Bacterial Vaginosis: What Do We Currently Know?
- 1Aix Marseille Univ, IRD, AP-HM, SSA, VITROME, Marseille, France
- 2IHU-Méditerranée Infection, Marseille, France
The vaginal microbiome is a well-defined compartment of the human microbiome. It has unique conditions, characterized by the dominance of one bacterial species, the Lactobacilli. This microbiota manifests itself by a low degree of diversity and by a strong dynamic of change in its composition under the influence of various exogenous and endogenous factors. The increase in diversity may paradoxically be associated with dysbiosis, such as bacterial vaginosis (BV). BV is the result of a disturbance in the vaginal ecosystem; i.e., a sudden replacement of Lactobacilli by anaerobic bacteria such as Gardnerella vaginalis, Atopobium vaginae, Ureaplasma urealyticum, Mycoplasma hominis, and others. It is the most common cause of vaginal discharge in women of childbearing age, approximately 30% of all causes. The etiology of this dysbiosis remains unknown, but its health consequences are significant, including obstetrical complications, increased risk of sexually transmitted infections and urogenital infections. Its diagnosis is based on Amsel’s clinical criteria and/or a gram stain based on the Nugent score. While both of these methods have been widely applied worldwide for approximately three decades, Nugent score are still considered the “gold standard” of BV diagnostic tools. Given the limitations of these tools, methods based on molecular biology have been developed as alternative rational strategies for the diagnosis of BV. The treatment of BV aims at restoring the balance of the vaginal flora to stop the proliferation of harmful microorganisms. Prescription of antibiotics such as metronidazole, clindamycin, etc. is recommended. Faced with the considerable uncertainty about the cause of BV, the high rate of recurrence, the unacceptable treatment options, and clinical management which is often insensitive and inconsistent, research on this topic is intensifying. Knowledge of its composition and its associated variations represents the key element in improving the therapeutic management of patients with the most suitable treatments possible.
1 Introduction
The vaginal microbial community is complex and dynamic, consisting of a group of bacteria typically characterized by abundant Lactobacilli that evolve during the life of the woman, depending on age, hormonal estrogen levels, sexual practices and the environment (Kumar et al., 2011; Bilardi et al., 2016b). The vaginal microbiota plays a crucial role in women’s health (infection, reproduction…), and that of their fetuses (Li et al., 2012).
BV is a dysbiosis of the vaginal microbiota characterized by a shift from Lactobacilli dominance to that of a mixture of various anaerobic bacteria (Mårdh, 1993; Hay, 2002). It is the most common vaginal disorder worldwide in women of childbearing age (Cristiano et al., 1996; Hogan et al., 2007; Trabert and Misra, 2007). BV is associated with significant adverse healthcare outcomes, including increased susceptibility to sexually transmitted infections, urogenital infections, pelvic inflammatory disease, and an increased risk of abnormal pregnancy (Marrazzo and Hillier, 2013). The etiology of BV is still unknown. Standard antibiotic therapy often fails, with an estimated relapse rate of 50% at six months follow-up (Bradshaw et al., 2006; Bretelle et al., 2015).
2 Normal Healthy Vaginal Flora
The vaginal ecosystem is colonized from the very first hours of the birth of a female and remains throughout her life until death (Romero et al., 2014). Women of childbearing age produce about 1 to 4 mL of vaginal fluid, containing 108 to 109 bacterial cells per mL (Danielsson et al., 2011).
2.1 Composition of Normal Vaginal Flora
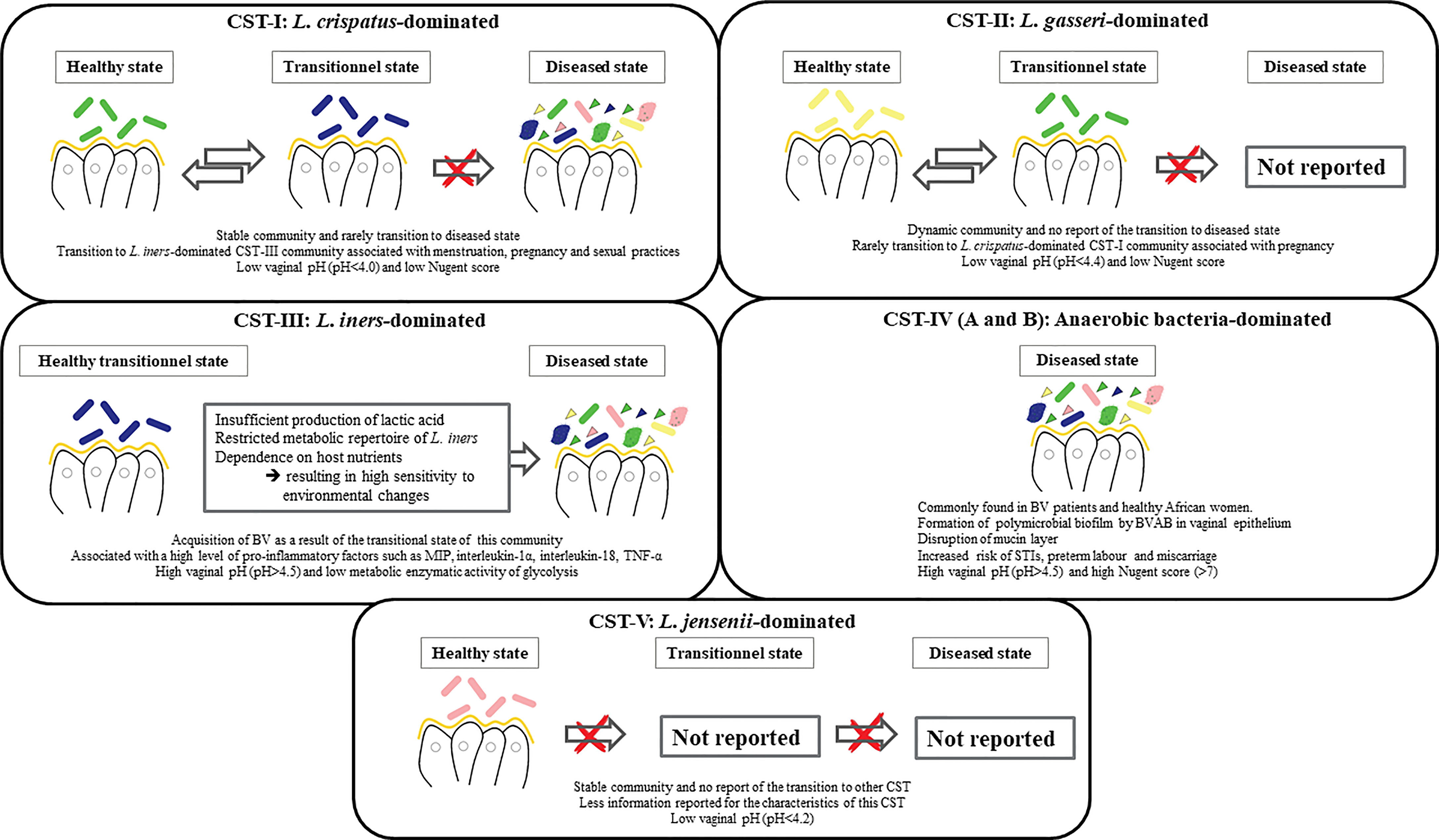
The vaginal flora was first described by the German gynecologist Albert Döderlein in 1892, who reported a homogeneous vaginal flora of gram-positive bacilli in healthy women (Lepargneur and Rousseau, 2002). They were named “Döderlein’s bacilli” and were later identified as members of the Lactobacillus genus by Beijerink in 1901 (Lepargneur and Rousseau, 2002). Under normal conditions, 70-90% of the vaginal bacterial species in healthy premenopausal women are Lactobacilli (Africa et al., 2014). As molecular techniques have advanced, our understanding of the diversity and complexity of the vaginal bacterial community has broadened (Fredricks et al., 2005). Among more than 200 Lactobacillus species with standing in the nomenclature, over 20 species have been found in the vaginal flora (Huang et al., 2014). Sequencing of the 16 rRNA gene revealed that the vaginal bacterial community, mainly composed of Lactobacilli, is classified into five groups named community state types, namely I, II, III, IV and V (Ravel et al., 2011). Four of these groups are dominated by Lactobacillus. The first is dominated by L. crispatus, the second by L. gasseri, the third by L. iners, and the fifth by L. jensenii, while the fourth contains a smaller proportion of Lactobacilli but is composed of a polymicrobial mixture of strict and facultative anaerobes (Gardnerella, Atopobium, Mobiluncus, Prevotella…). Although there is always a temporal transition between vaginal bacterial communities (Figure 1) (Gajer et al., 2012).
Thus, many other bacteria are present at lower concentrations in healthy vaginal flora, such as Peptostreptococcus, Bacteroides, Corynebacterium, Streptococcus, and Peptococcus (Kumar et al., 2011).
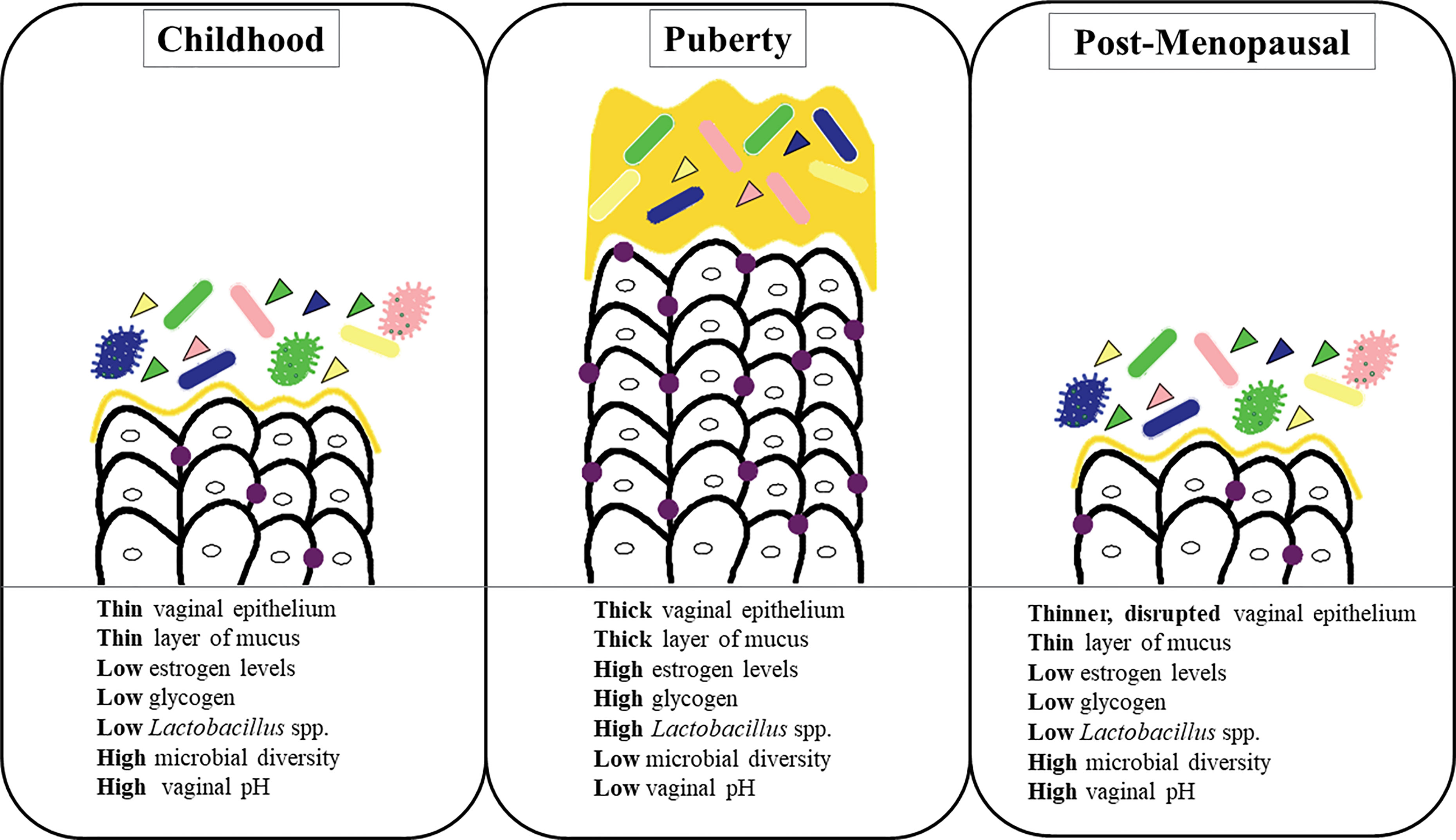
The composition of the vaginal microbiota evolves throughout a woman’s lifespan. Various physical and hormonal changes occur in the vagina biotope during these different stages of a woman’s life (Muhleisen and Herbst-Kralovetz, 2016; Nuriel-Ohayon et al., 2016) (Figure 2).
