliage projective cover (Ff), crown cover (Fc) and porosity (ϕ). Statistically significant lower LAI, Ff and higher ϕ were observed in basal defoliation groups (TB-v and TB-m), compared to the non-defoliated group (Ctrl; Figure 1). This study clearly quantifies the impacts of basal defoliation, with around 33% reduction in LAI, 27% reduction in Ff and 23% increase in ϕ (Figure 1). However, no significant differences were observed between Ctrl and apical defoliation groups (TA-v and TA-m) in the same parameters. No significant differences were observed amongst all experimental groups in Fc in the 2015–2016 growing season.
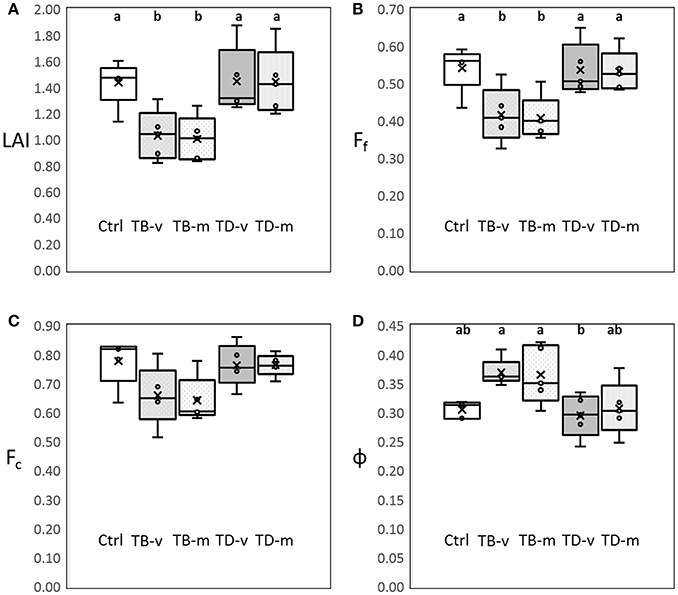
Figure 1. Influence of leaf trimming on canopy structure parameters measured at mid-ripeness in the 2015–2016 growing season: (A) leaf area index (LAI); (B) Foliage projective cover (Ff); (C) Crown cover (Fc); (D) Porosity (ϕ) (p < 0.05), no unites for each parameter. One-way ANOVA were conducted to compare different parameters at p < 0.05, and a, b were used to indicate statistically significant differences. Boxplot shows median value and standard deviation for each treatment groups. Control group (Ctrl), basal leaves removal at veraison (TB-v) at mid ripeness (TB-m), apical leaves removal at veraison (TD-v) at mid ripeness (TD-m).
LAI and Ff explains the area of leave tissue and plant foliage per unit ground surface, respectively, while ϕ explains the light penetration rate through the canopy (Fuentes et al., 2008, 2014). Basal defoliation decreases the leaf area in the central part of the canopy and increase light penetration, and therefore explains the decrease in LAI and Ff, and increase in ϕ. Minimal influences were observed in apical treatment groups, this was likely due to the VSP trellis system of the grapevine, where apical leaves mainly stay on top of the canopy. Therefore, apical defoliation has minimal impacts on grapevine canopy shading and canopy structure parameters. Fc represented area of the canopy per unit ground surface (Fuentes et al., 2008, 2014), and defoliation within the canopy does not influence the outer size of the canopy and therefore no significant differences were observed.