2.2 Variability of Vaginal Flora According to Ethnicity
Vaginal bacterial communities of women of childbearing age may vary between women from different regions, but also between women of different ethnicities living in the same geographical area (Hickey et al., 2012). In 2011, a study by Ravel et al. characterized the vaginal microbiota of asymptomatic North American women with pyrosequencing, showing that the vaginal flora of Asian and white American women was dominated by Lactobacilli, unlike Hispanic and African-American women, of whom only 60% had a Lactobacillus-dominated vaginal flora (Ravel et al., 2011). In addition, Caucasian and Asian women tend to have high levels of L. crispatus and lower levels of L. iners compared to African women (Green et al., 2015). In another study using 16S rRNA gene sequencing, Fettweis et al. demonstrated that the vaginal microbiota of European-ancestry women was dominated by Lactobacilli, as opposed to African-American women, who presented a mixed vaginal community containing, among others, Mycoplasma hominis, Aerococcus, L. iners and numerous strict anaerobes, including gram-positive anaerobic cocci, BV-associated bacteria, Sneathia, Prevotella amnii, Megasphaera, Atopobium, and Gardnerella vaginalis (Fettweis et al., 2014). The vaginal pH also differs between racial groups. African-American and Hispanic women had a vaginal pH (4.7 and 5.0, respectively) higher than what is considered the norm (<4.5) (Hickey et al., 2012).
2.3 Role of the Vaginal Microbiota in Women’s Health
The vaginal flora presents one of the most important defense mechanisms for reproductive function and maintaining a healthy environment. The stability of this flora prevents the proliferation of commensal microorganisms and colonization by pathogens, thereby preventing infection (Deidda et al., 2016; Donders et al., 2017). Bacteria form a adhered monolayer on the vaginal mucosa and produce antimicrobial compounds that maintain this health equilibrium, such as hydrogen peroxide (antimicrobial product protecting against deleterious microorganisms) (Cherpes et al., 2008; Sgibnev and Kremleva, 2015), lactic acid (which maintains the normal vaginal pH between 3.5 to 4.5) (O’Hanlon et al., 2013; Tachedjian et al., 2017), bacteriocins (antibiotics that inhibit the growth of harmful microorganisms within the vagina) (Stoyancheva et al., 2014), and arginine deaminase enzyme (metabolizes arginine into citrulline and ammonia (NH3), depriving anaerobic pathogens of this amino acid necessary for their growth) (Rousseau et al., 2005; Makarova et al., 2006).
Notably, L. crispatus and L. jensenii may produce hydrogen peroxide, an oxidizing agent, toxic for catalase-negative bacteria and also capable in vitro of inhibiting HIV-1 and herpes simplex virus type 2 (Aldunate et al., 2013; Borges et al., 2014). The vaginal acids produced can, in the presence of viral RNA, stimulate the maturation of dendritic cells, activation of 17 subclasses of T helper lymphocytes, and the production of protective inflammatory cytokines and interferon-γ (Witkin, 2015).
In addition to the role of Lactobacilli, cervical mucus is mainly composed of mucin, which protects the vaginal mucosa and optimizes its barrier role against microbial colonization. Analyses of the composition of cervical mucus and vaginal secretions have demonstrated the presence of several proteins with antimicrobial activities that act independently of the presence of antibodies, such as lactoferrin, lysosyme, calprotectin [also known as MRP8/MRP14 (“myeloid related protein”)], cathelicidin LL-37 (Nasioudis et al., 2017).
3 Bacterial Vaginosis
3.1 Background
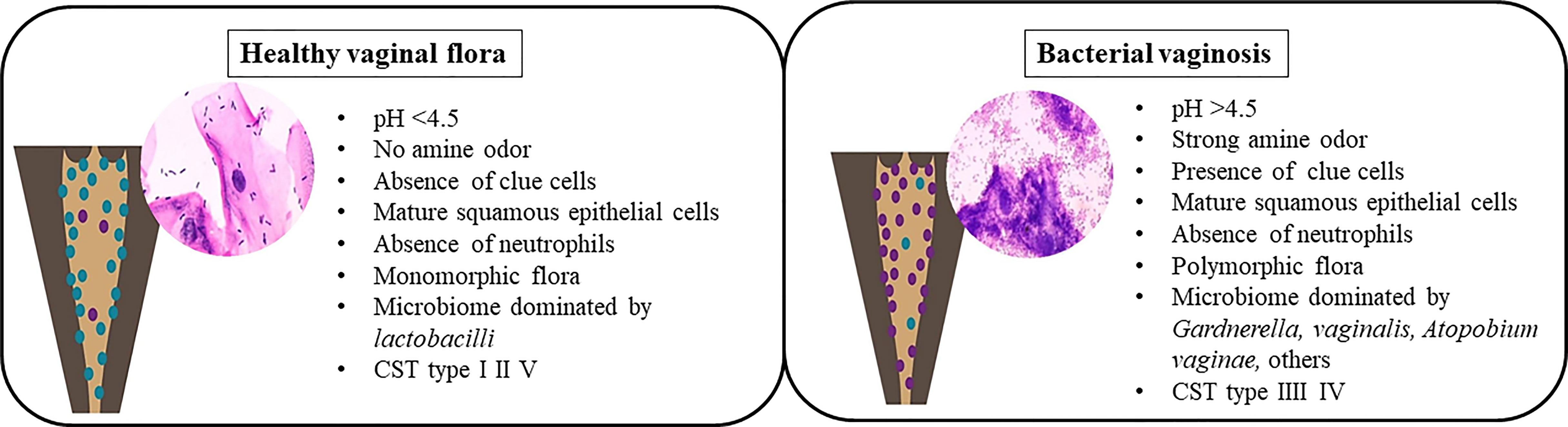
Formerly known as non-specific vaginitis (Amsel et al., 1983), BV is characterized by a change in the vaginal flora composition, with a dramatic depletion of Lactobacilli due to a significant overgrowth of obligate or facultative anaerobes previously a minority in the vagina (Mårdh, 1993; Marrazzo and Hillier, 2013), such as Gardnerella vaginalis, Atopobium vaginae, Ureaplasma urealyticum, Mycoplasma hominis, Prevotella, Peptoniphilus, Megasphaera, Mobiluncus, and several fastidious and uncultured bacteria, including BV-associated bacteria (BVAB-1 to 3) (Romero et al., 2014; Margolis and Fredricks, 2015; Zozaya et al., 2016). The factor triggering this overgrowth of anaerobic bacteria is unknown. It is linked to an alkaline vaginal ecosystem due to an increase of vaginal pH following the loss of Lactobacilli protective effects (Figure 3).
The diversity of the vaginal flora in patients with BV was described in 1921 by Schröder (Kumar et al., 2011). In 1955, Gardner and Dukes claimed that the etiological agent of BV was Haemophilus vaginalis (Gardner and Dukes, 1955), a gram-negative rod later renamed Gardnerella vaginalis (Green et al., 2015; Mendling, 2016). The bacteria present in the microbiota of BV form a biofilm on the vaginal epithelium and secrete a cytotoxin capable of killing the epithelial cells (Huang et al., 2014). In addition, G.vaginalis produce proteolytic enzymes able to degrade proteins and decarboxylases that convert amino acids. Not being degraded, the amine compounds become malodorous (fishy odor: “Whiff test”) thank to an increase of the vaginal pH (Gonzalez Pedraza Aviles et al., 1999). Subsequently, cytotoxicity resulting from the combination of organic acids present in the vagina during BV and bacterial polyamines leads to the production of a vaginal discharge caused by the exfoliation of vaginal epithelial cells (Sobel, 2017). Furthermore, this bacterium, cover the vaginal epithelial cells, causing the formation of “clue-cells”, a specific characteristic of BV (Khan et al., 2007).
A few years later, G. vaginalis was found in 40% of healthy women, and its role was disputed (Machado et al., 2016). Thus, colonization of G. vaginalis does not always promote BV (Hickey and Forney, 2014), suggesting that this bacterium alone may be essential but not sufficient for BV development. In addition to G. vaginalis, some anaerobic bacteria are highly associated with BV, indicating that BV is a polymicrobial syndrome, which does not follow Koch’s postulates (Kumar et al., 2011).
Recent progress in research of BV pathogenesis have determined the existence of 13 different species within the genus Gardnerella (Vaneechoutte et al., 2019). Although these Gardnerella species may be closely linked genetically, only a few of them may be implicated in disease such as BV (Vaneechoutte et al., 2019). Healthy women may be colonized by non-pathogenic Gardnerella species, whereas virulent strains are involved in the development of BV. Advances in technology, particularly next-generation sequencing, have clarified much of this issue. Arguably, all papers dealing with G. vaginalis prior to that of Vaneechoutte et al. are not specifically about G. vaginalis but rather about Gardnerella spp (Vaneechoutte et al., 2019).
Based on a recent prospective study, an updated conceptual model on the pathogenesis of BV was outlined (Alves et al., 2014; Schellenberg et al., 2016; Castro et al., 2017; Muzny et al., 2019). The potential synergistic relationship between G. vaginalis, P. bivia, A. vaginae was studied (Muzny et al., 2018; Gilbert et al., 2019). After sexual exposure to virulent strains of G. vaginalis, these strains displace vaginal Lactobacilli and begin to form a BV biofilm on the vaginal epithelium (Machado and Cerca, 2015; Beebout et al., 2019). Subsequently, proteolysis by G. vaginalis occurs which promotes the growth of P. bivia. This bacterium produces an ammonia product which in turn promotes the growth of G. vaginalis and the biofilm develops (Gilbert et al., 2019; Castro et al., 2019). These 2 bacteria then produce sialidase that degrades the biofilm and P. bivia may thus degrade the mucin layer of the vaginal epithelium (Briselden et al., 1992; Gilbert et al., 2019). After the loss of the protective mucus layer, increased adhesion of other BV-associated bacteria, including A. vaginae, to the polymicrobial biofilm will occur (Hardy et al., 2016). The role of the other bacteria remains unknown (Muzny et al., 2019). Further research focusing on the complex interactions between bacteria during BV is needed.
3.2 Diagnosis
BV ranges from asymptomatic to an increase in vaginal discharge with or without a fishy odor (Gardner and Dukes, 1955; Majigo et al., 2021). The collection of a specimen for diagnosis can be performed using a speculum during the pelvic exam. When there is no reason for a pelvic exam as part of the clinical evaluation, a self-collected vaginal swab may also be provided (Menard et al., 2012; Camus et al., 2021).
3.2.1 Amsel Criteria and Nugent’s Score
Two main categories of diagnostic strategies for BV exist: the “bedside” method introduced in 1983, mainly based on real-time clinical criteria –”Amsel’s criteria” (Amsel et al., 1983), and laboratory-based testing developed in 1991, relying on the evaluation of morphotypes on gram staining – “ Nugent’s score” (Nugent et al., 1991). Amsel’s criteria and Nugent’s score are the most common diagnostic methods used for BV. Furthermore, the World Health Organization (WHO) has considered the Nugent’s score as a gold standard for studies. However, the current recommended best clinical practice for diagnosing BV in women is gram staining microscopy according to the Hay-Ison criteria, as it is easier and faster to use (Sherrard et al., 2018). The Hay-Ison criteria were analogous to the Nugent’s scores. Hay’s grades I, II, and III were similar to Nugent’s scores 0-3, 4-6, and 7-10 (Ison and Hay, 2002).
Even if Nugent’s score is considered as a gold standard by the WHO, it has some pitfalls. In fact, intermediate flora is so far an uncharacterized category and is a challenge in the diagnosis of BV. In addition, the identification of morphotypes is subjective and technician-dependent, thus the diagnosis may be influenced by individual skills and experience (Menard et al., 2008; Antonucci et al., 2017).
Recently, a study made by Wang et al. provided a proof of concept for a deep learning-based model to quantify Gram staining and, consequently, automated Nugent score classification. Deep learning methods, particularly convolutional neural network (CNN) models, have demonstrated excellent performance in computer vision tasks. This model outperformed human healthcare professionals in terms of accuracy and stability for three diagnostic categories of Nugent scores. The deep learning model may offer translational applications in automating the diagnosis of BV with appropriate supporting hardware (Wang et al., 2020).
3.2.2 Molecular Diagnostic Technique
The diagnosis of BV is problematic and challenging because of its intricate polymicrobial features and a wide range of clinical features (Money, 2005). In order to overcome these diagnostic problems, alternative diagnostic strategies have been attempted, such as molecular, enzymatic and chromatographic techniques.
A molecular technique used in the diagnosis of BV is specific quantitative real-time PCR (qPCR) test. It is a quantitative, reproducible and reliable molecular biology tool that measures the presence of bacteria presents in BV, such as Atopobium vaginae, BVAB2, Gardnerella vaginalis, Leptotrichia/Sneathia spp., Megasphaera spp., and Mobiluncus spp…. (Breding et al., 2020). Many studies proposed objective molecular cut-off values from bacterial load to predict BV (Menard et al., 2008; Redelinghuys et al., 2017).
Several commercially molecular diagnostic assays have been reported for the diagnosis of BV in women including the NuSwab R multiplex quantitative PCR (Cartwright et al., 2012; Cartwright et al., 2018), SureSwab BV DNA real-time quantitative assay, BD Max vaginal panel (Gaydos et al., 2017) and BV multiplex assay (Hilbert et al., 2016). The quantification of these bacteria therefore makes it possible to establish a precise diagnosis of BV, with a sensitivity ranging from 90.5% to 96.7% and a specificity ranging from 85.8% to 95% compared to Amsel criteria and Nugent score (Coleman and Gaydos, 2018).
Even though these tests have a higher sensitivity and specificity than the currently available diagnostic tools, they are not point-of-care tests and are more expensive. However, Dessai et al. are the first to report on the performance of the BD AffirmTM VPIII test as a POCT in a prenatal population. But the test has been shown to be inadequate as a screening test for vaginal infections in pregnancy (Dessai et al., 2020).
Finally, POC tests for BV are not available or are simply too expensive to be used routinely. It is therefore mandatory that the development and evaluation of new diagnostic tests include a cost analysis.
3.2.3 Other Emerging Strategies
As the presence of sialidase is currently considered a key indicator of BV in the clinical examination, an enzymatic approach has been developed: The OSOM R BVBlue R test as a POC diagnostic test for BV. It is based on the qualitative detection of a high level of sialidase produced by anaerobic pathogens in vaginal fluid samples. It has been shown to be reliable compared to conventional methods such as Amsel criteria and Nugent score (Myziuk et al., 2003; Shujatullah et al., 2010; Khatoon et al., 2013; Madhivanan et al., 2014)
In addition, a recent study by Liu et al., showed the feasibility of turn-on tetravalent sialic acid-coated tetraphenylethene luminogen (TPE4S) as a powerful diagnostic tool for high-throughput fluorescence-guided diagnosis of BV. This study uses light signal intensity to detect and measure the relative concentration of sialidase in a vaginal sample. All reagents are present in a reagent bead and sample buffer, essentially allowing for a one-step test. The test is highly sensitive and quantitative, with a sensitivity and specificity of 95.40% and 94.94%, respectively, compared to the Amsel method and 92.5% and 91.8% compared to the BVBlue diagnostic results. Notably, this method gives a more accurate classification and quantification of BV severity based on relative fluorescence intensity (I/I0). Thus this test can be a potential tool for diagnosis of BV, and risk assessment of patients with BV based on sialidase activity levels and monitoring of antibiotic therapy (Liu et al., 2018).
Another new approach based on immunodetection also targeting sialidase has been developed for the diagnosis of BV. The nanophotonic operating principle of this biodetection method allows a cheaper, faster and simpler analysis than the indirect enzyme-linked immunosorbent assay (ELISA). This nanotechnology has a high sensitivity and specificity (96,29%, respectively). This method offers an original approach to perform a very rapid diagnosis of BV (Rodríguez-Nava et al., 2021).
3.3 Epidemiology and Risk Factors
BV may appear at any reproductive age (between 15 to 44 years-old). Its prevalence rates vary considerably among the geographic regions of the world, within the same country, and even within the same population, depending on ethnic origin and socioeconomic status. Although its exact prevalence remains difficult to determine, BV occurs between 4-75%, depending on the population studied (Onderdonk et al., 2016; Bitew et al., 2017). Intermediate in the USA (29%), the prevalence of BV is estimated to be low in Europe, with a maximum (> 20%) in Poland and Norway (Kenyon et al., 2013). In Africa, the estimated prevalence tends to be high. However, BV prevalence is lowest in west Africa (6-8% in Burkina Faso, 14.2% in Nigeria) than southern and eastern Africa: 32.5% in Zimbabwe, 37% in Kenya, 38% in Botswana, and 68.3% in Mozambique (Kenyon et al., 2013; Afolabi et al., 2016; Bitew et al., 2017).
3.3.1 Sexual Practices
Although the absence of a known causal agent makes it difficult to characterize BV as a sexually transmitted infection (STI) (Morris et al., 2001; Reid, 2018), it is strongly associated with sexual activities and has some characteristics of a sexually transmitted disease not by microorganism transfer, but by mechanical or chemical interaction such as contact with highly alkaline semen (Guédou et al., 2013; Muzny et al., 2013; Lewis et al., 2017). Overall, BV is diagnosed in post-pubertal women who have never been sexually active, but at a lower prevalence than those who are sexually active (Cherpes et al., 2008). The prevalence varies with the number of sex partners. It has been evaluated at 18.8% for non-sexually active women, 22.4% for women with one lifetime partner and 43.4% and 58% for women having 2-3 lifetime sex partners and those having ≥ 4-lifetime sex partners, respectively (Koumans et al., 2007).
In this dynamic, sex workers had a higher bacterial vaginal diversity but a much lower abundance of Lactobacillus species than women who are not engaged in sex work (Wessels et al., 2017). Compared with male partners of healthy women, BV-related bacteria can be found in the penile skin, urethra (Zozaya et al., 2016), spermatozoa, and prostatic fluid microbiota (Gallo et al., 2011; Hou et al., 2013) of male partners of women with BV. Furthermore, biofilm fragments have been found in their urine and sperm (Swidsinski et al., 2010a; Swidsinski et al., 2010b), suggesting that male partners are a reservoir, and also that heterosexual transmission may occur. Nevertheless, there is no corresponding illness in male partners (Verstraelen et al., 2010). Use of condoms by male partners also prevents acquisition and recurrence of BV (Verstraelen et al., 2010). Also, since the preputial area of some men hosts BV-associated microorganisms, male circumcision may reduce the risk of BV (Margolis and Fredricks, 2015).
Prevalence rates also depend on the nature of the couple and their sexual practices. In fact, BV prevalence varies between 10-30% in heterosexual women, and is more frequent, 25-50%, in women who have sex with women (WSW) (Forcey et al., 2015; Bilardi et al., 2016b). The reasons for this difference in prevalence are not clear, although sexual activities involving the transmission of vaginal fluid increase the risk of BV acquisition (Marrazzo and Hillier, 2013). Several studies have indicated that certain sexual behaviors, including non-coital sexual practices such as digital and penile penetration, anal and oral intercourse followed by vaginal penetration, enhance the risk of BV (Kenyon and Osbak, 2014). In WSW, a symptomatic female sexual partner, receptive oral sex, and the use and sharing of unwashed sex toys constitute risk factors for BV (Cherpes et al., 2008). These observations have led some to consider BV as not an infection, but rather a taxonomic change in the vaginal microbiota resulting from translocation of oral (Africa et al., 2014) or fecal (Fenollar and Raoult, 2016) microbiota during non-coital sexual practices.
3.3.2 Other Bacterial Vaginosis Risk Factors
Additionally, genital hygiene can also promote disequilibrium in the vaginal microbiota. One study found that patients who did not wash their vaginal region were more susceptible to BV than those who often washed the vaginal region, a prevalence of 53.9% and 40.2%, respectively. Similarly, the prevalence of BV is higher in patients who do not change their underpants frequently compared to those who change it more frequently (57.6% versus 36.9%) (Bitew et al., 2017). In addition, other sexual sanitary habits, including vaginal douching and washing (Aslan and Bechelaghem, 2018), as well as cigarette smoking (Nelson et al., 2018), certain contraceptive methods like disposable intra-uterine devices (Achilles et al., 2018) and stress (Marrazzo and Hillier, 2013) may also enhance the risk of developing BV.
3.4 Bacterial Vaginosis Complications and Women’s Health
Women with BV are vulnerable: the presence of BV-related bacteria and/or sexually transmissible microorganisms in the BV microbiota can lead to opportunistic infections (Wiesenfeld et al., 2003; Brotman et al., 2014; Rumyantseva et al., 2019; Shipitsyna et al., 2020). During this imbalance, 10-30% of pregnant women with BV give birth prematurely, a preterm delivery often accompanied by perinatal mortality, up to 70% worldwide (Svare et al., 2006; Afolabi et al., 2016). During pregnancy, BV increases the risk of preterm labor, late miscarriage, intrauterine fetal death, preterm rupture of the membranes, amniotic fluid infections, chorioamnionitis, post-abortion and postpartum infections in these women (Wilson et al., 2011; Nelson et al., 2015a; Kairys and Garg, 2017; Brown et al., 2018).
In non-pregnant women, bacteria involved in BV can initially cause cervicitis, endometritis, salpingitis, and urinary tract infections (Georgijević et al., 2000). After damage of the cervix, bacteria can migrate from the lower to upper genital tract, reaching the uterus and fallopian tubes and causing illnesses such as pelvic inflammatory disease (Wilson et al., 2011; Sharma et al., 2014), post-hysterectomy infections (Marrazzo and Hillier, 2013), and even cervical cancer or tubal infertility (van van Oostrum et al., 2013; Babu et al., 2017). Likewise, BV is associated with significantly increased rates of acquiring herpes simplex virus (Nardis et al., 2013), human immunodeficiency virus (McClelland et al., 2018), papillomavirus (Kero et al., 2017) and transmission of the pathogens causing syphilis, chancroid, gonorrhea, trichomoniasis, and chlamydia (Brotman, 2011; Bitew et al., 2017).