Grape and Wine Composition Analysis
Due to large variations amongst replicates, no statistically significant differences could be established among treatment groups in berry weigh and brix at the four sampling points (Table 1). However, there is a trend that apical defoliation tends to lead to slightly lower TSS (°Brix) at harvest in both seasons (2010–2011: Ctrl 22.00, TD-v 21.20; 2015–2016: Ctrl 23.2 ± 0.45, TD-v 22.9 ± 0.74, TD-m 22.5 ± 0.47). This trend can be confirmed by observing the alcohol strength (%v/v) of the finished wine (2010–2011: Ctrl 12.40, TD-v 11.72; 2015–2016: Ctrl 13.17 ± 0.69, TD-v 12.94 ± 0.43, TD-m 13.06 ± 0.17). Significant increases in the pH of harvested grape samples were observed in basal defoliation treatment at veraison compared to Ctrl (Table 1). Significantly decrease in TA was observed in all treatments in the 2015–2016 growing season and a similar trend was observed in the 2011–2012 growing season. The pH and TA was adjusted during winemaking process, and therefore pH and TA of the wine were not compared amongst experimental groups for finished wine. No significant changes were observed amongst experimental groups in the total anthocyanins and phenolics of grape berries at harvest in the 2015–2016 growing season, which is further confirmed in the wine profile where no differences were observed in wine color density, total phenolics, red pigments, color hue, and degree of red pigment coloration. However, reduction in grape total anthocyanins, wine color density and total red pigments were observed in TD-v treatment group compared to Ctrl in the 2010–2011 growing season.
The impacts of defoliation on the composition of harvested grape has had varying reports and most of the research has focused on basal defoliation. Baiano et al. (2015) reported increased berry TSS and decreased berry TA, anthocyanins and phenolics in basal defoliation treatments at veraison and intermediate ripeness in Nero di Troia grapes. This is consistent to another study in Sauvignon Blanc and Riesling showing that basal leaf removal increase TSS and decrease TA and pH (Kozina et al., 2008). However, Di Profio et al. (2011) observed slightly increase of minimal changes in TSS, lower TA and higher pH in basal defoliation treatments at veraison in Merlot, Cabernet Franc and Cabernet Sauvignon. The same study also observed inconsistent results in berry anthocyanins and phenolics over three different vintages. A recent research reported that basal leaf removal at veraison did not significantly modify grape TSS, TA, anthocyanins and phenolics content in Merlot and Teran, but did slightly increase TSS and decrease TA in Plavac mali in one of the 2-year study (Osrečak et al., 2016). Similarly, another research in Merlot with basal leaf removal a pre-veraison showed no significantly differences in TSS, pH and TA, and increased anthocyanins in one season but decreased in another (King et al., 2012). Minimal changes in grape TSS, pH, and TA of basal defoliation treatment at pre-veraison and veraison were also observed in Pinot and Grenache (Tardáguila et al., 2008; Feng et al., 2015). Tardáguila et al. (2008) also reported minimal changes to total phenolics concent, while inconsistent results were observed over the 3 year trial in Pinot (Feng et al., 2015). Very few reports have studied the influences of apical leaf removal on grape content. Lanari et al. (2013) removed the apical part the canopy using mechanical leaf stripper at intermediate ripeness in Sangiovese and Montepulciano, and observed lower TSS in harvested grapes, which is consistent with the results presented here. The same study also observed lower anthocyanins and polyphenols in harvested Montepulciano, but not in Sangiovese. Another study in Sangiovese also observed lower berry TSS and wine alcohol level in treatment with defoliation in medium top part of the canopy, but minimal change in anthocyanins and phenolics, which is consistent to current results (Palliotti et al., 2013).