3.5 Treatment and Management of Bacterial Vaginosis
Considering that clinical cure corresponds to the disappearance of all symptoms, the treatment of BV is currently focused on stopping the proliferation of BV-associated microorganisms and restoring the normal vaginal flora (Marrazzo and Hillier, 2013). Typically, clinical therapies include the use of antibiotics with broad activity against anaerobic microbes and protozoa: clindamycin and the nitroimidazoles (metronidazole and tinidazole) and/or use of probiotics (Kumar et al., 2011; Bacterial Vaginosis, 2015; Bradshaw and Sobel, 2016).
3.5.1 Antibiotic Therapies
The first line of therapy recommended by the World Health Organization (WHO) is 500 mg oral metronidazole twice daily for one week (Bacterial Vaginosis, 2015; World Health Organization, 2021). However, treatment with metronidazole may cause side effects such as gastrointestinal pain, nausea, and vomiting (Machado et al., 2016). Other proposed therapeutic regimens include 300 mg oral clindamycin twice daily for one week, 100 mg of intravaginal clindamycin ovule daily for 5 days and an application of 0.75% intravaginal metronidazole gel for 5 days or 2% of intravaginal clindamycin cream at bedtime for one week (Donders et al., 2014; World Health Organization, 2021). However, it should be noted that local application of clindamycin may damage latex-based products such as condoms and may also trigger pseudomembranous colitis (Machado et al., 2016).
In addition, the use of tinidazole, a drug similar to metronidazole, has been approved and proposed as an alternative therapy in an oral regimen (either 2 g/day for 2 days or 1g/day for 5 days) if metronidazole and clindamycin are not tolerated (Bacterial Vaginosis, 2015; Dickey et al., 2009).
Some researchers have evaluated the efficacy of other antimicrobial agents, such as azithromycin, secnidazole or ornidazole and rifaximin (Schwebke and Desmond, 2007; Thulkar et al., 2012; Laghi et al., 2014). Secnidazole has shown activity similar to that of the recommended nitroimidazoles and also spares Lactobacilli, a beneficial characteristic in BV treatment. Even for Rifaximin, it acts on BV by restoring Lactobacilli and increasing lactic acid in patients (Bagnall and Rizzolo, 2017).
Taken locally or orally, these antimicrobial agents have almost similar efficacy, with cure rates around 58% to 92% after 1 month of treatment (Donders et al., 2014). Nevertheless, these results are temporary, leading to recurrence or re-infection at rates above 50% within 6-12 months of treatment (Bilardi et al., 2016a; Bradshaw and Sobel, 2016). The reasons for this high relapse rate remain unclear. However, it appears that, with the formation of bacterial biofilms, these recommended therapies only temporarily eradicated BV-associated microorganisms, or these bacteria are reintroduced in the vagina by their sex partners (Marrazzo et al., 2012; Bradshaw and Brotman, 2015; Margolis and Fredricks, 2015). Further, the presence of some BV-associated bacteria such as Peptoniphilus lacrimalis, Megasphaera type 2 and BVAB-1 to 3 at the beginning of treatment is strongly related to BV recurrence, thus causing antibiotic failure (Marrazzo et al., 2008). To this end, two recent studies have examined the acceptability, tolerability, and especially the efficacy of concomitant partner treatment to improve the cure of BV (Schwebke et al., 2021; Plummer et al., 2021). The first study by Schwebke et al, showed no significant reduction in BV recurrence in female partners after treatment of the male partner with multidose metronidazole, although women whose partners adhered to multidose metronidazole were less likely to fail treatment (Schwebke et al., 2021). The second study showed that simultaneous partner treatment had a significant change in the overall composition of genital microbiota in both partners immediately after treatment (Plummer et al., 2021).
3.5.2 Non-Antibiotic Therapies
As antibiotic treatments can have a negative impact on the stability of the vaginal flora, Lactobacillus probiotics, an alternative and complementary therapy to antibiotic treatment, has been developed to help restore and maintain the healthy vaginal flora (Bradshaw et al., 2012). Probiotics consist of living microorganisms that confer a health benefit on the host when they are administered in an appropriate quantity (Borges et al., 2014). Nine studies from 1989 to 2014 tested the effectiveness of vaginal or oral Lactobacillus probiotics (Fredricsson et al., 1989; Hallén et al., 1992; Wewalka et al., 2002; Mastromarino et al., 2009; Hummelen et al., 2010; Hemalatha et al., 2012; Ling et al., 2013; Vujic et al., 2013; Vicariotto et al., 2014). The results showed that both oral and vaginal Lactobacillus treatments were effective in curing acute BV. On balance, the application of Lactobacillus in the form of vaginal capsules (containing≥108 CFU of Lactobacillus strains per dose) or a fermented milk product (containing≥5×109 CFU of Lactobacillus strains per dose) may be an equally effective alternative to standard antibiotic capsules. Only the strains L. reuteri RC-14 and L. rhamnonus GR-1 have positive clinical effects (Kumar et al., 2011; Ouarabi et al., 2017). Administrated orally (twice daily) or vaginally (once weekly), probiotics may restore the normal Lactobacillus-dominated microbiota and reduce BV recurrence (Daliri and Lee, 2015). Nevertheless, probiotics have had minimal success in African women (Margolis and Fredricks, 2015).
In the same context, a new study on the applicability of three strains of Lactobacillus spp. (Lactobacillus delbrueckii DM8909, Lactiplantibacillus plantarum ATCC14917 and Lactiplantibacillus plantarum ZX27) according to their in vitro probiotic capacities. These three Lactobacillus spp. strains have shown efficacy in the treatment of BV by limiting the growth, adhesion, biofilm formation and virulence properties of G. vaginalis (Qian et al., 2021).
In addition, sucrose-containing products may promote recolonization, since sucrose is metabolized by Lactobacilli. A triple-blind randomized clinical trial compared the efficacy of a sucrose vaginal gel versus a metronidazole vaginal gel for 5 days in 70 women with diagnosed BV (Khazaeian et al., 2018). The sucrose vaginal gel was as effective as metronidazole treatment, according to this individual RCT.
Another option is to combine Lactobacilli with estriol. Two studies, a PC-RCT and a head-to-head RCT, were conducted on the efficacy of vaginal capsules containing L. acidophilus and 0.03 mg estriol (Gynofor®) in BV (Parent et al., 1996; Donders et al., 2010). Compared to placebo, the cure rate of BV was significantly higher in the treated group, but according to the comparative study, the combination of estriol and Lactobacilli is equivalent, but not better, results than antibiotic treatment.
In terms of method of application, vaginal suppositories deposit Lactobacillus strains directly on the vaginal mucosa, whereas oral probiotics survive gastrointestinal transit and increase the number of strains in the colon and feces. In turn, this may promote recolonization of the vagina due to the proximity of the rectum and vagina (Tidbury et al., 2020).
3.5.3 New Emerging Therapies
The management of BV urgently requires implementation of new therapeutic strategies. To disrupt BV-associated biofilms, studies are underway to investigate the role of novel agents such as DNases, retrocyclines, antiseptics, and plant-derived compounds in the treatment of BV (Machado et al., 2016). Dequalinium chloride, an antiseptic, has reported similar efficacy to clindamycin intravaginal cream (Weissenbacher et al., 2012). Thymol, a molecule found in thyme essential oil, has shown an inhibitory effect on biofilms in vitro (Braga et al., 2010). The application of acidifying agents, such as vitamin C or buffering agents (polycarbophil or boric acid), in combination with a nitroimidazole antibiotic, has been demonstrated to reduce the recurrence of BV, potentially by destructuring the vaginal biofilm (Machado et al., 2016).
In this regard, other promising therapeutic agents for the treatment of BV are under investigation. Including DNase agents that can disrupt vaginal biofilms by targeting extracellular DNA essential for their structural integrity (Hymes et al., 2013), retrocyclin 101, a synthetic cyclic antimicrobial peptide that inhibits the growth and development of G. vaginalis in vitro (Hooven et al., 2012), and the amphoteric tenside pessary (WO3191), which disrupts biofilms after metronidazole treatment and promotes the growth of Lactobacillus species (Gottschick et al., 2017; Algburi et al., 2017).
Furthermore, given the success demonstrated by fecal microbiota transplant (FMT) in the management of various intestinal disorders and diseases such as recurrent Clostridium difficile infection, pseudomembranous colitis, inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), the use of vaginal microbiota transplantation (VMT) in BV treatment is a new therapeutic approach that modulates the vaginal microbiota in order to eradicate this scourge and reduce adverse gynecological outcomes (DeLong et al., 2019).
Given the high rates of recurrence and relapse, research is needed to identify and evaluate these novel biofilm-disrupting treatment strategies.
4 Conclusions and Perspectives
The taxonomic composition and bacterial proportion of the vaginal microbiota are under the influence of intrinsic and external factors over the female lifespan. In the last decades, understanding of the bacterial diversity of this ecosystem has been increased by molecular methods. Dominated by Lactobacilli that protect against infection, the vaginal flora of healthy women is less complex than that of patients with BV, which presents a diverse microbiota containing numerous obligate anaerobic and uncultivable species. This polymicrobial condition is associated with relatively uncomplicated clinical symptoms that do not occur in all affected women, thus complicating the determination of its etiology. Treatment is usually unsuccessful, with a high rate of relapse. Future studies that thoroughly examine the vaginal bacterial community are needed to cultivate the bacteria associated with BV and its treatment failure, in order to study antibiotic resistance and to establish more effective alternative therapeutic strategies that reduce BV symptoms as well as its associated complications. Overall, unlocking the enigma of the pathogenesis of BV is key for the prevention and management of this public health problem.
Author Contributions
LAC, FF, and KD have equally contributed to the preparation of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency under the “Investissements d’avenir” programme, reference ANR-10-IAHU-03, the Region Provence Alpes Côte d’Azur and European FEDER PRIMI funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the foundation Mediterranean Infection and the National Research Agency under the program “Investissements d’avenir”, reference ANR-10-IAHU-03, for funding this study. The funding source had no other input in this article.
References
Achilles, S. L., Austin, M. N., Meyn, L. A., Mhlanga, F., Chirenje, Z. M., Hillier, S. L. (2018). Impact of Contraceptive Initiation on Vaginal Microbiota. Am. J. Obstet. Gynecol. 218 (6), 622–e15. doi: 10.1016/j.ajog.2018.02.017
Afolabi, B. B., Moses, O. E., Oduyebo, O. O. (2016). “Bacterial Vaginosis and Pregnancy Outcome in Lagos, Nigeria,” in Open Forum Infectious Diseases, vol. 3. (Oxford: Oxford University Press), ofw030. doi: 10.1093/ofid/ofw030
Africa, C. W. J., Nel, J., Stemmet, M. (2014). Anaerobes and Bacterial Vaginosis in Pregnancy: Virulence Factors Contributing to Vaginal Colonisation. Int. J. Environ. Res. Public Health 11 (7), 6979–70005. doi: 10.3390/ijerph110706979
Aldunate, M., Tyssen, D., Johnson, A., Zakir, T., Sonza, S., Moench, T., et al. (2013). Vaginal Concentrations of Lactic Acid Potently Inactivate HIV. J. Antimicrob. Chemother. 68 (9), 2015–2255. doi: 10.1093/jac/dkt156
Algburi, A., Zhang, Y., Weeks, R., Comito, N., Zehm, S., Pinto, J., et al. (2017). Gemini Cationic Amphiphiles Control Biofilm Formation by Bacterial Vaginosis Pathogens. Antimicrob. Agents Chemother. 61 (12), e00650–e00175. doi: 10.1128/AAC.00650-17
Alves, P., Castro, J., Sousa, C., Cereija, T. B., Cerca, N. (2014). Gardnerella Vaginalis Outcompetes 29 Other Bacterial Species Isolated From Patients With Bacterial Vaginosis, Using in an in Vitro Biofilm Formation Model. J. Infect. Dis. 210 (4), 593–965. doi: 10.1093/infdis/jiu131
Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C. S., Eschenbach, D., Holmes, K. K. (1983). Nonspecific Vaginitis: Diagnostic Criteria and Microbial and Epidemiologic Associations. Am. J. Med. 74 (1), 14–225. doi: 10.1016/0002-9343(83)91112-9
Antonucci, F., Mirandola, W., Fontana, C., Fontana, C. (2017). Comparison Between Nugent’s and Hay/Ison Scoring Criteria for the Diagnosis of Bacterial Vaginosis in WASP Prepared Vaginal Samples. Clin. Investig. (Lond.) 7, 89–93. doi: 10.4172/Clinical-Investigation.1000116
Aslan, E., Bechelaghem, N. (2018). To ‘Douche’or Not to ‘Douche’: Hygiene Habits May Have Detrimental Effects on Vaginal Microbiota. J. Obstet. Gynaecol. 38 (5), 678–815. doi: 10.1080/01443615.2017.1395398
Babu, G., Ganvelu Singaravelu, B., Srikumar, R., Reddy, S. V. (2017). Comparative Study on the Vaginal Flora and Incidence of Asymptomatic Vaginosis Among Healthy Women and in Women With Infertility Problems of Reproductive Age. J. Clin. Diagn. Res. 11 (8), DC18. doi: 10.7860/JCDR/2017/28296.10417
Bacterial Vaginosis (2015) STD Treatment Guidelines. Available at: https://www.cdc.gov/std/tg2015/bv.html (Accessed April 14, 2018).