HS-SPME-GC-MS Analysis of Wine Volatiles
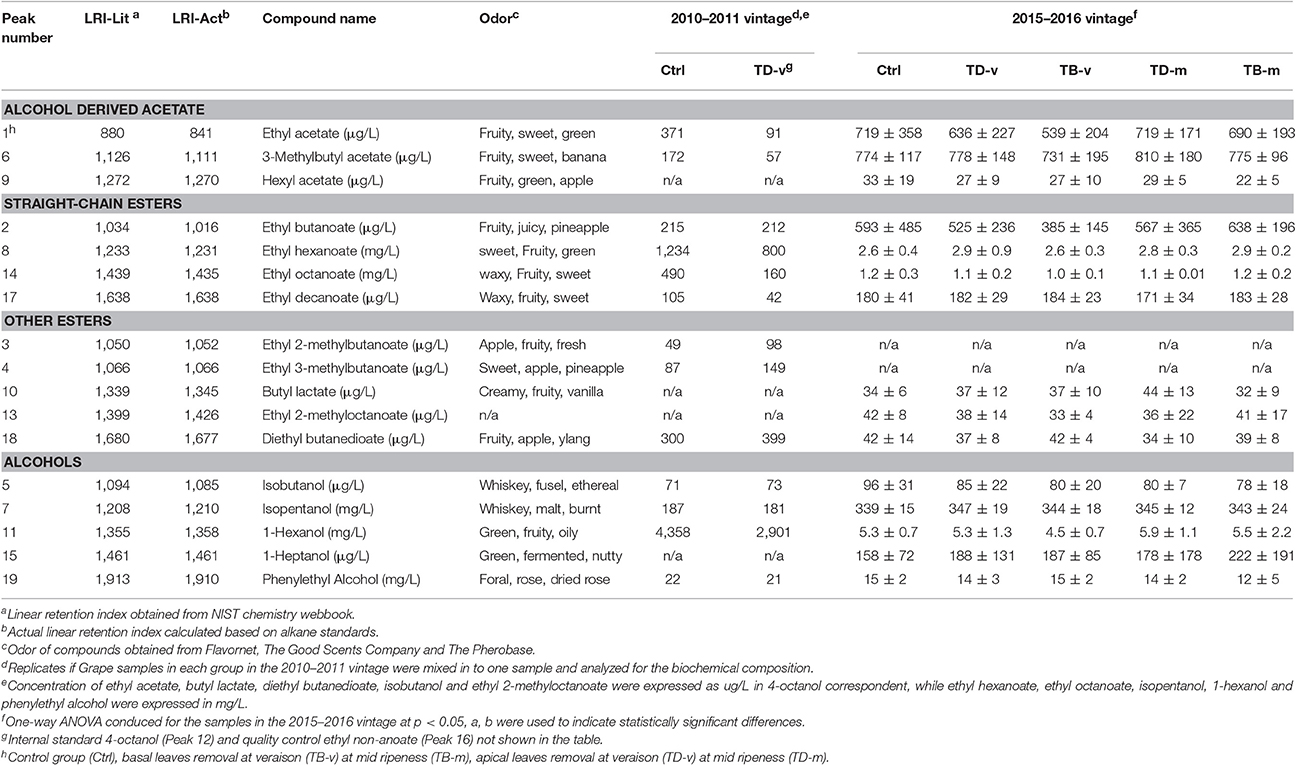
The volatile compounds in the wine samples of both vintage were analyzed using an HS-SPME-GC-MS method, which analyses 62 commonly found wine aroma compounds, and here only 17 volatile compounds were found of high concentrations in the sample wine (Table 2). The volatile compounds identified can classified as: fatty acid derived esters, other esters, alcohol derived acetate, and miscellaneous compounds. No statistically significant differences were observed amongst different treatment groups in the concentration of these volatile compounds, and large differences were observed between two vintages. Despite this, apical defoliaton resulted in much lower concentration of ethyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, 3-methylbutyl acetate, and 1-hexanol in 2010–2011 vintage, but fewer differences were observed between Ctrl and TD-v in the 2015–2016 vintage. Higher concentration of diethyl butanedioate, ethyl 2-methylbutanoate, and ethyl 3-methylbutanoate were also observed in the apical defoliation group at veraison in the 2010–2011 vintage. However, in the 2015–2016 vintage the concentration of diethyl butanedioate in TD-v was lower than that of Ctrl, while ethyl 2-methylbutanoate and ethyl 3-methylbutanoate were not detected. All defoliation treatments had slightly higher average concentration of 1-heptanol and slightly lower average concentration of hexyl acetate, diethyl butanedioate, isobutanol, isopentanol, and ethyl 2-methylbutanoate, compared to non-defoliated group in the 2015–2016 vintage.