Bagnall, P., Rizzolo, D. (2017). Bacterial Vaginosis: A Practical Review. J. Am. Acad. Physician Assist. 30 (12), 15–215. doi: 10.1097/01.JAA.0000526770.60197.fa
Beebout, C. J., Eberly, A. R., Werby, S. H., Reasoner, S. A., Brannon, J. R., De, S., et al. (2019). Respiratory Heterogeneity Shapes Biofilm Formation and Host Colonization in Uropathogenic Escherichia Coli. MBio 10 (2), e02400–e02185. doi: 10.1128/mBio.02400-18
Bilardi, J., Walker, S., McNair, R., Mooney-Somers, J., Temple-Smith, M., Bellhouse, C., et al. (2016a). Women’s Management of Recurrent Bacterial Vaginosis and Experiences of Clinical Care: A Qualitative Study. PLoS One 11 (3), e01517945. doi: 10.1371/journal.pone.0151794
Bilardi, J., Walker, S., Mooney-Somers, J., Temple-Smith, M., McNair, R., Bellhouse, C., et al. (2016b). Women’s Views and Experiences of the Triggers for Onset of Bacterial Vaginosis and Exacerbating Factors Associated With Recurrence. PLoS One 11 (3), e01502725. doi: 10.1371/journal.pone.0150272
Bitew, A., Abebaw, Y., Bekele, D., Mihret, A. (2017). Prevalence of Bacterial Vaginosis and Associated Risk Factors Among Women Complaining of Genital Tract Infection. Int. J. Microbiol. 2017, 4919404. doi: 10.1155/2017/4919404
Borges, S., Silva, J., Teixeira, P. (2014). The Role of Lactobacilli and Probiotics in Maintaining Vaginal Health. Arch. Gynecol. Obstet. 289 (3), 479–895. doi: 10.1007/s00404-013-3064-9
Bradshaw, C. S., Brotman, R. M. (2015). Making Inroads Into Improving Treatment of Bacterial Vaginosis–Striving for Long-Term Cure. BMC Infect. Dis. 15 (1), 1–125. doi: 10.1186/s12879-015-1027-4
Bradshaw, C. S., Pirotta, M., De Guingand, D., Hocking, J. S., Morton, A. N., Garland, S. M., et al. (2012). Efficacy of Oral Metronidazole With Vaginal Clindamycin or Vaginal Probiotic for Bacterial Vaginosis: Randomised Placebo-Controlled Double-Blind Trial. PLoS One 7 (4), e345405. doi: 10.1371/journal.pone.0034540
Bradshaw, C. S., Sobel, J. D. (2016). Current Treatment of Bacterial Vaginosis—Limitations and Need for Innovation. J. Infect. Dis. 214 (suppl_1), S14–S20. doi: 10.1093/infdis/jiw159
Bradshaw, C. S., Tabrizi, S. N., Fairley, C. K., Morton, A. N., Rudland, E., Garland, S. M. (2006). The Association of Atopobium Vaginae and Gardnerella Vaginalis With Bacterial Vaginosis and Recurrence After Oral Metronidazole Therapy. J. Infect. Dis. 194 (6), 828–365. doi: 10.1086/506621
Braga, P. C., Dal Sasso, M., Culici, M., Spallino, A. (2010). Inhibitory Activity of Thymol on Native and Mature Gardnerella Vaginalis Biofilms: In Vitro Study. Arzneimittelforschung 60 (11), 675–815. doi: 10.1055/s-0031-1296346
Breding, K., Selbing, A., Farnebäck, M. (2020). Diagnosis of Bacterial Vaginosis Using a Novel Molecular Real-Time PCR Test. J. Womens Health Gynecol. 7, 1–7.
Bretelle, F., Rozenberg, P., Pascal, A., Favre, R., Bohec, C., Loundou, A., et al. (2015). High Atopobium Vaginae and Gardnerella Vaginalis Vaginal Loads Are Associated With Preterm Birth. Clin. Infect. Dis. 60 (6), 860–675. doi: 10.1093/cid/ciu966
Briselden, A. M., Moncla, B. J., Stevens, C. E., Hillier, S. L. (1992). Sialidases (Neuraminidases) in Bacterial Vaginosis and Bacterial Vaginosis-Associated Microflora. J. Clin. Microbiol. 30 (3), 663–665. doi: 10.1128/jcm.30.3.663-666.1992
Brotman, R. M. (2011). Vaginal Microbiome and Sexually Transmitted Infections: An Epidemiologic Perspective. J. Clin. Invest. 121 (12), 4610–4617. doi: 10.1172/JCI57172
Brotman, R. M., Shardell, M. D., Gajer, P., Kathleen Tracy, J., Zenilman, J. M., Ravel, J., et al. (2014). Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 210 (11), 1723–1335. doi: 10.1093/infdis/jiu330
Brown, R. G., Marchesi, J. R., Lee, Y. S., Smith, A., Lehne, B., Kindinger, L. M., et al. (2018). Vaginal Dysbiosis Increases Risk of Preterm Fetal Membrane Rupture, Neonatal Sepsis and Is Exacerbated by Erythromycin. BMC Med. 16 (1), 95. doi: 10.1186/s12916-017-0999-x
Camus, C., Penaranda, G., Khiri, H., Camiade, S., Molet, L., Lebsir, M., et al. (2021). Acceptability and Efficacy of Vaginal Self-Sampling for Genital Infection and Bacterial Vaginosis: A Cross-Sectional Study. PLoS One 16 (11), e02600215. doi: 10.1371/journal.pone.0260021
Cartwright, C. P., Lembke, B. D., Ramachandran, K., Body, B. A., Nye, M. B., Rivers, C. A., et al. (2012). Development and Validation of a Semiquantitative, Multitarget PCR Assay for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 50 (7), 2321–2295. doi: 10.1128/JCM.00506-12
Cartwright, C. P., Pherson, A. J., Harris, A. B., Clancey, M. S., Nye, M. B. (2018). Multicenter Study Establishing the Clinical Validity of a Nucleic-Acid Amplification–Based Assay for the Diagnosis of Bacterial Vaginosis. Diagn. Microbiol. Infect. Dis. 92 (3), 173–785. doi: 10.1016/j.diagmicrobio.2018.05.022
Castro, J., França, A., Bradwell, K. R., Serrano, M. G., Jefferson, K. K., Cerca, N. (2017). Comparative Transcriptomic Analysis of Gardnerella Vaginalis Biofilms vs. Planktonic Cultures Using RNA-Seq. NPJ Biofilms Microbiomes 3 (1), 1–75. doi: 10.1038/s41522-017-0012-7
Castro, J., Machado, D., Cerca, N. (2019). Unveiling the Role of Gardnerella Vaginalis in Polymicrobial Bacterial Vaginosis Biofilms: The Impact of Other Vaginal Pathogens Living as Neighbors. ISME J. 13 (5), 1306–1175. doi: 10.1038/s41396-018-0337-0
Cherpes, T. L., Hillier, S. L., Meyn, L. A., Busch, J. L., Krohn, M. A. (2008). A Delicate Balance: Risk Factors for Acquisition of Bacterial Vaginosis Include Sexual Activity, Absence of Hydrogen Peroxide-Producing Lactobacilli, Black Race, and Positive Herpes Simplex Virus Type 2 Serology. Sex. Transm. Dis. 35 (1), 78–835. doi: 10.1097/OLQ.0b013e318156a5d0
Coleman, J. S., Gaydos, C. A. (2018). Molecular Diagnosis of Bacterial Vaginosis: An Update. J. Clin. Microbiol. 56 (9), e00342-18. doi: 10.1128/JCM.00342-18
Cristiano, L., Rampello, S., Noris, C., Valota, V. (1996). Bacterial Vaginosis: Prevalence in an Italian Population of Asymptomatic Pregnant Women and Diagnostic Aspects. Eur. J. Epidemiol. 12 (4), 383–905. doi: 10.1007/BF00145302
Daliri, E. B. M., Lee, B. H. (2015). New Perspectives on Probiotics in Health and Disease. Food Sci. Hum. Wellness 4 (2), 56–65. doi: 10.1016/j.fshw.2015.06.002
Danielsson, D., Teigen, P. K., Moi, H. (2011). The Genital Econiche: Focus on Microbiota and Bacterial Vaginosis. Ann. N. Y. Acad. Sci. 1230(1), 48–58. doi: 10.1111/j.1749-6632.2011.06041.x
Deidda, F., Amoruso, A., Allesina, S., Pane, M., Graziano, T., Del Piano, M., et al. (2016). In Vitro Activity of Lactobacillus Fermentum LF5 Against Different Candida Species and Gardnerella Vaginalis: A New Perspective to Approach Mixed Vaginal Infections? J. Clin. Gastroenterol. 50, S168–S170. doi: 10.1097/MCG.0000000000000692
DeLong, K., Bensouda, S., Zulfiqar, F., Zierden, H. C., Hoang, T. M., Abraham, A. G., et al. (2019). Conceptual Design of a Universal Donor Screening Approach for Vaginal Microbiota Transplant. Front. Cell. Infect. Microbiol. 9, 306. doi: 10.3389/fcimb.2019.00306
Dessai, F., Nyikjrenda, M., Sebitloane, M., Abbai, N. (2020). Diagnostic Evaluation of the BD Affirm VPIII Assay as a Point-Of-Care Test for the Diagnosis of Bacterial Vaginosis, Trichomoniasis and Candidiasis. Int. J. STD AIDS 31 (4), 303–115. doi: 10.1177/0956462419895684
Dickey, L. J., Nailor, M. D., Sobel, J. D. (2009). Guidelines for the Treatment of Bacterial Vaginosis: Focus on Tinidazole. Ther. Clin. Risk Manage. 5, 485. doi: 10.2147/TCRM.S3777
Donders, G., Bellen, G., Donders, F., Pinget, J., Vandevelde, I., Michiels, T., et al. (2017). Improvement of Abnormal Vaginal Flora in Ugandan Women by Self-Testing and Short Use of Intravaginal Antimicrobials. Eur. J. Clin. Microbiol. Infect. Dis. 36, (4). doi: 10.1007/s10096-016-2856-9
Donders, G. G. G., Van Bulck, B., Van de Walle, P., Kaiser, R. R., Pohlig, G., Gonser, S., et al. (2010). Effect of Lyophilized Lactobacilli and 0.03 Mg Estriol (Gynoflor®) on Vaginitis and Vaginosis With Disrupted Vaginal Microflora: A Multicenter, Randomized, Single-Blind, Active-Controlled Pilot Study. Gynecol. Obstet. Invest. 70 (4), 264–725. doi: 10.1159/000314016
Donders, G. G. G., Zodzika, J., Rezeberga, D. (2014). Treatment of Bacterial Vaginosis: What We Have and What We Miss. Expert Opin. Pharmacother. 15 (5), 645–575. doi: 10.1517/14656566.2014.881800
Fenollar, F., Raoult, D. (2016). Does Bacterial Vaginosis Result From Fecal Transplantation? J. Infect. Dis. 214 (11), 17845. doi: 10.1093/infdis/jiw472
Fettweis, J. M., Brooks, J. P., Serrano, M. G., Sheth, N. U., Girerd, P. H., Edwards, D. J., et al. (2014). Differences in Vaginal Microbiome in African American Women Versus Women of European Ancestry. Microbiology 160Pt 10), 2272. doi: 10.1099/mic.0.081034-0
Forcey, D. S., Vodstrcil, L. A., Hocking, J. S., Fairley, C. K., Law, M., McNair, R. P., et al. (2015). Factors Associated With Bacterial Vaginosis Among Women Who Have Sex With Women: A Systematic Review. PLoS One 1012), e01419055. doi: 10.1371/journal.pone.0141905
Fredricks, D. N., Fiedler, T. L., Marrazzo, J. M. (2005). Molecular Identification of Bacteria Associated With Bacterial Vaginosis. N. Engl. J. Med. 353 (18), 1899–19115. doi: 10.1056/NEJMoa043802
Fredricsson, B., Englund, K., Weintraub, L., Ölund, A., Nord, C.-E. (1989). Bacterial Vaginosis Is Not a Simple Ecological Disorder. Gynecol. Obstet. Invest. 28 (3), 156–605. doi: 10.1159/000293556
Gajer, P., Brotman, R. M., Bai, G., Sakamoto, J., Schütte, U. M., Zhong, X., et al. (2012). Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 4, 132ra52. doi: 10.1126/scitranslmed.3003605
Gallo, M. F., Warner, L., King, C. C., Sobel, J. D., Klein, R. S., Cu-Uvin, S., et al. (2011). Association Between Semen Exposure and Incident Bacterial Vaginosis. Infect. Dis. Obstet. Gynecol. 2011, 842652. doi: 10.1155/2011/842652
Gardner, H. L., Dukes, C. D. (1955). Haemophilus Vaginalis Vaginitis: A Newly Defined Specific Infection Previously Classified ‘Nonspecific’ Vaginitis. Am. J. Obstet. Gynecol. 69 (5), 962–765. doi: 10.1016/0002-9378(55)90095-8
Gaydos, C. A., Beqaj, S., Schwebke, J. R., Lebed, J., Smith, B., Davis, T. E., et al. (2017). Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet. Gynecol. 130 (1), 1815. doi: 10.1097/AOG.0000000000002090
Georgijević, A., Cjukić-Ivancević, S., Bujko, M. (2000). Bacterial Vaginosis. Epidemiology and Risk Factors. Srp. Arh. Celok. Lek. 128 (1–2), 29–33.