The influences of defoliation on wine volatiles profile are associated with five major factors, the first being different cultivars and clones having different responses to defoliation treatment. Significant reductions in the concentration of most wine volatile esters were observed in basal defoliated Sauvignon Blanc and Riesling, however Sauvignon Blanc is more sensitive to defoliation treatment and has much higher percentage of reduction (Kozina et al., 2008). Significant differences in wine volatile were reported amongst defoliation treatment groups of two different Sauvignon blanc clone (Šuklje et al., 2016). The timing of defoliation may have dramatic influence on grape composition, as shown by Šuklje et al. (2014) who showed that the influence of basal defoliation on Sauvignon Blanc was similar as that of Kozina et al. (2008) (treatment at veraison), but when performed at an earlier berry development stage (treatment at pepper core size), observed higher concentration of volatile esters in Sauvignon Blanc. Similarly, another study in Tempranillo reported much higher total acetates and lower wine alcohols in defoliation group at pre-bloom compared to fruit-set (Vilanova et al., 2012). Defoliation by mechanical or manual methods are another factor may affect volatiles profile of the resultant wine. Dramatic reduction of wine volatile esters were only observed in manual defoliation treatment, but not in mechanical treatment groups in Tempranillo (Vilanova et al., 2012). Further harvest time can also alter the result of defoliation. Significantly higher concentrations of wine esters were observed in the defoliation treatment group at first harvest, but no significant differences when a second harvest was conducted 12 days later (Verzera et al., 2016). Finally, the location of the defoliation as given in the current study is another cause of variation in wine volatiles. This study is the first to study the effect of apical defoliation, but together with two studies shows that apical leaf removal modifies grape berry composition differently compared to basal leaf removal and therefore influences wine volatiles differently (Lanari et al., 2013; Palliotti et al., 2013).
The influences of apical defoliation on grape volatiles such as terpenes, have yet to be elucidated. This is especially true when terpenoid compounds, such as rotundone, are associated with wine quality (Herderich et al., 2013, 2015). Since terpenes may be produced differently within individual bunches, individual grapevines, and individual vineyard blocks (Zhang et al., 2013, 2015a, 2016c), it is essential to consider the location and aspects of the vineyard when conducting defoliation treatments. Additionally, canopy defoliation is associated with other factors, such as solar exposure, which is further associated with bunch zone microclimate (Zhang et al., 2015b). Thus, these factors have the potential to influence the defoliation outcome and therefore should be considered separately as suggested previously (Spayd et al., 2002). More importantly, terpenoids in grape berries are actively produced in different phenological stages (Zhang et al., 2016a,b), and therefore apical defoliation at different phenological stages should also be investigated.
Conclusions
Defoliation is a commonly used technique to manipulate grape and wine composition, and the present study compared both basal defoliation and the less studied apical defoliation and its influences on grape and wine composition. Compared to basal defoliation, apical defoliation has less influence on the grapevine canopy and therefore less potential effect on the bunch zone microclimate. Apical defoliation near veraison resulted in slightly increased pH and decreased TA in harvested grape berries, and could lower the alcoholic strength in the resultant wine. Reduction of wine anthocyanins and color profile in apical treatment groups were observed in one season, but not the other. Minimal changes were observed in major wine volatile compounds amongst non-defoliation and defoliation treatments. These results demonstrate that apical defoliation is a novel and effective way to moderate wine alcohol with minimal influenced on wine aromatic properties, and therefore may be a technique to mitigate the influences of global warming on increasing wine alcohol level. Further research on apical defoliation is needed to study the influence on other important aromatic compounds. The timing and location of apical defoliation and its interaction with other viticulture and climate factors need to be investigated to determine the optimal apical defoliation technique suitable for commercial vineyards.
Author Contributions
PZ and KH conceived and designed the study, analyzed the data, and wrote the paper. SF and DL contributed analytical methods and finalized the data. XW, SN, and PZ performed the experiments. PZ, KH, SF, SN, and XW participated in the design and critical revision. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was sponsored by the Rathbone Wine Group and The University of Melbourne. The work was performed at the University of Melbourne and Yering Station Winery. Funding was received from the Faculty of Veterinary and Agricultural Sciences, University of Melbourne. The authors thank Mr. Andy Clark, Mr. Willy Lunn, and the late Mr. Nathan Scarlett (Rathbone Wine Group) for their support and assistance with the field trials.
References
Ashenfelter, O., and Storchmann, K. (2016). Climate change and wine: a review of the economic implications. J. Wine Econ. 11, 105–138. doi: 10.1017/jwe.2016.5
Baiano, A., De Gianni, A., Previtali, M. A., Del Nobile, M. A., Novello, V., and De Palma, L. (2015). Effects of defoliation on quality attributes of Nero di Troia (Vitis vinifera L.) grape and wine. Food Res. Int. 75, 260–269. doi: 10.1016/j.foodres.2015.06.007
Biyela, B., Du Toit, W., Divol, B., Malherbe, D., and Van Rensburg, P. (2009). The production of reduced-alcohol wines using Gluzyme Mono® 10.000 BG-treated grape juice. South Afr. J. Enol. Vitic. 30, 124–132. doi: 10.21548/30-2-1432
Coulter, A., Henschke, P., Simos, C., and Pretorius, I. (2008). When the heat is on, yeast fermentation runs out of puff. Aust. N.Z. Wine Ind. J. 23:26.
Di Profio, F., Reynolds, A., and Kasimos, A. (2011). Canopy management and enzyme impacts on Merlot, Cabernet Franc, and Cabernet Sauvignon. I. Yield and berry composition. Am. J. Enol. Viticult. 62, 139–151. doi: 10.5344/ajev.2010.10024
Diban, N., Athes, V., Bes, M., and Souchon, I. (2008). Ethanol and aroma compounds transfer study for partial dealcoholization of wine using membrane contactor. J. Memb. Sci. 311, 136–146. doi: 10.1016/j.memsci.2007.12.004
Erasmus, D., Van Der Merwe, G., and Van Vuuren, H. (2003). Genolewide expression analyses: metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res. 3, 375–399. doi: 10.1016/S1567-1356(02)00203-9
Feng, H., Yuan, F., Skinkis, P. A., and Qian, M. C. (2015). Influence of cluster zone leaf removal on Pinot noir grape chemical and volatile composition. Food Chem. 173, 414–423. doi: 10.1016/j.foodchem.2014.09.149
Fuentes, S., Palmer, A., Taylor, D., Zeppel, M., Whitely, R., and Eamus, D. (2008). An automated procedure for estimating the leaf area index (LAI) of woodland ecosystems using digital imagery, MATLAB programming and its application to an examination of the relationship between remotely sensed and field measurements of LAI. Funct. Plant Biol. 2008, 1070–1079. doi: 10.1071/FP08045
Fuentes, S., Poblete-Echeverría, C., Ortega-Farias, S., Tyerman, S., and De Bei, R. (2014). Automated estimation of leaf area index from grapevine canopies using cover photography, video and computational analysis methods. Aust. J. Grape Wine Res. 20, 465–473. doi: 10.1111/ajgw.12098
Herderich, M. J., Siebert, T. E., Parker, M., Capone, D. L., Mayr, C., Zhang, P., et al. (2013). Synthesis of the ongoing works on Rotundone, an aromatic compound responsible for the peppery notes in wines. Internet J. Enol. Vitic. 6/1, 1–6.
Herderich, M., Barter, S., Black, C. A., Bramley, R., Capone, D., Dry, P., et al. (2015). “Terroir effects on grape and wine aroma compounds,” in Advances in Wine Research, eds S. B. Ebeler, G. Sacks, S. Vidal, and P. Winterhalter (American Chemical Society), 131–146.
Iland, P. (2004). Chemical Analysis of Grapes and Wine: Techniques and Concepts. Campbelltown, SA: Patrick Iland Wine Promotions.