Gilbert, N. M., Lewis, W. G., Li, G., Sojka, D. K., Bernard Lubin, J., Lewis, A. L. (2019). Gardnerella Vaginalis and Prevotella Bivia Trigger Distinct and Overlapping Phenotypes in a Mouse Model of Bacterial Vaginosis. J. Infect. Dis. 220 (7), 1099–11085. doi: 10.1093/infdis/jiy704
Gonzalez Pedraza Aviles, P., Ortiz Zaragoza, M. C., Irigoyen Coria, A. (1999). Bacterial Vaginosis a Broad Overview. Rev. Latinoam. Microbiol. Mexico 41 (1), 25–345.
Gottschick, C., Deng, Z.-L., Vital, M., Masur, C., Abels, C., Pieper, D. H., et al. (2017). Treatment of Biofilms in Bacterial Vaginosis by an Amphoteric Tenside Pessary-Clinical Study and Microbiota Analysis. Microbiome 5 (1), 1–155. doi: 10.1186/s40168-017-0326-y
Green, K. A., Zarek, S. M., Catherino, W. H. (2015). Gynecologic Health and Disease in Relation to the Microbiome of the Female Reproductive Tract. Fertil. Steril. 104 (6), 1351–1575. doi: 10.1016/j.fertnstert.2015.10.010
Guédou, F. A., Van Damme, L., Deese, J., Crucitti, T., Becker, M., Mirembe, F., et al. (2013). Behavioural and Medical Predictors of Bacterial Vaginosis Recurrence Among Female Sex Workers: Longitudinal Analysis From a Randomized Controlled Trial. BMC Infect. Dis. 131, 1–115. doi: 10.1186/1471-2334-13-208
Hallén, A., Jarstrand, C., Påhlson, C. (1992). Treatment of Bacterial Vaginosis With Lactobacilli. Sex. Transm. Dis. 19 (3), 146–148. doi: 10.1097/00007435-199205000-00007
Hardy, L., Jespers, V., Abdellati, S., De Baetselier, I., Mwambarangwe, L., Musengamana, V., et al. (2016). A Fruitful Alliance: The Synergy Between Atopobium Vaginae and Gardnerella Vaginalis in Bacterial Vaginosis-Associated Biofilm. Sex. Transm. Infect. 92 (7), 487–915. doi: 10.1136/sextrans-2015-052475
Hay, P. E. (2002). Bacterial Vaginosis as a Mixed Infection. In: Brogden, K., Guthmiller, J., editors. Polymicrobial Diseases. Washington (DC). doi: 10.1128/9781555817947.ch7
Hemalatha, R., Mastromarino, P., Ramalaxmi, B. A., Balakrishna, N. V., Sesikeran, B. (2012). Effectiveness of Vaginal Tablets Containing Lactobacilli Versus PH Tablets on Vaginal Health and Inflammatory Cytokines: A Randomized, Double-Blind Study. Eur. J. Clin. Microbiol. Infect. Dis. 31 (11), 3097–3105. doi: 10.1007/s10096-012-1671-1
Hickey, R. J., Forney, L. J. (2014). Gardnerella Vaginalis Does Not Always Cause Bacterial Vaginosis. J. Infect. Dis. 210 (10), 1682–1835. doi: 10.1093/infdis/jiu303
Hickey, R. J., Zhou, X., Pierson, J. D., Ravel, J., Forney, L. J. (2012). Understanding Vaginal Microbiome Complexity From an Ecological Perspective. Trans. Res. 160 (4), 267–825. doi: 10.1016/j.trsl.2012.02.008
Hilbert, D. W., Smith, W. L., Chadwick, S. G., Toner, G., Mordechai, E., Adelson, M. E., et al. (2016). Development and Validation of a Highly Accurate Quantitative Real-Time PCR Assay for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 54 (4), 1017–1245. doi: 10.1128/JCM.03104-15
Hogan, V. K., Culhane, J. F., Hitti, J., Rauh, V. A., McCollum, K. F., Agnew, K. J. (2007). Relative Performance of Three Methods for Diagnosing Bacterial Vaginosis During Pregnancy. Matern. Child Health J. 11 (6), 532–395. doi: 10.1007/s10995-007-0205-4
Hooven, T. A., Randis, T. M., Hymes, S. R., Rampersaud, R., Ratner, A. J. (2012). Retrocyclin Inhibits Gardnerella Vaginalis Biofilm Formation and Toxin Activity. J. Antimicrob. Chemother. 67 (12), 2870–2725. doi: 10.1093/jac/dks305
Hou, D., Zhou, X., Zhong, X., Settles, M. L., Herring, J., Wang, L., et al. (2013). Microbiota of the Seminal Fluid From Healthy and Infertile Men. Fertil. Steril. 100 (5), 1261–1695. doi: 10.1016/j.fertnstert.2013.07.1991
Huang, B., Fettweis, J. M., Brooks, J. P., Jefferson, K. K., Buck, G. A. (2014). The Changing Landscape of the Vaginal Microbiome. Clin. Lab. Med. 34 (4), 747–615. doi: 10.1016/j.cll.2014.08.006
Hummelen, R., Hemsworth, J., Reid, G. (2010). Clinical Study Comparing Probiotic Lactobacillus GR-1 and RC-14 With Metronidazole Vaginal Gel to Treat Symptomatic Bacterial Vaginosis. Nutrients 2 (6), 626. doi: 10.3390/nu2060626
Hymes, S. R., Randis, T. M., Sun, T. Y., Ratner, A. J. (2013). DNase Inhibits Gardnerella Vaginalis Biofilms In Vitro and In Vivo. J. Infect. Dis. 207 (10), 1491–1975. doi: 10.1093/infdis/jit047
Ison, C. A., Hay, P. E. (2002). Validation of a Simplified Grading of Gram Stained Vaginal Smears for Use in Genitourinary Medicine Clinics. Sex. Transm. Infect. 78 (6), 413–415. doi: 10.1136/sti.78.6.413
Kairys, N., Garg, M. (2017). Bacterial Vaginosis. In: StatPearls. (Treasure Island (FL): StatPearls Publishing); 2021.
Kenyon, C., Colebunders, R., Crucitti, T. (2013). The Global Epidemiology of Bacterial Vaginosis: A Systematic Review. Am. J. Obstet. Gynecol. 209 (6), 505–235. doi: 10.1016/j.ajog.2013.05.006
Kenyon, C. R., Osbak, K. (2014). Recent Progress in Understanding the Epidemiology of Bacterial Vaginosis. Curr. Opin. Obstet. Gynecol. 26 (6), 448–545. doi: 10.1097/GCO.0000000000000112
Kero, K., Rautava, J., Syrjänen, K., Grenman, S., Syrjänen, S. (2017). Association of Asymptomatic Bacterial Vaginosis With Persistence of Female Genital Human Papillomavirus Infection. Eur. J. Clin. Microbiol. Infect. Dis. 36 (11), 2215–2219. doi: 10.1007/s10096-017-3048-y
Khan, K., Shah, R., Gautam, M., Patil, S. (2007). Clue Cells. Indian J. Sex. Transm. Dis. 28 (2), 1085. doi: 10.4103/0253-7184.39018
Khatoon, R., Ahmad, S., Jahan, N. (2013). OSOM BV Blue Test: A New Point-Of-Care Test for Diagnosing Bacterial Vaginosis and Its Comparison With Gram Staining. Afr. J. Microbiol. Res. 7 (32), 4103–4106. doi: 10.5897/AJMR2013.5957
Khazaeian, S., Navidian, A., Navabi-Rigi, S.-d., Araban, M., Mojab, F., Khazaeian, S. (2018). Comparing the Effect of Sucrose Gel and Metronidazole Gel in Treatment of Clinical Symptoms of Bacterial Vaginosis: A Randomized Controlled Trial. Trials 19 (1), 1–85. doi: 10.1186/s13063-018-2905-z
Koumans, E. H., Sternberg, M., Bruce, C., McQuillan, G., Kendrick, J., Sutton, M., et al. (2007). The Prevalence of Bacterial Vaginosis in the United States 2001–2004; Associations With Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 34 (11), 864–695. doi: 10.1097/OLQ.0b013e318074e565
Kumar, N., Behera, B., Sagiri, S. S., Pal, K., Ray, S. S., Roy, S. (2011). Bacterial Vaginosis: Etiology and Modalities of Treatment—a Brief Note. J. Pharm. Bioallied Sci. 3 (4), 496. doi: 10.4103/0975-7406.90102
Laghi, L., Picone, G., Cruciani, F., Brigidi, P., Calanni, F., Donders, G., et al. (2014). Rifaximin Modulates the Vaginal Microbiome and Metabolome in Women Affected by Bacterial Vaginosis. Antimicrob. Agents Chemother. 58 (6), 3411–3205. doi: 10.1128/AAC.02469-14
Lepargneur, J.-P., Rousseau, V. (2002). Rôle Protecteur De La Flore De Doderleïn. J. Gynécol. Obstét. Biol. Reprod. 31 (5), 485–494.
Lewis, F. M. T., Bernstein, K. T., Aral, S. O. (2017). Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 129 (4), 6435. doi: 10.1097/AOG.0000000000001932
Li, J., McCormick, J., Bocking, A., Reid, G. (2012). Importance of Vaginal Microbes in Reproductive Health. Reprod. Sci. 19 (3), 235–425. doi: 10.1177/1933719111418379
Ling, Z., Liu, X., Chen, W., Luo, Y., Yuan, L., Xia, Y., et al. (2013). The Restoration of the Vaginal Microbiota After Treatment for Bacterial Vaginosis With Metronidazole or Probiotics. Microb. Ecol. 65 (3), 773–805. doi: 10.1007/s00248-012-0154-3
Liu, G.-j., Wang, B., Zhang, Y., Xing, G.-w., Yang, X., Wang, S. (2018). A Tetravalent Sialic Acid-Coated Tetraphenylethene Luminogen With Aggregation-Induced Emission Characteristics: Design, Synthesis and Application for Sialidase Activity Assay, High-Throughput Screening of Sialidase Inhibitors and Diagnosis of Bacterial Va. Chem. Commun. 54 (76), 10691–10945. doi: 10.1039/C8CC06300A
Machado, D., Castro, J., Palmeira-de-Oliveira, A., Martinez-de-Oliveira, J., Cerca, N. (2016). Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 6, 1528. doi: 10.3389/fmicb.2015.01528
Machado, A., Cerca, N. (2015). Influence of Biofilm Formation by Gardnerella Vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 212 (12), 1856–1615. doi: 10.1093/infdis/jiv338
Madhivanan, P., Krupp, K., Li, T., Ravi, K., Selezneva, J., Srinivas, V., et al. (2014). Performance of BVBlue Rapid Test in Detecting Bacterial Vaginosis Among Women in Mysore, India. Infect. Dis. Obstet. Gynecol. 2014, 908313. doi: 10.1155/2014/908313
Majigo, M. V., Kashindye, P., Mtulo, Z. (2021). Bacterial Vaginosis, the Leading Cause of Genital Discharge Among Women Presenting With Vaginal Infection in Dar Es Salaam, Tanzania. Afr. Health Sci. 21 (2), 531–375. doi: 10.4314/ahs.v21i2.7
Makarova, K., Slesarev, A., Wolf, Y., Sorokin, A., Mirkin, B., Koonin, E., et al. (2006). Comparative Genomics of the Lactic Acid Bacteria. Proc. Natl. Acad. Sci. 103 (42), 15611–15165. doi: 10.1073/pnas.0607117103
Mårdh, P. A. (1993). The Definition and Epidemiology of Bacterial Vaginosis. Rev. Fr. Gynecol. Obstet. 88 (3 Pt 2), 195–197.
Margolis, E., Fredricks, D. N. (2015). “Bacterial Vaginosis-Associated Bacteria,” in Molecular Medical Microbiology (Bonston: Elsevier), 1487–1496.
Marrazzo, J. M., Fiedler, T. L., Srinivasan, S., Thomas, K. K., Liu, C., Ko, D., et al. (2012). Extravaginal Reservoirs of Vaginal Bacteria as Risk Factors for Incident Bacterial Vaginosis. J. Infect. Dis. 205 (10), 1580–1885. doi: 10.1093/infdis/jis242
Marrazzo, J. M., Hillier, S. L. (2013). Bacterial Vaginosis. Sex. Transm. Dis., 463–498. doi: 10.1016/B978-0-12-391059-2.00018-8
Marrazzo, J. M., Thomas, K. K., Fiedler, T. L., Ringwood, K., Fredricks, D. N. (2008). Relationship of Specific Vaginal Bacteria and Bacterial Vaginosis Treatment Failure in Women Who Have Sex With Women. Ann. Intern. Med. 149 (1), 20–285. doi: 10.7326/0003-4819-149-1-200807010-00006
Mastromarino, P., Macchia, S., Meggiorini, L., Trinchieri, V., Mosca, L., Perluigi, M., et al. (2009). Effectiveness of Lactobacillus-Containing Vaginal Tablets in the Treatment of Symptomatic Bacterial Vaginosis. Clin. Microbiol. Infect. 15 (1), 67–74. doi: 10.1111/j.1469-0691.2008.02112.x
McClelland, R. S., Lingappa, J. R., Srinivasan, S., Kinuthia, J., John-Stewart, G. C., Jaoko, W., et al. (2018). Evaluation of the Association Between the Concentrations of Key Vaginal Bacteria and the Increased Risk of HIV Acquisition in African Women From Five Cohorts: A Nested Case-Control Study. Lancet Infect. Dis. 18 (5), 554–645. doi: 10.1016/S1473-3099(18)30058-6
Menard, J. P., Fenollar, F., Henry, M., Bretelle, F., Raoult, D. (2008). Molecular Quantification of Gardnerella Vaginalis and Atopobium Vaginae Loads to Predict Bacterial Vaginosis. Clin. Infect. Dis. 47 (1), 33–435. doi: 10.1086/588661
Menard, J.-P., Fenollar, F., Raoult, D., Boubli, L., Bretelle, F. (2012). Self-Collected Vaginal Swabs for the Quantitative Real-Time Polymerase Chain Reaction Assay of Atopobium Vaginae and Gardnerella Vaginalis and the Diagnosis of Bacterial Vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 31 (4), 513–185. doi: 10.1007/s10096-011-1341-8
Mendling, W. (2016). Vaginal Microbiota. In: Schwiertz, A. (eds). Microbiota of the Human Body. Advances in Experimental Medicine and Biology. Cham: Springer, vol. 902, p. 83–93. doi: 10.1007/978-3-319-31248-4_6
Money, D. (2005). The Laboratory Diagnosis of Bacterial Vaginosis. Can. J. Infect. Dis. Med. Microbiol. 16 (2), 77–79. doi: 10.1155/2005/230319
Morris, M. C., Rogers, P. A., Kinghorn, G. R. (2001). Is Bacterial Vaginosis a Sexually Transmitted Infection? Sex. Transm. Infect. 77 (1), 63–68. doi: 10.1136/sti.77.1.63
Muhleisen, A. \. L., Herbst-Kralovetz, M. M. (2016). Menopause and the Vaginal Microbiome. Maturitas 91, 42–50. doi: 10.1016/j.maturitas.2016.05.015
Muzny, C. A., Blanchard, E., Taylor, C. M., Aaron, K. J., Talluri, R., Griswold, M. E., et al. (2018). Identification of Key Bacteria Involved in the Induction of Incident Bacterial Vaginosis: A Prospective Study. J. Infect. Dis. 218 (6), 966–785. doi: 10.1093/infdis/jiy243
Muzny, C. A., Sunesara, I. R., Austin, E. L., Mena, L. A., Schwebke, J. R. (2013). Bacterial Vaginosis Among African American Women Who Have Sex With Women. Sex. Transm. Dis. 40 (9), 751–555. doi: 10.1097/OLQ.0000000000000004
Muzny, C. A., Taylor, C. M., Swords, W. E., Tamhane, A., Chattopadhyay, D., Cerca, N., et al. (2019). An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 220 (9), 1399–14055. doi: 10.1093/infdis/jiz342
Myziuk, L., Romanowski, B., Johnson, S. C. (2003). BVBlue Test for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 41 (5), 1925–1285. doi: 10.1128/JCM.41.5.1925-1928.2003
Nardis, C., Mosca, L., Mastromarino, P. (2013). Vaginal Microbiota and Viral Sexually Transmitted Diseases. Ann. Ig. 25 (5), 443–456. doi: 10.7416/ai.2013.1946
Nasioudis, D., Linhares, I. M., Ledger, W. J., Witkin, S. S. (2017). Bacterial Vaginosis: A Critical Analysis of Current Knowledge. BJOG 124 (1), 61–695. doi: 10.1111/1471-0528.14209
Nelson, T. M., Borgogna, J. C., Michalek, R. D., Roberts, D. W., Rath, J. M., Glover, E. D., et al. (2018). Cigarette Smoking Is Associated With an Altered Vaginal Tract Metabolomic Profile. Sci. Rep. 8 (1), 1–135. doi: 10.1038/s41598-017-14943-3
Nelson, D. B., Hanlon, A. L., Wu, G., Liu, C., Fredricks, D. N. (2015a). First Trimester Levels of BV-Associated Bacteria and Risk of Miscarriage Among Women Early in Pregnancy. Matern. Child Health J. 19 (12), 2682–2875. doi: 10.1007/s10995-015-1790-2
Nugent, R. P., Krohn, M. A., Hillier, S. L. (1991). Reliability of Diagnosing Bacterial Vaginosis Is Improved by a Standardized Method of Gram Stain Interpretation. J. Clin. Microbiol. 29 (2), 297–3015. doi: 10.1128/jcm.29.2.297-301.1991
Nuriel-Ohayon, M., Neuman, H., Koren, O. (2016). Microbial Changes During Pregnancy, Birth, and Infancy. Front. Microbiol. 7, 1031. doi: 10.3389/fmicb.2016.01031
O’Hanlon, D. E., Moench, T. R., Cone, R. A. (2013). Vaginal PH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS One 8 (11), e800745. doi: 10.1371/journal.pone.0080074
Onderdonk, A. B., Delaney, M. L., Fichorova, R. N. (2016). The Human Microbiome During Bacterial Vaginosis. Clin. Microbiol. Rev. 29 (2), 223–385. doi: 10.1128/CMR.00075-15
Parent, D., Bossens, M., Bayot, D., Kirkpatrick, C., Graf, F., Wilkinson, F. E., et al. (1996). Therapy of Bacterial Vaginosis Using Exogenously-Applied Lactobacilli Acidophili and a Low Dose of Estriol: A Placebo-Controlled Multicentric Clinical Trial. Arzneimittelforschung 46 (1), 68–735.