Jones, G. V. (2007). Climate change: observations, projections, and general implications for viticulture and wine production. Econ. Dep. Work. Paper 7, 15. doi: 10.1787/177754708811
Jones, G. V., and Webb, L. B. (2010). Climate change, viticulture, and wine: challenges and opportunities. J. Wine Res. 21, 103–106. doi: 10.1080/09571264.2010.530091
Keller, M. (2010). Managing grapevines to optimise fruit development in a challenging environment: a climate change primer for viticulturists. Aust. J. Grape Wine Res. 16, 56–59. doi: 10.1111/j.1755-0238.2009.00077.x
Kilmister, R. L., Mazza, M., Baker, N. K., Faulkner, P., and Downey, M. O. (2014). A role for anthocyanin in determining wine tannin concentration in Shiraz. Food Chem. 152, 475–482. doi: 10.1016/j.foodchem.2013.12.007
King, P. D., McClellan, D. J., and Smart, R. E. (2012). Effect of severity of leaf and crop removal on grape and wine composition of Merlot vines in Hawke's Bay vineyards. Am. J. Enol. Viticult. 2012:12020. doi: 10.5344/ajev.2012.12020
Kozina, B., Karoglan, M., Herjavec, S., Jeromel, A., and Orlic, S. (2008). Influence of basal leaf removal on the chemical composition of Sauvignon Blanc and Riesling wines. J. Food Agric. Environ. 6, 28–33.
Lanari, V., Lattanzi, T., Borghesi, L., Silvestroni, O., and Palliotti, A. (2013). Post-veraison mechanical leaf removal delays berry ripening on ‘Sangiovese’ and ‘Montepulciano’ grapevines. ISHS Acta Hoticult. 978, 327–334. doi: 10.17660/ActaHortic.2013.978.38
Milanovic, V., Ciani, M., Oro, L., and Comitini, F. (2012). Starmerella bombicola influences the metabolism of Saccharomyces cerevisiae at pyruvate decarboxylase and alcohol dehydrogenase level during mixed wine fermentation. Microb. Cell Fact. 11:18. doi: 10.1186/1475-2859-11-18
Mira De Orduna, R. (2010). Climate change associated effects on grape and wine quality and production. Food Res. Int. 43, 1844–1855. doi: 10.1016/j.foodres.2010.05.001
Mozell, M. R., and Thach, L. (2014). The impact of climate change on the global wine industry: challenges & solutions. Wine Econ. Policy 3, 81–89. doi: 10.1016/j.wep.2014.08.001
Osrečak, M., Karoglan, M., and Kozina, B. (2016). Influence of leaf removal and reflective mulch on phenolic composition and antioxidant activity of Merlot, Teran and Plavac mali wines (Vitis vinifera L.). Sci. Hortic. 209, 261–269. doi: 10.1016/j.scienta.2016.07.005
Ozturk, B., and Anli, E. (2014). Different techniques for reducing alcohol levels in wine: a review? BIO Web Confer. 3:02012. doi: 10.1051/bioconf/20140302012
Palliotti, A., Panara, F., Silvestroni, O., Lanari, V., Sabbatini, P., Howell, G. S., et al. (2013). Influence of mechanical postveraison leaf removal apical to the cluster zone on delay of fruit ripening in Sangiovese (Vitis vinifera L.) grapevines. Aust. J. Grape Wine Res. 19, 369–377. doi: 10.1111/ajgw.12033
Siebert, T. E., Smyth, H. E., Capone, D. L., Neuwohner, C., Pardon, K. H., Skouroumounis, G. K., et al. (2005). Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 381, 937–947. doi: 10.1007/s00216-004-2992-4
Spayd, S. E., Tarara, J. M., Mee, D. L., and Ferguson, J. C. (2002). Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot Berries. Am. J. Enol. Vitic. 53, 171–182.