Plummer, E. L., Vodstrcil, L. A., Doyle, M., Danielewski, J. A., Murray, G. L., Fehler, G., et al. (2021). A Prospective, Open-Label Pilot Study of Concurrent Male Partner Treatment for Bacterial Vaginosis. Mbio 12 (5), e02323–e02215. doi: 10.1128/mBio.02323-21
Qian, Z., Zhu, H., Zhao, D., Yang, P., Gao, F., Lu, C., et al. (2021). Probiotic Lactobacillus Sp. Strains Inhibit Growth, Adhesion, Biofilm Formation, and Gene Expression of Bacterial Vaginosis-Inducing Gardnerella Vaginalis. Microorganisms 9 (4), 728. doi: 10.3390/microorganisms9040728
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S. (2011). Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. U. S. A. 108, 4680–4687. doi: 10.1073/pnas.1002611107
Redelinghuys, M. J., Ehlers, M. M., Bezuidenhoudt, J. E., Becker, P. J., Kock, M. M. (2017). Assessment of Atopobium Vaginae and Gardnerella Vaginalis Concentrations in a Cohort of Pregnant South African Women. Sex. Transm. Infect. 93 (6), 410–55. doi: 10.1136/sextrans-2016-052883
Reid (2018). Is Bacterial Vaginosis a Disease? Appl. Microbiol. Biotechnol. 102 (2), 553–558. doi: 10.1007/s00253-017-8659-9
Rodríguez-Nava, C., Cortés-Sarabia, K., Avila-Huerta, M. D., Ortiz-Riaño, E. J., Estrada-Moreno, A. K., del C Alarcón-Romero, L., et al. (2021). Nanophotonic Sialidase Immunoassay for Bacterial Vaginosis Diagnosis. ACS Pharmacol. Trans. Sci. 4 (1), 365–715. doi: 10.1021/acsptsci.0c00211
Romero, R., Hassan, S. S., Gajer, P., Tarca, A. L., Fadrosh, D. W., Nikita, L., et al. (2014). The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different From That of Non-Pregnant Women. Microbiome 2, 1–19. doi: 10.1186/2049-2618-2-4
Rousseau, V., Lepargneur, J. P., Roques, C., Remaud-Simeon, M., Paul, F. (2005). Prebiotic Effects of Oligosaccharides on Selected Vaginal Lactobacilli and Pathogenic Microorganisms. Anaerobe 11 (3), 145–153. doi: 10.1016/j.anaerobe.2004.12.002
Rumyantseva, T., Khayrullina, G., Guschin, A., Donders, G. (2019). Prevalence of Ureaplasma Spp. And Mycoplasma Hominis in Healthy Women and Patients With Flora Alterations. Diagn. Microbiol. Infect. Dis. 93 (3), 227–315. doi: 10.1016/j.diagmicrobio.2018.10.001
Schellenberg, J. J., Jayaprakash, T. P., Gamage, N. W., Patterson, M. H., Vaneechoutte, M., Hill, J. E. (2016). Gardnerella Vaginalis Subgroups Defined by Cpn 60 Sequencing and Sialidase Activity in Isolates From Canada, Belgium and Kenya. PLoS One 11 (1), e01465105. doi: 10.1371/journal.pone.0146510
Schwebke, J. R., Desmond, R. A. (2007). A Randomized Trial of the Duration of Therapy With Metronidazole Plus or Minus Azithromycin for Treatment of Symptomatic Bacterial Vaginosis. Clin. Infect. Dis. 44 (2), 213–219. doi: 10.1086/509577
Schwebke, J. R., Lensing, S. Y., Lee, J., Muzny, C. A., Pontius, A., Woznicki, N., et al. (2021). Treatment of Male Sexual Partners of Women With Bacterial Vaginosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 73 (3), e672–e795. doi: 10.1093/cid/ciaa1903
Sgibnev, A. V., Kremleva, E. A. (2015). Vaginal Protection by H2O2-Producing Lactobacilli. Jundishapur J. Microbiol. 8 (10), e22913–e22913. doi: 10.5812/jjm.22913
Sharma, H., Tal, R., Clark, N. A., Segars, J. H. (2014). Microbiota and Pelvic Inflammatory Disease. in Seminars in Reproductive Medicine (New York: Thieme Medical Publishers), 43–49. doi: 10.1055/s-0033-1361822
Sherrard, J., Wilson, J., Donders, G., Mendling, W., Jensen, J. S. (2018). 2018 European (IUSTI/WHO) International Union Against Sexually Transmitted Infections (IUSTI) World Health Organisation (WHO) Guideline on the Management of Vaginal Discharge. Int. J. STD AIDS 29 (13), 1258–1725. doi: 10.1177/0956462418785451
Shipitsyna, E., Khusnutdinova, T., Budilovskaya, O., Krysanova, A., Shalepo, K., Savicheva, A., et al. (2020). Bacterial Vaginosis-Associated Vaginal Microbiota Is an Age-Independent Risk Factor for Chlamydia Trachomatis, Mycoplasma Genitalium and Trichomonas Vaginalis Infections in Low-Risk Women, St. Petersburg, Russia. Eur. J. Clin. Microbiol. Infect. Dis. 39 (7), 1221–1305. doi: 10.1007/s10096-020-03831-w
Shujatullah, F., Khan, H. M., Khatoon, R., Rabbani, T., Malik, A. (2010). An Evaluation of OSOM BV Blue Test in the Diagnosis of Bacterial Vaginosis. Asian Pac. J. Trop. Med. 3 (7), 574–765. doi: 10.1016/S1995-7645(10)60139-3
Sobel, J. D. (2017). “Vaginitis, Vulvitis, Cervicitis and Cutaneous Vulval Lesions,” in Infectious Diseases (St. Louis: Elsevier), 483–491.
Stoyancheva, G., Marzotto, M., Dellaglio, F., Torriani, S. (2014). Bacteriocin Production and Gene Sequencing Analysis From Vaginal Lactobacillus Strains. Arch. Microbiol. 196 (9), 645–535. doi: 10.1007/s00203-014-1003-1
Svare, J. A., Schmidt, H., Hansen, B. B., Lose, G. (2006). Bacterial Vaginosis in a Cohort of Danish Pregnant Women: Prevalence and Relationship With Preterm Delivery, Low Birthweight and Perinatal Infections. BJOG 113 (12), 1419–1255. doi: 10.1111/j.1471-0528.2006.01087.x
Swidsinski, A., Doerffel, Y., Loening-Baucke, V., Swidsinski, S., Verstraelen, H., Vaneechoutte, M., et al. (2010a). Gardnerella Biofilm Involves Females and Males and Is Transmitted Sexually. Gynecol. Obstet. Invest. 70 (4), 256–635. doi: 10.1159/000314015
Swidsinski, A., Dörffel, Y., Loening-Baucke, V., Mendling, W., Verstraelen, H., Dieterle, S., et al. (2010b). Desquamated Epithelial Cells Covered With a Polymicrobial Biofilm Typical for Bacterial Vaginosis Are Present in Randomly Selected Cryopreserved Donor Semen. FEMS Immunol. Med. Microbiol. 59 (3), 399–4045. doi: 10.1111/j.1574-695X.2010.00688.x
Tachedjian, G., Aldunate, M., Bradshaw, C. S., Cone, R. A. (2017). The Role of Lactic Acid Production by Probiotic Lactobacillus Species in Vaginal Health. Res. Microbiol. 168 (9–10), 782–792. doi: 10.1016/j.resmic.2017.04.001
Thulkar, J., Kriplani, A., Agarwal, N. (2012). A Comparative Study of Oral Single Dose of Metronidazole, Tinidazole, Secnidazole and Ornidazole in Bacterial Vaginosis. Indian J. Pharmacol. 44 (2), 2435. doi: 10.4103/0253-7613.93859
Tidbury, F. D., Langhart, A., Weidlinger, S., Stute, P. (2020). Non-Antibiotic Treatment of Bacterial Vaginosis—a Systematic Review. Arch. Gynecol. Obstet. 303, 1–9. doi: 10.1007/s00404-020-05821-x
Trabert, B., Misra, D. P. (2007). Risk Factors for Bacterial Vaginosis During Pregnancy Among African American Women. Am. J. Obstet. Gynecol. 197 (5), 477–e15. doi: 10.1016/j.ajog.2007.03.085
Vaneechoutte, M., Guschin, A., Van Simaey, L., Gansemans, Y., Van Nieuwerburgh, F., Cools, P. (2019). Emended Description of Gardnerella Vaginalis and Description of Gardnerella Leopoldii Sp. Nov., Gardnerella Piotii Sp. Nov. And Gardnerella Swidsinskii Sp. Nov., With Delineation of 13 Genomic Species Within the Genus Gardnerella. Int. J. Syst. Evol. Microbiol. 69 (3), 679–687. doi: 10.1099/ijsem.0.003200
van Oostrum, N., De Sutter, P., Meys, J., Verstraelen, H. (2013). Risks Associated With Bacterial Vaginosis in Infertility Patients: A Systematic Review and Meta-Analysis. Hum. Reprod. 28 (7), 1809–1155. doi: 10.1093/humrep/det096
Verstraelen, H., Verhelst, R., Vaneechoutte, M., Temmerman, M. (2010). The Epidemiology of Bacterial Vaginosis in Relation to Sexual Behaviour. BMC Infect. Dis. 10 (1), 1–115. doi: 10.1186/1471-2334-10-81
Vicariotto, F., Mogna, L., Del Piano, M. (2014). Effectiveness of the Two Microorganisms Lactobacillus Fermentum LF15 and Lactobacillus Plantarum LP01, Formulated in Slow-Release Vaginal Tablets, in Women Affected by Bacterial Vaginosis: A Pilot Study. J. Clin. Gastroenterol. 48, S106–S112. doi: 10.1097/MCG.0000000000000226
Vujic, G., Knez, A. J., Despot Stefanovic, V., Kuzmic Vrbanovic, V. (2013). Efficacy of Orally Applied Probiotic Capsules for Bacterial Vaginosis and Other Vaginal Infections: A Double-Blind, Randomized, Placebo-Controlled Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 168 (1), 75–795. doi: 10.1016/j.ejogrb.2012.12.031
Wang, Z., Zhang, L., Zhao, M., Wang, Y., Bai, H., Wang, Y., et al. (2020). Deep Neural Networks Offer Morphologic Classification and Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 59 (2), e02236–20. doi: 10.1128/JCM.02236-20
Weissenbacher, E. R., Donders, G., Unzeitig, V., Martinez De Tejada, B., Gerber, S., Halaška, M., et al. (2012). A Comparison of Dequalinium Chloride Vaginal Tablets (Fluomizin®) and Clindamycin Vaginal Cream in the Treatment of Bacterial Vaginosis: A Single-Blind, Randomized Clinical Trial of Efficacy and Safety. Gynecol. Obstet. Invest. 73 (1), 8–155. doi: 10.1159/000332398
Wessels, J. M., Lajoie, J., Vitali, D., Omollo, K., Kimani, J., Oyugi, J., et al. (2017). Association of High-Risk Sexual Behaviour With Diversity of the Vaginal Microbiota and Abundance of Lactobacillus. PLoS One 12 (11), e01876125. doi: 10.1371/journal.pone.0187612
Wewalka, G., Stary, A., Bosse, B., Duerr, H. E., Reimer, K. (2002). Efficacy of Povidone-Iodine Vaginal Suppositories in the Treatment of Bacterial Vaginosis. Dermatology 204 (Suppl. 1), 79–85. doi: 10.1159/000057731
Wiesenfeld, H. C., Hillier, S. L., Krohn, M. A., Landers, D. V., Sweet, R. L. (2003). Bacterial Vaginosis Is a Strong Predictor of Neisseria Gonorrhoeae and Chlamydia Trachomatis Infection. Clin. Infect. Dis. 36 (5), 663–685. doi: 10.1086/367658
Wilson, B. A., Thomas, S. M., Ho, M. (2011). “The Human Vaginal Microbiome,” in Metagenomics of the Human Body (New York: Springer), 91–115.
Witkin, S. S. (2015). The Vaginal Microbiome, Vaginal Anti-Microbial Defence Mechanisms and the Clinical Challenge of Reducing Infection-Related Preterm Birth. BJOG 122 (2), 213–218. doi: 10.1111/1471-0528.13115
World Health Organization (2021). Guidelines for the Management of Symptomatic Sexually Transmitted Infections. (Geneva, Switerzerland: World Health Organization).
Keywords: vaginal microbiome, Lactobacillus, dysbiosis, bacterial vaginosis, sexually transmitted infection, bacterial vaginosis-associated bacteria
Citation: Abou Chacra L, Fenollar F and Diop K (2022) Bacterial Vaginosis: What Do We Currently Know? Front. Cell. Infect. Microbiol. 11:672429. doi: 10.3389/fcimb.2021.672429
Received: 25 February 2021; Accepted: 17 December 2021;
Published: 18 January 2022.
Edited by:
Mariya Ivanova Petrova, KU Leuven, BelgiumReviewed by:
Jack David Sobel, Wayne State University, United StatesNuno Cerca, University of Minho, Portugal
Copyright © 2022 Abou Chacra, Fenollar and Diop. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khoudia Diop, khouj_diop@yahoo.fr
 Linda Abou Chacra
Linda Abou Chacra Florence Fenollar
Florence Fenollar Khoudia Diop
Khoudia Diop