Šuklje, K., Antalick, G., Buica, A., Langlois, J., Coetzee, Z. A., Gouot, J., et al. (2016). Clonal differences and impact of defoliation on Sauvignon blanc (Vitis vinifera L.) wines: a chemical and sensory investigation. J. Sci. Food Agric. 96, 915–926. doi: 10.1002/jsfa.7165
Šuklje, K., Antalick, G., Coetzee, Z., Schmidtke, L. M., Baša Česnik, H., Brandt, J., et al. (2014). Effect of leaf removal and ultraviolet radiation on the composition and sensory perception of Vitis vinifera L. cv. Sauvignon Blanc wine. Aust. J. Grape Wine Res. 20, 223–233. doi: 10.1111/ajgw.12083
Tardáguila, J., Diago, M. P., De Toda, F. M., Poni, S., and Vilanova, M. (2008). Effects of timing of leaf removal on yield, berry maturity, wine composition and sensory properties of cv. Grenache grown under non irrigated conditions. OENO One 42, 221–229. doi: 10.20870/oeno-one.2008.42.4.810
Van Leeuwen, C., and Darriet, P. (2016). The impact of climate change on viticulture and wine quality. J. Wine Econ. 11, 150–167. doi: 10.1017/jwe.2015.21
Verzera, A., Tripodi, G., Dima, G., Condurso, C., Scacco, A., Cincotta, F., et al. (2016). Leaf removal and wine composition of Vitis vinifera L. cv. Nero d'Avola: the volatile aroma constituents. J. Sci. Food Agric. 96, 150–159. doi: 10.1002/jsfa.7075
Vilanova, M., Diago, M. P., Genisheva, Z., Oliveira, J., and Tardaguila, J. (2012). Early leaf removal impact on volatile composition of Tempranillo wines. J. Sci. Food Agricult. 92, 935–942. doi: 10.1002/jsfa.4673
Zhang, P., Barlow, S., Krstic, M., Herderich, M., Fuentes, S., and Howell, K. (2015a). Within-Vineyard, Within-Vine, and Within-Bunch Variability of the Rotundone Concentration in Berries of Vitis vinifera L. cv. Shiraz. J. Agricult. Food Chem. 63, 4276–4283. doi: 10.1021/acs.jafc.5b00590
Zhang, P., Fuentes, S., Siebert, T., Krstic, M., Herderich, M., Barlow, E. W. R., et al. (2016a). Comparison data of common and abundant terpenes at different grape development stages in Shiraz wine grapes. Data Brief 8, 1127–1136. doi: 10.1016/j.dib.2016.07.010
Zhang, P., Fuentes, S., Siebert, T., Krstic, M., Herderich, M., Barlow, E. W. R., et al. (2016b). Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem. 204, 463–474. doi: 10.1016/j.foodchem.2016.02.125
Zhang, P., Fuentes, S., Wang, Y., Deng, R., Krstic, M., Herderich, M., et al. (2016c). Distribution of rotundone and possible translocation of related compounds amongst grapevine tissues in Vitis vinifera L. cv. Shiraz. Front. Plant Sci. 7:859. doi: 10.3389/fpls.2016.00859
Zhang, P., Howell, K., Krstic, M., Herderich, M., Barlow, E. W. R., and Fuentes, S. (2015b). Environmental factors and seasonality affect the concentration of rotundone in Vitis vinifera L. cv. Shiraz Wine. PLoS ONE 10:e0133137. doi: 10.1371/journal.pone.0133137
Zhang, P., Scarlett, N., Sheehan, D., Barlow, S., Krstic, M., Herderich, M., et al. (2013). “Intra-bunch variability of rotundone concentration in Vitis vinifera cv. Shiraz wine grapes at harvest,” in Australian Wine Industry Technical Conference, eds. E. M. C. Robinson, P. W. Godden, and D. L. Johnson (Adelaide, SA: Australian Wine Industry Technical Conference Inc.), 158.
Keywords: canopy management, defoliation, canopy structure, image analysis, shiraz, wine, aroma profile
Citation: Zhang P, Wu X, Needs S, Liu D, Fuentes S and Howell K (2017) The Influence of Apical and Basal Defoliation on the Canopy Structure and Biochemical Composition of Vitis vinifera cv. Shiraz Grapes and Wine. Front. Chem. 5:48. doi: 10.3389/fchem.2017.00048
Received: 07 April 2017; Accepted: 21 June 2017;
Published: 07 July 2017.
Edited by:
Andrea Buettner, Fraunhofer Institute for Process Engineering and Packaging (FHG), GermanyReviewed by:
Marcin Szymański, Poznan University of Medical Sciences, PolandMichael Rychlik, Technische Universität München, Germany
Copyright © 2017 Zhang, Wu, Needs, Liu, Fuentes and Howell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Howell, khowell@unimelb.edu.au
†These authors have contributed equally to this work.
 Pangzhen Zhang
Pangzhen Zhang Xiwen Wu†
Xiwen Wu†  Sigfredo Fuentes
Sigfredo Fuentes Kate Howell
Kate Howell